Biomechanical Assessment of Design Parameters on a Self-Developed 3D-Printed Titanium-Alloy Reconstruction/Prosthetic Implant for Mandibular Segmental Osteotomy Defect
Abstract
1. Introduction
2. Materials and methods
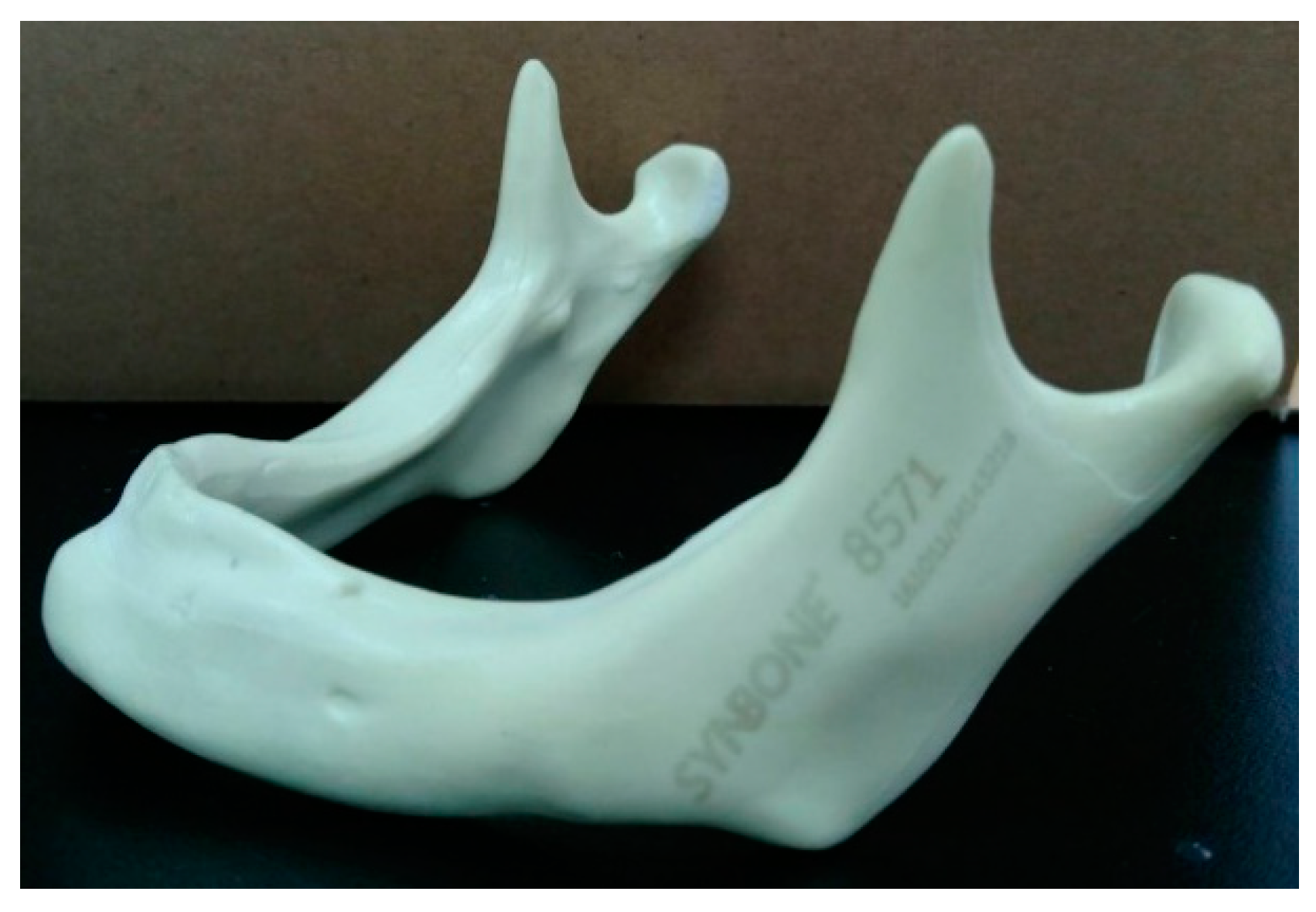
2.1. Processing of 3D STL (Stereolithography) Mandibular Model
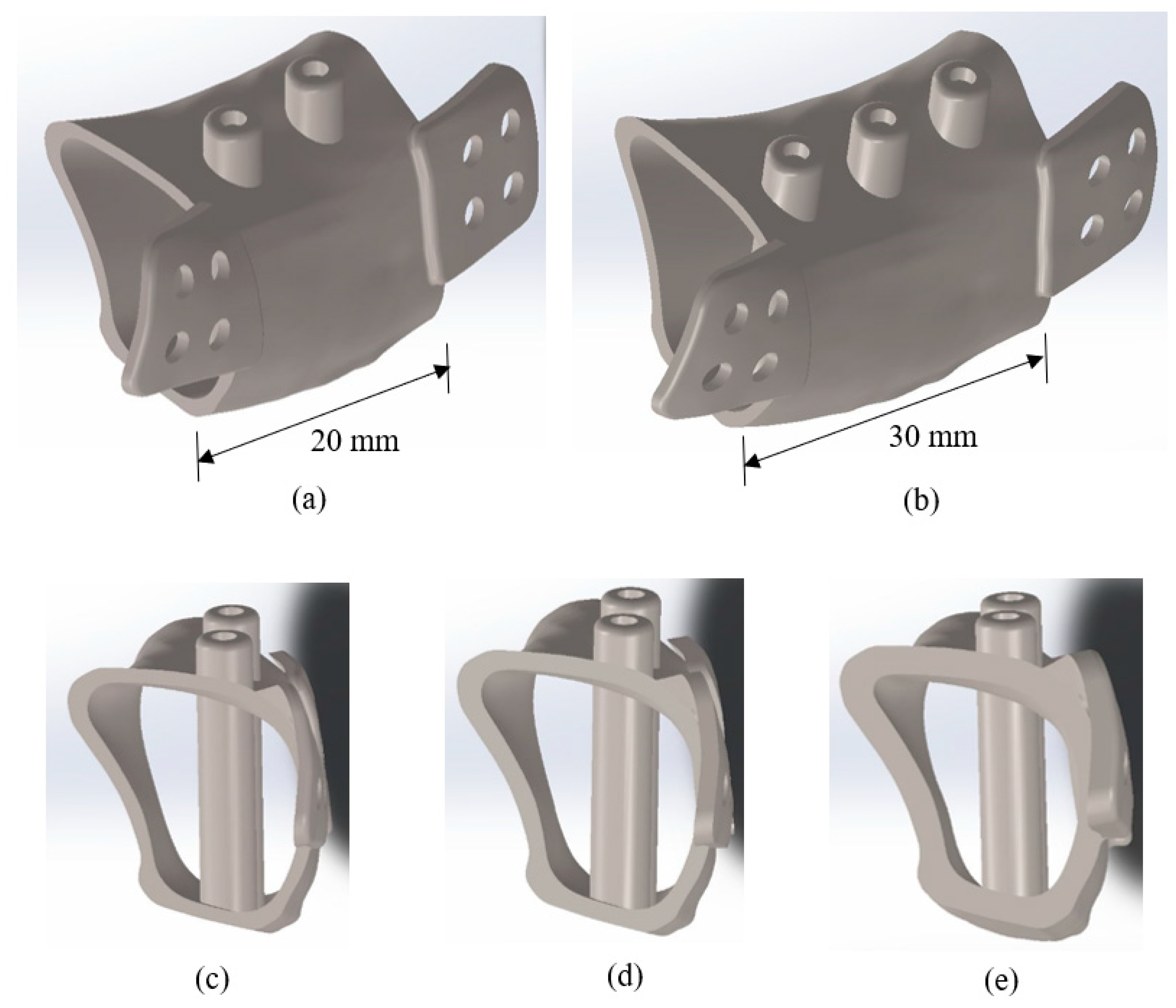
2.2. 3D Modeling of Computer-Aided Design (CAD) Solid Model
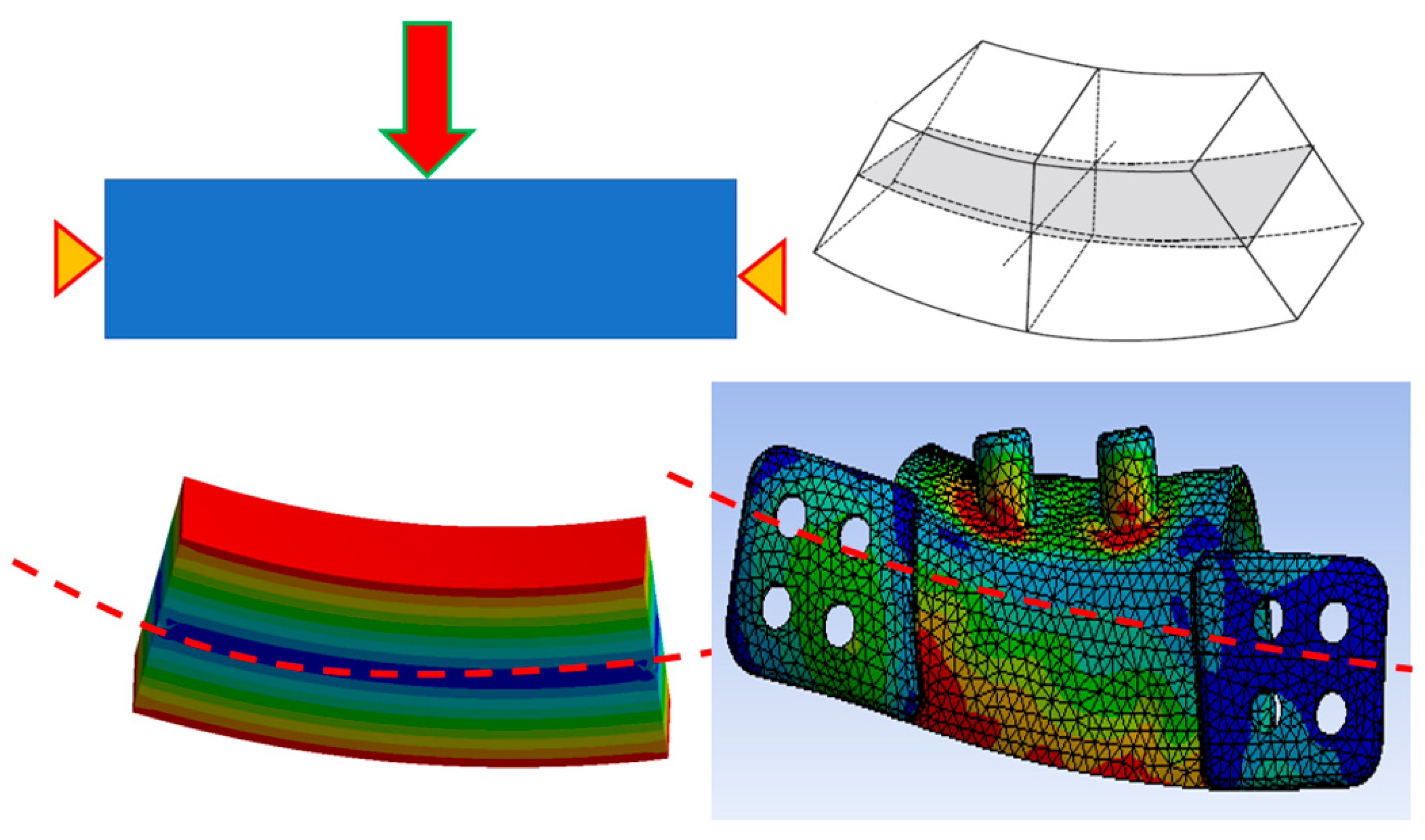
2.3. Finite Element Simulation
3. Results
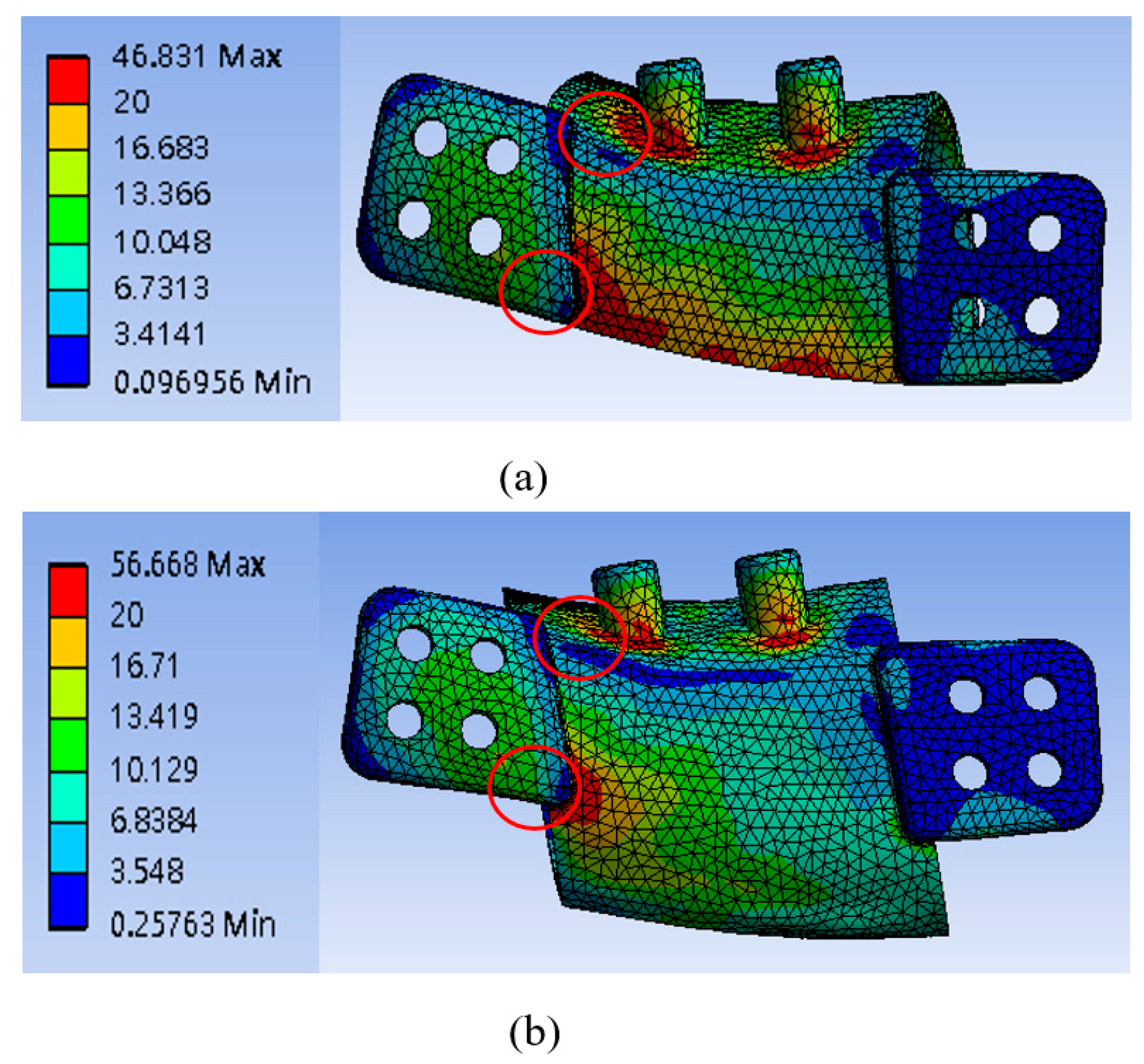
3.1. Mandibular Implant Stress
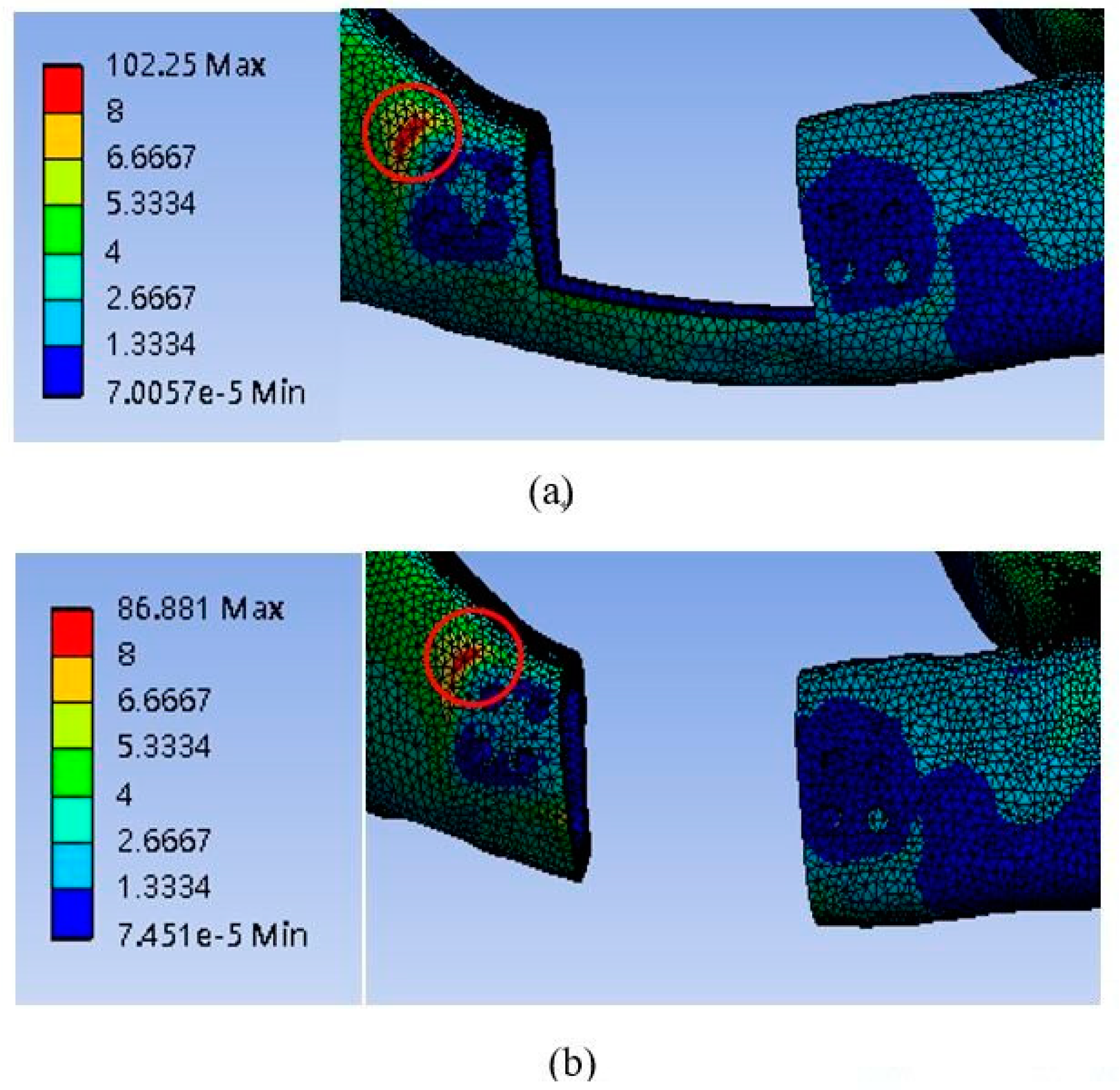
3.2. Cortical Bone Stress
4. Discussion
4.1. Comparing the Two Implant Designs
4.2. Implant Body Length and Body Thickness
4.3. Limitations and Future Work
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Foster, R.D.; Anthony, J.P.; Sharma, A.; Pogrel, M.A. Vascularized bone flaps versus nonvascularized bone grafts for mandibular reconstruction: An outcome analysis of primary bony union and endosseous implant success. Head Neck 1999, 21, 66–71. [Google Scholar] [CrossRef]
- Hidalgo, D.A.; Swartz, W.M. Aesthetic Improvements in Free-Flap Mandible Reconstruction. Plast. Reconstr. Surg. 1991, 88, 574–585. [Google Scholar] [CrossRef] [PubMed]
- Goh, B.T.; Lee, S.; Tideman, H.; Stoelinga, P.J. Mandibular reconstruction in adults: a review. Int. J. Oral Surg. 2008, 37, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.; Laviv, A.; Berman, P.; Nashef, R.; Abu-Tair, J. Mandibular reconstruction using stereolithographic 3-dimensional printing modeling technology. Oral Surg., Oral Med., Oral Pathol., Oral Radiol., Endod. 2009, 108, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Mehra, P.; Miner, J.; D’Innocenzo, R.; Nadershah, M. Use of 3-D Stereolithographic Models in Oral and Maxillofacial Surgery. J. Oral Surg. 2011, 10, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, J. 3D modeling, custom implants and its future perspectives in craniofacial surgery. Ann. Surg. 2014, 4, 9–18. [Google Scholar] [CrossRef] [PubMed]
- BBC. Transplant Jaw Made by 3D Printer Claimed as First. Available online: http://www.bbc.co.uk/news/technology-16907104 (accessed on 8 March 2012).
- Mohammed, M.I.; Fitzpatrick, A.P.; Gibson, I. Customised design of a patient specific 3D printed whole mandible implant. KnE Eng. 2017, 2, 104–111. [Google Scholar] [CrossRef]
- Popov, V.V.; Muller-Kamskii, G.; Kovalevsky, A.; Dzhenzhera, G.; Strokin, E.; Kolomiets, A.; Ramon, J. Design and 3D-printing of titanium bone implants: Brief review of approach and clinical cases. Biomed. Eng. Lett. 2018, 8, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Ackland, D.C.; Robinson, D.; Redhead, M.; Lee, P.V.S.; Moskaljuk, A.; Dimitroulis, G. A personalized 3D-printed prosthetic joint replacement for the human temporomandibular joint: From implant design to implantation. J. Mech. Biomed. Mater. 2017, 69, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Dawood, A.; Marti, B.M.; Sauret-Jackson, V.; Darwood, A. 3D printing in dentistry. Br. Dent. J. 2015, 219, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Sutradhar, A.; Park, J.; Carrau, D.; Nguyen, T.H.; Miller, M.J.; Paulino, G.H. Designing patient-specific 3D printed craniofacial implants using a novel topology optimization method. Med. Biol. Eng. Comput. 2016, 54, 1123–1135. [Google Scholar] [CrossRef] [PubMed]
- Shaoki, A.; Xu, J.-Y.; Sun, H.; Chen, X.-S.; Ouyang, J.; Zhuang, X.-M.; Deng, F.-L. Osseointegration of three-dimensional designed titanium implants manufactured by selective laser melting. Biofabrication 2016, 8, 045014. [Google Scholar] [CrossRef] [PubMed]
- Jahadakbar, A.; Moghaddam, N.S.; Amerinatanzi, A.; Dean, D.; Karaca, H.E.; Elahinia, M.; Wang, G.-J. Finite Element Simulation and Additive Manufacturing of Stiffness-Matched NiTi Fixation Hardware for Mandibular Reconstruction Surgery. Bioengineering 2016, 3, 36. [Google Scholar] [CrossRef] [PubMed]
- Dahake, S.; Kuthe, A.; Kulkarni, S.; Mawale, M. Finite element analysis of customized implant in mandibular reconstruction after tumor resection with and without using customized surgical osteotomy guide. Int. J. Med. Rob. Comput. Assisted Surg. 2018, 14, e1854. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.-P.; Tan, K.B.; Liu, G.-R. Application of finite element analysis in implant dentistry: A review of the literature. J. Prosthet. Dent. 2001, 85, 585–598. [Google Scholar] [CrossRef] [PubMed]
- Renishaw. Ti6Al4V ELI-0406 Powder for Additive Manufacturing. Available online: https://resources.renishaw.com/en/details/data-sheet-ti6al4v-eli-0406-powder-for-additive-manufacturing--94700 (accessed on 22 May 2019).
- Huang, H.-L.; Huang, J.-S.; Ko, C.-C.; Hsu, J.-T.; Chang, C.-H.; Chen, M.Y.C. Effects of splinted prosthesis supported a wide implant or two implants: A three-dimensional finite element analysis. Clin. Oral Implant. 2005, 16, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Nagels, J.; Stokdijk, M.; Rozing, P.M. Stress shielding and bone resorption in shoulder arthroplasty. J. Shoulder Elbow Surg. 2003, 12, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Sumner, D. Long-term implant fixation and stress-shielding in total hip replacement. J. Biomech. 2015, 48, 797–800. [Google Scholar] [CrossRef] [PubMed]
- Sing, S.L.; An, J.; Yeong, W.Y.; Wiria, F.E. Laser and electron-beam powder-bed additive manufacturing of metallic implants: A review on processes, materials and designs. J. Orthop. Res. 2016, 34, 369–385. [Google Scholar] [CrossRef] [PubMed]



| Material | Young’s Modulus E (MPa) | Poisson’s Ratio ν |
|---|---|---|
| Cortical bone | 13,400 | 0.3 |
| Trabecular bone | 790 | 0.3 |
| Titanium alloy screw (Ti-6Al-4V) | 110,000 | 0.35 |
| 3D printing titanium alloy (Ti-6Al-4V) | 129,000 | 0.34 |
| Model # | High Stress Position | 20 mm | 25 mm | |
|---|---|---|---|---|
| Model 1 | Lower edge of the abutment | 0.8 mm | 35.53 MPa | 46.68 MPa |
| 1 mm | 32.35 MPa | 38.93 MPa | ||
| 1.5 mm | 25.18 MPa | 31.69 MPa | ||
| Lower edge of the implant body near right-wing plate | 0.8 mm | 46.83 MPa | 41.10 MPa | |
| 1 mm | 50.52 MPa | 40.34 MPa | ||
| 1.5 mm | 38.32 MPa | 35.03 MPa | ||
| Model 2 | Lower edge of the abutment | 0.8 mm | 33.56 MPa | 38.91 MPa |
| 1 mm | 30.17 MPa | 36.65 MPa | ||
| 1.5 mm | 24.80 MPa | 25.84 MPa | ||
| Lower edge of the implant body near left-wing plate | 0.8 mm | 56.67 MPa | 52.95 MPa | |
| 1 mm | 46.02 MPa | 42.75 MPa | ||
| 1.5 mm | 38.37MPa | 33.00 MPa |
| Model # | 20 mm | 25 mm | |
|---|---|---|---|
| Model 1 | 0.8 mm | 11.79 MPa | 10.59 MPa |
| 1 mm | 12.02 MPa | 11.16 MPa | |
| 1.5 mm | 13.37 MPa | 11.91 MPa | |
| Model 2 | 0.8 mm | 10.51 MPa | 10.07 MPa |
| 1 mm | 11.29 MPa | 11.05 MPa | |
| 1.5 mm | 12.16 MPa | 11.51 MPa |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.-N.; Shie, M.-Y.; Shen, Y.-W.; Hsu, J.-T.; Huang, H.-L.; Fuh, L.-J. Biomechanical Assessment of Design Parameters on a Self-Developed 3D-Printed Titanium-Alloy Reconstruction/Prosthetic Implant for Mandibular Segmental Osteotomy Defect. Metals 2019, 9, 597. https://doi.org/10.3390/met9050597
Huang S-N, Shie M-Y, Shen Y-W, Hsu J-T, Huang H-L, Fuh L-J. Biomechanical Assessment of Design Parameters on a Self-Developed 3D-Printed Titanium-Alloy Reconstruction/Prosthetic Implant for Mandibular Segmental Osteotomy Defect. Metals. 2019; 9(5):597. https://doi.org/10.3390/met9050597
Chicago/Turabian StyleHuang, Sheng-Ni, Ming-You Shie, Yen-Wen Shen, Jui-Ting Hsu, Heng-Li Huang, and Lih-Jyh Fuh. 2019. "Biomechanical Assessment of Design Parameters on a Self-Developed 3D-Printed Titanium-Alloy Reconstruction/Prosthetic Implant for Mandibular Segmental Osteotomy Defect" Metals 9, no. 5: 597. https://doi.org/10.3390/met9050597
APA StyleHuang, S.-N., Shie, M.-Y., Shen, Y.-W., Hsu, J.-T., Huang, H.-L., & Fuh, L.-J. (2019). Biomechanical Assessment of Design Parameters on a Self-Developed 3D-Printed Titanium-Alloy Reconstruction/Prosthetic Implant for Mandibular Segmental Osteotomy Defect. Metals, 9(5), 597. https://doi.org/10.3390/met9050597






