Effect of Electron Beam Method on Processing of Titanium Technogenic Material
Abstract
:1. Introduction
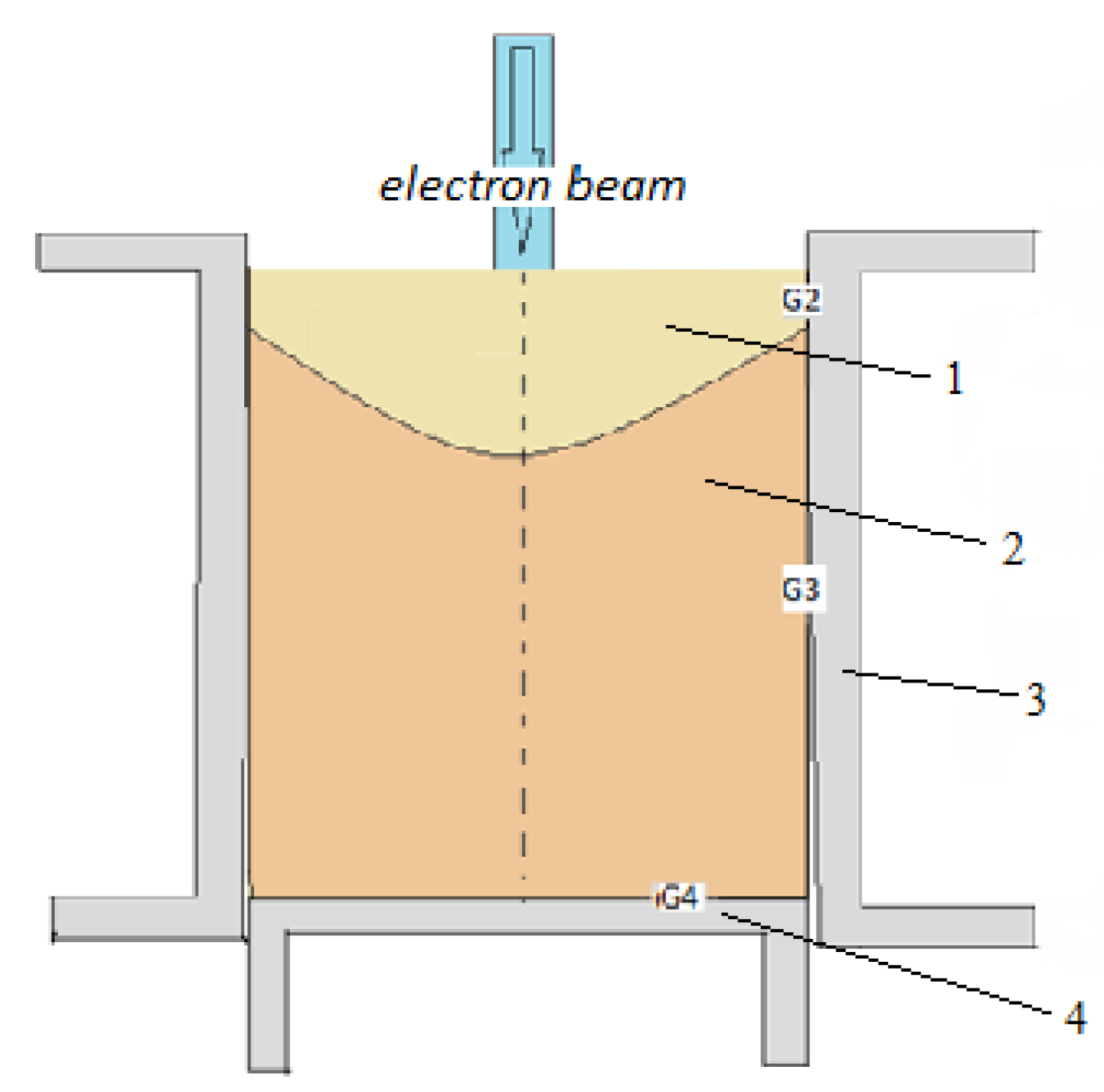
2. Materials and Methods
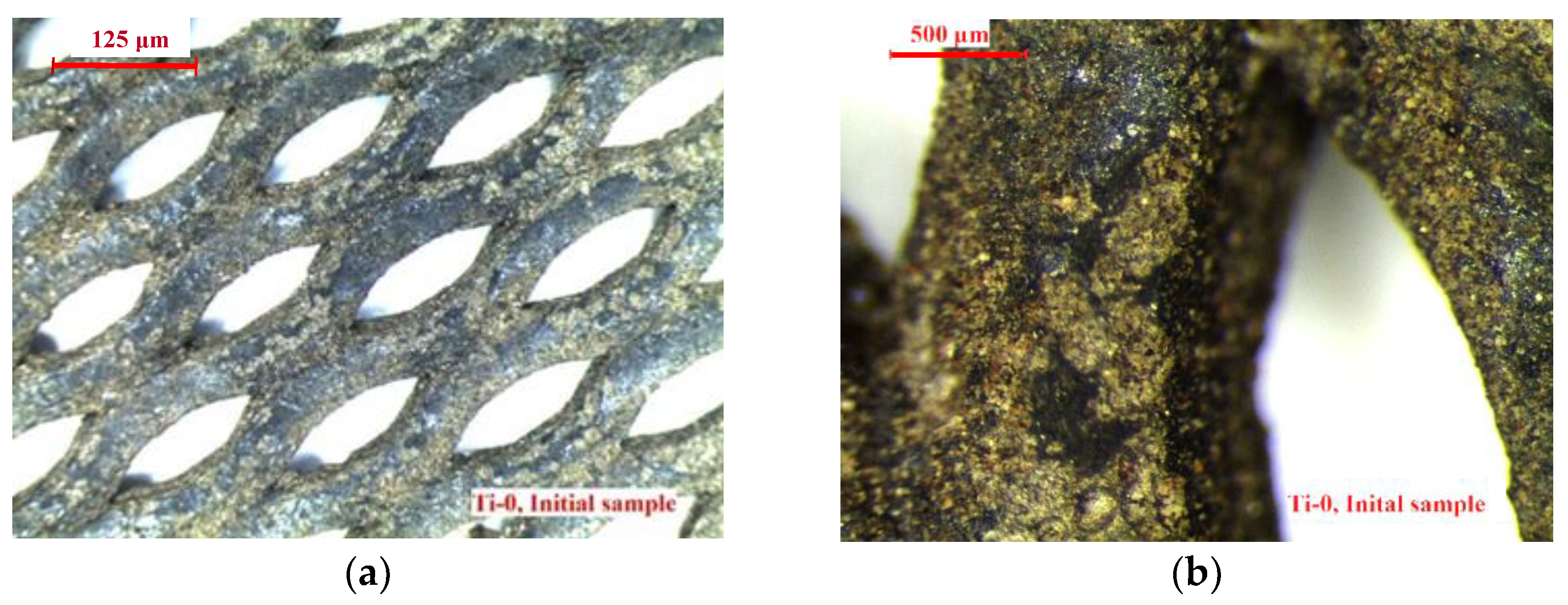
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgements
Conflicts of Interest
References
- Boyer, R.R. An overview on the use of titanium in the aerospace industry. Mater. Sci. Eng. A 1996, 213, 103–114. [Google Scholar] [CrossRef]
- Lutjering, G.; Williams, J.C. Titanium, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2007; p. 442. [Google Scholar]
- Banerjee, D.; Williams, J.C. Perspectives on Titanium Science and Technology. Acta Mater. 2013, 61, 844–879. [Google Scholar] [CrossRef]
- Huo, D.; Liang, J.; Li, H.; Xie, S.; Yang, Y. Progress of Research and Application of Titanium Alloy. Foundry Technol. 2016, 10, 2065–2066. [Google Scholar]
- Gao, L.; Huang, H.; Zhang, Y.; Zhang, H.; Shi, Z.; Jiang, Y.; Zhou, R. Numerical Modeling of EBCHM for Large-Scale TC4 Alloy Round Ingots. JOM 2018, 70, 2934–2942. [Google Scholar] [CrossRef]
- Roh, K.-M.; Suh, C.-Y.; Oh, J.-M.; Kim, W.; Kwon, H.; Lim, J.-W. Comparison of deoxidation capability for preparation of low oxygen content powder from TiNi alloy scraps. Powder Technol. 2014, 253, 266–269. [Google Scholar] [CrossRef]
- Zhao, Y.Q.; Ge, P. Current situation and development of new titanium alloys invented in China. J. Aeronaut. Mater. 2014, 34, 51–61. [Google Scholar]
- An, H.; Liu, J.L.; Fan, L.Y. Quality control in smelting titanium ingots in vacuum arc-melting furnace. World Nonferr. Met. 2007, 8, 25–27. [Google Scholar]
- Jin, H.X.; Wei, K.X.; Li, J.M.; Zhou, J.Y.; Peng, W.J. Research development of titanium alloy in aerospace industry. Chin. J. Nonferr. Met. 2015, 25, 280–292. [Google Scholar]
- Mitchell, A. The electron beam melting and refining of titanium alloys. Mater. Sci. Eng. A 1999, 263, 217–223. [Google Scholar] [CrossRef]
- Tan, Y.; Shi, S. Progress in research and development of electron beam technology in metallurgy refining field. J. Mater. Eng. 2013, 8, 92–100. [Google Scholar]
- Vassileva, V.; Vutova, K.; Mladenov, G. An Investigation on the Heat Transfer Influence on the Crystallisation Processes during the Electron Beam Melting and Casting of Metals. Vacuum 2001, 62, 197–202. [Google Scholar] [CrossRef]
- Wang, X.; Liu, L.Q.; Li, X.M. Effect of crystallizer’s three-dimension on the solid-liquid interface morphology of the large-scale Ti64 during EBCHM. Mater. Res. Express 2019. [Google Scholar] [CrossRef]
- Liu, Q.L.; Li, X.M.; Jiang, Y.H. Microstructure evolution of large-scale titanium slab ingot based on CAFE method during EBCHM. J. Mater. Res. 2017, 32, 3175–3182. [Google Scholar] [CrossRef]
- Park, H.K.; Ahn, Y.K.; Lee, B.S.; Jung, K.H.; Lee, C.W.; Kim, H.G. Refining effect of electron beam melting on additive manufacturing of pure titanium products. Mater. Lett. 2017, 187, 98–100. [Google Scholar] [CrossRef]
- Dolimont, A.; Michotte, S.; Riviére-Lorphéver, E.; Ducobu, F.; De Formanoir, C.; Godet, S.; Filippi, E. Characterization of electron beam melting process (EBM): Capability approach. In Proceedings of the ASPE/Euspen 2016 Summer Topical Meeting: Dimensional Accuracy and Surface Finish in Additive Manufacturing, Raleigh, NC, USA, 27–30 July 2016; pp. 16–21. [Google Scholar]
- Vassileva, V.; Mladenov, G.; Vutova, K.; Nikolov, T.; Georgieva, E. Oxygen removal during electron beam drip melting and refining. Vacuum 2005, 77, 429–436. [Google Scholar] [CrossRef]
- You, Q.; Yuan, H.; You, X.; Li, J.; Zhao, L.; Shi, S.; Tan, Y. Segregation behavior of nickel-based superalloy after electron beam smelting. Vacuum 2017, 145, 116–122. [Google Scholar] [CrossRef]
- Vutova, K.; Vassileva, V.; Naplatanova, M.; Tanaka, T. Refining effect of electron beam melting on recycling of nickel wastes. In Proceedings of the International Spring Seminar on Electronics Technology, Sofia, Bulgaria, 10–14 May 2017. [Google Scholar]
- Liu, Q.L.; Li, X.M.; Jiang, Y.H. Research progress of electron beam cold hearth melting for titanium and titanium alloys. Hot Work. Technol. 2016, 45, 9–14. [Google Scholar]
- Dolimont, A.; Michotte, S.; Rivière-Lorphèvre, E.; Ducobu, F.; Formanoir, C.D.; Godet, S.; Filippi, E. Characterisation of electron beam melting process on Ti6Al4V in order to guide finishing operation. Int. J. Rapid Manuf. 2015, 5, 320–338. [Google Scholar] [CrossRef]
- Chen, X.; Li, X.; Jiang, Y.; Zhou, R. Effects of drawing velocity and casting temperature on solidification interface of the flat titanium ingot. Spec. Cast. Nonferr. Alloys 2016, 36, 412–415. [Google Scholar]
- Liu, Q.; Jiang, Y.; Li, X. The effect of bulk nucleation parameters on the formation of macroscopic grain of the large-scale titanium slab ingot during EBCHM. Mater. Sci. Technol. 2018, 34, 1649–1656. [Google Scholar] [CrossRef]
- Oh, J.-M.; Lee, B.-K.; Choi, G.-S.; Kim, H.-S.; Lim, J.-W. Preparation of ultrahigh purity cylindrical tantalum ingot by electron beam drip melting without sintering process. Mater. Sci. Technol. 2013, 29, 542–546. [Google Scholar] [CrossRef]
- Vutova, K.; Vassileva, V.; Koleva, E.; Georgieva, E.; Mladenov, G.; Mollov, D.; Kardjiev, M. Investigation of electron beam melting and refining of titanium and tantalum scrap. J. Mater. Process. Technol. 2010, 210, 1089–1094. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, Y.; Liu, W.; Long, L. Preparation procedure of high purity tungsten via electron beam side surface melting. Mater. Sci. Technol. 2014, 22, 30–35. [Google Scholar]
- Vutova, K.; Vassileva, V.; Koleva, E.; Munirathnam, N.; Amalnerkar, DP.; Tanaka, T. Investigation of Tantalum Recycling by Electron Beam Melting. Metals 2016, 6, 287. [Google Scholar] [CrossRef]
- Su, B.; Chen, D.M.; Wang, Z.H.; Ma, R.; Li, Y.F.; Xia, S.Q. Numerical Simulation of Electron Beam Melting Vanadium Alloys by Finite Element Method. Rare Met. Mater. Eng. 2019, 48, 711–715. [Google Scholar]
- Yao, K.; Min, X.; Shi, S.; Tan, Y. Volatilization Behavior of β-Type Ti-Mo Alloy Manufactured by Electron Beam Melting. Metals 2018, 8, 206. [Google Scholar] [CrossRef]
- Choi, S.H.; Jang, B.Y.; Lee, J.S.; Ahn, Y.S.; Yoon, W.Y.; Joo, J.H. Effects of electron beam patterns on melting and refining of silicon for photovoltaic applications. Renew. Energy 2013, 54, 40–45. [Google Scholar] [CrossRef]
- Tan, Y.; Guo, X.L.; Shi, S.; Dong, W.; Jiang, D.C. Study on the removal process of phosphorus from silicon by electron beam melting. Vacuum 2013, 93, 65–70. [Google Scholar] [CrossRef]
- You, X.G.; Tan, Y.; You, Q.F.; Shi, S.; Li, J.Y.; Ye, F.; Wei, X. Preparation of Inconel 740 superalloy by electron beam smelting. J. Alloys Compd. 2016, 676, 202–208. [Google Scholar] [CrossRef]
- You, Q.; Yuan, H.; Zhaoa, L.; Lia, J.; Youa, X.; Shia, S.; Tana, Y.; Ding, X. Removal inclusions from nickel-based superalloy by induced directional solidification during electron beam smelting. Vacuum 2018, 156, 39–47. [Google Scholar] [CrossRef]
- Voort, G.V. Preparation of Titanium and its Alloys. Tech. Notes 2015, 3, 1–5. [Google Scholar]
- Pederson, R. Microstructure and Phase Transformation of Ti-6Al-4V. Licentiate Thesis, Lulea University of Technology, Lulea, Sweden, 2002. [Google Scholar]
- Murr, L.E.; Quinones, S.A.; Gaytan, S.M.; Lopez, M.I.; Rodela, A.; Martinez, E.Y.; Hernandez, D.H.; Martinez, E.; Medina, F.; Wicker, R.B. Microstructure and mechanical behavior of Ti–6Al–4V produced by rapid-layer manufacturing, for biomedical applications. J. Mech. Behav. Biomed. Mater. 2009, 2, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Murr, L.E.; Amato, K.N.; Li, S.J.; Tian, Y.X.; Cheng, X.Y.; Gaytan, S.M.; Martinez, E.; Shindo, P.W.; Medina, F.; Wicker, R.B. Microstructure and mechanical properties of open-cellular biomaterials prototypes for total knee replacement implants fabricated by electron beam melting. J. Mech. Behav. Biomed. Mater. 2011, 4, 1396–1411. [Google Scholar] [CrossRef] [PubMed]
- Al-Bermani, S.; Blackmore, M.; Zhang, W.; Todd, I. The Origin of Microstructural Diversity, Texture, and Mechanical Properties in Electron Beam Melted Ti6Al4V. Metall. Mater. Trans. A 2010, 41, 3422–3434. [Google Scholar] [CrossRef]
- Brandl, E.; Schoberth, A.; Leyens, Ch. Morphology, microstructure, and hardness of titanium (Ti-6Al-4V) blocks deposited by wire-feed additive layer manufacturing (ALM). Mater. Sci. Eng. A 2012, 532, 295–307. [Google Scholar] [CrossRef]


| Pb/τ | 15 min | 25 min | 40 min |
|---|---|---|---|
| 2.5 kW | 92.5% | 94.79% | 99.84% |
| 3.5 kW | 96.9% | 97.9% | 99.97% |
| 5.5 kW | 98.71% | 99.8% | 99.975% |
| Pb/τ | 15 min | 25 min | 40 min |
|---|---|---|---|
| 2.5 kW | 1.7 g | 2.1 g | 3.8 g |
| 3.5 kW | 2.6 g | 3.2 g | 4.4 g |
| 5.5 kW | 3.2 g | 5.4 g | 5.8 g |
| Sample № | Process Parameters | Ci (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| P (kW) | τ (min) | Si | W | Ta | Al | Cu | Cd | Fe | Ir | |
| Ti-02 | 2.5 | 25 | 0.001 | 0.02 | 0.2 | 0.002 | 0.0003 | 0.3 | 0.3 | 0.01 |
| Ti-04 | 2.5 | 40 | 0.002 | 0.01 | 0 | 0.002 | 0.002 | 0.01 | 0 | 0 |
| Ti-03 | 3.5 | 25 | 0 | 0.02 | 0.2 | 0.003 | 0.0003 | 0.01 | 0.1 | 0.003 |
| Ti-06 | 3.5 | 40 | 0.002 | 0 | 0 | 0.003 | 0 | 0 | 0 | 0 |
| Ti-01 | 5.5 | 15 | 0.003 | 0.1 | 0.1 | 0.003 | 0.0001 | 0 | 0 | 0 |
| Ti-05 | 5.5 | 40 | 0.002 | 0 | 0 | 0.002 | 0 | 0 | 0 | 0 |
| Concentrations before EBM | 4.94 | 0.1 | 0.03 | 3.28 | 1.52 | 0.1 | 2.36 | 3.66 | ||
| Sample № | Process Parameters | HBav (kg/mm2) | Standard Deviation | |
|---|---|---|---|---|
| P (kW) | τ (min) | |||
| Ti-02 | 2.5 | 25 | 282 | 28 |
| Ti-04 | 2.5 | 40 | 161 | 23 |
| Ti-03 | 3.5 | 25 | 292 | 25 |
| Ti-06 | 3.5 | 40 | 100 | 7 |
| Ti-01 | 5.5 | 15 | 205 | 17 |
| Ti-05 | 5.5 | 40 | 120 | 11 |
| before electron beam melting | 127 | 10 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vutova, K.; Vassileva, V.; Stefanova, V.; Amalnerkar, D.; Tanaka, T. Effect of Electron Beam Method on Processing of Titanium Technogenic Material. Metals 2019, 9, 683. https://doi.org/10.3390/met9060683
Vutova K, Vassileva V, Stefanova V, Amalnerkar D, Tanaka T. Effect of Electron Beam Method on Processing of Titanium Technogenic Material. Metals. 2019; 9(6):683. https://doi.org/10.3390/met9060683
Chicago/Turabian StyleVutova, Katia, Vania Vassileva, Vladislava Stefanova, Dinesh Amalnerkar, and Takeshi Tanaka. 2019. "Effect of Electron Beam Method on Processing of Titanium Technogenic Material" Metals 9, no. 6: 683. https://doi.org/10.3390/met9060683
APA StyleVutova, K., Vassileva, V., Stefanova, V., Amalnerkar, D., & Tanaka, T. (2019). Effect of Electron Beam Method on Processing of Titanium Technogenic Material. Metals, 9(6), 683. https://doi.org/10.3390/met9060683





