Statistical Analysis of the Combined ECAP and Heat Treatment for Recycling Aluminum Chips Without Remelting
Abstract
1. Introduction
2. Design of Experiments
3. Materials and Methods
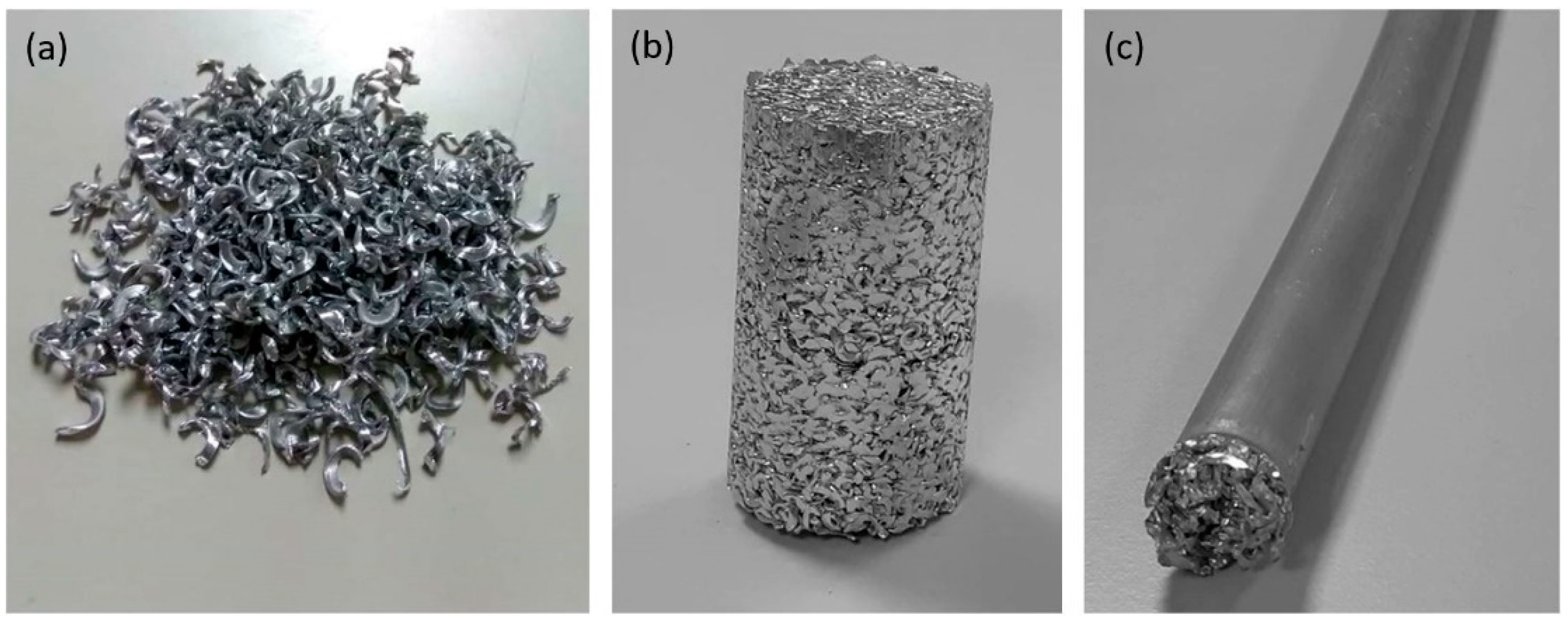
3.1. Machining Chips Fabrication and Cold Compaction
3.2. Direct Hot Extrusion
3.3. Combination of ECAP Process and Heat Treatment
4. Results Analysis and Discussion
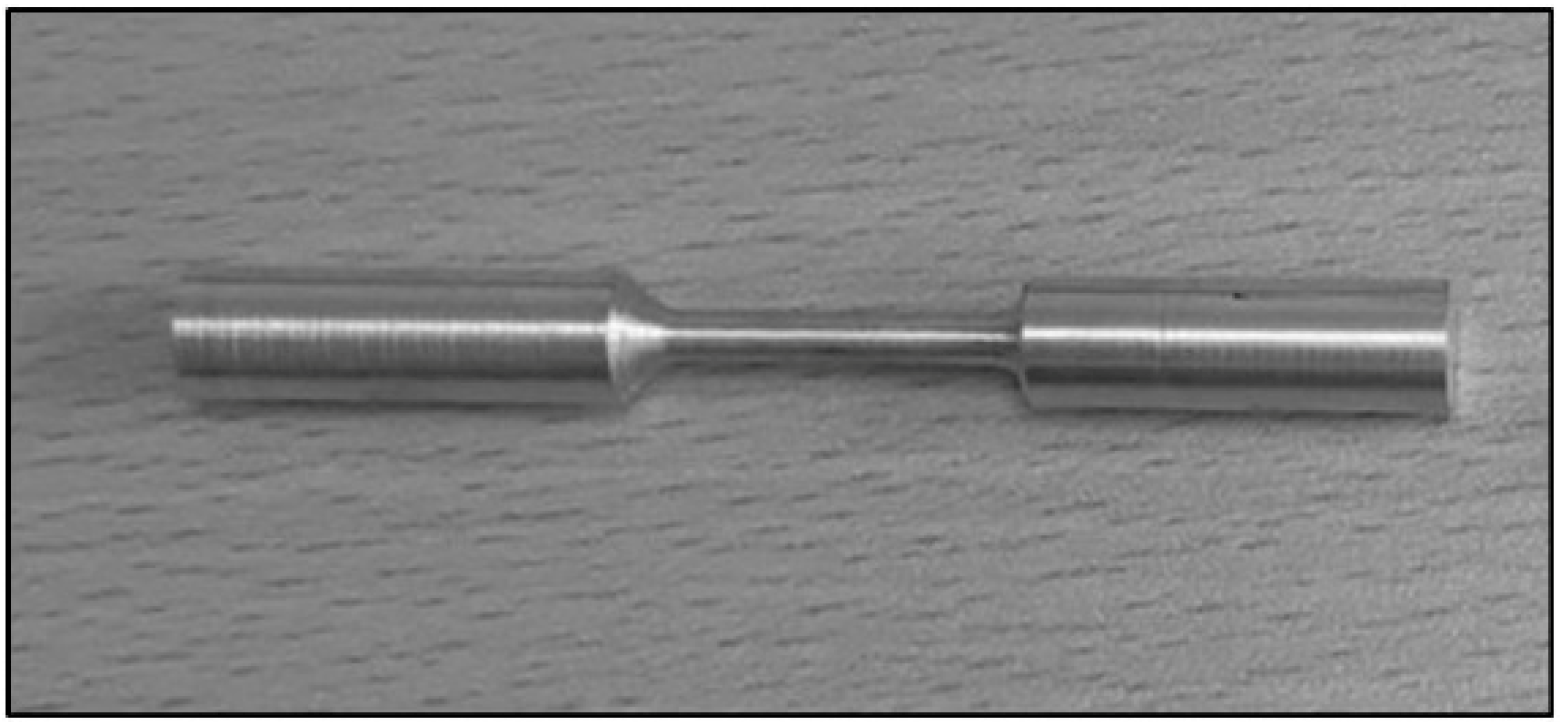
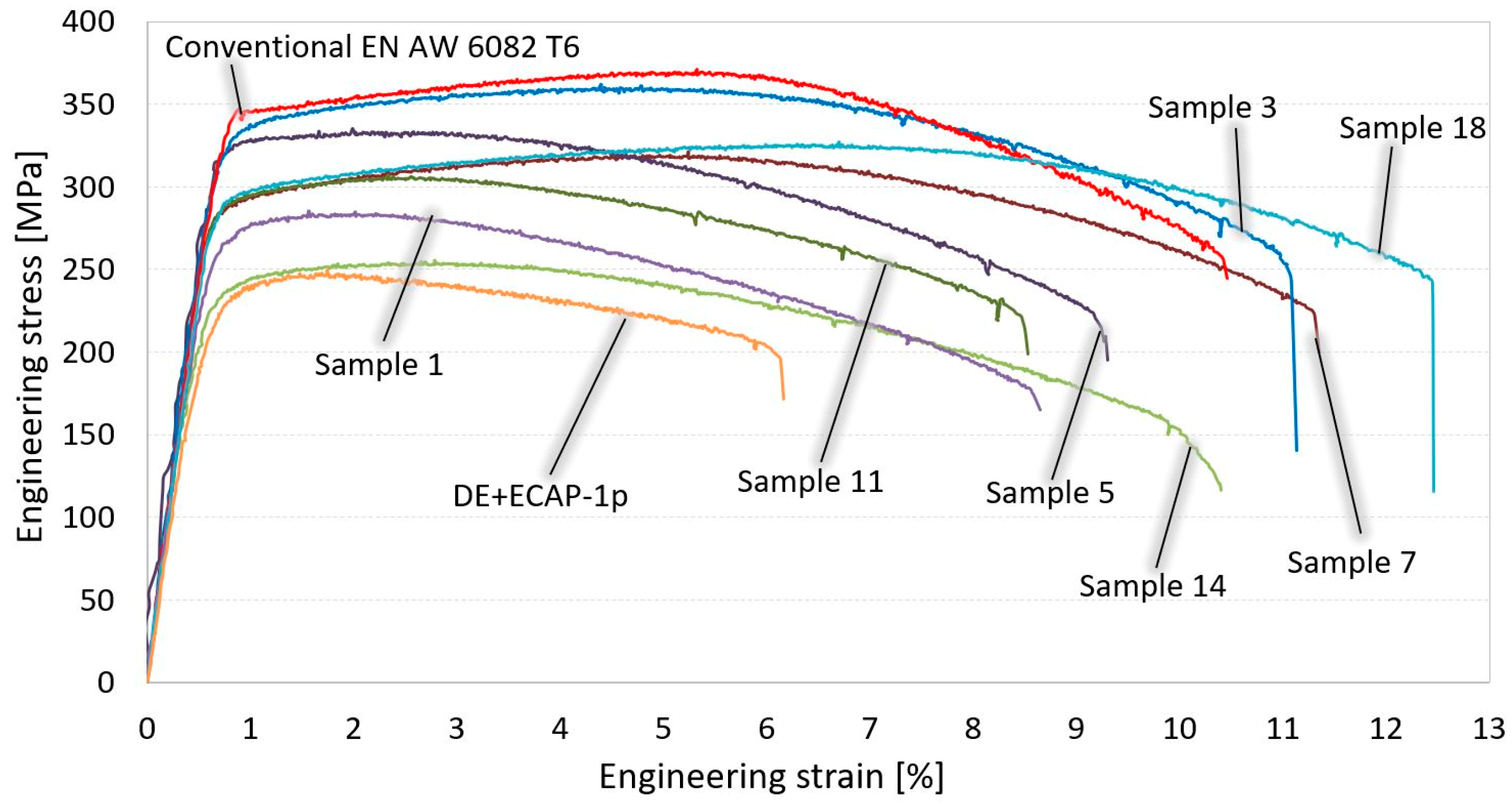
4.1. Tensile Testing Results
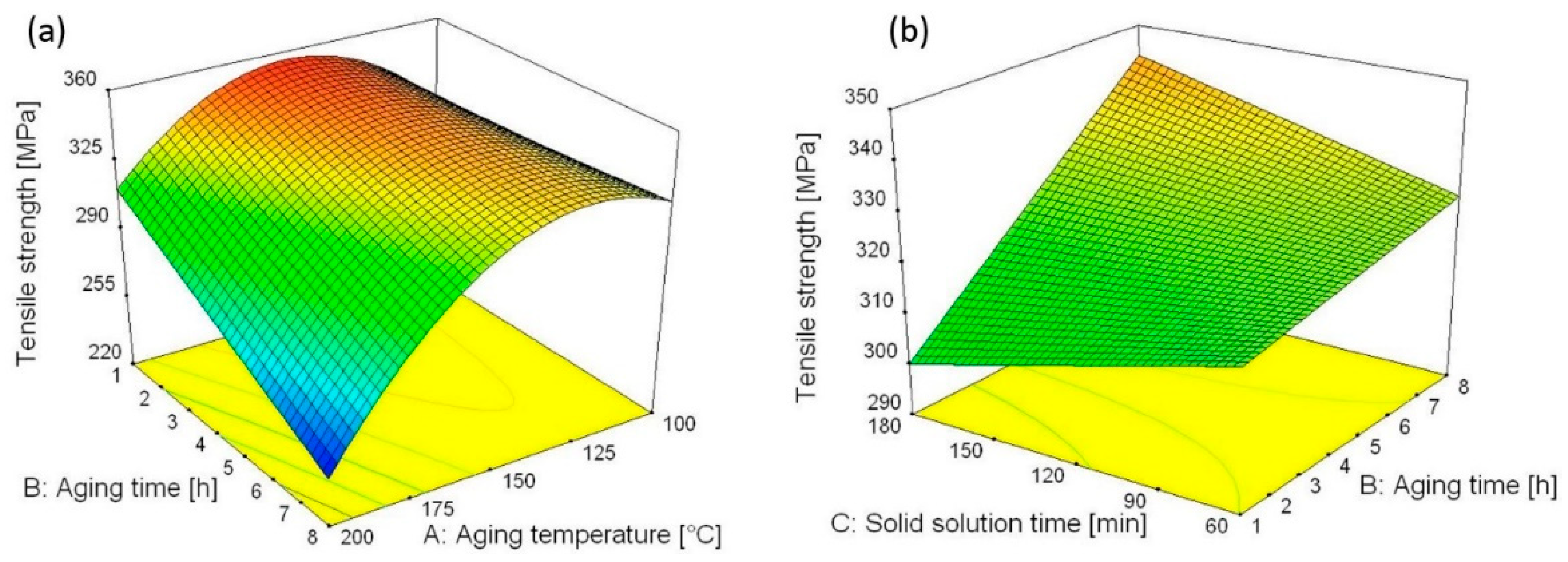
4.2. Regression Analysis for Ultimate Tensile Strength
4.3. Regression Analysis for Yield Strength
4.4. Percentage Elongation
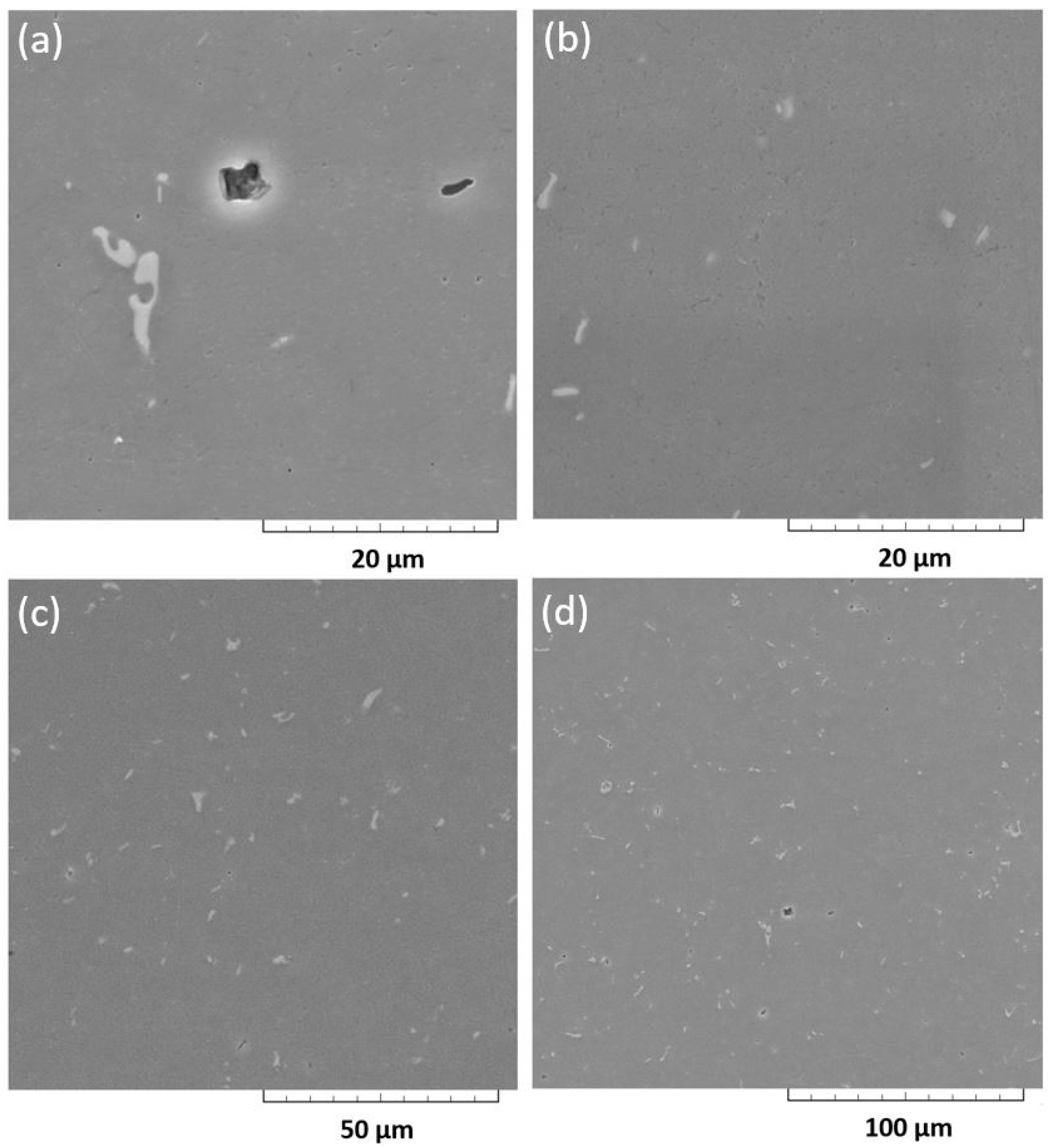
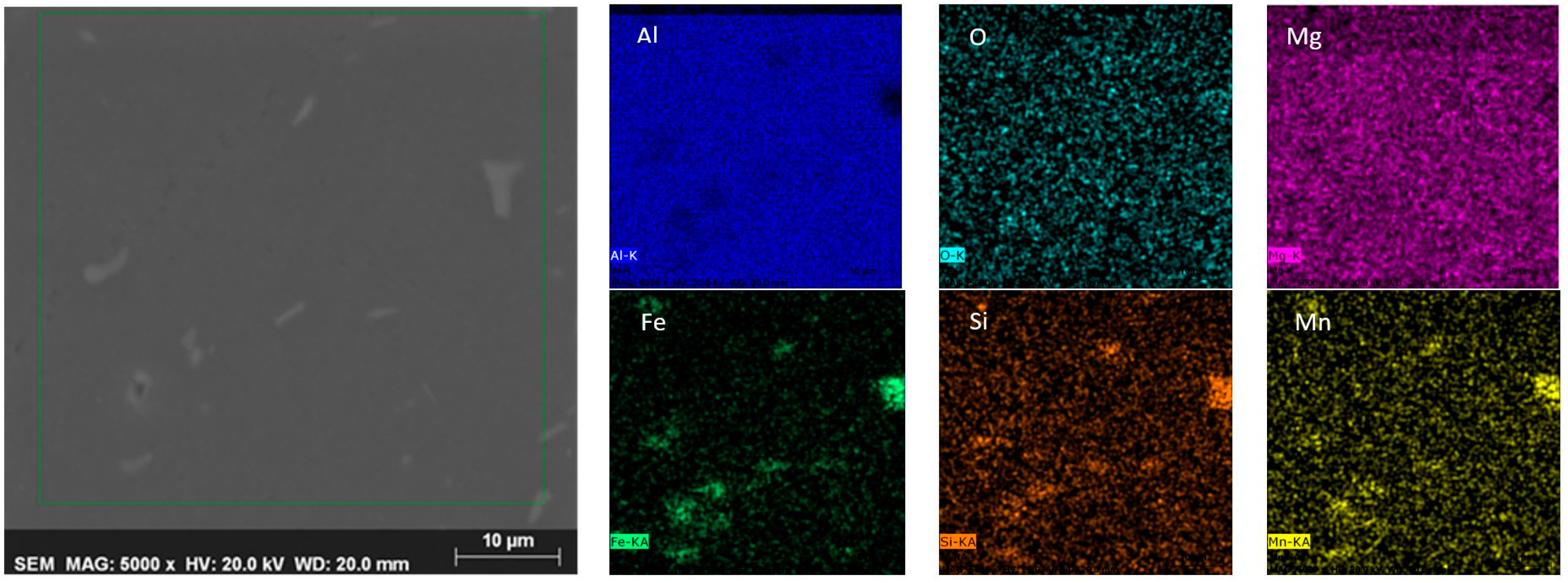
4.5. Metallographic Analysis
5. Conclusions
- (1)
- The lower extrusion ratio could be used for SSR process if afterwards ECAP process in combination with heat treatment will be applied. Combination of the heat treatment and ECAP after DE significantly improved mechanical properties of the SSR samples compared with SSR sample obtained only with DE and afterwards one ECAP pass.
- (2)
- Description and prediction of the influence of heat treatment parameters on Rm and Rp0.2 was successfully performed utilizing statistical analysis approach. Quadratic mathematical models were suggested for description of the influence of heat treatment parameters on Rm and Rp0.2 of SSR samples. Increase in aging time slightly increases Rm and Rp0.2 for lower aging temperature (100 °C). However, for higher aging temperature (200 °C), increase in aging time decreases Rm and Rp0.2. For constant artificial aging temperature at 100 °C and higher solid solution time (180 min), increase in artificial aging time, also increases Rm value. For lower solid solution time (60 min), increase in aging time increases Rm just slightly. For constant aging temperature at 150 °C, shorter solid solution time (60 min) and artificial aging time (1 h) Rp0.2 values significantly increases. According to the statistical analysis, the influence of heat treatment parameters on percentage elongation cannot be described with statistically significant mathematical model and therefore, mean value of the percentage elongation was taken as final (PEmean = 9.7%).
- (3)
- Heat treatment assisted in the improvement of SSR samples quality by the simultaneous interparticle diffusion bonding, recovery and precipitation hardening. However, too high artificial aging temperature or to long artificial aging time leads to decrement of the SPD effects and precipitation over-aging which caused mechanical properties decrement. Finally, according to the optimization results maximal values for Rm and Rp0.2 of 356 and 336.6 MPa would be obtained if artificial aging temperature, artificial aging time, and solid solution time were set as 148.2 °C, 1 h, and 60 min, respectively.
- (4)
- According to the SEM + EDX analysis microstructure of the recycled samples was very homogeneous and there were not any visible cracks, voids or porosity. Qualitative chemical analysis performed by elemental mapping showed that visible intermetallic phase particles were consisted of the same elements for the SSR samples and for conventionally obtained sample.
Author Contributions
Funding
Conflicts of Interest
References
- Stacey, M. Aluminium Recyclability and Recycling; Towards Sustainable Cities; Cwningen Press, International Aluminium Institute: Nottingham/Llundain, UK, 2015; Available online: http://www.world-aluminium.org/media/filer_public/2016/10/03/tsc_report2_arr_72dpi_release_locked_1016.pdf (accessed on 15 May 2019).
- International Aluminium Institute. Global Aluminium Recycling: A Cornerstone of Sustainable Development. Available online: http://world-aluminium.org/media/filer_public/2013/01/15/fl0000181.pdf (accessed on 15 May 2019).
- Cullen, J.M.; Allwood, J.M. Mapping the Global Flow of Aluminum: From Liquid Aluminum to End-Use Goods. Environ. Sci. Technol. 2013, 47, 3057–3064. [Google Scholar] [CrossRef] [PubMed]
- Allwood, J.M.; Cullen, J.M. Sustainable Materials with Both Eyes Open; UIT Cambridge Ltd.: Cambridge, UK, 2012. [Google Scholar]
- Duflou, J.R.; Tekkaya, A.E.; Haase, M.; Welo, T.; Vanmeensel, K.; Kellens, K.; Dewulf, W.; Paraskevas, D. Environmental assessment of solid state recycling routes for aluminum alloys: Can solid state processes significantly reduce the environmental impact of aluminum recycling? CIRP Ann. Manuf. Technol. 2015, 64, 37–40. [Google Scholar] [CrossRef]
- Gronostajski, J.; Matuszak, A. The recycling of metals by plastic deformation: An example of recycling of aluminum and its alloy chips. J. Mater. Process. Technol. 1999, 92, 35–41. [Google Scholar] [CrossRef]
- Tekkaya, A.E.; Schikorra, M.; Becker, D.; Biermann, D.; Hammer, N.; Pantke, K. Hot profile extrusion of AA-6060 aluminum chips. J. Mater. Process. Technol. 2009, 209, 3343–3350. [Google Scholar] [CrossRef]
- Allwood, J.M.; Cullen, J.M.; Cooper, D.R.; Milford, R.L.; Patel, A.C.H.; Carruth, M.A.; McBrien, M. Conserving Our Metal Energy: Avoiding Melting Steel and Aluminum Scrap to Save Energy and Carbon. WellMet2050 Report, University of Cambridge. Available online: https://www.uselessgroup.org/files/wellmet2050-conserving-our-metal-energy-sept-2010-web.pdf (accessed on 19 July 2018).
- Paraskevas, D.; Kellens, K.; Renaldi, W.D.; Duflou, J.R. 4.10 Resource Efficiency in Manufacturing: Identifying Low Impact Paths. In Proceedings of the 10th Global Conference on Sustainable Manufacturing (GCSM2012), Instabul, Turkey, 31 October–2 November 2012; pp. 271–276. [Google Scholar]
- Shamsudin, S.; Lajis, M.A.; Zhong, Z.W. Solid-state recycling of light metals: A review. Adv. Mech. Eng. 2016, 8, 1–23. [Google Scholar] [CrossRef]
- Vrelinden, B. Severe plastic deformation of metals. Metal. J. Metall. 2003, 165–182. [Google Scholar] [CrossRef]
- Cui, J.; Roven, H.J. Recycling of automotive aluminum. Trans. Nonferrous Met. Soc. China 2010, 20, 2057–2063. [Google Scholar] [CrossRef]
- Luo, P.; McDonald, D.T.; Zhu, S.M.; Palanisamy, S.; Dargusch, M.S.; Xia, K. Analysis of microstructure and strenghtening in pure titanium recycled from machining chips by equal channel angular pressing using electron backscatter diffraction. Mater. Sci. Eng. A 2012, 538, 252–258. [Google Scholar] [CrossRef]
- Cui, J.R.; Guo, W.; Roven, H.J.; Wang, Q.D.; Chen, Y.J.; Peng, T. Recycling of Aluminum Scrap by Severe Plastic Deformation. Mater. Sci. Forum 2011, 667, 1177–1182. [Google Scholar] [CrossRef]
- Mohamed, I.A.E.A.; Eun, Y.Y.; Hyoung, S.K. Recycling of AlSi8Cu3 alloy chips via high pressure torsion. Mater. Sci. Eng. A 2013, 560, 121–128. [Google Scholar] [CrossRef]
- Tang, W.; Reynolds, A.P. Production of wire via friction extrusion of aluminum alloy machining chips. J. Mater. Process. Technol. 2010, 210, 2231–2237. [Google Scholar] [CrossRef]
- Li, X.; Baffari, D.; Reynolds, A.P. Friction stir consolidation of aluminum machining chips. Int. J. Adv. Manuf. Technol. 2018, 94, 2031–2042. [Google Scholar] [CrossRef]
- Ying, T.; Zheng, M.; Hu, X.; Wu, K. Recycling of AZ91 Mg alloy through consolidation of machined chips by extrusion and ECAP. Trans. Nonferrous Met. Soc. China 2010, 20, 604–607. [Google Scholar] [CrossRef]
- Krolo, J.; Lela, B.; Švagelj, Z.; Jozić, S. Adaptive neuro-fuzzy and regression models for predicting microhardness and electrical conductivity of solid-state recycled EN AW 6082. Int. J. Adv. Manuf. Technol. 2019, 100, 2981–2993. [Google Scholar] [CrossRef]
- Hasse, M.; Ben Khalifa, N.; Tekkaya, A.E.; Misiolek, W.Z. Improving mechanical properties of chip-based aluminum extrudates by integrated extrusion and equal channel angular pressing (iECAP). Mater. Sci. Eng. A 2012, 539, 194–204. [Google Scholar] [CrossRef]
- Werenskiold, J.C. Equal Channel Angular Pressing (ECAP) of AA6082: Mechanical Properties, Texture and Microstructural Development. Ph.D. Thesis, Norwegian University of Science and Technology, Trondheim, Norway, 2004. [Google Scholar]
- Dadbakhsha, S.; Karimi Taheria, A.; Smith, C.W. Strengthening study on 6082 Al alloy after combination of aging treatment and ECAP process. Mater. Sci. Eng. A 2010, 527, 4758–4766. [Google Scholar] [CrossRef]
- Shamsudin, S.; Zhong, Z.W.; Rahim, S.N.; Lajis, M.A. The influence of temperature and preheating time in extrudate quality of solid-state recycled aluminum. Int. J. Adv. Manuf. Technol. 2017, 90, 2631–2643. [Google Scholar] [CrossRef]
- Lela, B.; Krolo, J.; Jozić, S. Mathematical modeling of solid-state recycling of aluminum chips. Int. J. Adv. Manuf. Technol. 2016, 87, 1125–1136. [Google Scholar] [CrossRef]
- Ansari, M.A.; Behnagh, R.A.; Narvan, M.; Naeini, E.S.; Givi, M.K.B.; Ding, H. Optimization of Friction Stir Extrusion (FSE) Parameters through Taguchi Technique. Trans. Indian Inst. Met. 2016, 69, 1351–1357. [Google Scholar] [CrossRef]
- Khamis, S.S.; Lajis, M.A.; Albert, R.A.O. A Sustainable Direct Recycling of Aluminum Chip (AA6061) in Hot Press Forging Employing Response Surface Methodology. Procedia CIRP 2015, 26, 477–481. [Google Scholar] [CrossRef]
- Kurama, E.; Ozcelik, B.; Bayramoglu, M.; Demirbas, E.; Simsek, B.T. Optimization of cutting fluids and cutting parameters during end milling by using D-optimal design of experiments. J. Clean. Prod. 2013, 42, 159–166. [Google Scholar] [CrossRef]
- Behrens, B.A.; Frischkorn, C.; Bonhage, M. Reprocessing of AW2007, AW6082 and AW7075 aluminium chips by using sintering and forging operations. Prod. Eng. 2014, 8, 443–451. [Google Scholar] [CrossRef]
- Mindivan, H.; Cimenoglu, H.; Kayali, E.S. Production of the composite from 6082 Al alloy chips and fly ash particles by hot pressing. In Proceedings of the Minerals, Metals & Materials Society (TMS) Annual Meeting 2009, San Francisco, CA, USA, 15–19 February 2009. [Google Scholar]
- Paraskevas, D. Complex deformation routes for direct recycling aluminium alloy scrap via industrial hot extrusion. In Proceedings of the 21st International ESAFORM Conference on Material Forming, Palermo, Italy, 23–25 April 2018. [Google Scholar] [CrossRef]
- Güley, V.; Khalifa, N.B.; Tekkaya, A.E. The effect of extrusion ratio and material flow on the mechanical properties of aluminum profiles solid state recycled from 6060 aluminum alloy chips. In Proceedings of the 14th International ESAFORM Conference on Material Forming, Belfast, Northern Ireland, 27–29 April 2011; pp. 1609–1614. [Google Scholar] [CrossRef]
- Tańskia, T.; Snopińskia, P.; Prusikc, K.; Srokaa, M. The effects of room temperature ECAP and subsequent aging on the structure and properties of the Al-3%Mg aluminium alloy. Mater. Charact. 2017, 133, 185–195. [Google Scholar] [CrossRef]
- Tercelj, M.; Fazarinc, M.; Kugler, G.; Perus, I. Influence of the chemical composition and process parameters on the mechanical properties of an extruded aluminium alloy for highly loaded structural parts. Constr. Build. Mater. 2013, 44, 781–791. [Google Scholar] [CrossRef]
- Jadhav, S.; Singh, R.; Vinayak, P.; Mane, S. Influence of Heat Treatment on Mechanical Properties and Microstructure of EN AW 6082 Aluminum alloy. In Proceedings of the 8th International Conference on Mechanical and Aerospace Engineering, Prague, Czech Republic, 22–25 July 2017; pp. 184–187. [Google Scholar] [CrossRef]


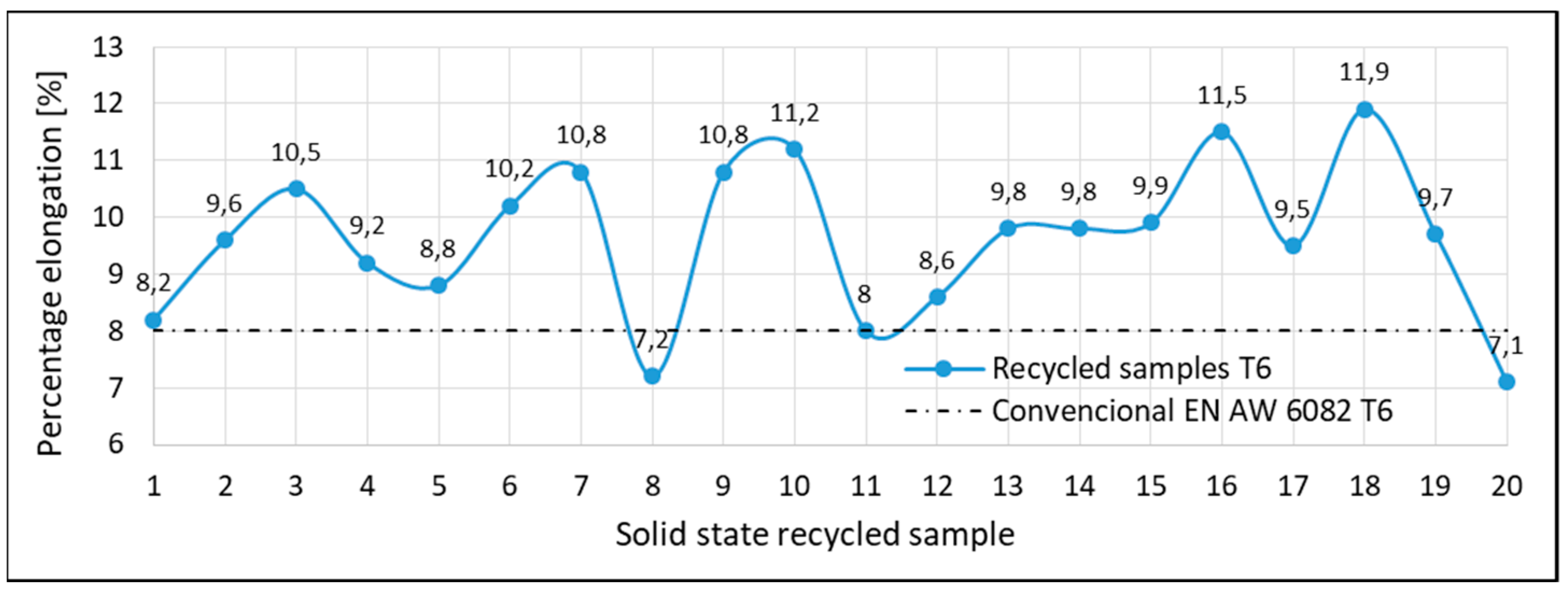
| Levels of Exp. Runs | Solid Solution Time (°C) | Artificial Aging Temperature (°C) | Artificial Aging Time (min) | Rm (MPa) | Rp0.2 (MPa) | PE (%) |
|---|---|---|---|---|---|---|
| 1 | 106.9 | 200.0 | 3.53 | 286 | 266 | 8.2 |
| 2 | 60.0 | 200.0 | 8.00 | 248 | 226 | 9.6 |
| 3 | 105.0 | 155.1 | 1.11 | 362 | 326 | 10.5 |
| 4 | 180.0 | 200.0 | 2.43 | 292 | 278 | 9.2 |
| 5 | 115.2 | 154.1 | 5.16 | 336 | 320 | 8.8 |
| 6 | 105.0 | 100.0 | 5.26 | 349 | 318 | 10.2 |
| 7 | 180.0 | 100.0 | 5.94 | 322 | 289 | 10.8 |
| 8 | 60.0 | 200.0 | 1.00 | 300 | 292 | 7.2 |
| 9 | 180.0 | 100.0 | 5.94 | 334 | 296 | 10.8 |
| 10 | 180.0 | 200.0 | 8.00 | 264 | 240 | 11.2 |
| 11 | 128.3 | 100.0 | 1.00 | 308 | 288 | 8 |
| 12 | 60.0 | 150.0 | 6.79 | 330 | 312 | 8.6 |
| 13 | 60.0 | 200.0 | 1.00 | 318 | 307 | 9.8 |
| 14 | 180.0 | 200.0 | 8.00 | 256 | 232 | 9.8 |
| 15 | 60.0 | 100.0 | 8.00 | 328 | 296 | 9.9 |
| 16 | 120.0 | 198.5 | 7.06 | 259 | 234 | 11.5 |
| 17 | 60.0 | 100.0 | 2.45 | 314 | 286 | 9.5 |
| 18 | 180.0 | 143.0 | 1.00 | 328 | 293 | 11.9 |
| 19 | 60.0 | 100.0 | 8.00 | 320 | 288 | 9.7 |
| 20 | 60.0 | 100.0 | 2.45 | 317 | 298 | 7.1 |
| Element | Si% | Fe% | Cu% | Mn% | Mg% | Zn% | Ti% | Cr% | Others% |
|---|---|---|---|---|---|---|---|---|---|
| Min.–Max. | 0.7–1.3 | 0–0.5 | 0–0.1 | 0.4–0.1 | 0.6–1.2 | 0–0.2 | 0–0.1 | 0–0.25 | 0–0.15 |
| Turning Parameters | Value |
|---|---|
| Cutting speed | vc = 350 m/min |
| Feed rate | f = 0.14 mm/rev |
| Cutting depth | ap = 0.5 mm |
| Chip geometry | - |
| Length | lavg = 10 mm |
| Width | wavg = 1.8 mm |
| Thickness | tavg = 0.8 mm |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krolo, J.; Lela, B.; Dumanić, I.; Kozina, F. Statistical Analysis of the Combined ECAP and Heat Treatment for Recycling Aluminum Chips Without Remelting. Metals 2019, 9, 660. https://doi.org/10.3390/met9060660
Krolo J, Lela B, Dumanić I, Kozina F. Statistical Analysis of the Combined ECAP and Heat Treatment for Recycling Aluminum Chips Without Remelting. Metals. 2019; 9(6):660. https://doi.org/10.3390/met9060660
Chicago/Turabian StyleKrolo, Jure, Branimir Lela, Ivana Dumanić, and Franjo Kozina. 2019. "Statistical Analysis of the Combined ECAP and Heat Treatment for Recycling Aluminum Chips Without Remelting" Metals 9, no. 6: 660. https://doi.org/10.3390/met9060660
APA StyleKrolo, J., Lela, B., Dumanić, I., & Kozina, F. (2019). Statistical Analysis of the Combined ECAP and Heat Treatment for Recycling Aluminum Chips Without Remelting. Metals, 9(6), 660. https://doi.org/10.3390/met9060660






