Hydrogen Trapping Behavior in Vanadium Microalloyed TRIP-Assisted Annealed Martensitic Steel
Abstract
1. Introduction
2. Experimental Procedure
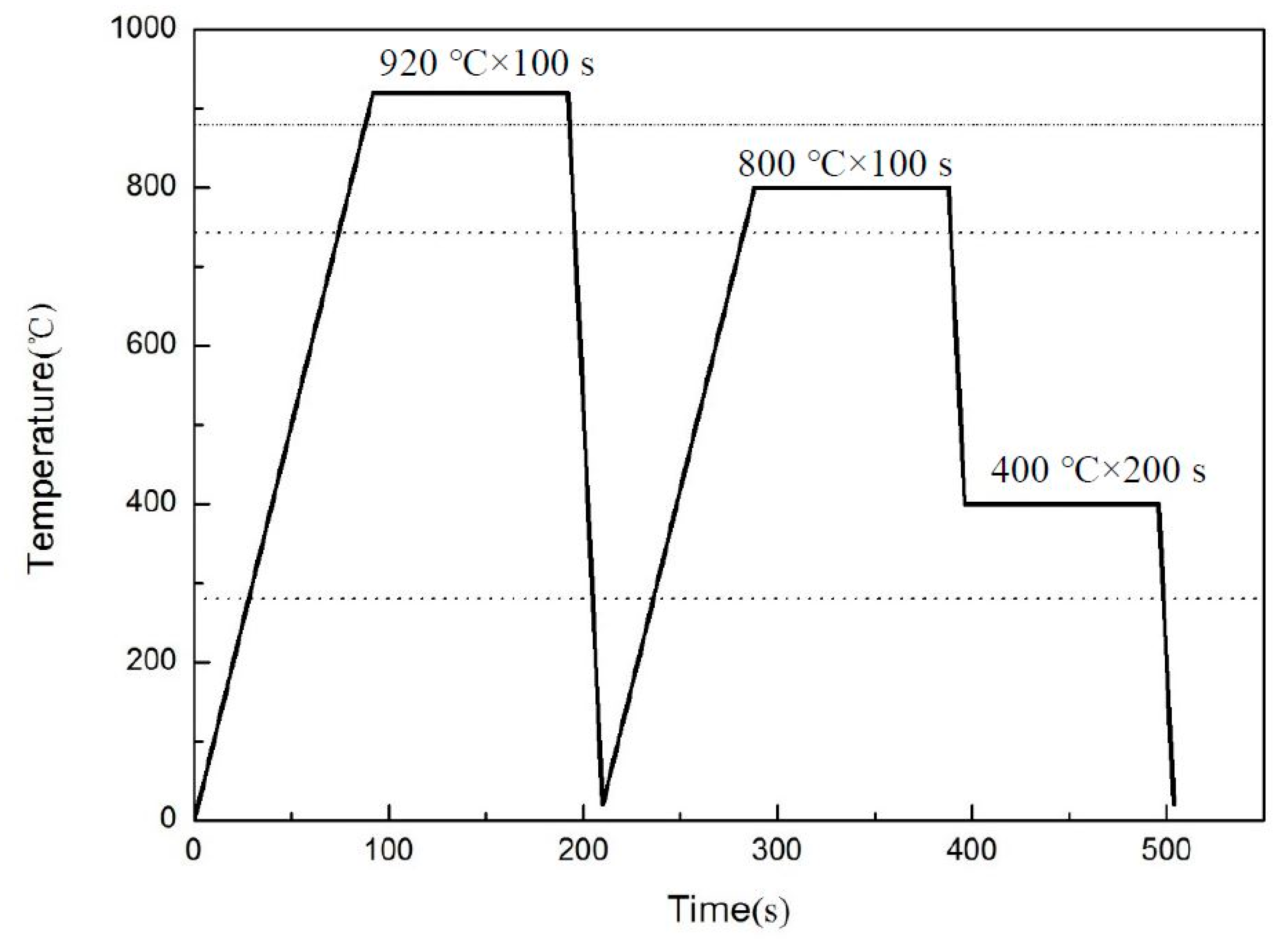
2.1. Materials
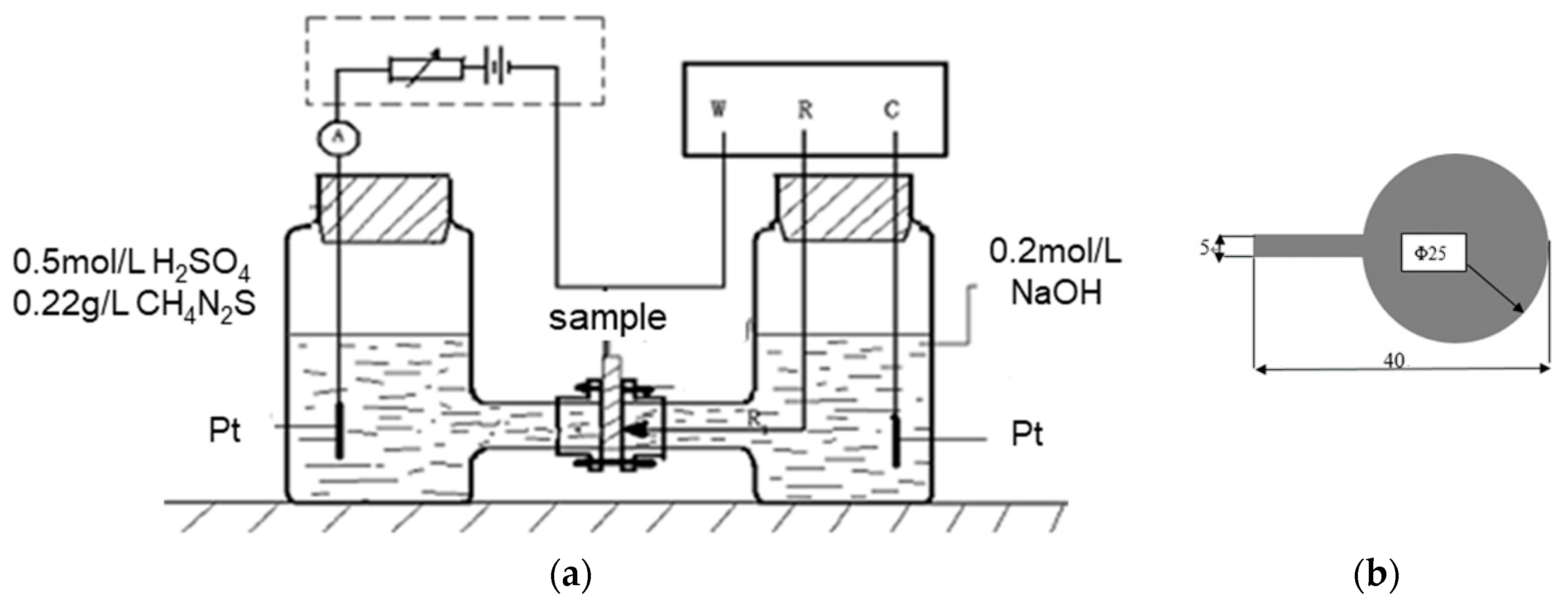
2.2. Hydrogen Charging Method
2.3. Slow Strain Rate Tensile Tests (SSRT)
2.4. Hydrogen Analysis
3. Results
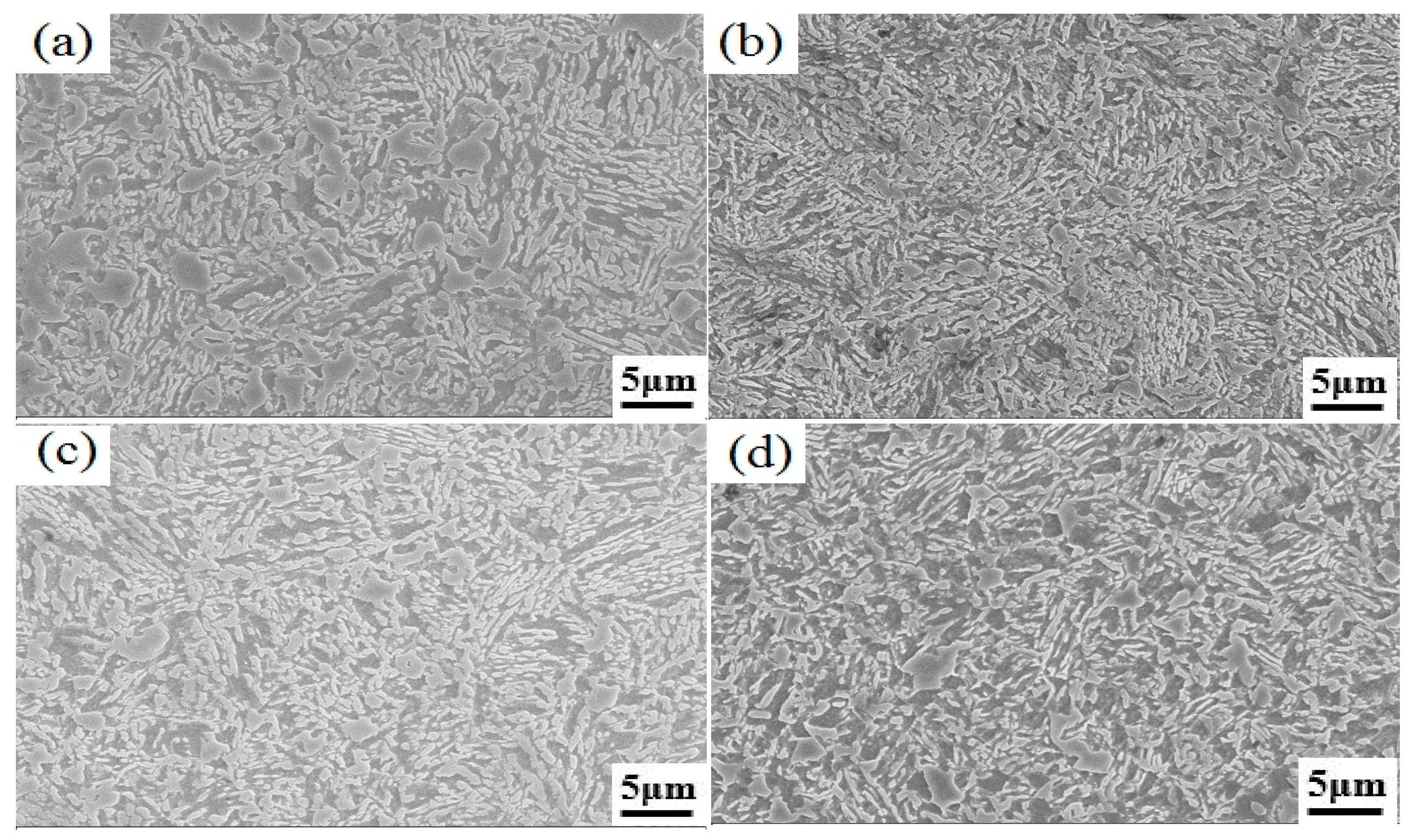
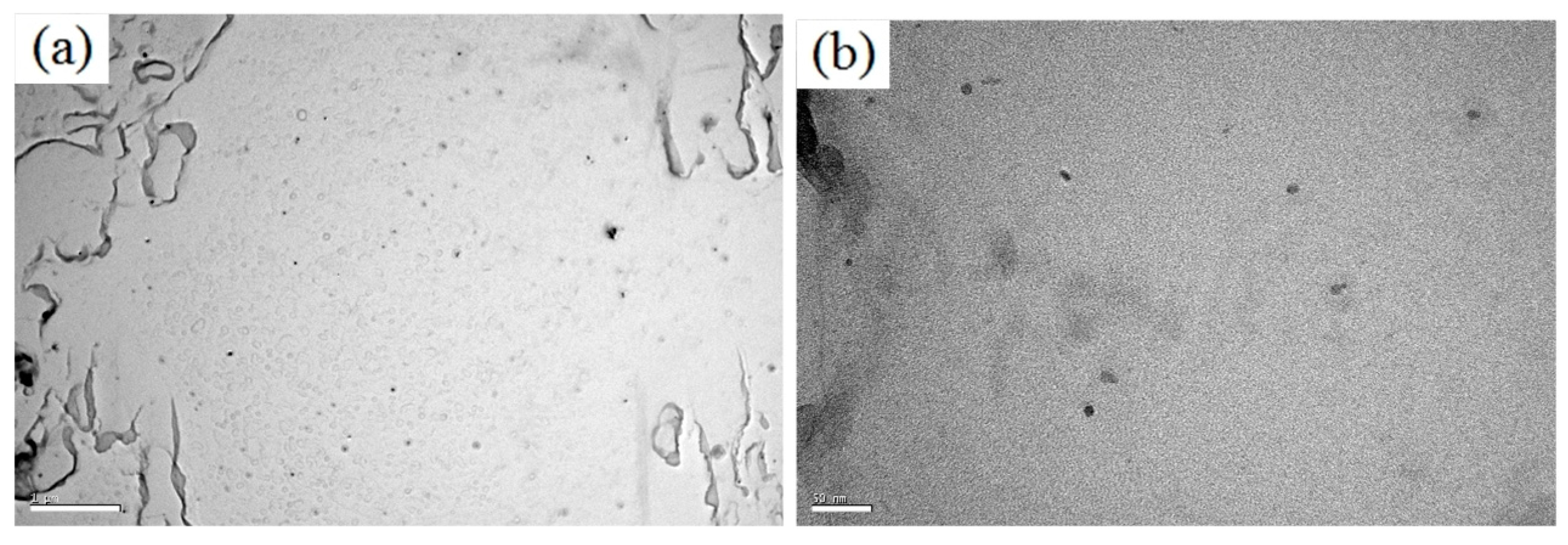
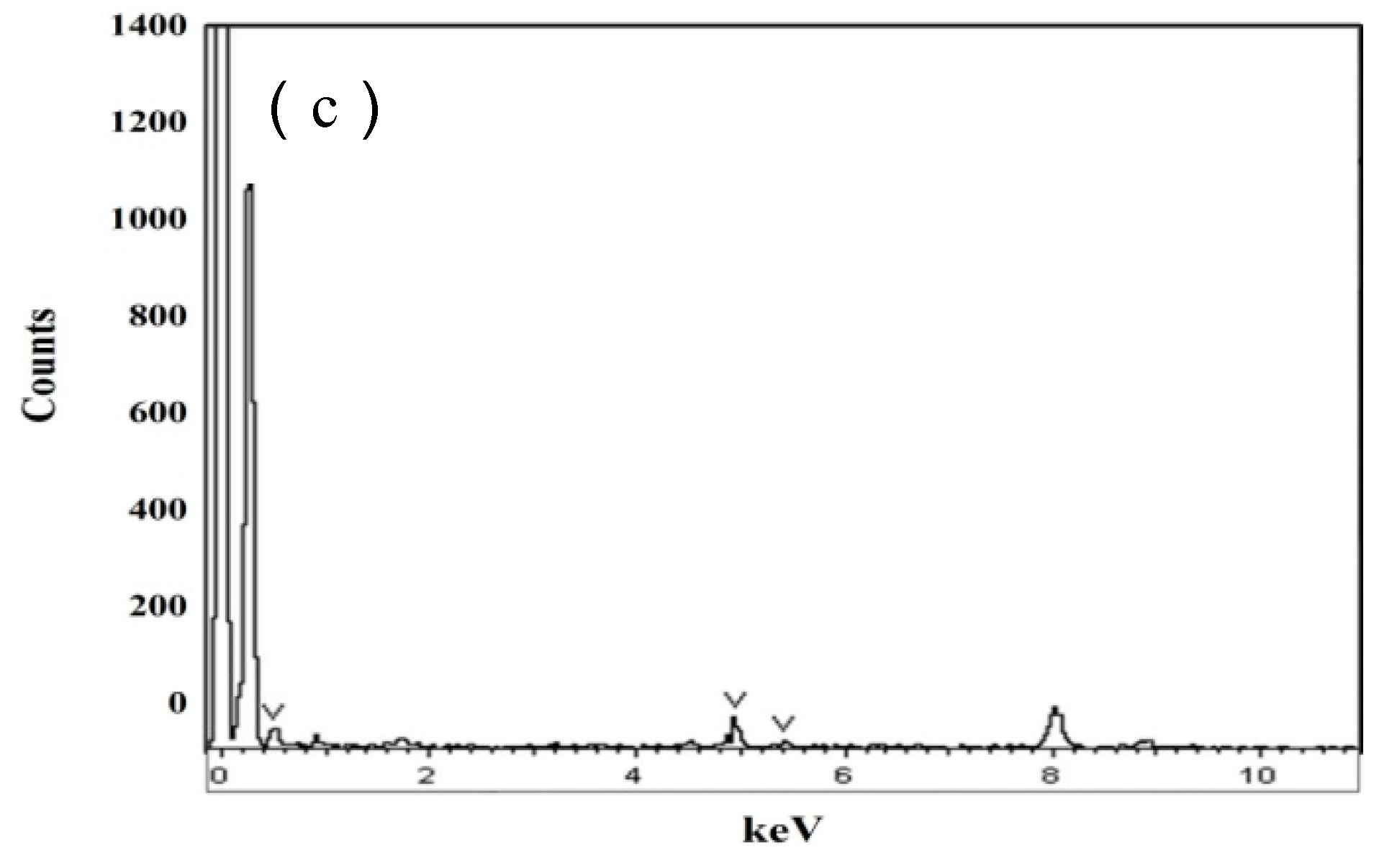
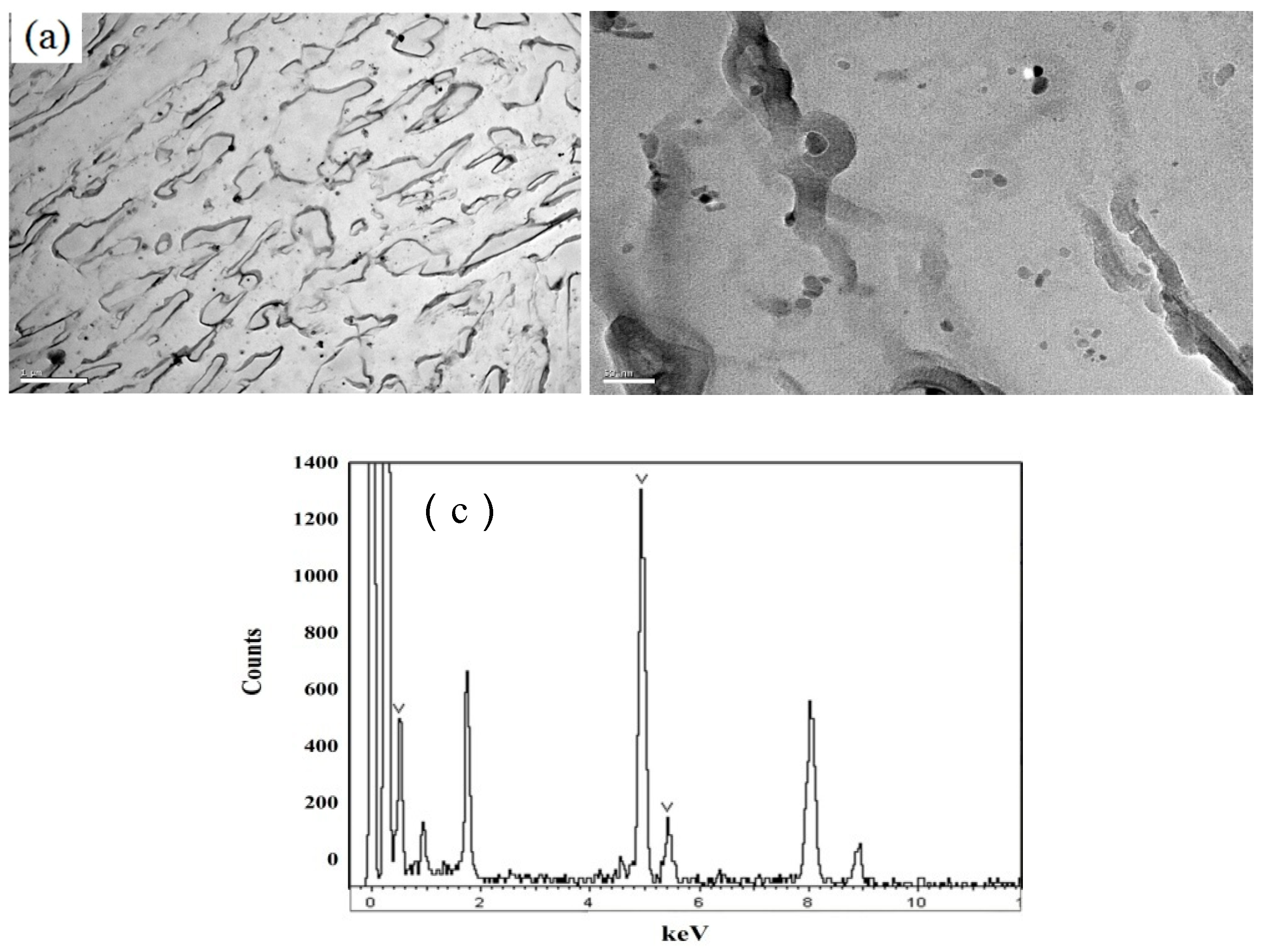
3.1. Microstructural
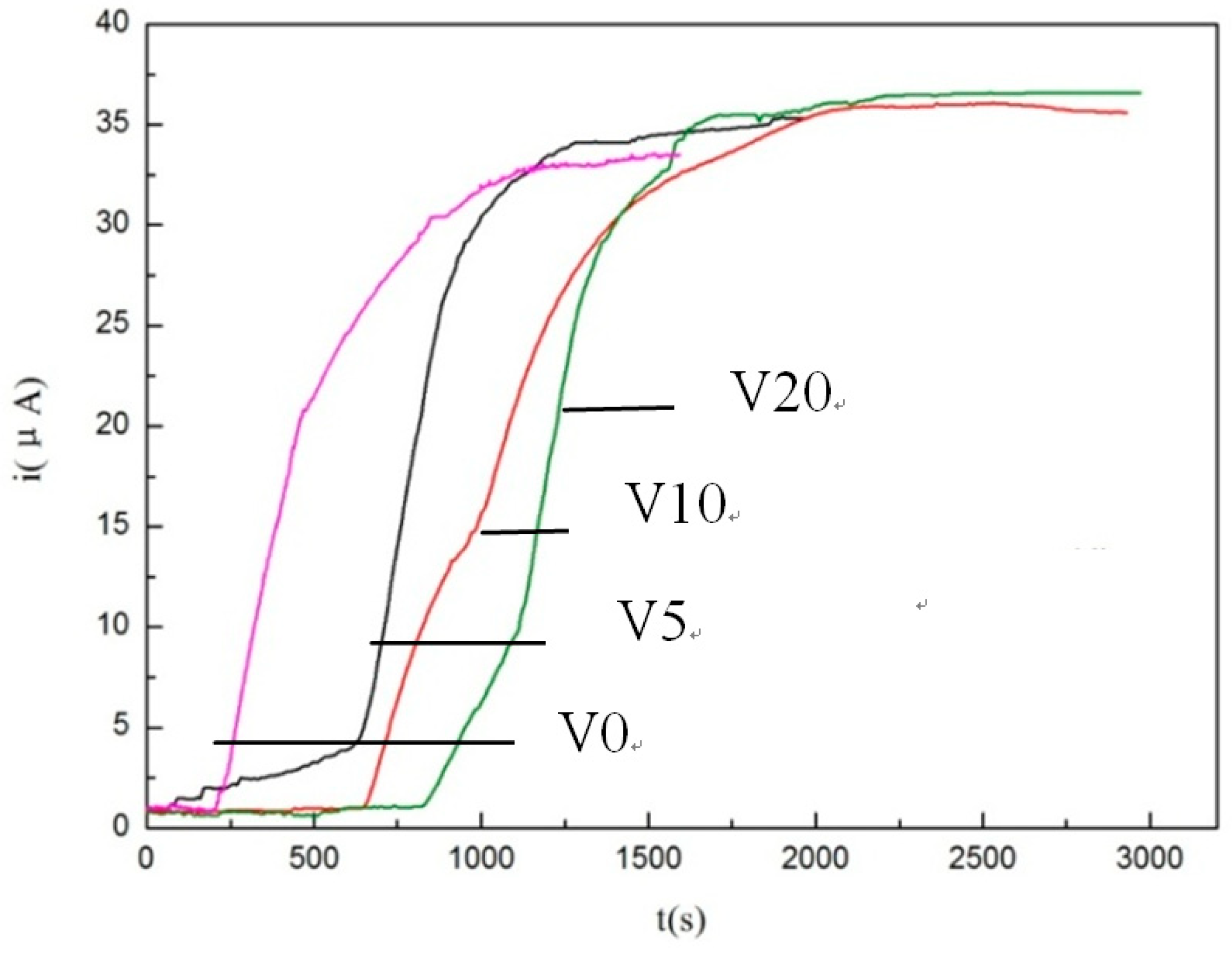
3.2. Hydrogen Permeation Test
3.3. SSRT Behaviors
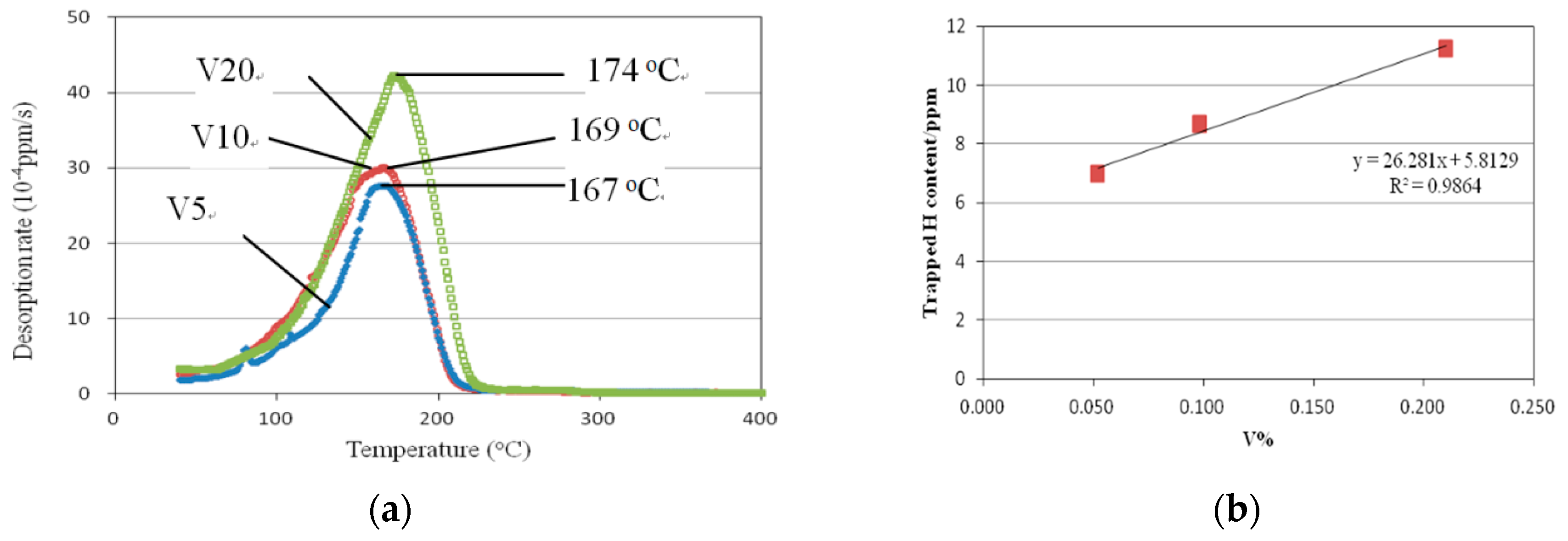
3.4. TDA Results
4. Discussion
4.1. Nature of Hydrogen Trapping
4.2. Synergistic Effect of Hydrogen Trap and RA on Ductility Loss
5. Conclusions
- (1)
- Hydrogen trap sites of V-added steel with annealled martensite structure are estimated to be vanadium carbide.This vanadium carbide is acting as reversible hydrogen traps. The peak temperature for de-tapping of hydrogen approximates 170 °C for all three vanadium-added steels.
- (2)
- Apparent hydrogen diffusive index Da and total trapped hydrogen content increasig with vanadium addition. Da was 1.94 × 10−7/cm2·s−1 for 0.21 wt.%V steel, while the value was 8.05 × 10−7/cm2·s−1 for V-free steel. The total trapped hydrogen are 7 ppm, 8.6 ppm, and 11.25 ppm for 0.052%V, 0.098%V, and 0.21%V steels, respectively. And there is a clear linear relation between Htotal and vanadium addition.
- (3)
- High volume fraction of effective-size precipatites are the essential for acting as hydrogen trapping sites.
- (4)
- The hydrogen induced ductility loss ID was 76.2% for 0.21 wt.%V steel, compared with 86.5% for V-free steel. However, the 0.098%V addition can achieve balancedcombination of high strength and elongation with excellent resistance to hydrogen induced delayed fracture.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, H.G.; Lee, J.Y. Hydrogen trapping by TiC particles in iron. Acta Metall. 1984, 32, 131–136. [Google Scholar] [CrossRef]
- Wei, F.G.; Tsuzaki, K. Quantitative analysis on hydrogen trapping of TiC particles in steel. Metall. Mater. Trans. A 2006, 37, 331–353. [Google Scholar] [CrossRef]
- Zhang, C.L.; Liu, Y.Z.; Jiang, C.; Xiao, J.F. Effects of Niobium and Vanadium on Hydrogen-Induced Delayed Fracture in High Strength Spring Steel. J. Iron Steel Res. 2011, 18, 49–53. [Google Scholar] [CrossRef]
- Szost, B.A.; Vegter, R.H.; Castillo, P.E.J.R. Developing bearing steels combining hydrogen resistance and improved hardness. Mater. Des. 2013, 43, 499–506. [Google Scholar] [CrossRef]
- Szost, B.A.; Vegter, R.H.; Castillo, P.E.J.R. Hydrogen-trapping mechanisms in nano-structured steels. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2013, 44, 4542–4550. [Google Scholar] [CrossRef]
- Hui, W.J.; Dong, H.; Wang, M.Q.; Chen, S.L.; Weng, Y.Q. Effect of Vanadium on Delyaed Fracture Resistance of High Strength Steel. Heat Treat. of Met. 2002, 27, 10–12. [Google Scholar]
- Asahi, H.; Hirakami, D.; Yamasaki, S. Hydrogen Trapping Behavior in Vanadium-added Steel. ISIJ Int. 2003, 43, 527–533. [Google Scholar] [CrossRef]
- Seong, B.S.; Shin, E.J.; Han, Y.S.; Lee, C.H.; Kim, Y.J.; Kim, S.J. Effect of retained austenite and solute carbon on the mechanical properties i n TRIP steels. Phys. B Condens. Matter 2004, 350, e467–e469. [Google Scholar] [CrossRef]
- Scott, C.; Cugy, P. Vanadium Additions in New Ultra High Strength and Ductility Steels. In Proceedings of the 2009 International Symposium on Automobile Steel (ISAS’09), Dalian, China, 6–8 September 2009. [Google Scholar]
- Enomoto, M.; Hirakami, D. Thermal desorption analysis of hydrogen in high strength martensitic steels. Metall. Mater. Trans. A 2011, 43A, 572–581. [Google Scholar] [CrossRef]
- Hagihara, Y.; Takai, K.; Hirai, K. Delayed fracture using CSRT and hydrogen trapping characteristic of V-bearing high-strength steel. ISIJ Int. 2012, 52, 298–306. [Google Scholar] [CrossRef]
- Yamasaki, S.; Bhadeshia, H.K.D.H. M4C3 precipitation in Fe-C-Mo-V steels and relationship to hydrogen trapping. Proc. Royal Soc. A 2006, 462, 2315–2330. [Google Scholar] [CrossRef]
- Sugimoto, K.; Yu, B.; Mukai, Y.; Ikeda, S. Microstructure and Formability of Aluminum Bearing TRIP-Aided Steel with Annealed Martensite Matrix. ISIJ Int. 2005, 45, 1194–1200. [Google Scholar] [CrossRef]
- Li, A.P.; Zhang, C.X. Hydrogen embrittlement of Ultra-high strength low alloyed TRIP steel. Heat Treat. Met. Abroad 2005, 26, 20–25. [Google Scholar]
- Ma, F.R.; Liu, J.X.; Chu, W.Y.; Zhang, W.L.; Yang, D.K. Study of Hydro Diffusion in Enamelled Steel Sheet. J. Chin. Soc. Corros. Prot. 2010, 30, 269–272. [Google Scholar]
- Hong, Q.; Chen, Y.X. Effects of Static and dynamic hydrogen charging on tensile properties of SM490B clean steel. Shanghai Metals 2012, 34, 25–28. [Google Scholar]
- Maroef, I. Hydrogen trapping in ferritic steel weld metal. Int. Mater. Rev. 2002, 4, 191–223. [Google Scholar] [CrossRef]
- Park, Y.D.; Maroef, A.; Landau, A. Retained austenite as a hydrogen trap in steel welds. Weld. J. 2002, 81, 27–35. [Google Scholar]

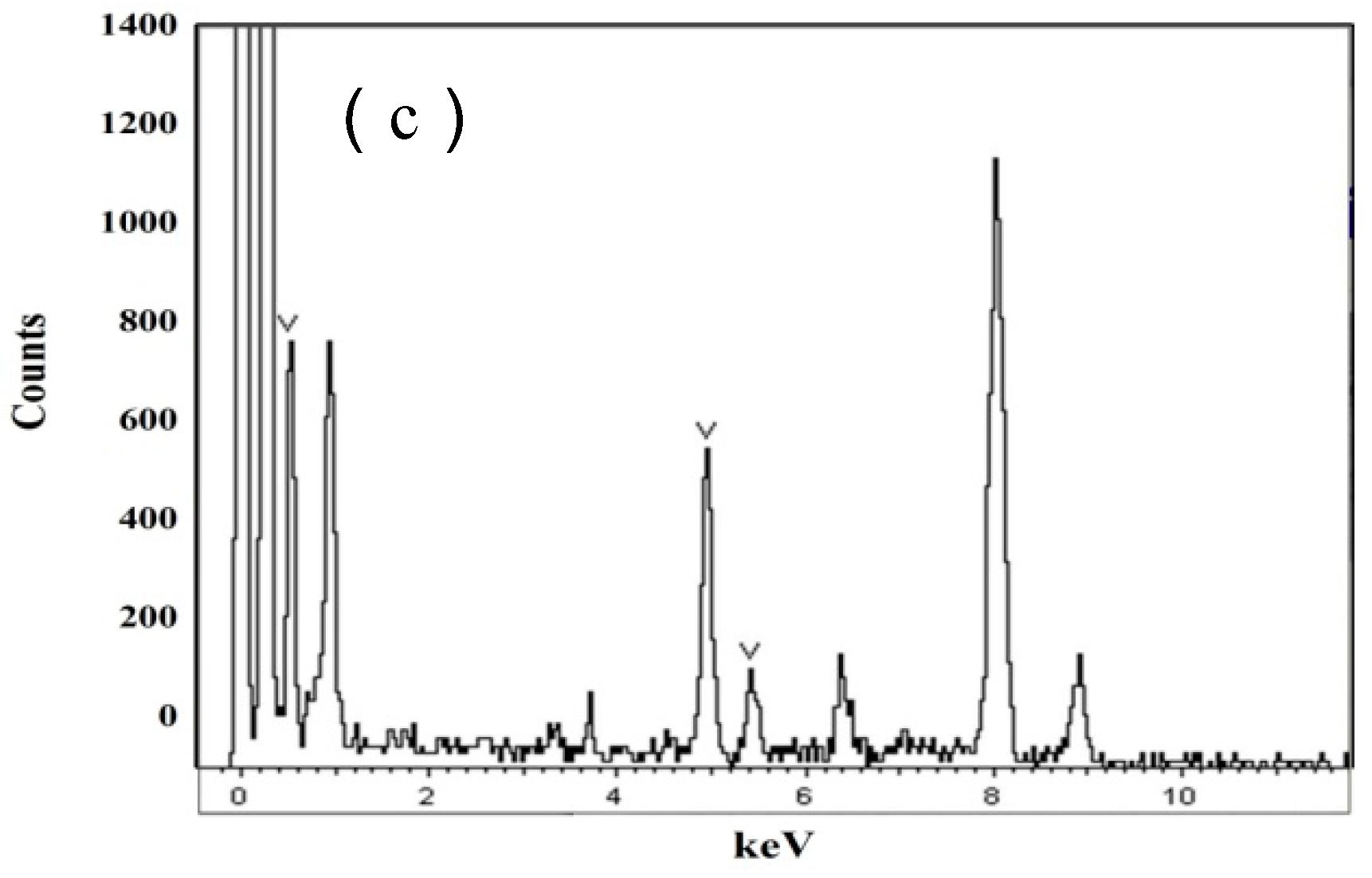
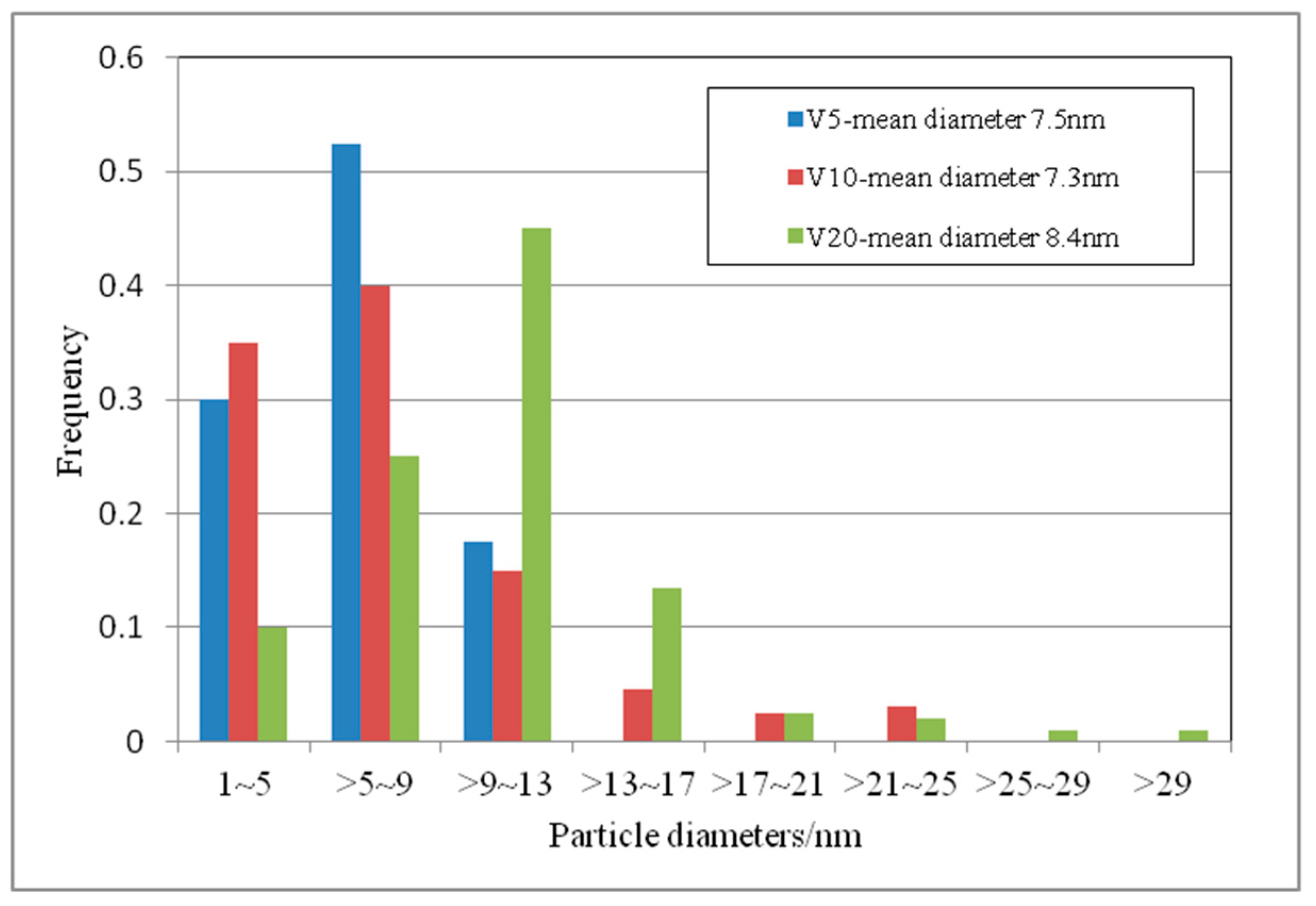
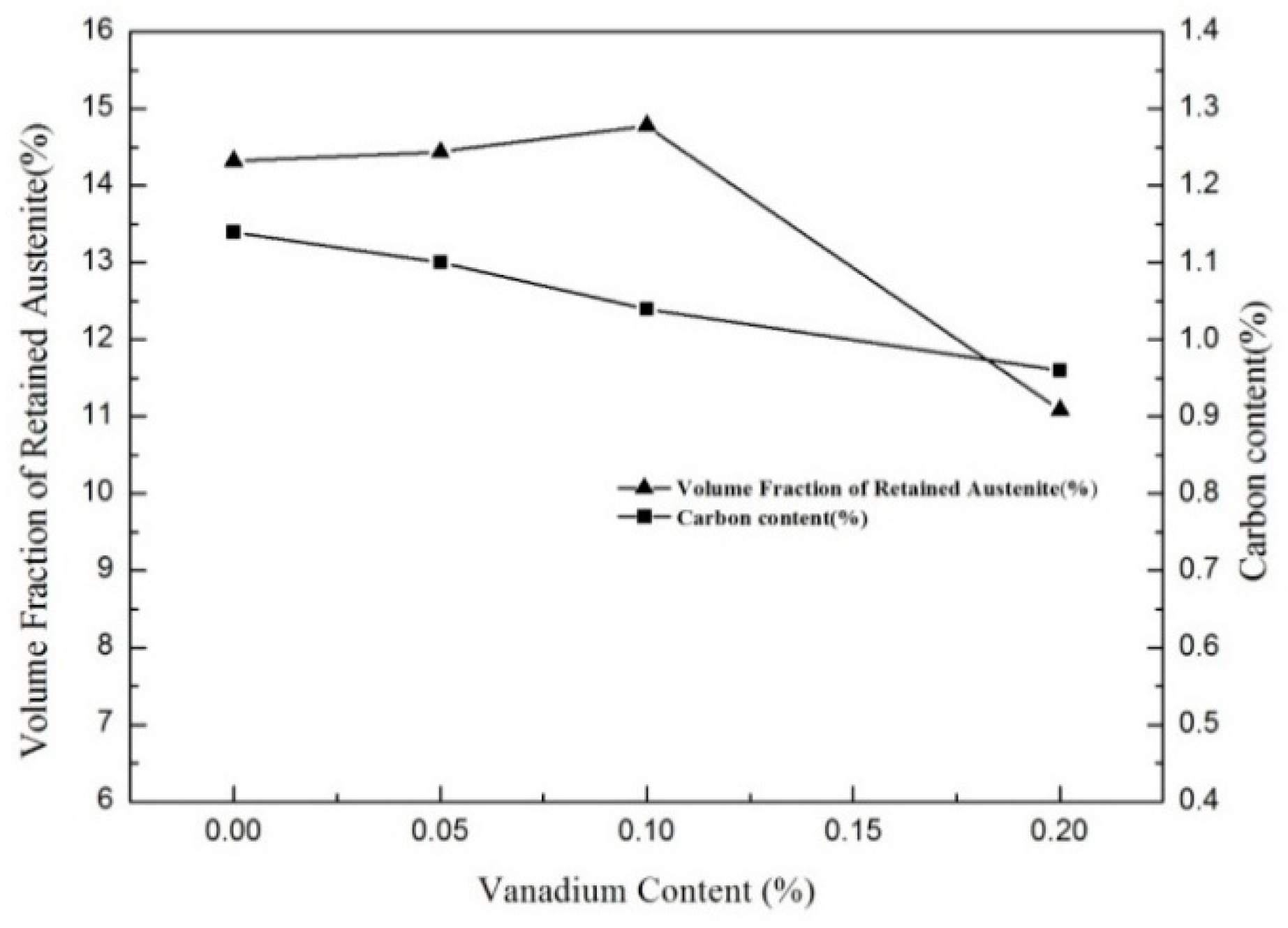
| Elements | C | Si | Mn | P | S | V | Ti | N |
|---|---|---|---|---|---|---|---|---|
| TAM-V0 | 0.19 | 1.42 | 2.02 | 0.007 | 0.005 | - | 0.030 | 0.0037 |
| TAM-V5 | 0.20 | 1.53 | 2.10 | 0.006 | 0.005 | 0.052 | 0.024 | 0.0036 |
| TAM-V10 | 0.20 | 1.50 | 2.10 | 0.005 | 0.006 | 0.098 | 0.030 | 0.0039 |
| TAM-V20 | 0.20 | 1.54 | 2.05 | 0.007 | 0.005 | 0.21 | 0.029 | 0.0045 |
| Critical Points | TAM-V0 | TAM-V5 | TAM-V10 | TAM-V20 |
|---|---|---|---|---|
| Ac1 | 744 | 740 | 745 | 750 |
| Ac3 | 865 | 870 | 875 | 878 |
| MsC | 402 | 398 | 397 | 398 |
| Steels | Sample Thickness L/mm | I∞/µA | 0.63I∞/µA | t0.63/s |
|---|---|---|---|---|
| TAM-V0 | 0.31 | 33.05 | 20.82 | 199 |
| TAM-V5 | 0.31 | 34.81 | 21.93 | 357 |
| TAM-V10 | 0.32 | 35.85 | 22.59 | 644 |
| TAM-V20 | 0.31 | 36.5 | 23.00 | 826 |
| Steels | Da × 10−7/cm2·s−1 | J∞ × 10−10/mol·cm−2·s−1 | |
|---|---|---|---|
| TAM-V0 | 8.05 | 4.36 | 1.68 |
| TAM-V5 | 4.49 | 4.59 | 3.18 |
| TAM-V10 | 2.65 | 4.73 | 5.72 |
| TAM-V20 | 1.94 | 4.82 | 7.70 |
| Steels | Charging Conditions | Rm/MPa | A/% | IT | ID |
|---|---|---|---|---|---|
| TAM-V0 | Uncharging | 1002 | 28.93 | 37.4 | 86.5 |
| 5 mA/cm2 | 760 | 3.87 | |||
| TAM-V5 | Uncharging | 1106 | 25.70 | 29.8 | 83.4 |
| 5 mA/cm2 | 776 | 4.26 | |||
| TAM-V10 | Uncharging | 1224 | 21.33 | 30.6 | 76.2 |
| 5 mA/cm2 | 850 | 5.08 | |||
| TAM-V20 | Uncharging | 1191 | 18.67 | 31.2 | 76.2 |
| 5 mA/cm2 | 819 | 4.45 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Yu, H.; Song, C.; Li, L. Hydrogen Trapping Behavior in Vanadium Microalloyed TRIP-Assisted Annealed Martensitic Steel. Metals 2019, 9, 741. https://doi.org/10.3390/met9070741
Yang X, Yu H, Song C, Li L. Hydrogen Trapping Behavior in Vanadium Microalloyed TRIP-Assisted Annealed Martensitic Steel. Metals. 2019; 9(7):741. https://doi.org/10.3390/met9070741
Chicago/Turabian StyleYang, Xiongfei, Hao Yu, Chenghao Song, and Lili Li. 2019. "Hydrogen Trapping Behavior in Vanadium Microalloyed TRIP-Assisted Annealed Martensitic Steel" Metals 9, no. 7: 741. https://doi.org/10.3390/met9070741
APA StyleYang, X., Yu, H., Song, C., & Li, L. (2019). Hydrogen Trapping Behavior in Vanadium Microalloyed TRIP-Assisted Annealed Martensitic Steel. Metals, 9(7), 741. https://doi.org/10.3390/met9070741






