Elastic Parameters of Paramagnetic Fe–20Cr–20Ni-Based Alloys: A First-Principles Study
Abstract
1. Introduction
2. Methodology
2.1. Total Energy Calculations
2.2. Elastic Constants
2.3. Numerical Details
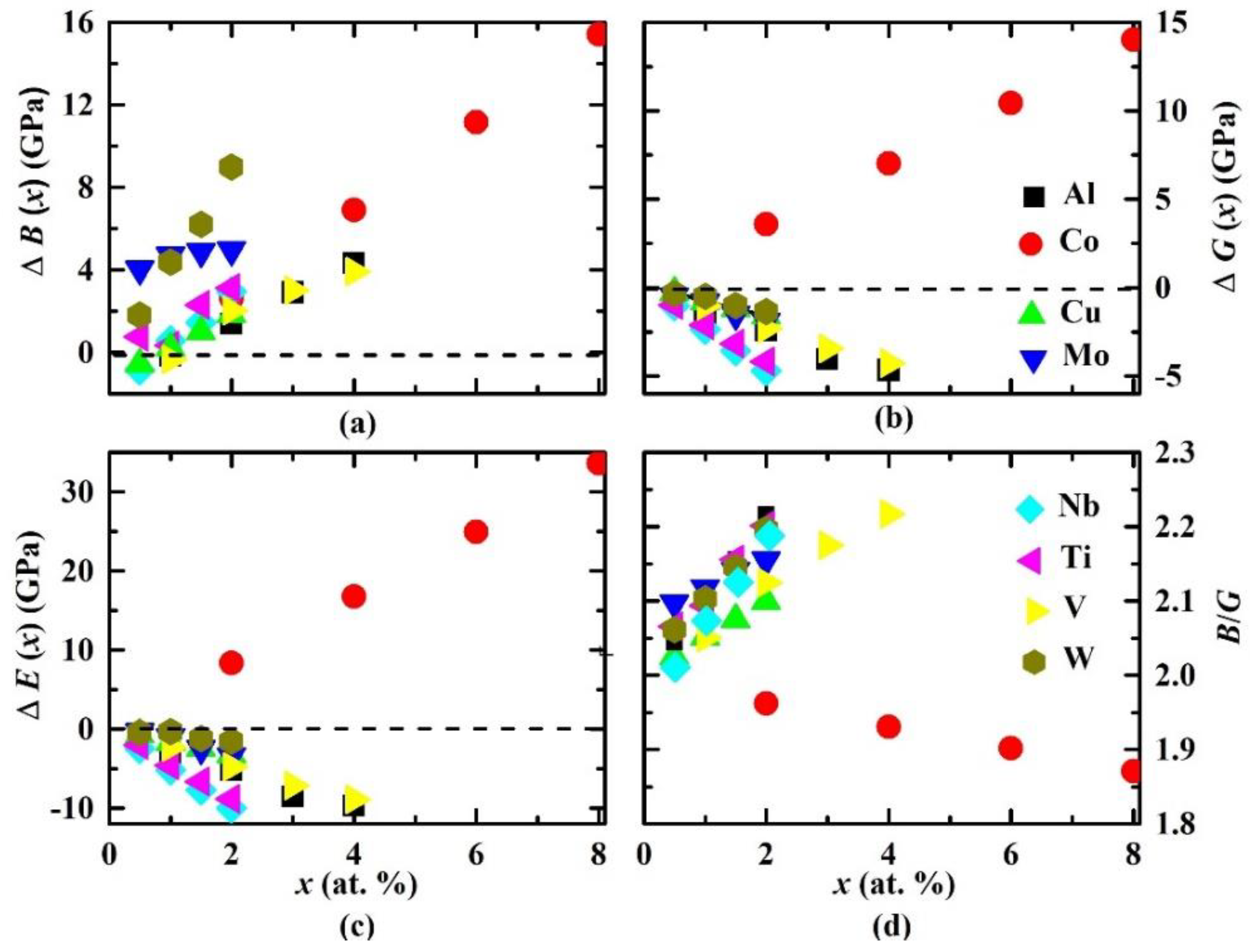
3. Results and Discussions
3.1. Assessing the Accuracy of the Paramagnetic fcc Fe–20Cr–20Ni Alloy
3.2. Single-Crystal Elastic Constants
3.3. Polycrystalline Elastic Constants
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Marshall, P. Austenitic Stainless Steel: Microstructure and Mechanical Properties; Elsevier Applied Science Publishers Ltd.: London, UK, 1984. [Google Scholar]
- Davis, J.R. AMS Specialty Handbook: Stainless Steels; AMS International: Geauga County, OH, USA, 1994. [Google Scholar]
- Wrangln, G. An Introduction to Corrosion and Protecting of Metals; Chapman and Hall: New York, NY, USA, 1985. [Google Scholar]
- Majumdar, A.K.; Blanckenhagen, P.V. Magnetic phase diagram of Fe80−xNixCr20 (10≤x≤30) alloys. Phys. Rev. B 1984, 29, 4079–4085. [Google Scholar]
- Ledbetter, H.M.; Kim, S.A. Molybdenum effect on Fe–Cr–Ni-alloy elastic constants. J. Mater. Res. 1988, 3, 40–44. [Google Scholar] [CrossRef]
- Ledbetter, H. Predicted monocrystal elastic constants of 304-type stainless steel. Physica 1985, 128, 1–4. [Google Scholar] [CrossRef]
- Ledbetter, H.M. Sound velocities and elastic constants of steels 304, 310, and 316. Met. Sci. 1980, 14, 595–596. [Google Scholar] [CrossRef]
- Teklu, A.; Ledbetter, H.; Kim, S.; Boatner, L.A.; McGuire, M.; Keppens, V. Single-crystal elastic constants of Fe-15Ni-15Cr alloy. Met. Mater. Trans. A 2004, 35, 3149–3154. [Google Scholar] [CrossRef]
- Dong, Z.; Schönecker, S.; Chen, D.; Li, W.; Long, M.; Vitos, L. Elastic properties of paramagnetic austenitic steel at finite temperature: Longitudinal spin fluctuations in multicomponent alloys. Phys. Rev. B 2017, 96, 174415. [Google Scholar] [CrossRef]
- Vitos, L. The EMTO Method and Applications, Computational Quantum Mechanics for Materials Engineers; Springer: London, UK, 2007. [Google Scholar]
- Vitos, L.; Korzhavyi, P.A.; Johansson, B. Elastic Property Maps of Austenitic Stainless Steels. Phys. Rev. Lett. 2002, 88, 155501. [Google Scholar] [CrossRef] [PubMed]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J.; Sham, L. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Andersen, O.K.; Jepsen, O.; Krier, G. Lectures on Methods of Electronic Structure Calculations; World Scientific Publishing: Singapore, 1994. [Google Scholar]
- Andersen, O.K.; Arcangeli, C.; Tank, R.W.; Saha-Dasgupta, T.; Krier, G.; Jepsen, O.; Dasgupta, I. Third-generation TB-LMTO. MRS Online Proc. Libr. Arch. 1998, 491. [Google Scholar] [CrossRef]
- Vitos, L.; Skriver, H.; Johansson, B.; Kollár, J. Application of the exact muffin-tin orbitals theory: The spherical cell approximation. Comput. Mater. Sci. 2000, 18, 24–38. [Google Scholar] [CrossRef]
- Vitos, L. Total-energy method based on the exact muffin-tin orbitals theory. Phys. Rev. B 2001, 64, 014107. [Google Scholar] [CrossRef]
- Vitos, L.; Abrikosov, I.A.; Johansson, B. Anisotropic Lattice Distortions in Random Alloys from First-Principles Theory. Phys. Rev. Lett. 2001, 87, 156401. [Google Scholar] [CrossRef] [PubMed]
- Soven, P. Coherent-Potential Model of Substitutional Disordered Alloys. Phys. Rev. 1967, 156, 809–813. [Google Scholar] [CrossRef]
- Györffy, B.L. Coherent-Potential Approximation for a Nonoverlapping-Muffin-Tin-Potential Model of Random Substitutional Alloys. Phys. Rev. B 1972, 5, 2382–2384. [Google Scholar] [CrossRef]
- Kollár, J.; Vitos, L.; Skriver, H.L. Electronic Structure and Physical Properties of Solids: The Uses of the LMTO Method; Lecture Notes in Physics; Springer: Berlin, Germany, 2000. [Google Scholar]
- Gyorffy, B.L.; Pindor, A.J.; Staunton, J.; Stocks, G.M.; Winter, H. A first-principles theory of ferromagnetic phase transitions in metals. J. Phys. F Met. Phys. 1985, 15, 1337–1386. [Google Scholar] [CrossRef]
- Zhang, H.L.; Al-Zoubi, N.; Johansson, B.; Vitos, L. Alloying effects on the elastic parameters of ferromagnetic and paramagnetic Fe from first-principles theory. J. Appl. Phys. 2011, 110, 073707. [Google Scholar] [CrossRef]
- Zhang, H.L.; Punkkinen, M.P.J.; Johansson, B.; Hertzman, S.; Vitos, L. Single-crystal elastic constants of ferromagnetic bcc Fe-based random alloys from first-principles theory. Phys. Rev. B 2010, 81, 184105. [Google Scholar] [CrossRef]
- Delczeg-Czirjak, E.K.; Delczeg, L.; Ropo, M.; Kokko, K.; Punkkinen, M.P.J.; Johansson, B.; Vitos, L. Ab initio study of the elastic anomalies in Pd-Ag alloys. Phys. Rev. B 2009, 79, 085107. [Google Scholar] [CrossRef]
- Al-Zoubi, N.; Johansson, B.; Nilson, G.; Vitos, L. The Bain path of paramagnetic Fe-Cr based alloys. J. Appl. Phys. 2011, 110, 013708. [Google Scholar] [CrossRef]
- Al-Zoubi, N.; Skorodumova, N.V.; Medvedeva, A.; Andersson, J.; Nilson, G.; Johansson, B.; Vitos, L. Tetragonality of carbon-doped ferromagnetic iron alloys: A first-principles study. Phys. Rev. B 2012, 85, 014112. [Google Scholar] [CrossRef]
- Al-Zoubi, N.; Li, X.; Schönecker, S.; Johansson, B.; Vitos, L. Influence of manganese on the bulk properties of Fe-Cr-Mn alloys: A first-principles study. Phys. Scr. 2014, 89, 125702. [Google Scholar] [CrossRef]
- Al-Zoubi, N.; Schönecker, S.; Johansson, B.; Vitos, L. Assessing the Exact Muffin-Tin Orbitals method for the Bain path of metals. Philos. Mag. 2017, 97, 1243–1264. [Google Scholar] [CrossRef]
- Al-Zoubi, N. First-principles study of the structural and elastic properties of AuxV1–x and AuxNb1–x alloys. Philos. Mag. 2018, 98, 1099–1113. [Google Scholar] [CrossRef]
- Hill, R. The Elastic Behavior of a Crystalline Aggregate. Proc. Phys. Soc. Sect. A 1952, 65, 349. [Google Scholar] [CrossRef]
- Voigt, W. Elasticitatsconstunten isotroper Korper. Ann. Phys. 1889, 38, 573. [Google Scholar] [CrossRef]
- Reuss, A.; Angew, Z. Berechnung der Fliehgrenze von Mischkristallen auf Grund der Plastizittitsbedingung fiir Einkristalle. Math. Phys. 1929, 9, 49–58. [Google Scholar]
- Korzhavyi, P.A.; Ruban, A.V.; Abrikosov, I.A.; Skriver, H.L.; Korzhavyi, P. Madelung energy for random metallic alloys in the coherent potential approximation. Phys. Rev. B 1995, 51, 5773–5780. [Google Scholar] [CrossRef]
- Moruzzi, V.L.; Janak, J.F.; Schwarz, K. Calculated thermal properties of metals. Phys. Rev. B 1988, 37, 790–799. [Google Scholar] [CrossRef]
- Dyson, D.J.; Holmes, B. Effect of alloying additions on the lattice parameter of Austenite. J. Iron Steel Inst. 1970, 208, 469–474. [Google Scholar]
- Bain, E.C.; Dunkirk, N.Y. The nature of martensite. Trans. Am. Inst. Min. Metall. Eng. 1924, 70, 25. [Google Scholar]
- Söderlind, P.; Eriksson, O.; Wills, J.M.; Boring, A.M. Theory of elastic constants of cubic transition metals and alloys. Phys. Rev. B 1993, 48, 5844–5851. [Google Scholar] [CrossRef] [PubMed]
- Söderlind, P.; Wills, J.M.; Boring, A.M.; Eriksson, O. Trends of the elastic constants of cubic transition metals. Phys. Rev. Lett. 1992, 68, 2802–2805. [Google Scholar]
- Craievich, P.J.; Sanchez, J.M.; Watson, R.E.; Weinert, M. Structural instabilities of excited phases. Phys. Rev. B 1997, 55, 787–797. [Google Scholar] [CrossRef]
- Craievich, P.J.; Weinert, M.; Sanchez, J.M.; Watson, R.E. Local stability of nonequilibrium phases. Phys. Rev. Lett. 1994, 72, 3076–3079. [Google Scholar] [CrossRef] [PubMed]
- Grimvall, G.; Magyari-Köpe, B.; Ozolins, V.; Persson, K.A. Lattice instabilities in metallic elements. Rev. Mod. Phys. 2012, 84, 945–986. [Google Scholar] [CrossRef]
- Al-Zoubi, N.; Schönecker, S.; Li, X.; Li, W.; Johansson, B.; Vitos, L. Elastic properties of 4d transition metal alloys: Values and trends. Comput. Mater. Sci. 2019, 159, 273–280. [Google Scholar] [CrossRef]
- Kollár, J.; Vitos, L.; Johansson, B.; Skriver, H. Metal Surfaces: Surface, Step and Kink Formation Energies. Phys. Status Solidi B 2000, 217, 405–418. [Google Scholar] [CrossRef]
- Brooks, M.S.S.; Johansson, B. Exchange integral matrices and cohesive energies of transition metal atoms. J. Phys. F Met. Phys. 1983, 13, L197–L202. [Google Scholar] [CrossRef]
- Vitos, L.; Korzhavyi, P.A.; Johansson, B. Stainless steel optimization from quantum mechanical calculations. Nat. Mater. 2003, 2, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Pugh, S.F. XCII. Relations between elastic moduli and plastic properties of polycrystalline pure metals. Philos. Mag. 1954, 45, 823–843. [Google Scholar] [CrossRef]
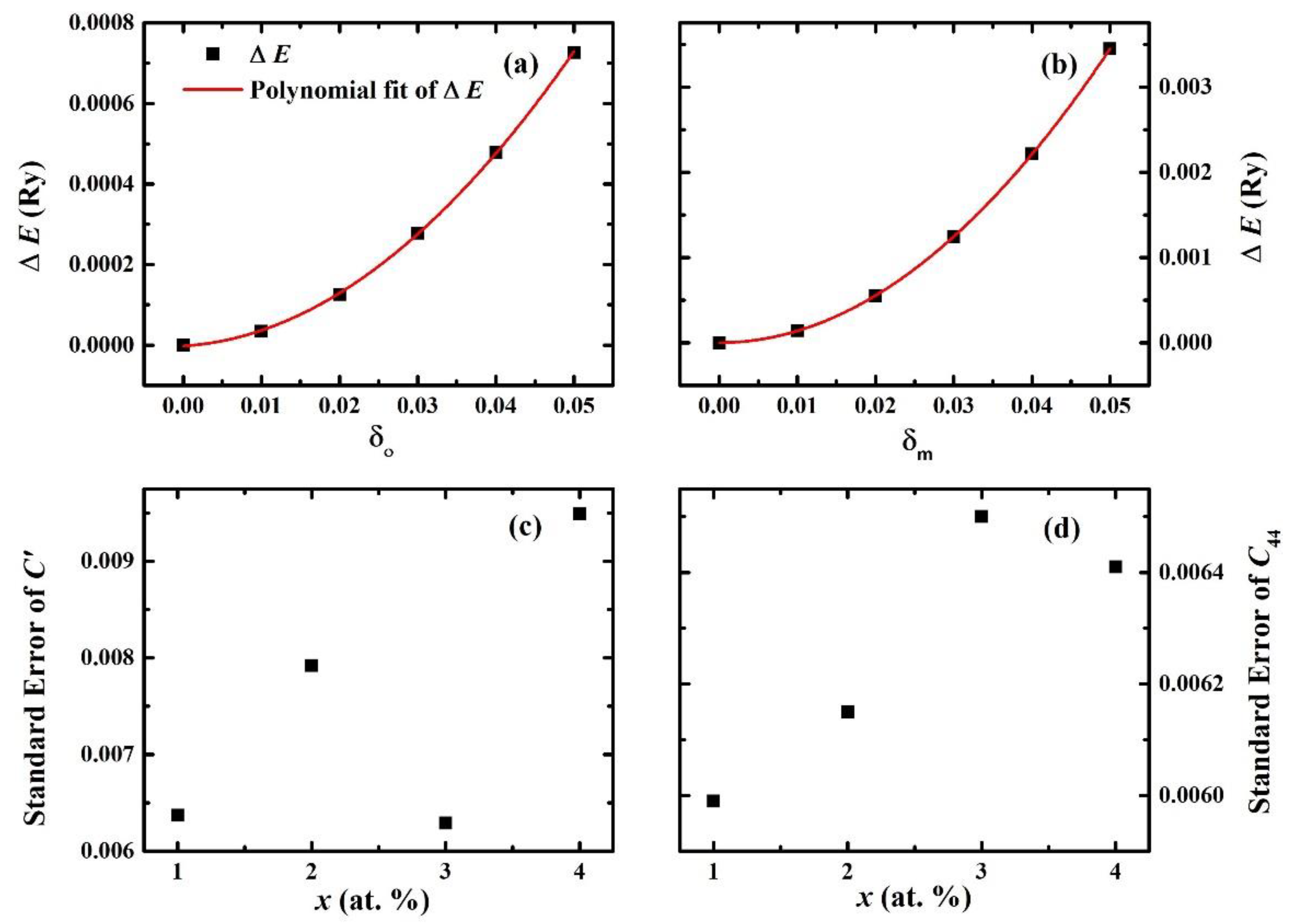
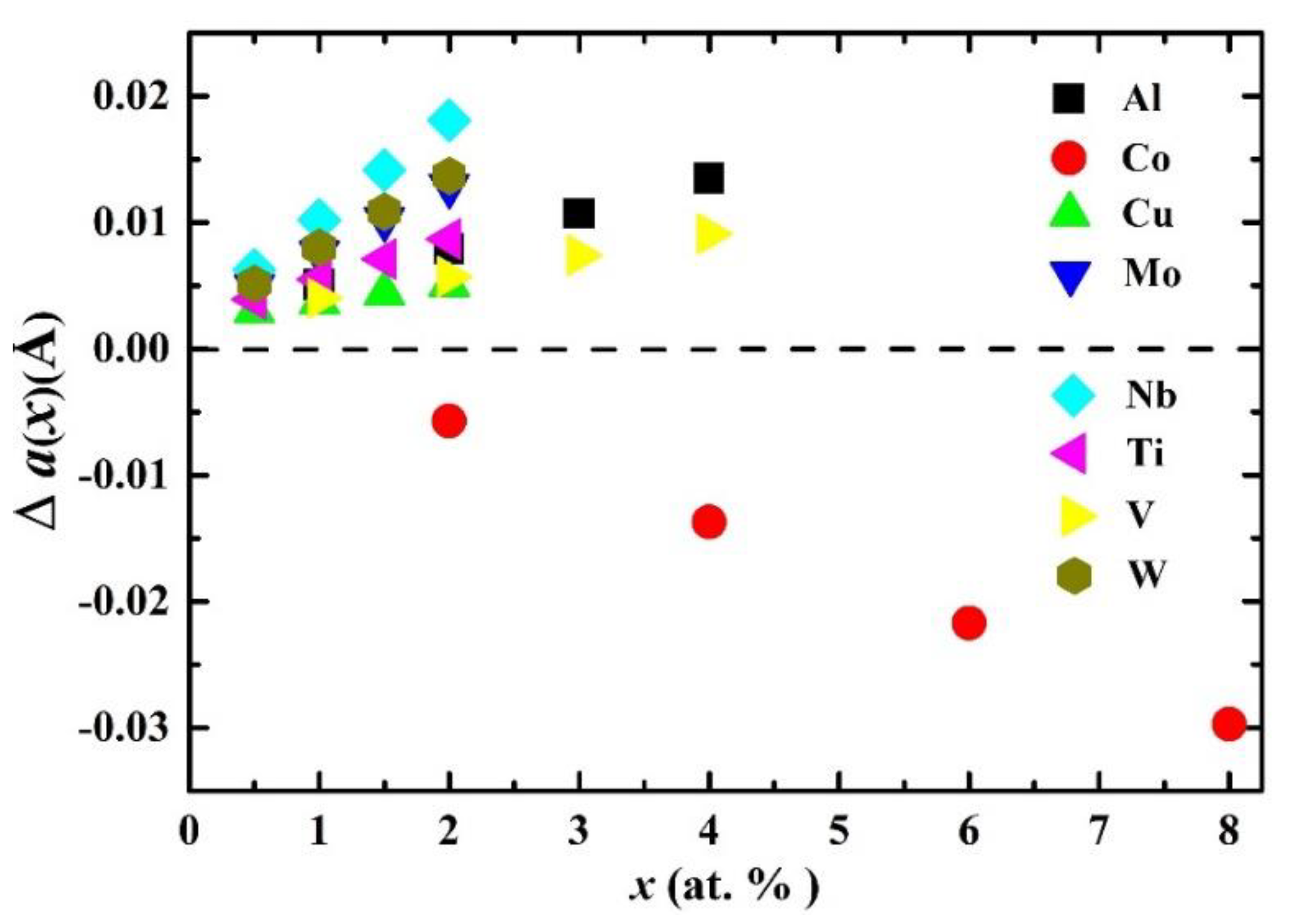
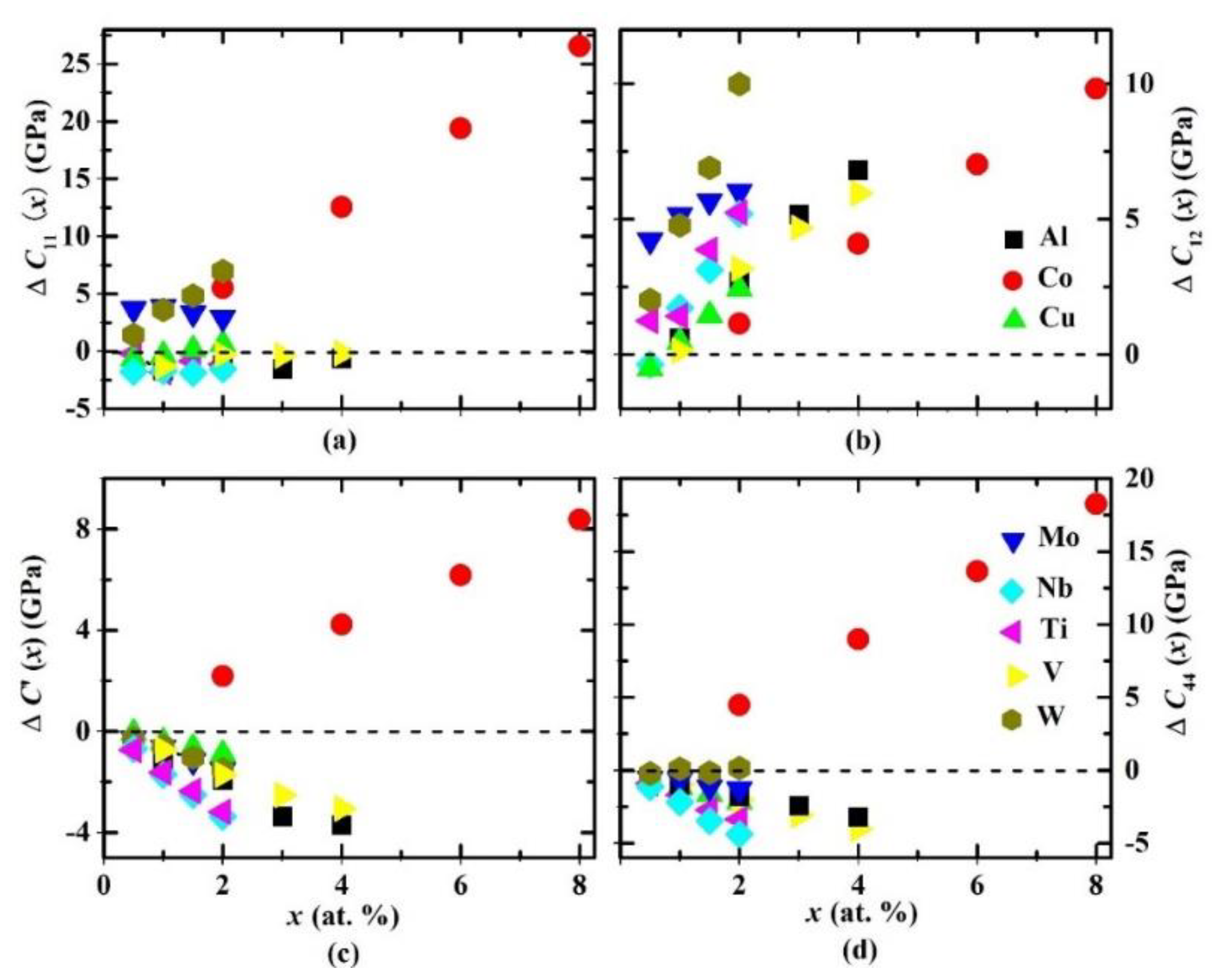
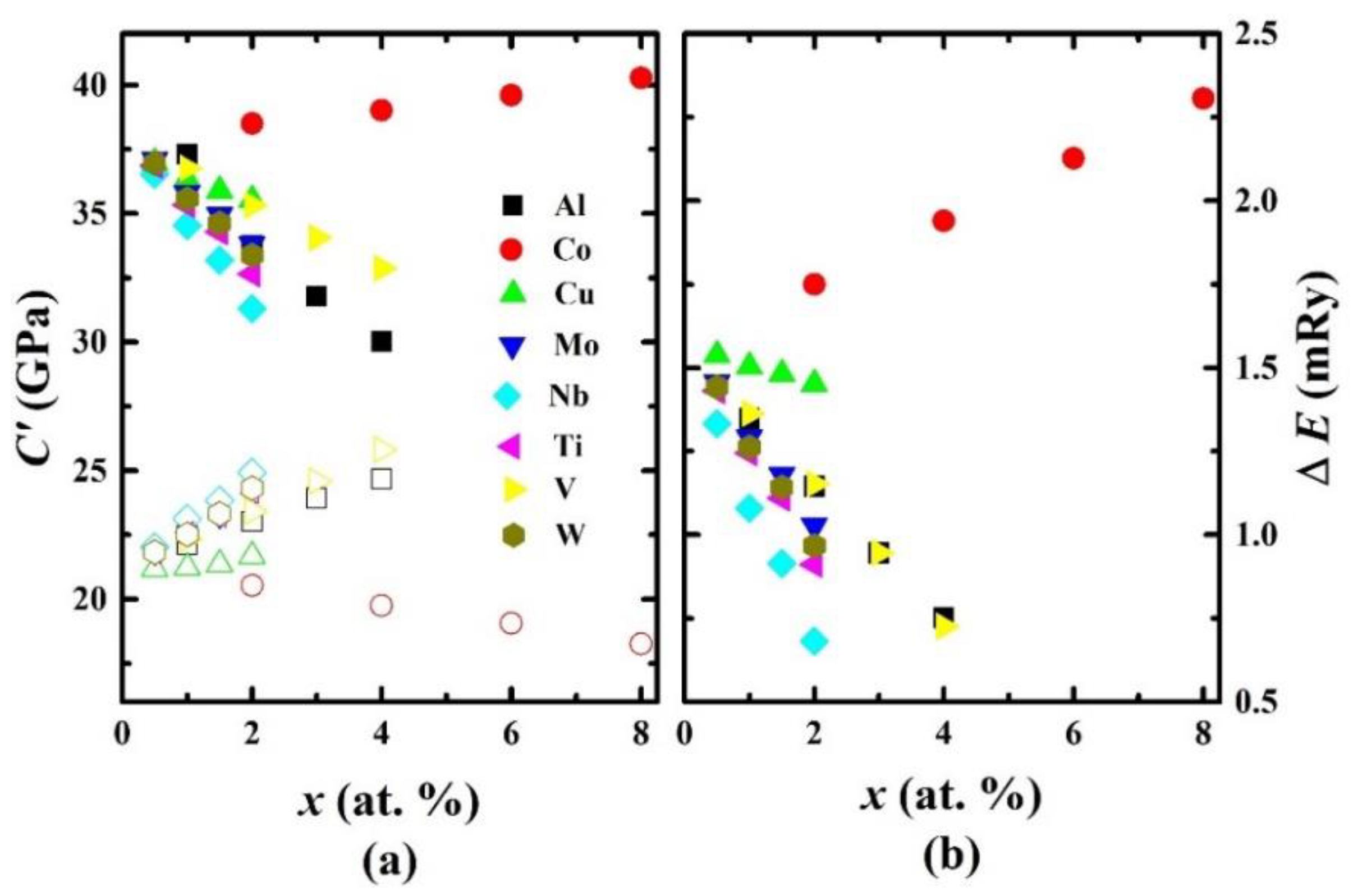

| System | C11 | C12 | C′ | C44 | B | E | G | B/G | ν |
|---|---|---|---|---|---|---|---|---|---|
| Fe0.6Cr0.2Ni0.2 (EMTO) | 177 | 124 | 26.6 | 131 | 142 | 181 | 70.1 | 2.03 | 0.288 |
| Fe0.62Cr0.19Ni0.19 (Exp) | 204 | 133 | 35.5 | 126 | 157 | 199 | 77.2 | 2.03 | 0.289 |
| Percentage error (%) | 13.2 | 6.8 | 25.1 | 3.9 | 9.4 | 9.2 | 9.2 | 0.27 | 0.17 |
| System | aexp | C11 | C12 | C′ | C44 |
|---|---|---|---|---|---|
| Fe–20Cr–20Ni | 3.583 | 177 | 124 | 26.6 | 131 |
| Fe–20Cr–20Ni–1.0Al | 3.588 | 176 | 125 | 25.4 | 130 |
| Fe–20Cr–20Ni–2.0Al | 3.591 | 176 | 127 | 24.6 | 129 |
| Fe–20Cr–20Ni–3.0Al | 3.593 | 176 | 129 | 23.2 | 129 |
| Fe–20Cr–20Ni–4.0Al | 3.596 | 177 | 131 | 22.8 | 128 |
| Fe–20Cr–20Ni–2.0Co | 3.577 | 183 | 125 | 28.7 | 136 |
| Fe–20Cr–20Ni–4.0Co | 3.569 | 190 | 128 | 30.8 | 140 |
| Fe–20Cr–20Ni–6.0Co | 3.561 | 197 | 131 | 32.7 | 145 |
| Fe–20Cr–20Ni–8.0Co | 3.553 | 204 | 134 | 34.9 | 149 |
| Fe–20Cr–20Ni–0.5Cu | 3.586 | 177 | 124 | 26.5 | 130 |
| Fe–20Cr–20Ni–1.0Cu | 3.586 | 177 | 125 | 26.1 | 130 |
| Fe–20Cr–20Ni–1.5Cu | 3.587 | 178 | 126 | 25.9 | 129 |
| Fe–20Cr–20Ni–2.0Cu | 3.588 | 178 | 127 | 25.6 | 129 |
| Fe–20Cr–20Ni–0.5Mo | 3.588 | 181 | 128 | 26.3 | 130 |
| Fe–20Cr–20Ni–1.0Mo | 3.590 | 181 | 129 | 25.9 | 130 |
| Fe–20Cr–20Ni–1.5Mo | 3.593 | 181 | 130 | 25.4 | 130 |
| Fe–20Cr–20Ni–2.0Mo | 3.596 | 180 | 130 | 25.0 | 130 |
| Fe–20Cr–20Ni–0.5Nb | 3.589 | 176 | 124 | 25.9 | 130 |
| Fe–20Cr–20Ni–1.0Nb | 3.593 | 176 | 126 | 24.8 | 129 |
| Fe–20Cr–20Ni–1.5Nb | 3.597 | 175 | 127 | 24.0 | 128 |
| Fe–20Cr–20Ni–2.0Nb | 3.601 | 176 | 129 | 23.2 | 127 |
| Fe–20Cr–20Ni–0.5Ti | 3.587 | 177 | 126 | 25.8 | 130 |
| Fe–20Cr–20Ni–1.0Ti | 3.588 | 176 | 126 | 24.9 | 129 |
| Fe–20Cr–20Ni–1.5Ti | 3.590 | 177 | 128 | 24.2 | 128 |
| Fe–20Cr–20Ni–2.0Ti | 3.591 | 176 | 129 | 23.4 | 128 |
| Fe–20Cr–20Ni–1.0V | 3.587 | 176 | 124 | 25.8 | 130 |
| Fe–20Cr–20Ni–2.0V | 3.588 | 177 | 127 | 24.8 | 129 |
| Fe–20Cr–20Ni–3.0V | 3.590 | 177 | 128 | 24.0 | 128 |
| Fe–20Cr–20Ni–4.0V | 3.592 | 177 | 130 | 23.5 | 127 |
| Fe–20Cr–20Ni–0.5W | 3.588 | 179 | 126 | 26.3 | 131 |
| Fe–20Cr–20Ni–1.0W | 3.591 | 181 | 129 | 26.0 | 131 |
| Fe–20Cr–20Ni–1.5W | 3.594 | 182 | 131 | 25.5 | 131 |
| Fe–20Cr–20Ni–2.0W | 3.596 | 184 | 134 | 25.0 | 131 |
| System | aexp | B | G | B/G | E | ν |
|---|---|---|---|---|---|---|
| Fe–20Cr–20Ni | 3.583 | 142 | 70.1 | 2.03 | 181 | 0.288 |
| Fe–20Cr–20Ni–1.0Al | 3.588 | 142 | 68.6 | 2.06 | 177 | 0.292 |
| Fe–20Cr–20Ni–2.0Al | 3.591 | 143 | 67.6 | 2.12 | 175 | 0.296 |
| Fe–20Cr–20Ni–3.0Al | 3.593 | 145 | 66.0 | 2.19 | 172 | 0.302 |
| Fe–20Cr–20Ni–4.0Al | 3.596 | 146 | 65.4 | 2.24 | 171 | 0.305 |
| Fe–20Cr–20Ni–2.0Co | 3.577 | 145 | 73.7 | 1.96 | 189 | 0.282 |
| Fe–20Cr–20Ni–4.0Co | 3.569 | 149 | 77.1 | 1.93 | 197 | 0.279 |
| Fe–20Cr–20Ni–6.0Co | 3.561 | 153 | 80.5 | 1.90 | 206 | 0.276 |
| Fe–20Cr–20Ni–8.0Co | 3.553 | 157 | 84.1 | 1.87 | 214 | 0.273 |
| Fe–20Cr–20Ni–0.5Cu | 3.586 | 141 | 69.8 | 2.03 | 180 | 0.288 |
| Fe–20Cr–20Ni–1.0Cu | 3.586 | 142 | 69.3 | 2.05 | 179 | 0.290 |
| Fe–20Cr–20Ni–1.5Cu | 3.587 | 143 | 68.9 | 2.07 | 178 | 0.292 |
| Fe–20Cr–20Ni–2.0Cu | 3.588 | 144 | 68.5 | 2.10 | 177 | 0.295 |
| Fe–20Cr–20Ni–0.5Mo | 3.588 | 146 | 69.6 | 2.10 | 180 | 0.294 |
| Fe–20Cr–20Ni–1.0Mo | 3.590 | 147 | 69.2 | 2.12 | 180 | 0.296 |
| Fe–20Cr–20Ni–1.5Mo | 3.593 | 147 | 68.6 | 2.14 | 178 | 0.298 |
| Fe–20Cr–20Ni–2.0Mo | 3.596 | 147 | 68.2 | 2.15 | 177 | 0.299 |
| Fe–20Cr–20Ni–0.5Nb | 3.589 | 141 | 69.0 | 2.04 | 178 | 0.290 |
| Fe–20Cr–20Ni–1.0Nb | 3.593 | 143 | 67.7 | 2.10 | 175 | 0.295 |
| Fe–20Cr–20Ni–1.5Nb | 3.597 | 143 | 66.5 | 2.16 | 173 | 0.299 |
| Fe–20Cr–20Ni–2.0Nb | 3.601 | 145 | 65.4 | 2.22 | 171 | 0.304 |
| Fe–20Cr–20Ni–0.5Ti | 3.587 | 143 | 69.1 | 2.07 | 178 | 0.292 |
| Fe–20Cr–20Ni–1.0Ti | 3.588 | 142 | 68.0 | 2.09 | 176 | 0.294 |
| Fe–20Cr–20Ni–1.5Ti | 3.590 | 144 | 66.9 | 2.16 | 174 | 0.299 |
| Fe–20Cr–20Ni–2.0Ti | 3.591 | 145 | 65.9 | 2.20 | 172 | 0.303 |
| Fe–20Cr–20Ni–1.0V | 3.587 | 142 | 69.0 | 2.05 | 178 | 0.290 |
| Fe–20Cr–20Ni–2.0V | 3.588 | 144 | 67.8 | 2.12 | 176 | 0.297 |
| Fe–20Cr–20Ni–3.0V | 3.590 | 145 | 66.6 | 2.18 | 173 | 0.301 |
| Fe–20Cr–20Ni–4.0V | 3.592 | 146 | 65.8 | 2.22 | 172 | 0.304 |
| Fe–20Cr–20Ni–0.5W | 3.588 | 144 | 69.7 | 2.06 | 180 | 0.291 |
| Fe–20Cr–20Ni–1.0W | 3.591 | 146 | 69.6 | 2.10 | 180 | 0.295 |
| Fe–20Cr–20Ni–1.5W | 3.594 | 148 | 69.1 | 2.15 | 179 | 0.298 |
| Fe–20Cr–20Ni–2.0W | 3.596 | 151 | 68.7 | 2.20 | 179 | 0.302 |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Zoubi, N. Elastic Parameters of Paramagnetic Fe–20Cr–20Ni-Based Alloys: A First-Principles Study. Metals 2019, 9, 792. https://doi.org/10.3390/met9070792
Al-Zoubi N. Elastic Parameters of Paramagnetic Fe–20Cr–20Ni-Based Alloys: A First-Principles Study. Metals. 2019; 9(7):792. https://doi.org/10.3390/met9070792
Chicago/Turabian StyleAl-Zoubi, Noura. 2019. "Elastic Parameters of Paramagnetic Fe–20Cr–20Ni-Based Alloys: A First-Principles Study" Metals 9, no. 7: 792. https://doi.org/10.3390/met9070792
APA StyleAl-Zoubi, N. (2019). Elastic Parameters of Paramagnetic Fe–20Cr–20Ni-Based Alloys: A First-Principles Study. Metals, 9(7), 792. https://doi.org/10.3390/met9070792




