Effects of Nb Content on the Mechanical Alloying Behavior and Sintered Microstructure of Mo-Nb-Si-B Alloys
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Mechanical Alloying Behavior
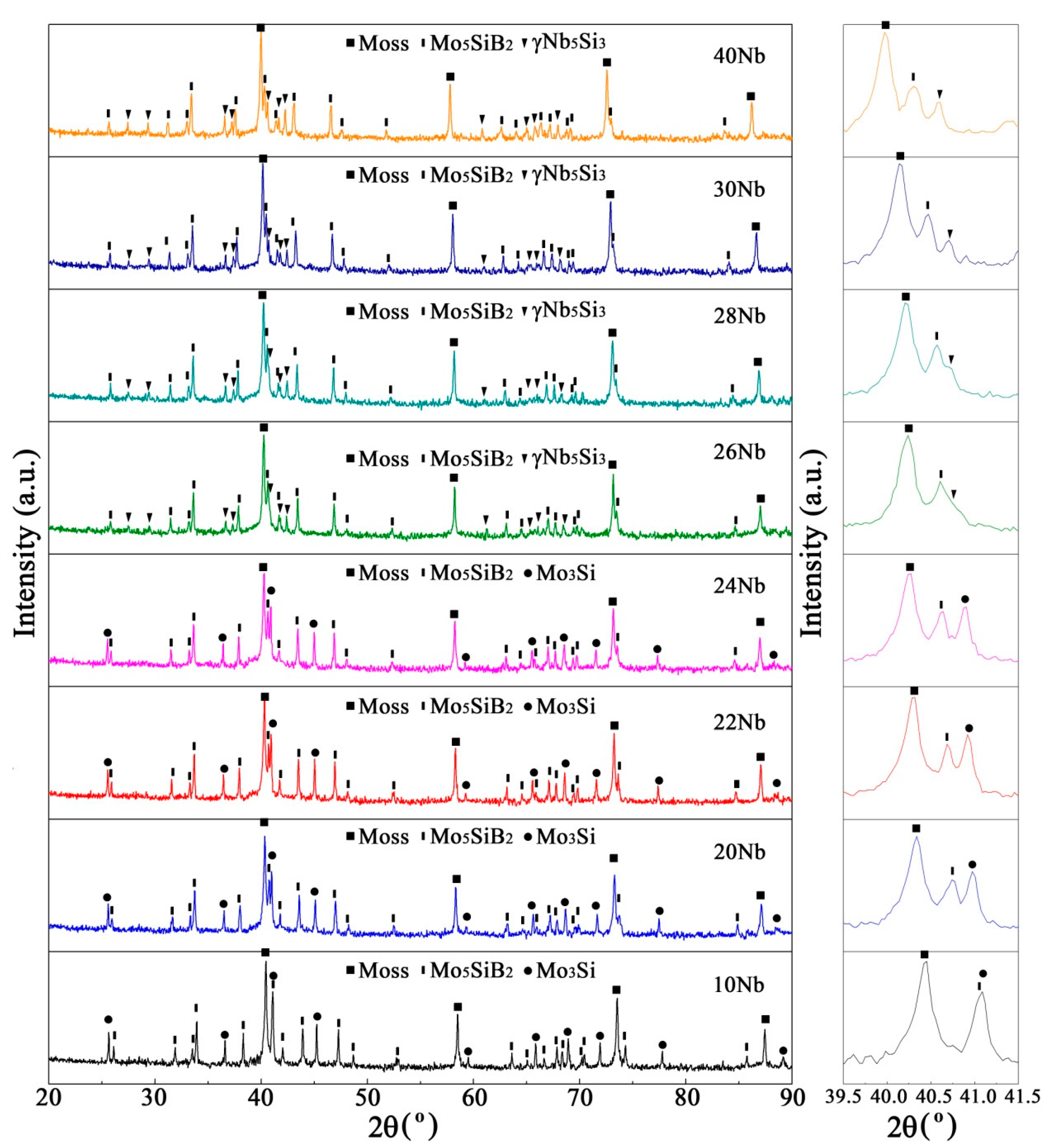
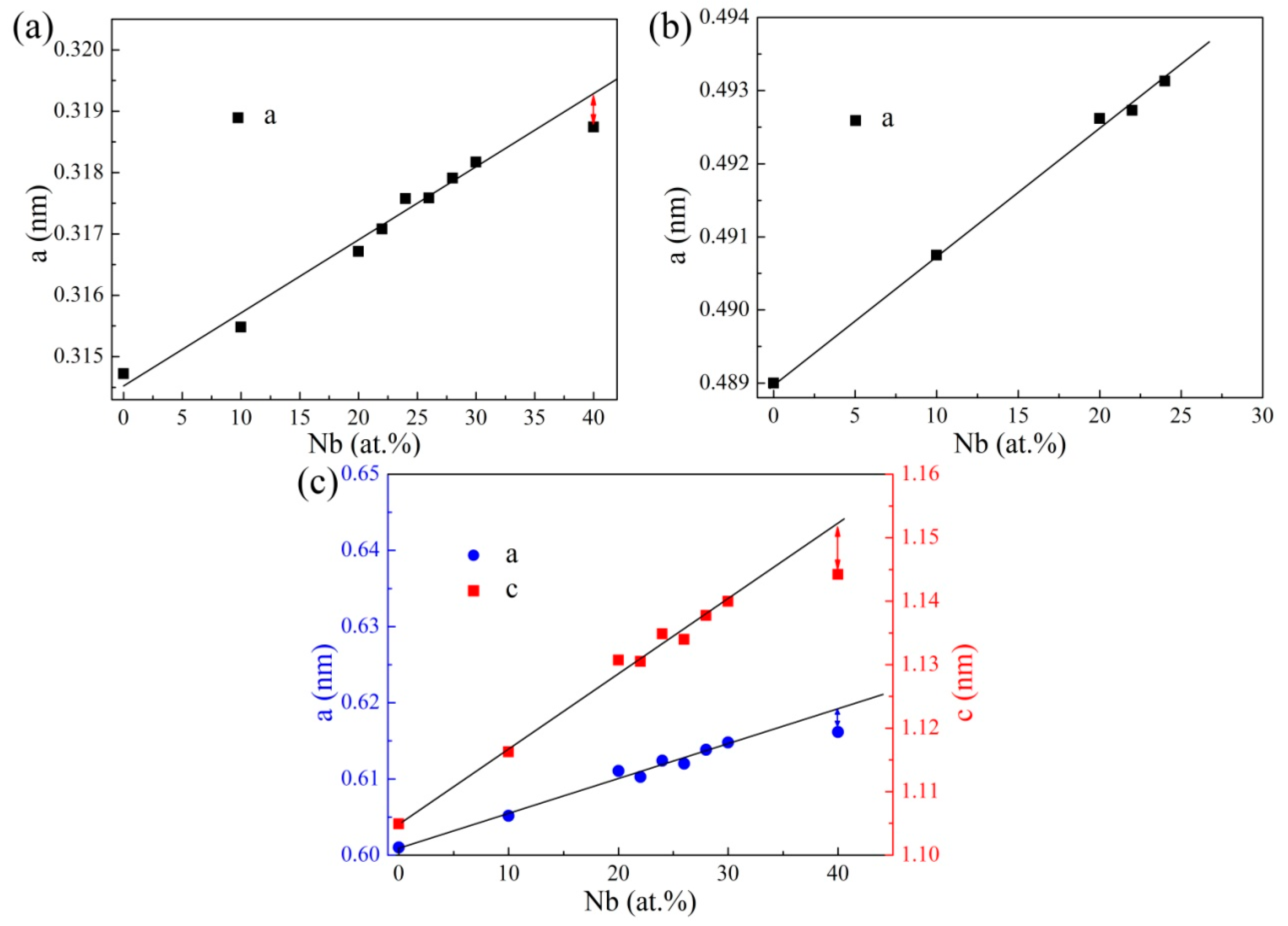
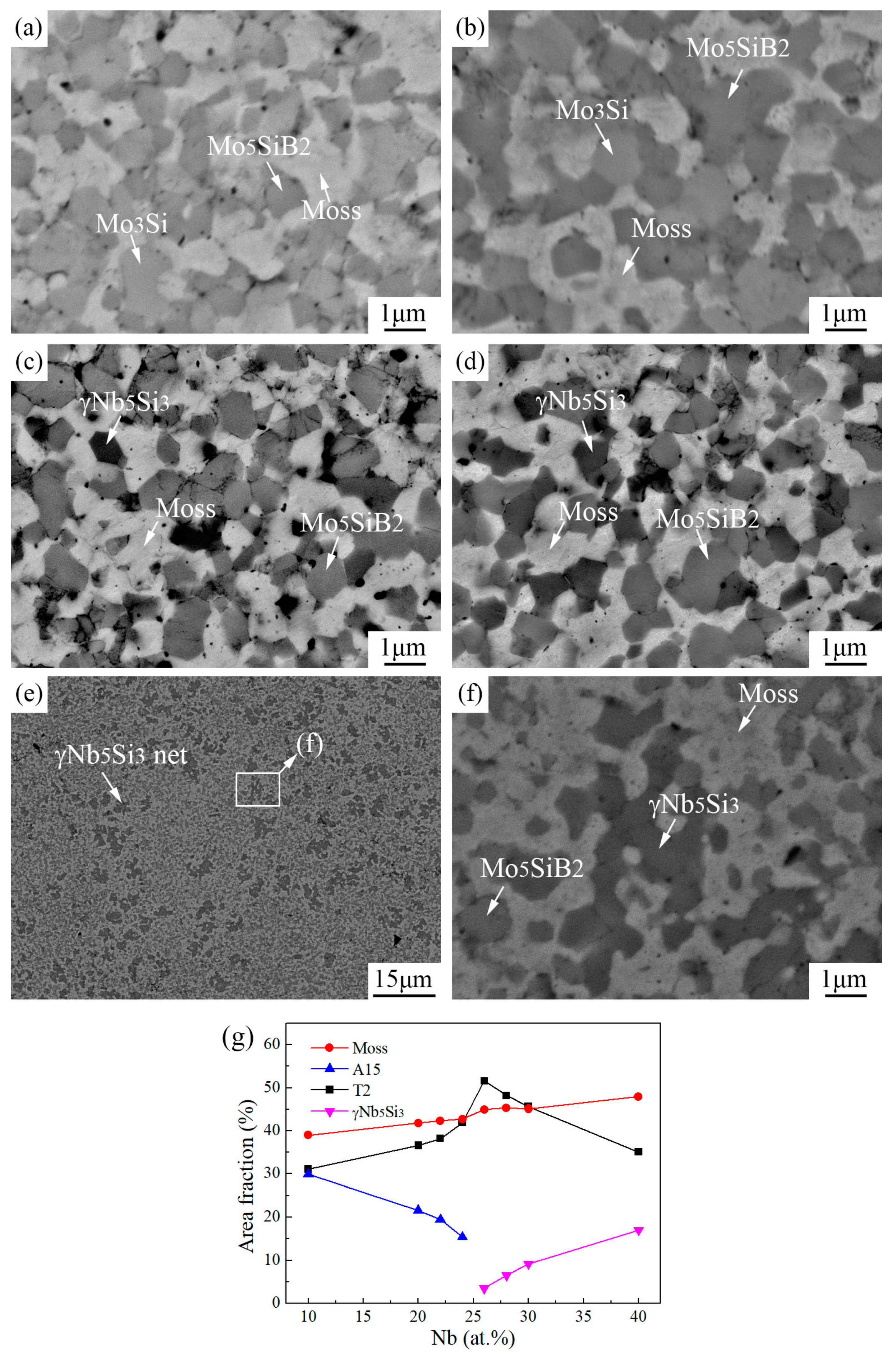
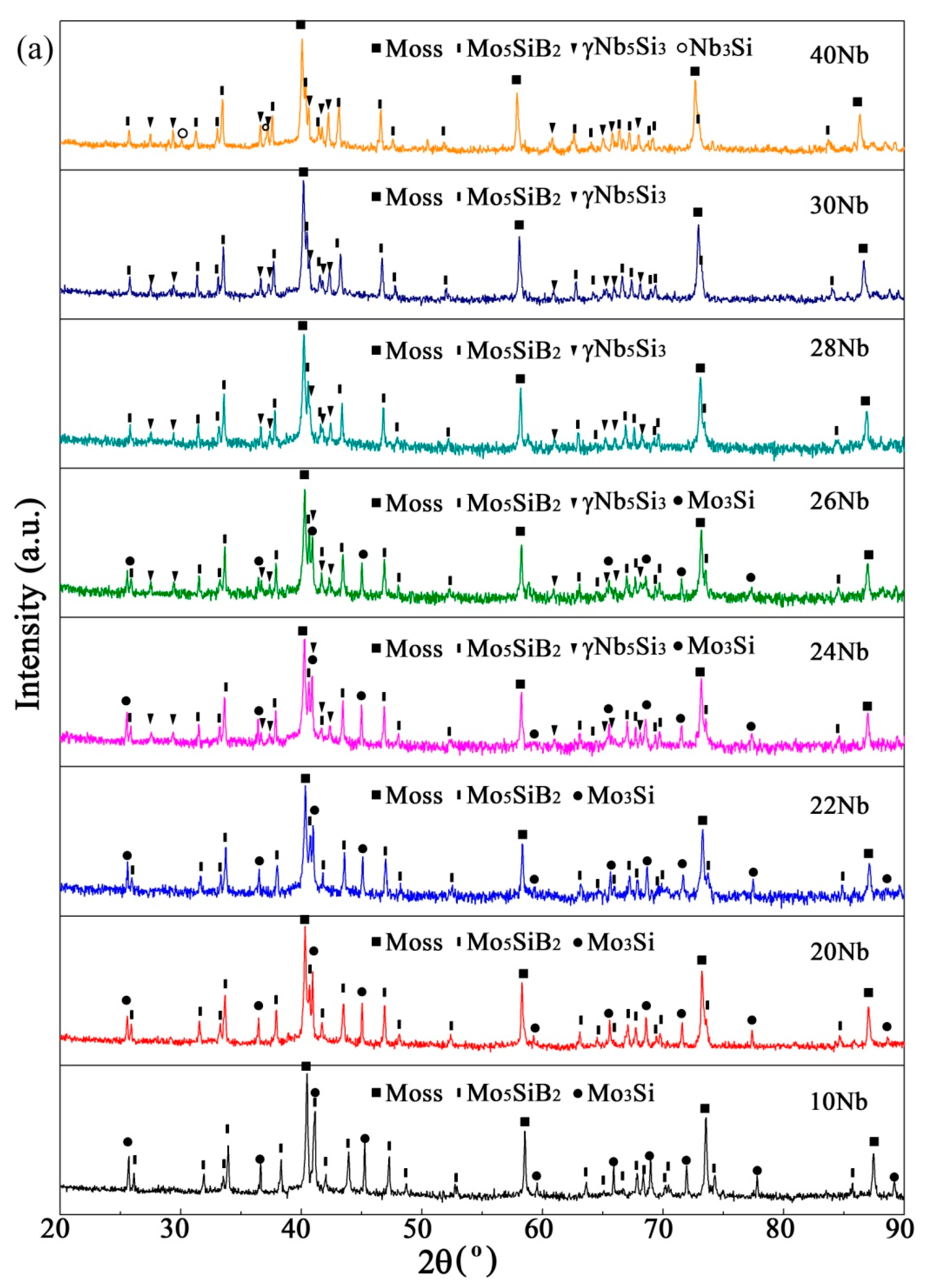
3.2. Sintered Microstructure
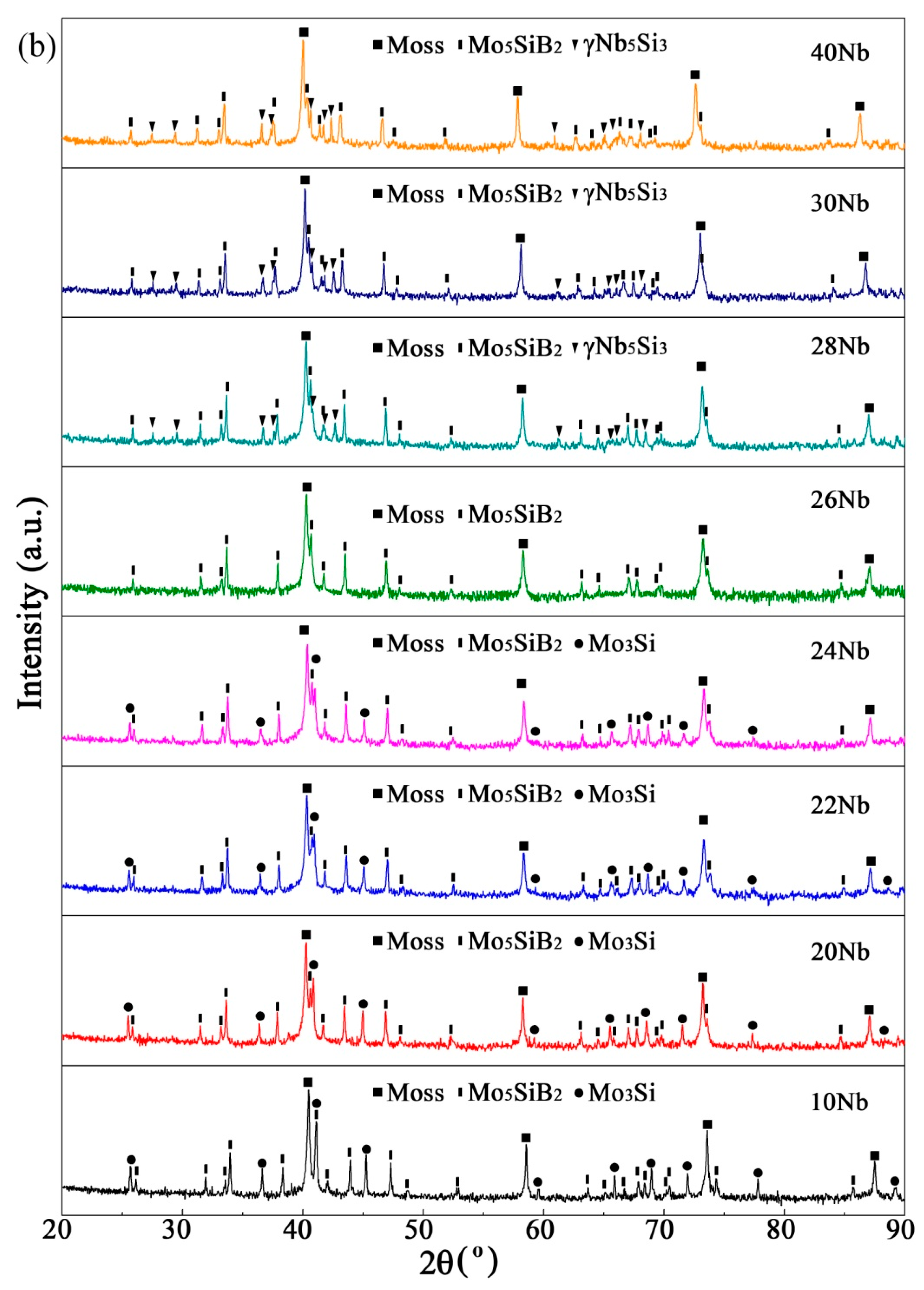
3.2.1. Effect of Nb Content
3.2.2. Effect of Prior MA Process
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dimiduk, D.M.; Perepezko, J.H. Mo-Si-B alloys: Developing a revolutionary turbine-engine material. MRS Bull. 2003, 28, 639–645. [Google Scholar] [CrossRef]
- Lemberg, J.A.; Ritchie, R.O. Mo-Si-B alloys for ultrahigh-temperature structural applications. Adv. Mater. 2012, 24, 3445–3480. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, S. Improvement of the oxidation-proof property and the scale structure of Mo3Si intermetallic alloy through the addition of chromium and aluminum elements. Intermetallics 2006, 14, 1351–1357. [Google Scholar] [CrossRef]
- Rioult, F.A.; Imhoff, S.D.; Sakidja, R.; Perepezko, J.H. Transient oxidation of Mo-Si-B alloys: Effect of the microstructure size scale. Acta Mater. 2009, 57, 4600–4613. [Google Scholar] [CrossRef]
- Rosales, I.; Martinez, H.; Bahena, D.; Ruiz, J.A.; Guardian, R.; Colin, J. Oxidation performance of Mo3Si with Al additions. Corros. Sci. 2009, 51, 534–538. [Google Scholar] [CrossRef]
- Yang, Y.; Bei, H.; Chen, S.; George, E.P.; Tiley, J.Y.; Chang, A. Effects of Ti, Zr, and Hf on the phase stability of Moss+Mo3Si+Mo5SiB2 alloys at 1600 °C. Acta Mater. 2010, 58, 541–548. [Google Scholar] [CrossRef]
- Akinc, M.; Meyer, M.K.; Kramer, M.J.; Thom, A.J.; Huebsch, J.J.; Cook, B. Boron-doped molybdenum silicides for structural applications. Mater. Sci. Eng. A 1999, 261, 16–23. [Google Scholar] [CrossRef]
- Nieh, T.G.; Wang, J.G.; Liu, C.T. Deformation of a multiphase Mo-9.4Si-13.8B alloy at elevated temperatures. Intermetallics 2001, 9, 73–79. [Google Scholar] [CrossRef]
- Ray, P.K.; Ye, Y.Y.; Akinc, M.; Kramer, M.J. Effect of Nb and W substitutions on the stability of the A15 Mo3Si phase. J. Alloy. Compd. 2012, 537, 65–70. [Google Scholar] [CrossRef]
- Sakidja, R.; Perepezko, J.H.; Kim, S.; Sekido, N. Phase stability and structural defects in high-temperature Mo-Si-B alloys. Acta Mater. 2008, 56, 5223–5244. [Google Scholar] [CrossRef]
- Geng, T.; Li, C.; Zhao, X.; Xu, H.; Du, Z.; Guo, C. Thermodynamic assessment of the Nb-Si-Mo system. J. Alloy. Compd. 2010, 34, 363–376. [Google Scholar] [CrossRef]
- Takata, N.; Sekido, N.; Takeyama, M.; Perepezko, J.H.; Follett-Figueroa, M.; Zhang, C. Solidification of Bcc/T1/T2 three-phase microstructure in Mo-Nb-Si-B alloys. Intermetallics 2016, 72, 1–8. [Google Scholar] [CrossRef]
- Nunes, C.A.; Sakidja, R.; Dong, Z.; Perepezko, J.H. Liquidus projection for the Mo-rich portion of the Mo-Si-B ternary system. Intermetallics 2000, 8, 327–337. [Google Scholar] [CrossRef]
- Katrych, S.; Grytsiv, A.; Bondar, A.; Rogl, P.; Velikanova, T.; Bohn, M. Structural materials: metal-silicon-boron: On the melting behavior of Mo-Si-B alloys. J. Alloy. Compd. 2002, 347, 94–100. [Google Scholar] [CrossRef]
- Krüger, M.; Franz, S.; Saage, H.; Heilmaier, M.; Schneibel, J.H.; Jéhanno, P.; Böning, M.; Kestler, H. Mechanically alloyed Mo-Si-B alloys with a continuous α-Mo matrix and improved mechanical properties. Intermetallics 2008, 16, 933–941. [Google Scholar] [CrossRef]
- Hassomeris, O.; Schumacher, G.; Krüger, M.; Heilmaier, M.; Banhart, J. Phase continuity in high temperature Mo-Si-B alloys: A FIB-tomography study. Intermetallics 2011, 19, 470–475. [Google Scholar] [CrossRef]
- Li, B.; Zhang, G.J.; Jiang, F.; Ren, S.; Liu, G.; Sun, J. Preparation of fine-grained Mo-12Si-8.5B alloys with improved mechanical properties via a mechanical alloying process. J. Alloy. Compd. 2014, 609, 80–85. [Google Scholar] [CrossRef]
- Byun, J.M.; Bang, S.-R.; Kim, S.H.; Choi, W.J.; Kim, Y.D. Mechanical properties of Mo-Nb-Si-B quaternary alloy fabricated by powder metallurgical method. Int. J. Refract. Met. Hard Mater. 2017, 65, 14–18. [Google Scholar] [CrossRef]
- Lei, R.S.; Wang, M.P.; Li, Z.; Wei, H.G.; Yang, W.C.; Jia, Y.L.; Gong, S. Structure evolution and solid solubility extension of copper-niobium powders during mechanical alloying. Mater. Sci. Eng. A 2011, 528, 4475–4481. [Google Scholar] [CrossRef]
- Yang, T.; Guo, X.P.; Luo, Y.C. Microstructural evolution of mechanically alloyed Mo-Si-B-Zr-Y powders. Int. J. Refract. Met. Hard Mater. 2016, 56, 35–43. [Google Scholar] [CrossRef]
- Zhong, Y.; Ping, D.; Song, X.; Yin, F. Determination of grain size by XRD profile analysis and TEM counting in nano-structured Cu. J. Alloy. Compd. 2009, 476, 113–117. [Google Scholar] [CrossRef]
- Ungár, T.; Tichy, G.; Gubicza, J.; Hellmig, R.J. Correlation between subgrains and coherently scattering domains. Powder Diffr. 2005, 20, 366–375. [Google Scholar] [CrossRef]
- Aguilar, C.; Ordoñez, S.; Guzman, D.; Rojas, P.A. Mechanical alloying of Cu–xCr (x = 3, 5 and 8 wt.%) alloys. J. Alloy. Compd. 2010, 504, 102–109. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Zhang, S.; Guo, X.P. Effects of B addition on the microstructure and properties of Nb silicide based ultrahigh temperature alloys. Intermetallics 2015, 57, 83–92. [Google Scholar] [CrossRef]
- Sakidja, R.; Kim, S.; Park, J.; Perepezko, J.H. Transition metal alloying and phase stability in the Mo-Si-B system. Symposium on Defect Properties and Related Phenomena in Intermetallic Alloys. In Proceedings of the 2002 MRS Fall Meeting, Boston, MA, USA, 3–5 December 2002. [Google Scholar]
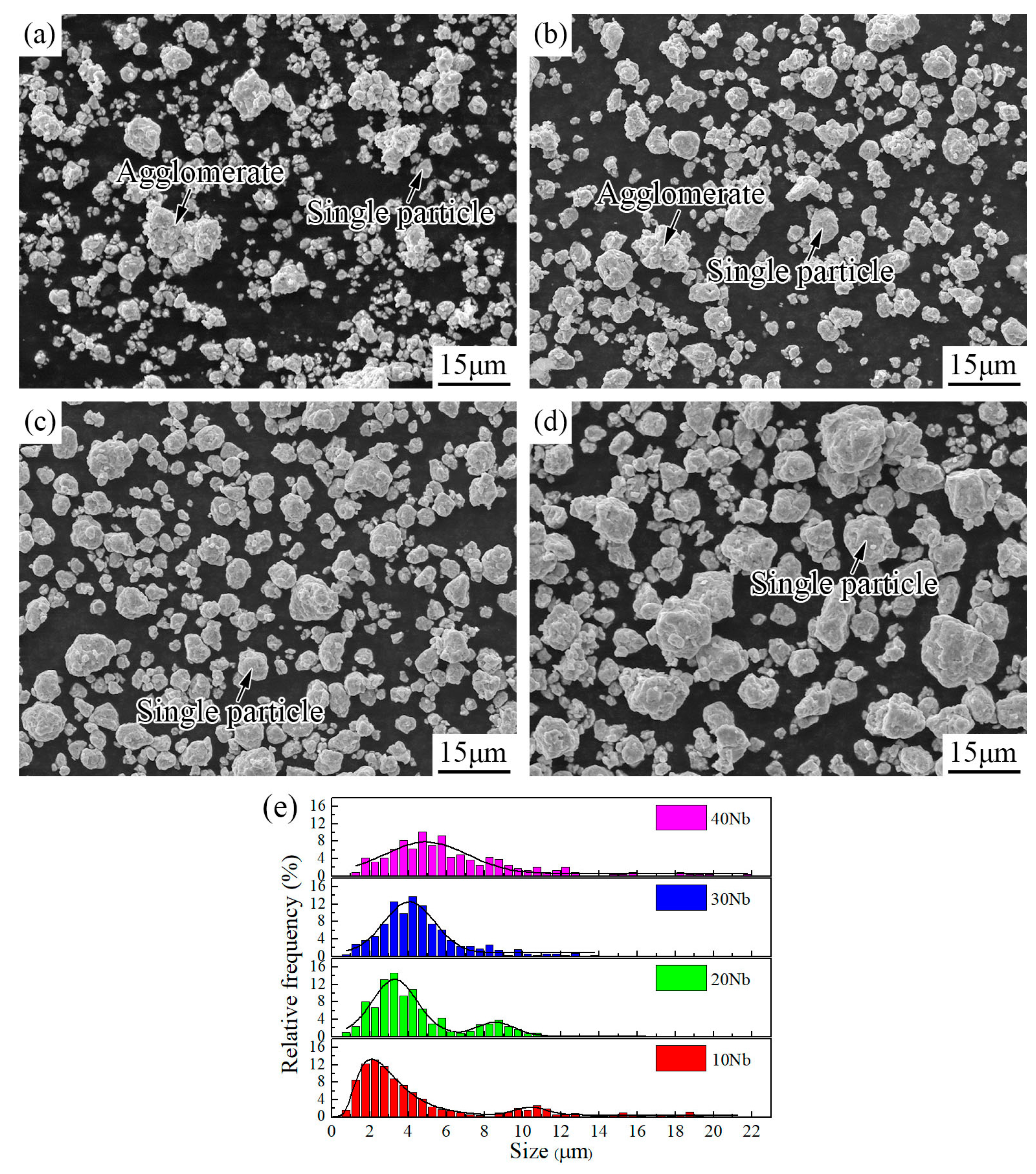

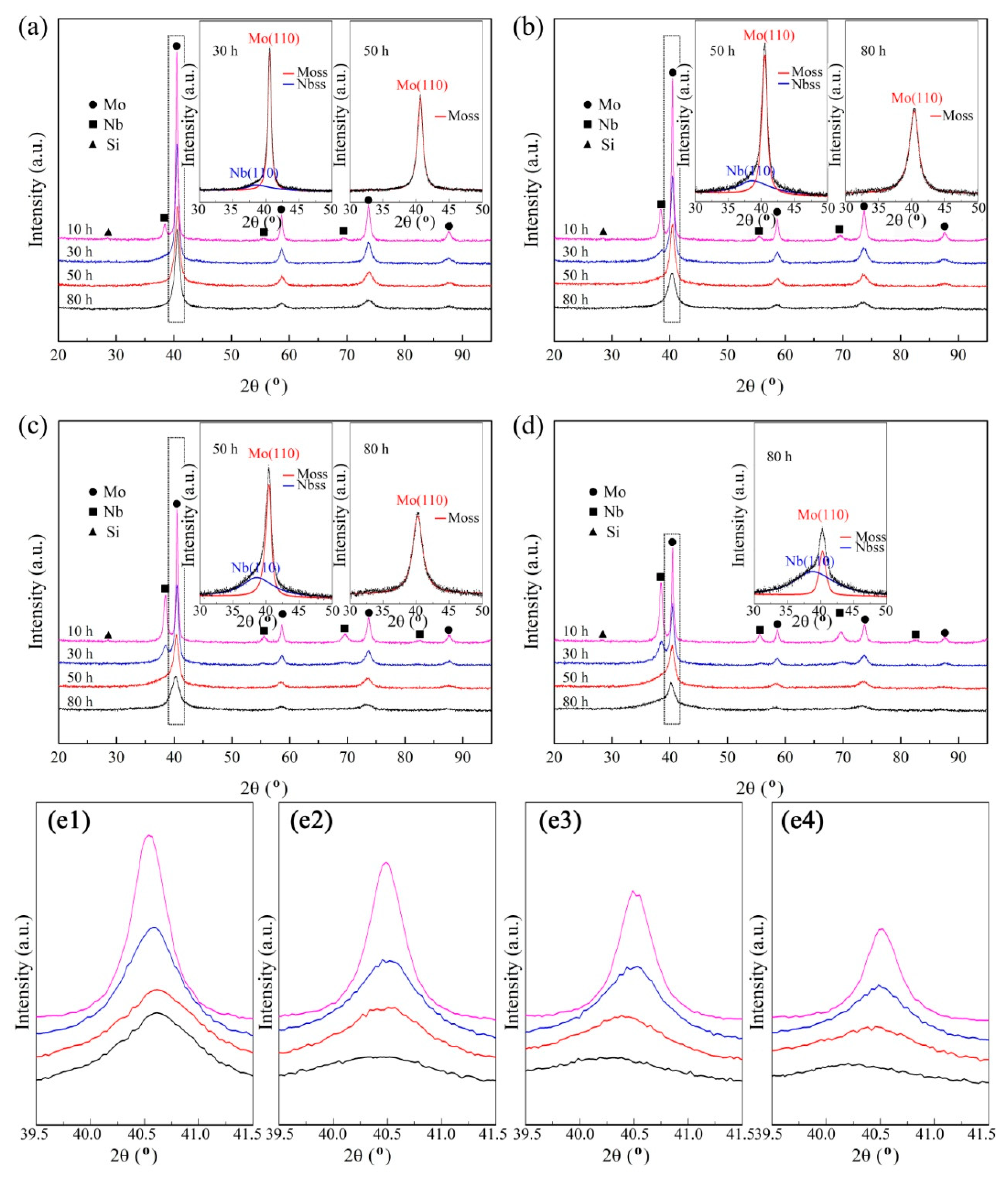
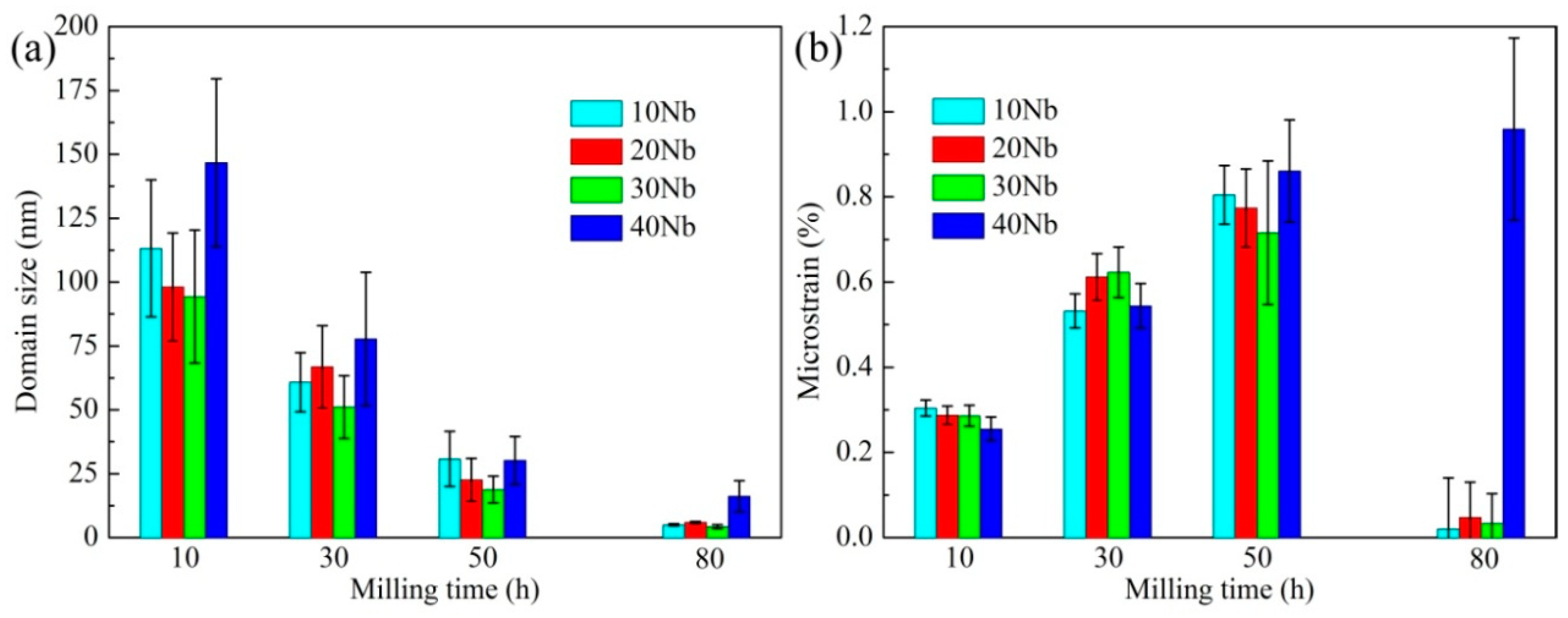
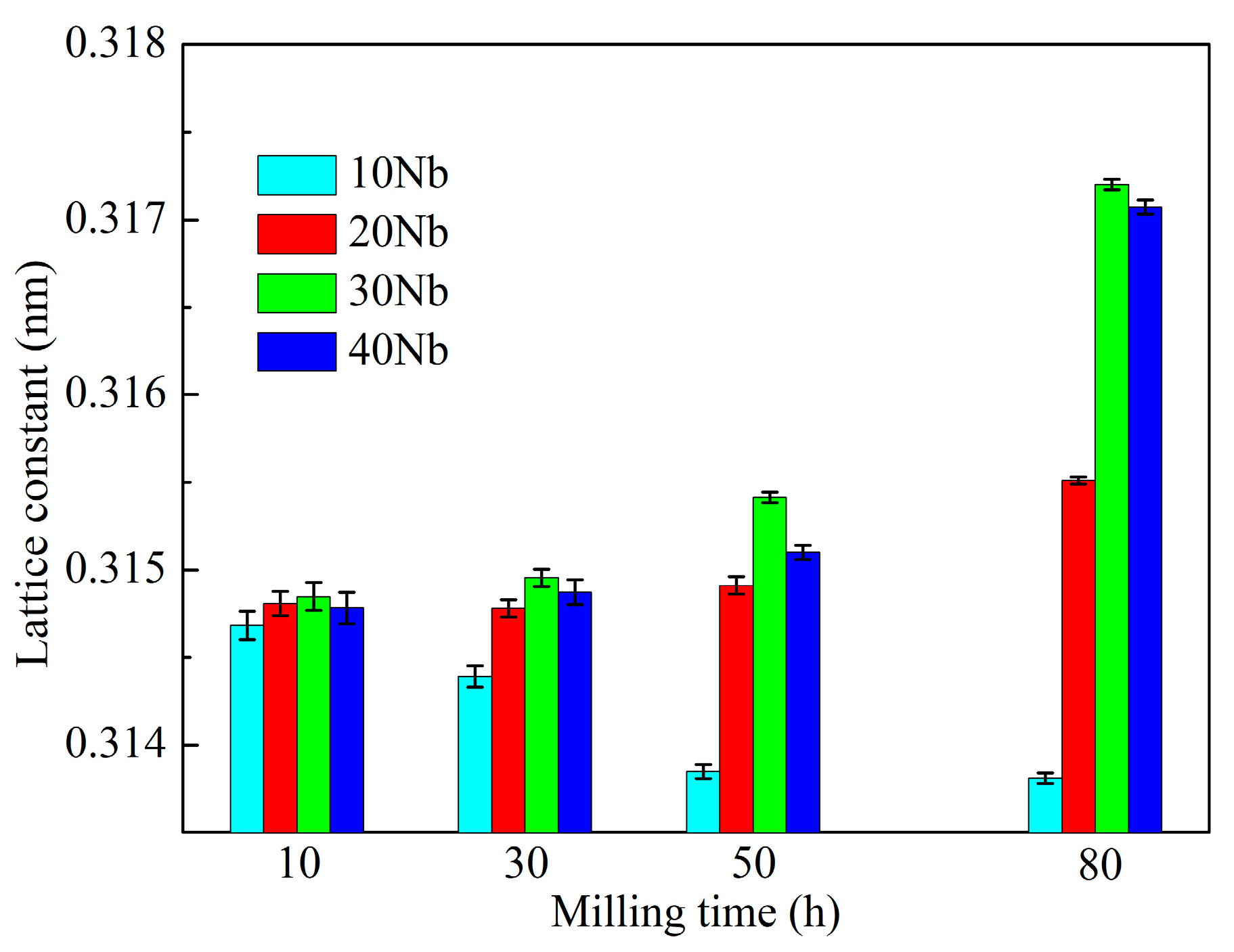
| Abbreviation of Products | Process Type | x | y |
|---|---|---|---|
| MA-xNb/yh | Mechanical alloying | Nb content (at.%) | Milling time (h) |
| S-xNb/yh | Sintering at 1773 K for 2h of milled powders |
| Sample | Phase | Composition (at.%) | ||
|---|---|---|---|---|
| Mo | Nb | Si | ||
| S-24Nb/30h | Moss | 78.3 | 20.2 | 1.5 |
| Mo3Si | 62.5 | 17.4 | 20.1 | |
| Mo5SiB2 ☆ | 55.1 | 32.5 | 12.4 | |
| S-26Nb/30h | Moss | 75.5 | 22.9 | 1.6 |
| γNb5Si3 | 30.7 | 39.2 | 30.1 | |
| Mo5SiB2 ☆ | 49.8 | 38.5 | 11.7 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, T.; Guo, X. Effects of Nb Content on the Mechanical Alloying Behavior and Sintered Microstructure of Mo-Nb-Si-B Alloys. Metals 2019, 9, 653. https://doi.org/10.3390/met9060653
Yang T, Guo X. Effects of Nb Content on the Mechanical Alloying Behavior and Sintered Microstructure of Mo-Nb-Si-B Alloys. Metals. 2019; 9(6):653. https://doi.org/10.3390/met9060653
Chicago/Turabian StyleYang, Tao, and Xiping Guo. 2019. "Effects of Nb Content on the Mechanical Alloying Behavior and Sintered Microstructure of Mo-Nb-Si-B Alloys" Metals 9, no. 6: 653. https://doi.org/10.3390/met9060653
APA StyleYang, T., & Guo, X. (2019). Effects of Nb Content on the Mechanical Alloying Behavior and Sintered Microstructure of Mo-Nb-Si-B Alloys. Metals, 9(6), 653. https://doi.org/10.3390/met9060653





