Effect of Carbide Precipitation on the Evolution of Residual Stress during Tempering
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
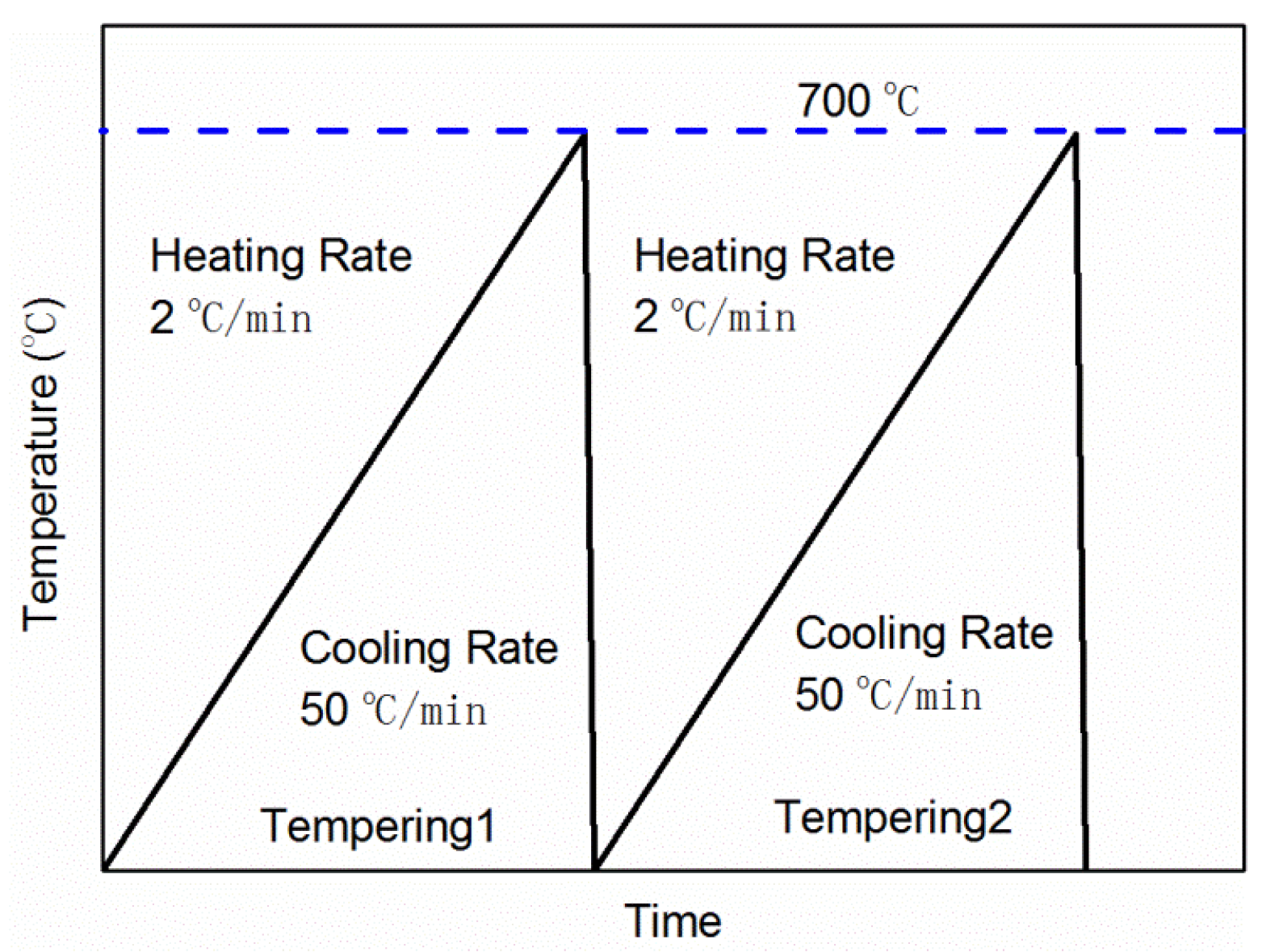
2.2. Non-Isothermal Tempering Test
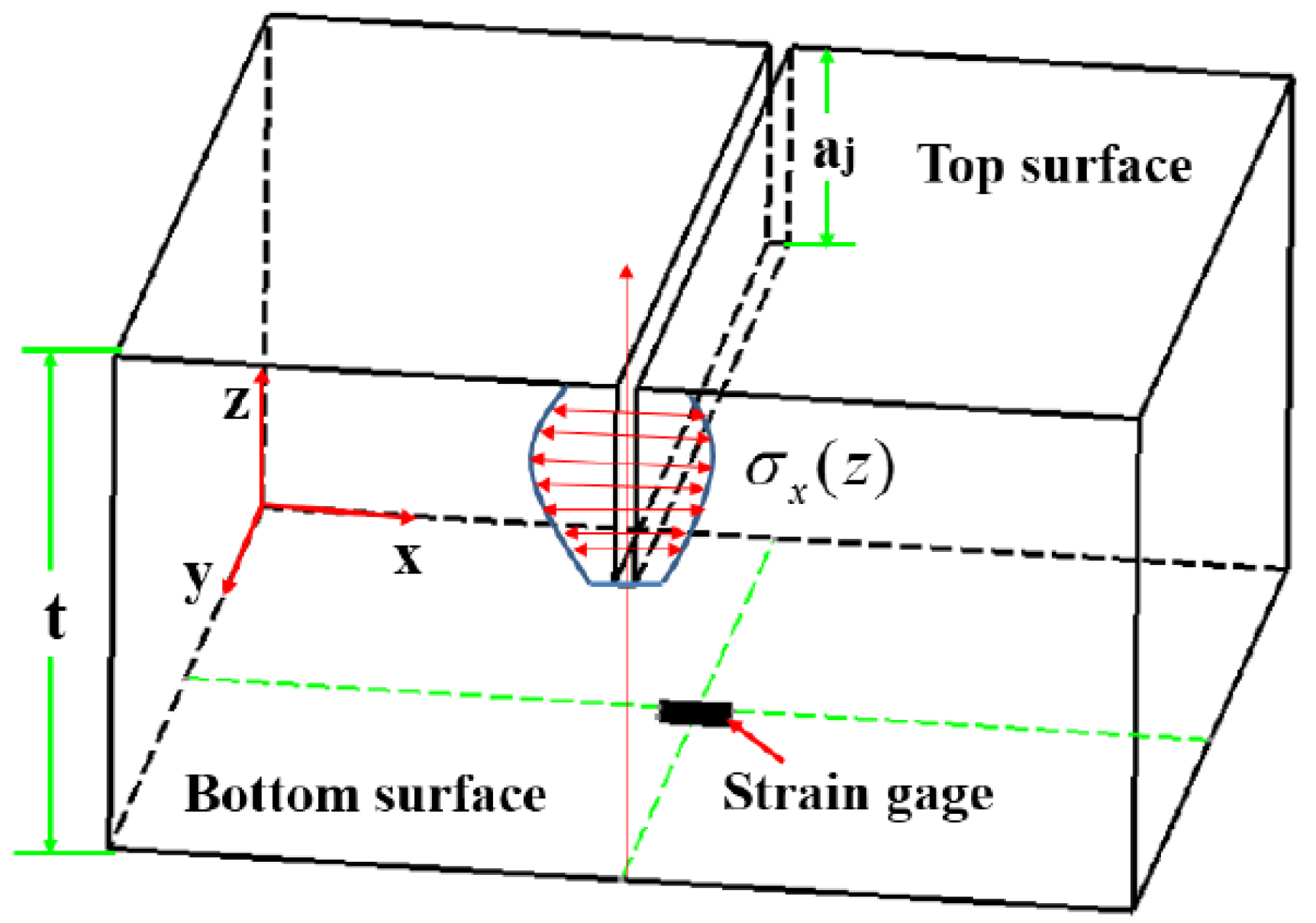
2.3. Measurement of Residual Stress
2.4. TEM Analysis
2.5. Vickers Micro-Hardness
3. Results
3.1. Evolution of 700L Microstructure during Tempering
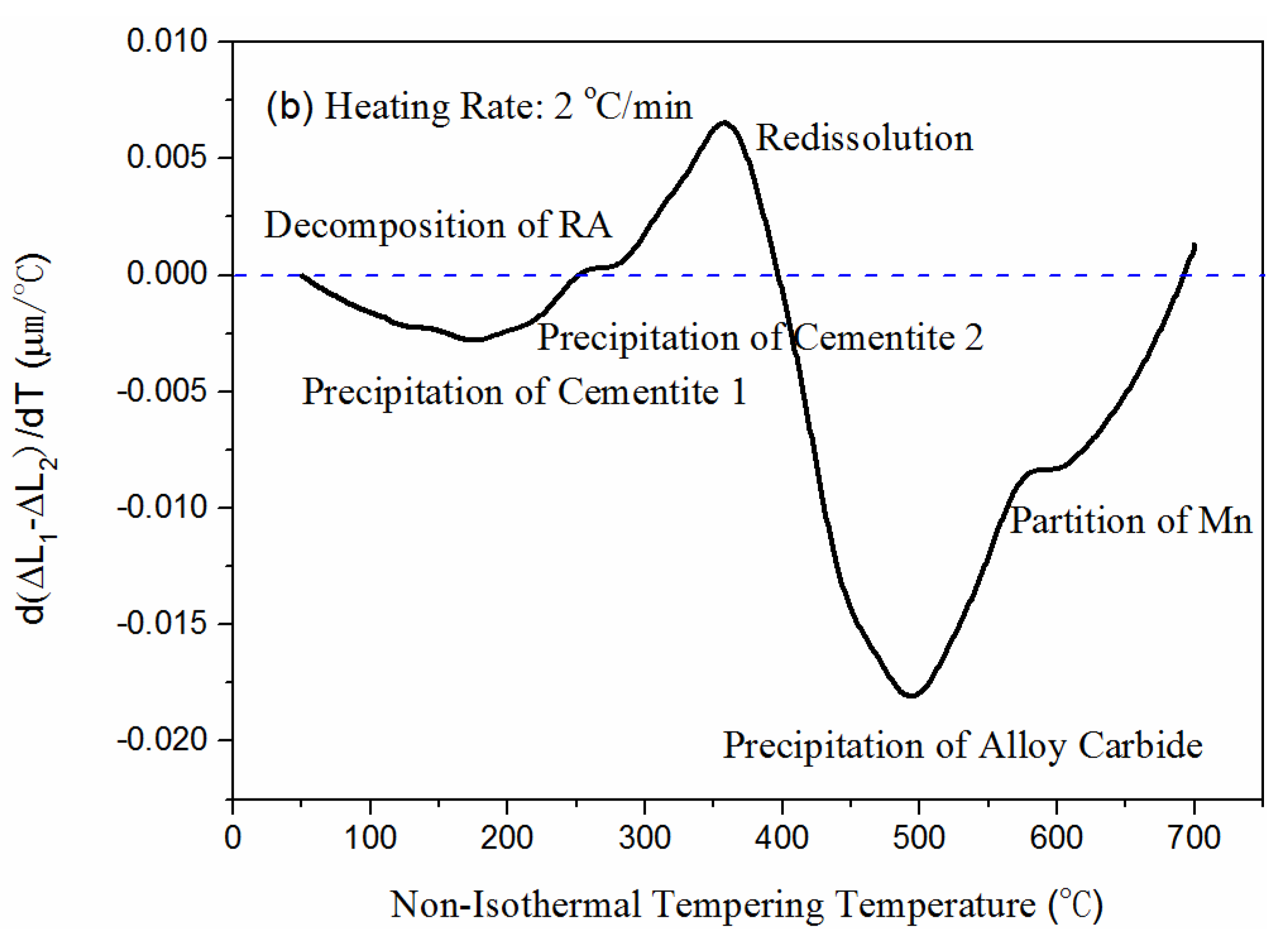
3.1.1. Stages in the Tempering Process
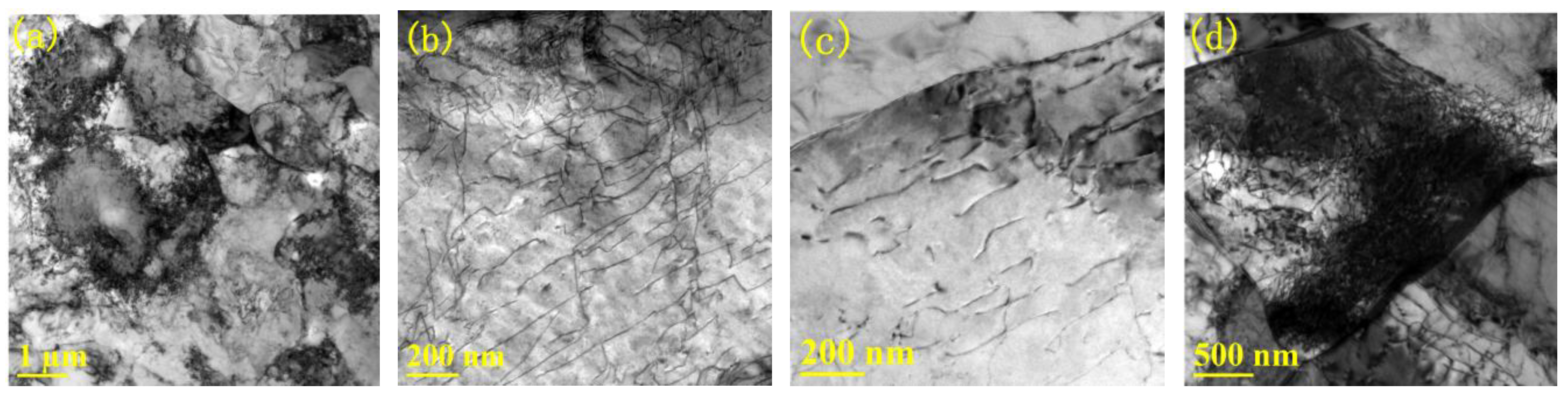
3.1.2. Evolution of Dislocation during Tempering
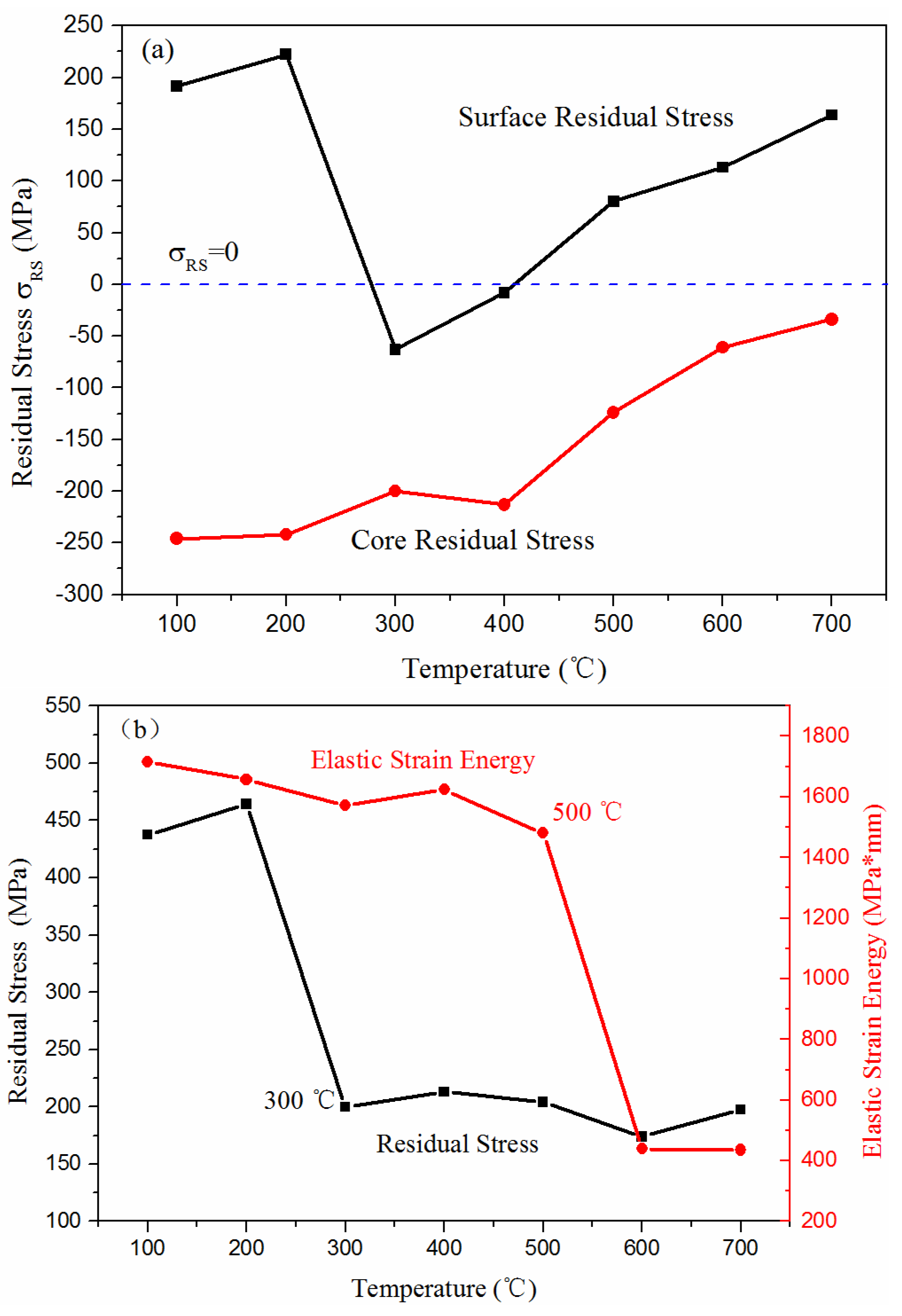
3.2. Residual Stress Test
3.2.1. Characterization Parameters of Residual Stress
3.2.2. Measurement Result of Residual Stress
3.3. Effect of Evolution of Microstructure on Residual Stress
4. Discussion
4.1. Transformation Plasticity during Tempering
4.2. Effect of Carbides Precipitation on Residual Stress
4.3. Regulation of Residual Stress by High-Temperature Creep
5. Conclusions
- (1)
- The tempering process of low-carbon 700 L included the following six stages—carbon segregation and cementite I precipitation, retained austenite decomposition, cementite II precipitation, cementite dissolution, alloy carbide precipitation, and Mn partitioning. In addition, three hardness peaks appeared in the cementite II precipitation, alloy carbide precipitation, and Mn partitioning stages.
- (2)
- 700 L has two distinct residual stress adjustment stages during tempering, which are related to the transformation of the microstructure. The first stage overlapped with cementite II precipitation, and the absolute value of residual stress was reduced from 487 MPa to 200 MPa; however, the elastic deformation energy remained unchanged. The second stage was for temperatures of 450–650 °C, and the absolute value of residual stress was reduced to 174 MPa. During this stage, the elastic deformation energy was reduced by 72.72%.
- (3)
- Precipitation plasticity was the main reason for the adjustment of residual stress during the first stage. The direction of the initial residual stress determined the direction of the precipitation plastic strain caused by the precipitation of carbides. This led to different trends in residual stress on the material’s surface and in the core during tempering. The adjustment of the residual stress at this stage reduced the micro-stress formed from the volume mismatch during the quenching process but had limited ability to adjust the macroscopic residual stress.
- (4)
- Although the second residual stress adjustment stage overlapped with the alloy carbide precipitation and Mn partitioning stages, the carbide precipitation only reduced the elastic strain energy by 8.7%. It is inferred that the activation energy for creep was the main driving force for the adjustment of the residual stress during the second stage. The initial residual stress provided an applied stress that generated creep, and the tempering temperature enhanced the driving force generated by the creep. Tempering at 600 °C could improve both the micro-stress and macro-stress in 700 L steel.
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Gong, P.; Palmiere, E.J.; Rainforth, W.M. Thermomechanical processing route to achieve ultrafine grains in low carbon micro-alloyed steels. Acta Mater. 2016, 119, 43–54. [Google Scholar] [CrossRef]
- Ariza, E.A.; Martorano, M.A.; De Lima, N.B.; Tschiptschin, A.P. Numerical Simulation with Thorough Experimental Validation to Predict the Build-up of Residual Stresses during Quenching of Carbon and Low-Alloy Steels. ISIJ Int. 2014, 54, 1396–1405. [Google Scholar] [CrossRef]
- Ding, W.; Liu, Y.; Xie, J.; Sun, L.; Liu, T. Influence of Stress on Kinetics and Transformation Plasticity of Ferrite Transformation Based on Hysteresis Effects. Metals 2019, 9, 73. [Google Scholar]
- Preciado, M.; Pellizzari, M. Influence of deep cryogenic treatment on the thermal decomposition of Fe-C martensite. J. Mater. Sci. 2014, 49, 8183–8191. [Google Scholar] [CrossRef]
- Lerchbacher, C.; Zinner, S.; Leitner, H. Direct or indirect: Influence of type of retained austenite decomposition during tempering on the toughness of a hot-work tool steel. Mater. Sci. Eng. A 2013, 564, 163–168. [Google Scholar] [CrossRef]
- Vieira, I.; Klemm-Toole, J.; Buchner, E.; Williamson, D.L.; Findley, K.O.; De Moor, E. A Dilatometric Study of Tempering Complemented by Mössbauer Spectroscopy and other Characterization Techniques. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Ritter, J.C.; Mcpherson, R. Anisothermal Stress Relaxation in a Carbon-maganese Steel. J. Iron Steel Inst. 1970, 10, 935–941. [Google Scholar]
- Chilukuru, H.; Durst, K.; Wadekar, S.; Schwienheer, M.; Scholz, A.; Berger, C.; Mayer, K.H.; Blum, W. Coarsening of precipitates and degradation of creep resistance in tempered martensite steels. Mater. Sci. Eng. A 2009, 510–511, 81–87. [Google Scholar] [CrossRef]
- Humphries, S.R.; Yeung, W.Y.; Callaghan, M.D. The effect of stress relaxation loading cycles on the creep behavior of 2.25Cr–1Mo pressure vessel steel. Mater. Sci. Eng. A 2011, 528, 1216–1220. [Google Scholar] [CrossRef]
- Greenwood, G.W.; Johnson, R.H. The Deformation of Metals under Small Stresses during Phase Transformations. Proc. R. Soc. Lond. Ser. A 1965, 283, 403–422. [Google Scholar]
- Denis, S.; Gautier, E.; Simon, A.; Beck, G. Stress-Phase-Transformation Interactions-Basic Principles, Modeling, and Calculation of Internal Stresses. Mater. Sci. Technol. 1985, 1, 805–814. [Google Scholar] [CrossRef]
- Morra, P.V.; Radelaar, S.; Yandouzi, M.; Chen, J.; Bottger, A.J. Precipitate coarsening-induced plasticity: Low temperature creep behaviour of tempered SAE 52100. Int. J. Plast. 2009, 25, 2331–2348. [Google Scholar] [CrossRef]
- Kaiser, D.; de Graaff, B.; Jung, A.M.; Dietrich, S.; Schulze, V. A dilatometric study on the influence of compressive stresses on the tempering of martensitic AISI 4140 steel-Evidence of transformation induced plasticity during cementite precipitation. Mater. Sci. Eng. A 2017, 705, 114–121. [Google Scholar] [CrossRef]
- Bensely, A.; Venkatesh, S.; Mohan, L.D.; Nagarajan, G.; Rajadurai, A.; Junik, K. Effect of cryogenic treatment on distribution of residual stress in case carburized En 353 steel. Mater. Sci. Eng. A 2008, 479, 229–235. [Google Scholar] [CrossRef]
- Chen, J.F.; Jiang, J.T.; Zhen, L.; Shao, W.Z. Stress relaxation behavior of an Al–Zn–Mg–Cu alloy in simulated age-forming process. J. Mater. Process. Technol. 2014, 214, 775–783. [Google Scholar] [CrossRef]
- Prime, M.B. Residual stress measurement by successive extension of a slot: The crack compliance method. Appl. Mech. Rev. 1999, 2, 75–96. [Google Scholar] [CrossRef]
- Prime, M.B. Uncertainty Analysis, Model Error, and Order Selection for Series-Expanded, Rwsidual Stress Inverse Solutions. J. Eng. Mater. Technol. 2006, 128, 175–185. [Google Scholar] [CrossRef]
- Mittemeijer, E.J. Analysis of the kinetics of phase transformations. J. Mater. Sci. 1992, 27, 3977–3987. [Google Scholar] [CrossRef]
- Waterschoot, T.; Verbeken, K.; De Cooman, B.C. Tempering Kinetics of the Martensitic Phase in DP Steel. ISIJ Int. 2006, 46, 138–146. [Google Scholar] [CrossRef]
- Leiva, J.A.V.; Morales, E.V.; Villar-Cociña, E.; Donis, C.A.; Bott, I.S. Kinetic parameters during the tempering of low-alloy steel through the non-isothermal dilatometry. J. Mater. Sci. 2010, 45, 418–428. [Google Scholar] [CrossRef]
- Talebi, S.H.; Ghasemi-Nanesa, H.; Jahazi, M.; Melkonyan, H. In Situ Study of Phase Transformations during Non-Isothermal Tempering of Bainitic and Martensitic Microstructures. Metals 2017, 7, 346. [Google Scholar] [CrossRef]
- Morra, P.V.; Böttger, A.J.; Mittemeijer, E.J. Decomposition of Iron-based Martensite. A kinetic analysis by means of differential scanning calorimetry and dilatometry. J. Therm. Anal. Calorim. 2001, 64, 905–914. [Google Scholar] [CrossRef]
- Ustinovshchikov, Y.I. Secondary hardening mechanism of alloy steels. Met. Sci. 1984, 18, 337–344. [Google Scholar] [CrossRef]
- Yamasaki, S.; Bhadeshia, H.K. Modelling and characterisation of Mo2C precipitation and cementite dissolution during tempering of Fe–C–Mo martensitic steel. Mater. Sci. Technol. 2013, 19, 723–731. [Google Scholar] [CrossRef]
- Clarke, A.J.; Miller, M.K.; Field, R.D.; Coughlin, D.R.; Gibbs, P.J.; Clarke, K.D.; Alexander, D.J.; Powers, K.A.; Papin, P.A.; Krauss, G. Atomic and nanoscale chemical and structural changes in quenched and tempered 4340 steel. Acta Mater. 2014, 77, 17–27. [Google Scholar] [CrossRef]
- Ande, A.K.; Sluiter, M.H. First-Principles Calculations on Stabilization of Iron Carbides (Fe3C, Fe5C2, and η-Fe2C) in Steels by Common Alloying Elements. Metall. Mater. Trans. A 2012, 43, 4436–4444. [Google Scholar] [CrossRef]
- Yang, S.; Shang, C.; He, X.; Wang, X.; Yuan, Y. Stability of Ultra-fine Microstructures during Tempering. J. Univ. Sci. Technol. Beijing 2001, 8, 119–122. [Google Scholar]
- Villa, M.; Niessen, F.; Somers, M.A. In Situ Investigation of the Evolution of Lattice Strain and Stresses in Austenite and Martensite During Quenching and Tempering of Steel. Metall. Mater. Trans. A 2018, 49, 28–40. [Google Scholar] [CrossRef]
- Schajer, G.S. Practical Residual Stress Measurement Methods; John Wiley & Sons, Ltd.: Vancouver, BC, Canada, 2013; pp. 89–106. [Google Scholar]
- Taleb, L.; Cavallo, N.; Waeckel, F. Experimental analysis of transformation plasticity. Int. J. Plast. 2001, 17, 1–20. [Google Scholar] [CrossRef]
- Taleb, L.; Petit, S. New investigations on transformation induced plasticity and its interaction with classical plasticity. Int. J. Plast. 2006, 22, 110–130. [Google Scholar] [CrossRef]
- Mukherjee, A.K.; Bird, J.E.; Dorn, J.E. Experimental Correlations for High-Temperature Creep. Trans. ASM 1969, 62, 155–179. [Google Scholar]





| C | Si | Mn | P | S | Nb | Al | Ti | Fe |
|---|---|---|---|---|---|---|---|---|
| 0.079 | 0.072 | 1.460 | 0.011 | 0.002 | 0.051 | 0.039 | 0.095 | Bal. |
| Residual Stress | Before Tempering | After 200 °C Tampering | After 300 °C Tampering | After 600 °C Tampering |
|---|---|---|---|---|
| (MPa) | 287 | 218 | 0 | 113 |
| (MPa) | −200 | −242 | −200 | −61 |
| (MPa) | 487 | 460 | 200 | 174 |
| % | 100% | 94.45% | 41.07% | 35.73% |
| Elastic Strain Energy | Before Tempering | After 200 °C Tampering | After 300 °C Tampering | After 600 °C Tampering |
|---|---|---|---|---|
| style="border-bottom:solid thin">Elastic strain energy (MPa∙mm) | 1609 | 1657 | 1572 | 439 |
| % | 100% | 102.98% | 97.70% | 27.28% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, W.; Liu, Y.; Xie, J.; Sun, L.; Liu, T.; Yuan, F.; Pan, J. Effect of Carbide Precipitation on the Evolution of Residual Stress during Tempering. Metals 2019, 9, 709. https://doi.org/10.3390/met9060709
Ding W, Liu Y, Xie J, Sun L, Liu T, Yuan F, Pan J. Effect of Carbide Precipitation on the Evolution of Residual Stress during Tempering. Metals. 2019; 9(6):709. https://doi.org/10.3390/met9060709
Chicago/Turabian StyleDing, Wenhong, Yazheng Liu, Jianxin Xie, Li Sun, Tianwu Liu, Fei Yuan, and Jin Pan. 2019. "Effect of Carbide Precipitation on the Evolution of Residual Stress during Tempering" Metals 9, no. 6: 709. https://doi.org/10.3390/met9060709
APA StyleDing, W., Liu, Y., Xie, J., Sun, L., Liu, T., Yuan, F., & Pan, J. (2019). Effect of Carbide Precipitation on the Evolution of Residual Stress during Tempering. Metals, 9(6), 709. https://doi.org/10.3390/met9060709





