Influence of Alloy Elements on Cracking in the Steel Ingot during Its Solidification
Abstract
1. Introduction
2. Calculation
3. Results and Discussion
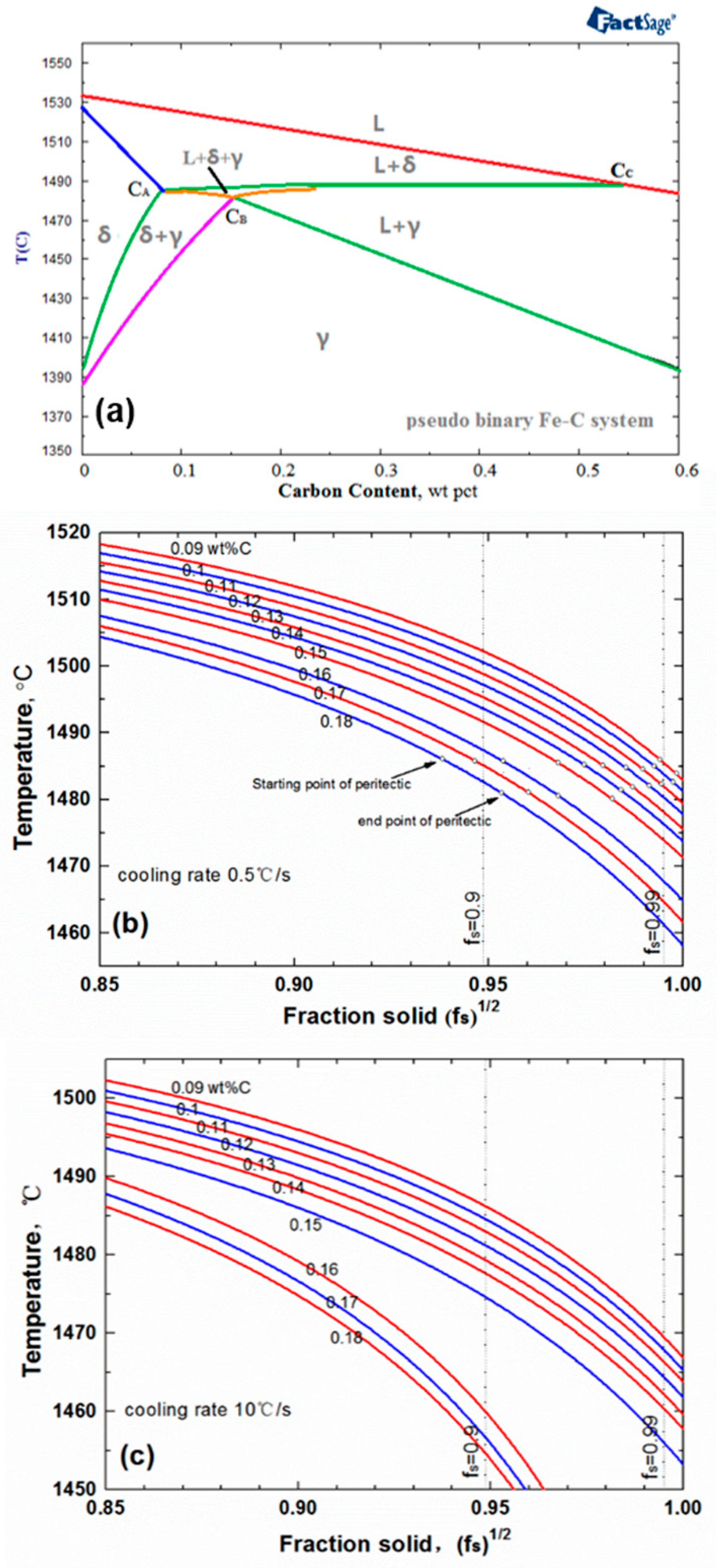
3.1. Carbon Contents
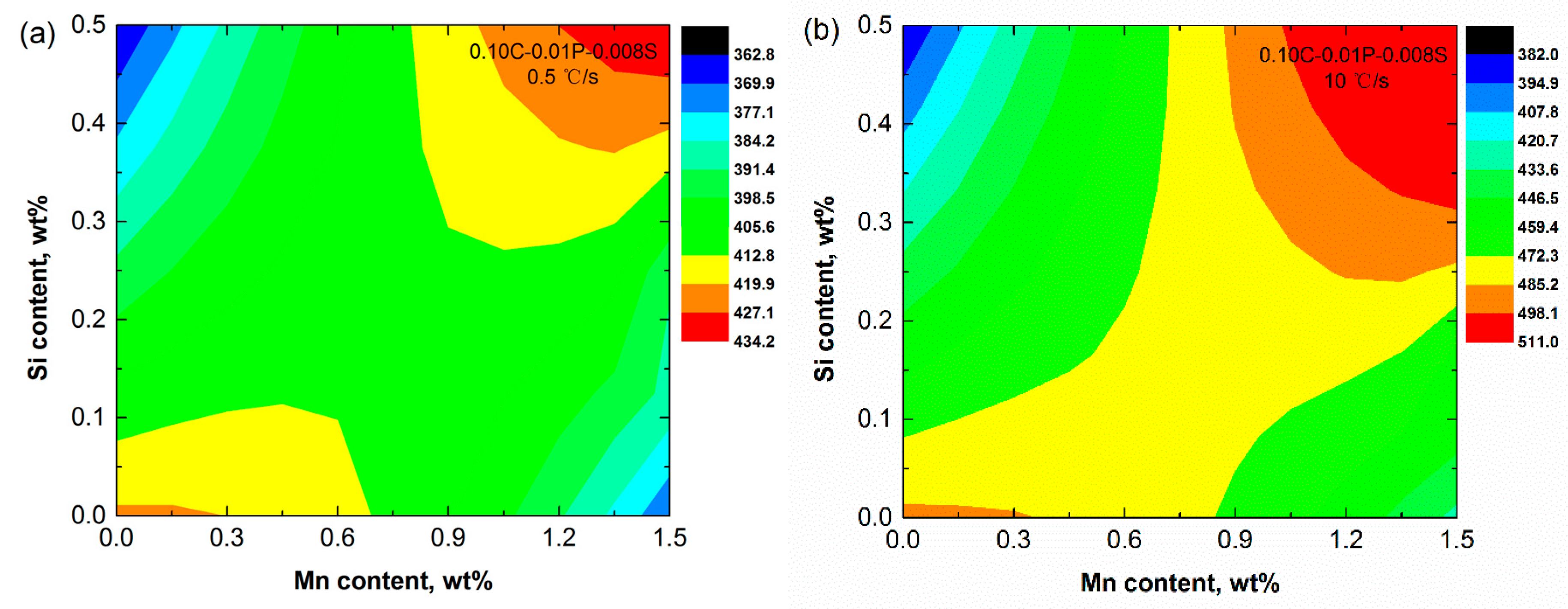
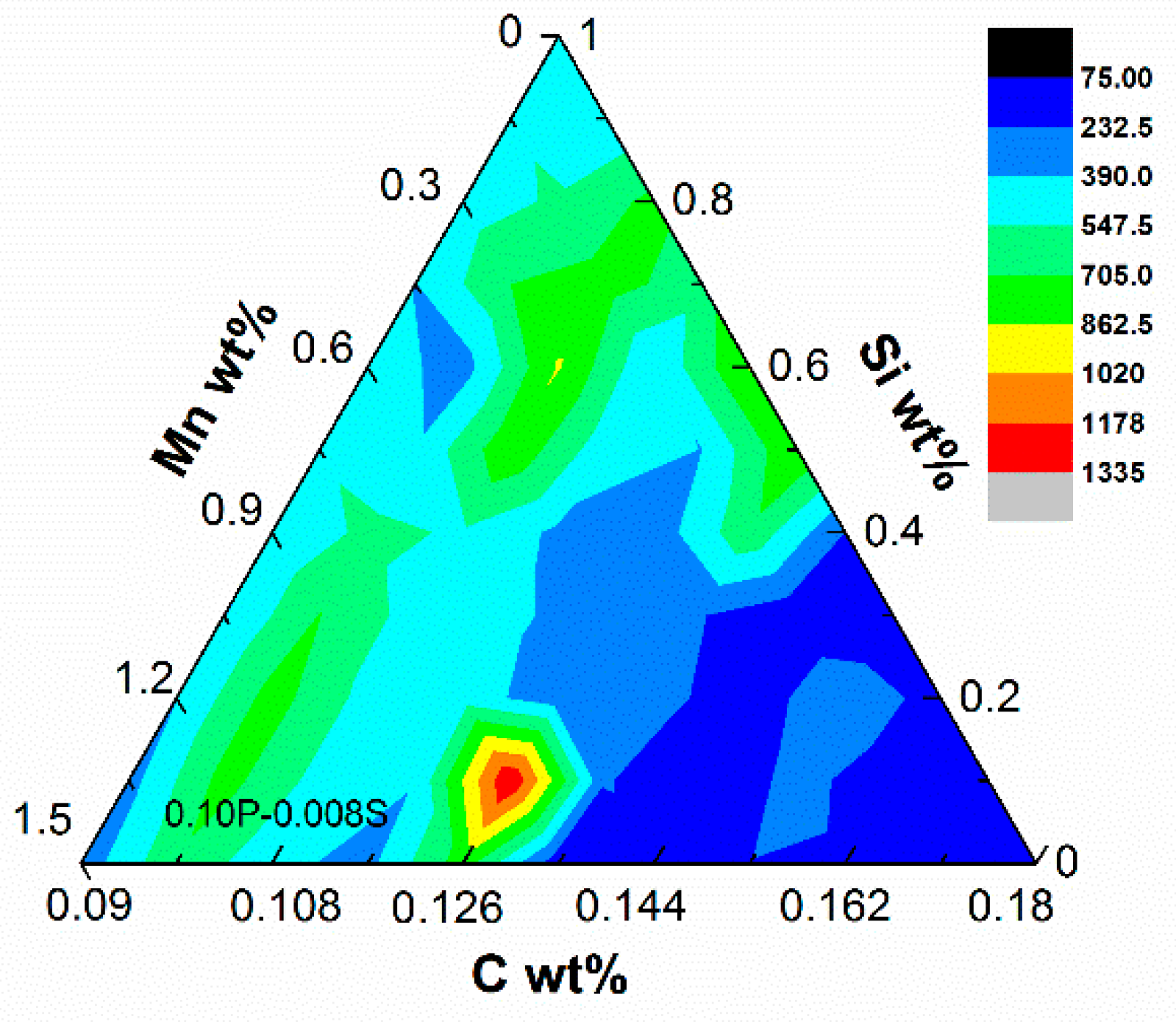
3.2. Mn and Si
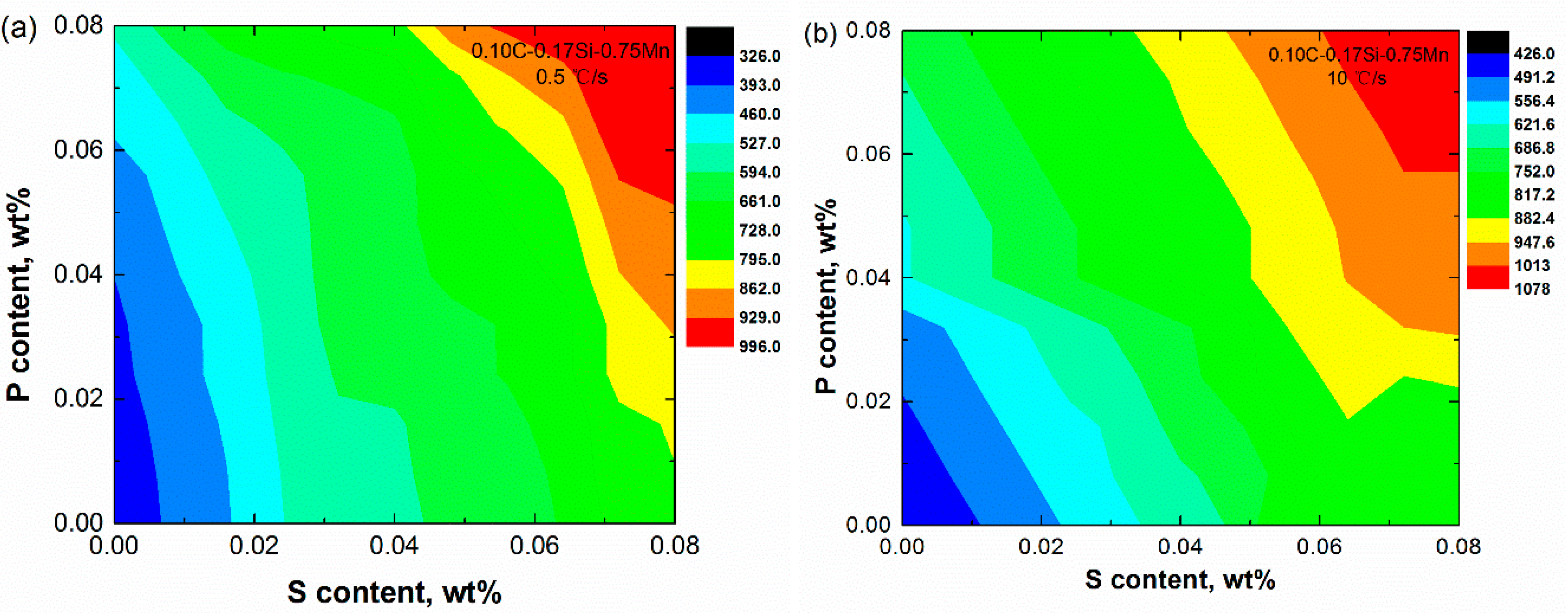
3.3. P and S
4. Conclusions
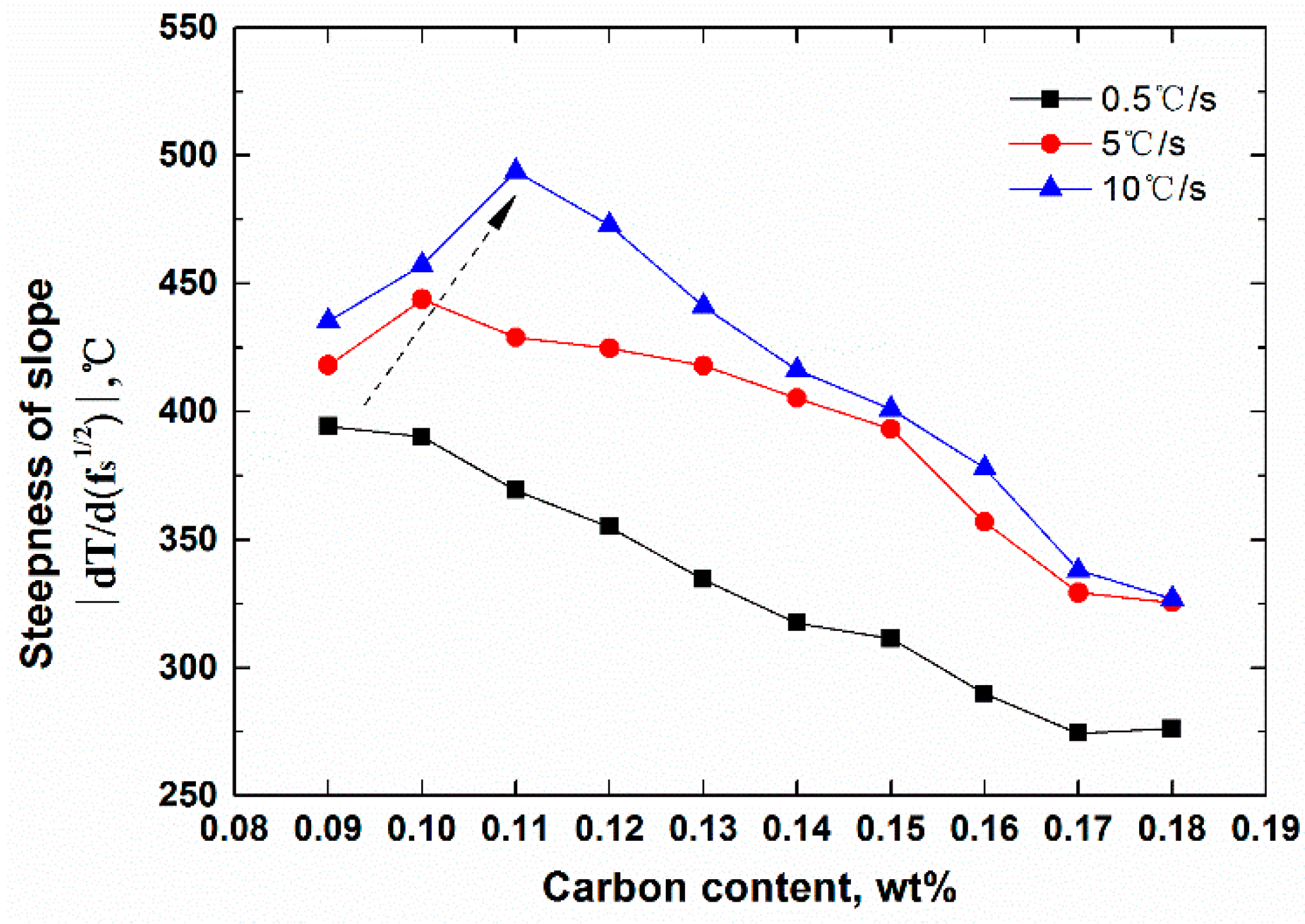
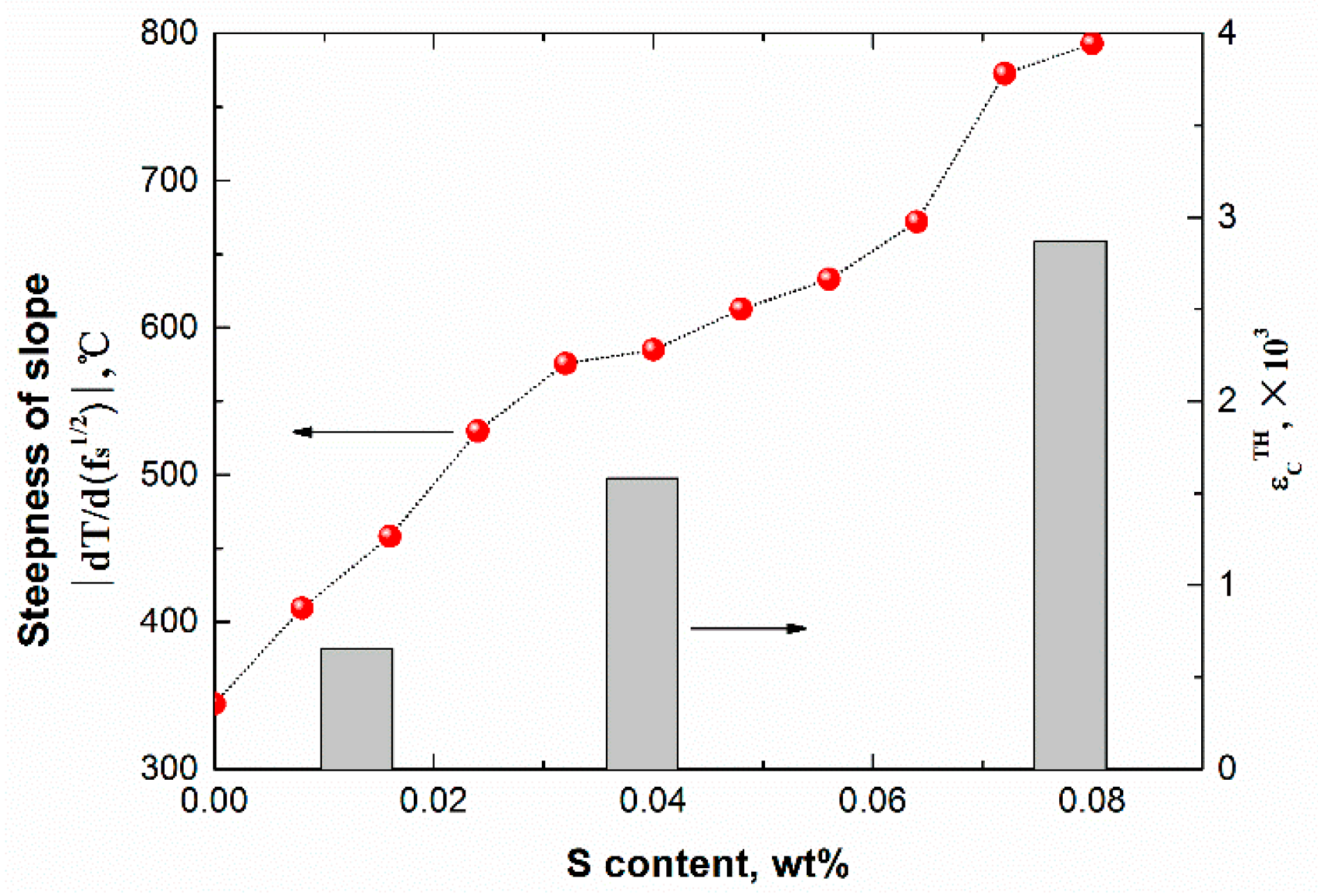
- The average steepness of |dT/d(fs)1/2| on the T − (fs)1/2 curve during peritectic solidification was proposed to estimate the solidification crack susceptibility for peritectic steels, which mainly reflected the capacity of liquid flow to compensate shrinkage during peritectic solidification. The cooling rate and peritectic solidification were considered.
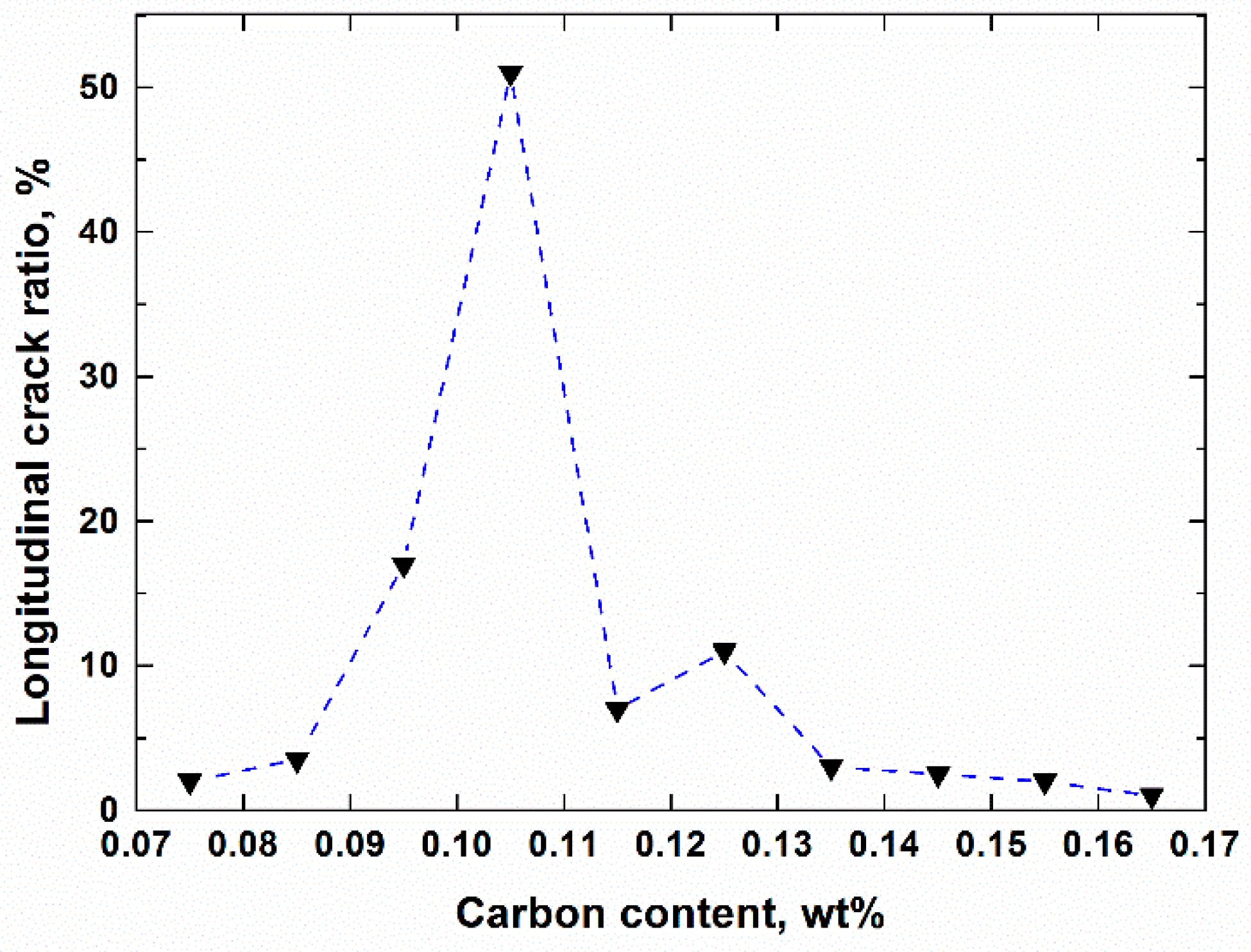
- The crack susceptibility index was calculated for steels with varying C contents at three cooling rates. The results indicate that the maximum level susceptibility changed from 0.09C wt.% to 0.11C wt.% with the increase of cooling rate. All of the |dT/d(fs)1/2| increased with increasing cooling rate, and the calculation index was verified compared with the date of industrial practice.
- The effects of Mn and Si elements on solidification crack susceptibility were calculated. Under the cooling rate of 10 °C/s, the crack susceptibility index was the highest at 0.5%Si–1.2%Mn: 511.0 °C. Regarding the crack susceptibility index in the ternary diagram (C–Si–Mn), the highest crack susceptibility was near 0.126%C–1.35%Mn–0.1%Si, 1334.7 °C. The effect of P and S elements on solidification crack susceptibility was also calculated. The crack susceptibility index was the highest at 0.08%P–0.08%S: 1076.0 °C. This method of estimating the crack susceptibility by the average steepness |dT/d(fs)1/2| of the T − (fs)1/2 curve during solidification may prove very helpful for the casting guidance of steels.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fredriksson, H. The mechanism of the peritectic reaction in iron-base alloys. Met. Sci. 1976, 10, 77–86. [Google Scholar] [CrossRef]
- Yang, G.; Zhu, L.; Chen, W.; Yu, X. He, B. Initiation of surface cracks on beam blank in the mold during continuous casting. Metals 2018, 8, 712. [Google Scholar] [CrossRef]
- Saleem, S.; Vynnycky, M.; Fredriksson, H. The influence of peritectic reaction/transformation on crack susceptibility in the continuous casting of steels. Metall. Mater. Trans. B 2017, 48, 1625–1635. [Google Scholar] [CrossRef]
- Trejo, M.H.; Lopez, E.A.; Mondragon, J.J.R.; Roman, M.J.C.; Tovar, H.S. Effect of solidification path and contraction on the cracking susceptibility of carbon peritectic steels. Met. Mater. Int. 2010, 16, 731–737. [Google Scholar] [CrossRef]
- Hechu, K.; Slater, C.; Santillana, B.; Srirangam, P.; Sridhar, S. Real-time measurement of contraction behaviour of peritectic steels during solidification. In Proceedings of the AISTech-2016: Proceedings of the Iron and Steel Technology Conference, Pittsburgh, PA, USA, 16–19 May 2016; pp. 132–138. [Google Scholar]
- Emi, T.; Fredriksson, H. High-speed continuous casting of peritectic carbon steels. Mater. Sci. Eng. A 2005, 413, 2–9. [Google Scholar] [CrossRef]
- Lopez, E.A.; Trejo, M.H.; Mondragon, J.J.R.; Roman, M.D.J.C.; Tovar, H.S. Effect of C and Mn variations upon the solidification mode and surface cracking susceptibility of peritectic steels. ISIJ Int. 2009, 49, 851–858. [Google Scholar] [CrossRef][Green Version]
- Ridolfi, M.R.; Vito, A.D.; Ferro, L. Effect of alloying elements on thermal contraction and crack susceptibility during in-mold solidification. Metal. Mater. Trans. B 2008, 39, 581–592. [Google Scholar] [CrossRef]
- Kerr, H.W.; Cisse, J.; Bolling, G.F. On equilibrium and non-equilibrium peritectic transformations. Acta Metall. 1974, 22, 677–686. [Google Scholar] [CrossRef]
- Wołczyński, W. Back-diffusion in crystal growth. Peritectics. Arch. Metall. Mater. 2015, 60, 2409–2414. [Google Scholar] [CrossRef]
- Dippenaar, R. In-Situ Analysis of the Peritectic Phase Transition-Relevant to the Continuous Casting of Steel. In Proceedings of the 5th International Congress on the Science and Technology of the Steelmaking, Dresden, Germany, 1–3 October 2012; pp. 1–9. [Google Scholar]
- Feurer, U. Quality control of engineering alloys and the role of metals science. Ph.D. Thesis, Delft University of Technology, Delft, The Netherlands, 1977. [Google Scholar]
- Clyne, T.W.; Wolf, M.; Kurz, W. The effect of melt composition on solidification cracking of steel, with particular reference to continuous casting. Metall. Trans. B 1982, 13B, 259–266. [Google Scholar] [CrossRef]
- Rappaz, M.; Drezet, J.M.; Gremaud, M. A new hot-tearing criterion. Metal. Mater. Trans. A 1999, 30, 449–455. [Google Scholar] [CrossRef]
- Kou, S. A criterion for cracking during solidification. Acta Mater. 2015, 88, 366–374. [Google Scholar] [CrossRef]
- Kou, S. A simple index for predicting the susceptibility to solidification cracking. Weld. J. 2015, 94, 374–388. [Google Scholar]
- Won, Y.M.; Yeo, T.J.; Seol, D.J.; Oh, K.H. A new criterion for internal crack formation in continuously cast steels. Metal. Mater. Trans. B 2000, 31, 779–794. [Google Scholar] [CrossRef]
- Asai, S. Transport Phenomena in Materials Processing, Electromagnetic Processing of Materials; Springer: Dordrecht, The Netherlands, 2012; pp. 321–329. [Google Scholar]
- Ueshima, Y.; Mizoguchi, S.; Matsumiya, T.; Kajioka, H. Analysis of solute distribution in dendrites of carbon steel with δ/γ transformation during solidification. Metall. Trans. B 1986, 17, 845–859. [Google Scholar] [CrossRef]
- Kim, K.H.; Yeo, T.J.; Oh, K.H.; Lee, D.N. Effect of carbon and sulfur in continuously cast strand on longitudinal surface cracks. ISIJ Int. 1996, 36, 284–289. [Google Scholar] [CrossRef]
- El-Bealy, M.; Thomas, B.G. Prediction of dendrite arm spacing for low alloy steel casting processes. Metal. Mater. Trans. B 1996, 27, 689–693. [Google Scholar] [CrossRef]
- Harste, K.; Schwerdtfeger, K. Shrinkage of round iron-carbon ingots during solidification and subsequent cooling. ISIJ Int. 2007, 43, 1011–1020. [Google Scholar] [CrossRef][Green Version]
- Bernhard, C.; Xia, G. Influence of alloying elements on the thermal contraction of peritectic steels during initial solidification. Ironmak. Steelmak. 2006, 33, 52–56. [Google Scholar] [CrossRef]
- Wolf, M.M. Mold Heat Transfer and Lubrication Control-Two Major Functions of Caster Productivity and Quality Assurance. In Proceedings of the 13th Process Technology Conference Proceedings, Nashvile, TN, USA, 2–5 April 1995; pp. 99–117. [Google Scholar]
- Xia, G.; Narzt, H.P.; Fürst, C.; Mörwald, K.; Moertl, J.; Reisinger, P.; Lindenberger, L. Investigation of mould thermal behaviour by means of mould instrumentation. Ironmak. Steelmak. 2004, 31, 364–370. [Google Scholar] [CrossRef]
- Sarkar, R.; Sengupta, A.; Kumar, V.; Choudhar, S.K. Effects of alloying elements on the ferrite potential of peritectic and ultra-low carbon steels. ISIJ Int. 2015, 55, 781–790. [Google Scholar] [CrossRef][Green Version]



| Element | kδ/L | kγ/L | kδ/γ | Dδ, m2·s−1 | Dγ, m2·s−1 |
|---|---|---|---|---|---|
| C | 0.19 | 0.34 | 1.79 | 1.27 × 10−6exp(−81,379/RT) | 7.6 × 10−6exp(−134,557/RT) |
| Si | 0.77 | 0.52 | 0.68 | 8.0 × 10−4exp(−248,948/RT) | 3.0 × 10−5exp(−251,458/RT) |
| Mn | 0.76 | 0.78 | 1.03 | 7.6 × 10−4exp(−224,430/RT) | 5.5 × 10−6exp(−249,366/RT) |
| P | 0.23 | 0.13 | 0.57 | 2.9 × 10−4exp(−230,120/RT) | 1.0 × 10−6exp(−182,841/RT) |
| S | 0.05 | 0.035 | 0.70 | 4.56 × 10−4exp(−214,639/RT) | 2.4 × 10−6exp(−223,425/RT) |
| Equation | Equation Parameters |
|---|---|
| λ1 = K(CR)m(C0)n μm, | 0 ≤ C0 ≤ 0.15 (wt.%C), n = −0.316225 + 2.0325C0 |
| K = 278.748, m = −0.206277638, CR is the cooling rate | 0.15 ≤ C0 ≤ 1 (wt.%C), n = −0.0189 + 0.49166C0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Wen, G. Influence of Alloy Elements on Cracking in the Steel Ingot during Its Solidification. Metals 2019, 9, 836. https://doi.org/10.3390/met9080836
Guo J, Wen G. Influence of Alloy Elements on Cracking in the Steel Ingot during Its Solidification. Metals. 2019; 9(8):836. https://doi.org/10.3390/met9080836
Chicago/Turabian StyleGuo, Junli, and Guanghua Wen. 2019. "Influence of Alloy Elements on Cracking in the Steel Ingot during Its Solidification" Metals 9, no. 8: 836. https://doi.org/10.3390/met9080836
APA StyleGuo, J., & Wen, G. (2019). Influence of Alloy Elements on Cracking in the Steel Ingot during Its Solidification. Metals, 9(8), 836. https://doi.org/10.3390/met9080836




