Materials Properties and Liquid Flow in the Hearth of the Experimental Blast Furnace
Abstract
1. Introduction
2. Methodology
2.1. EBF Campaign 32
2.2. Copper Injection in the End of EBF Campaign 32
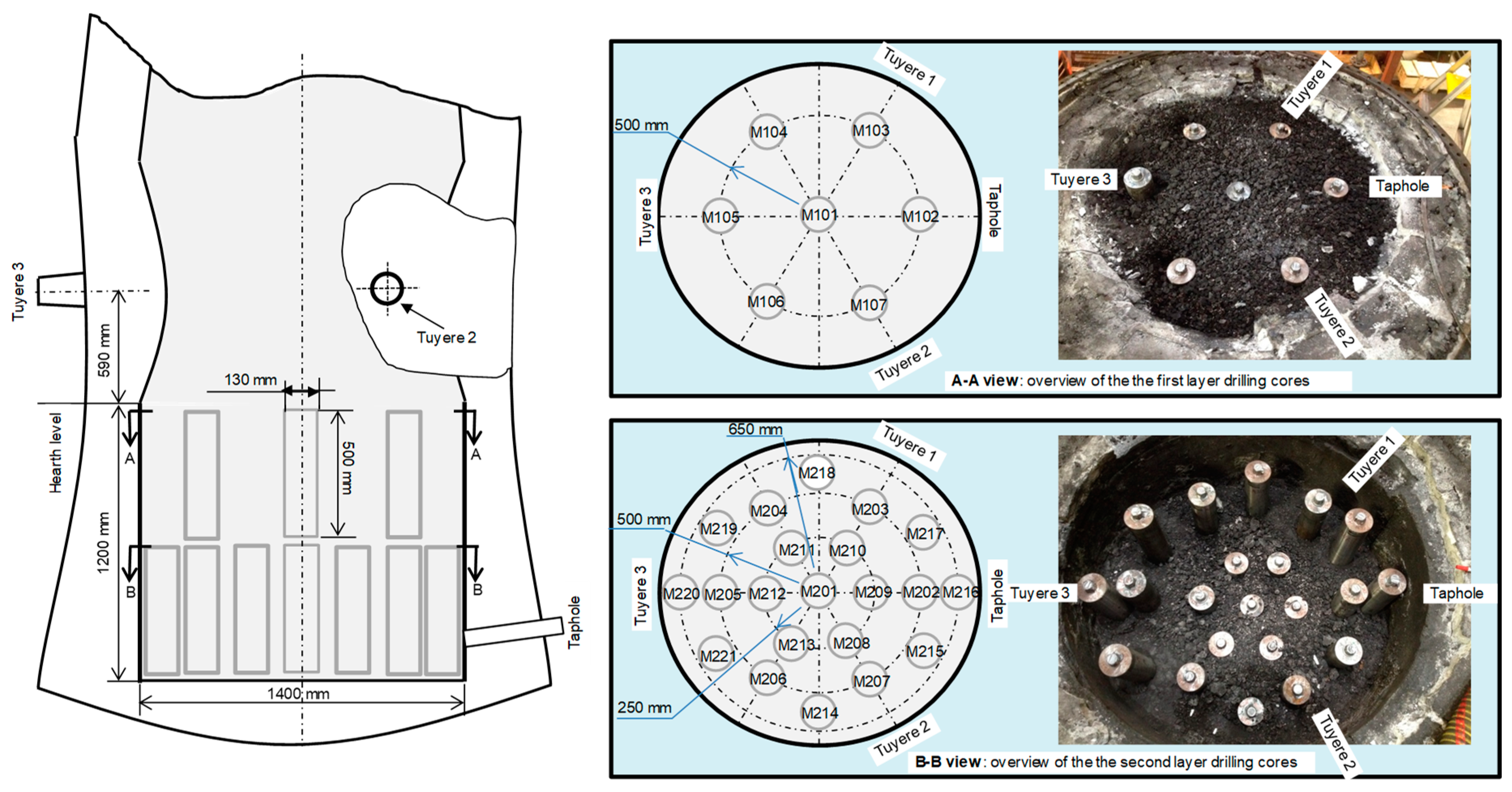
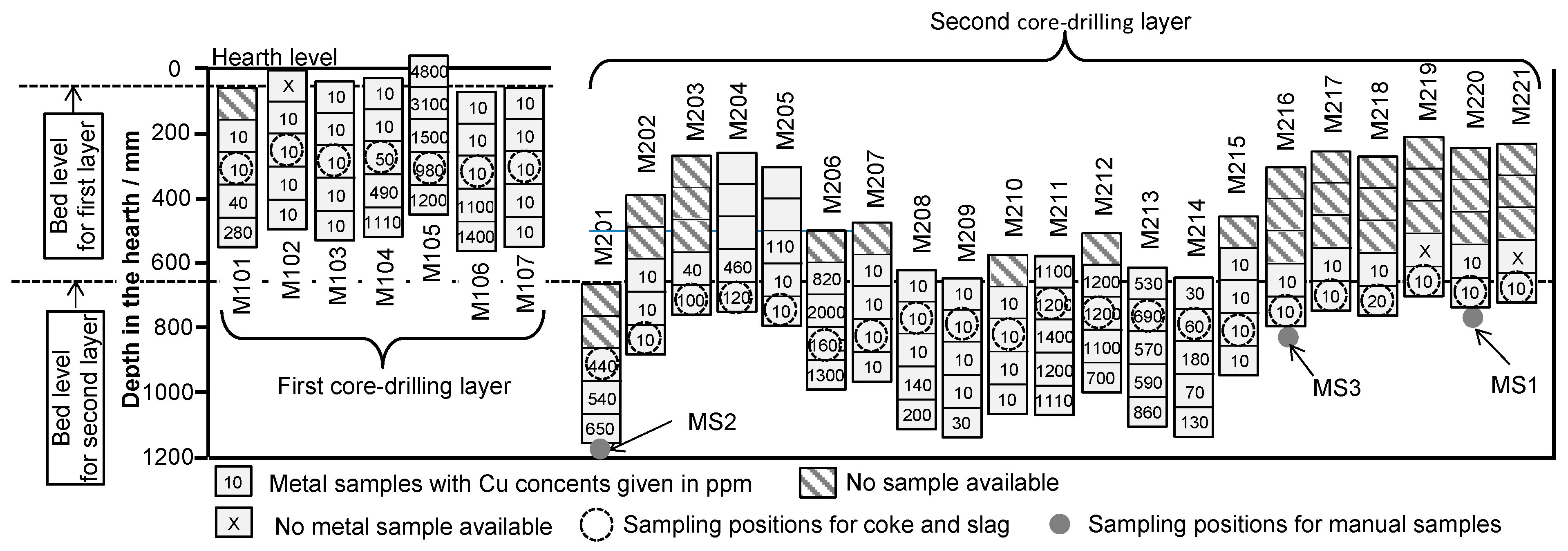
2.3. Collection of Samples from the EBF Hearth
2.4. Analysis of the Samples
3. Results and Discussion
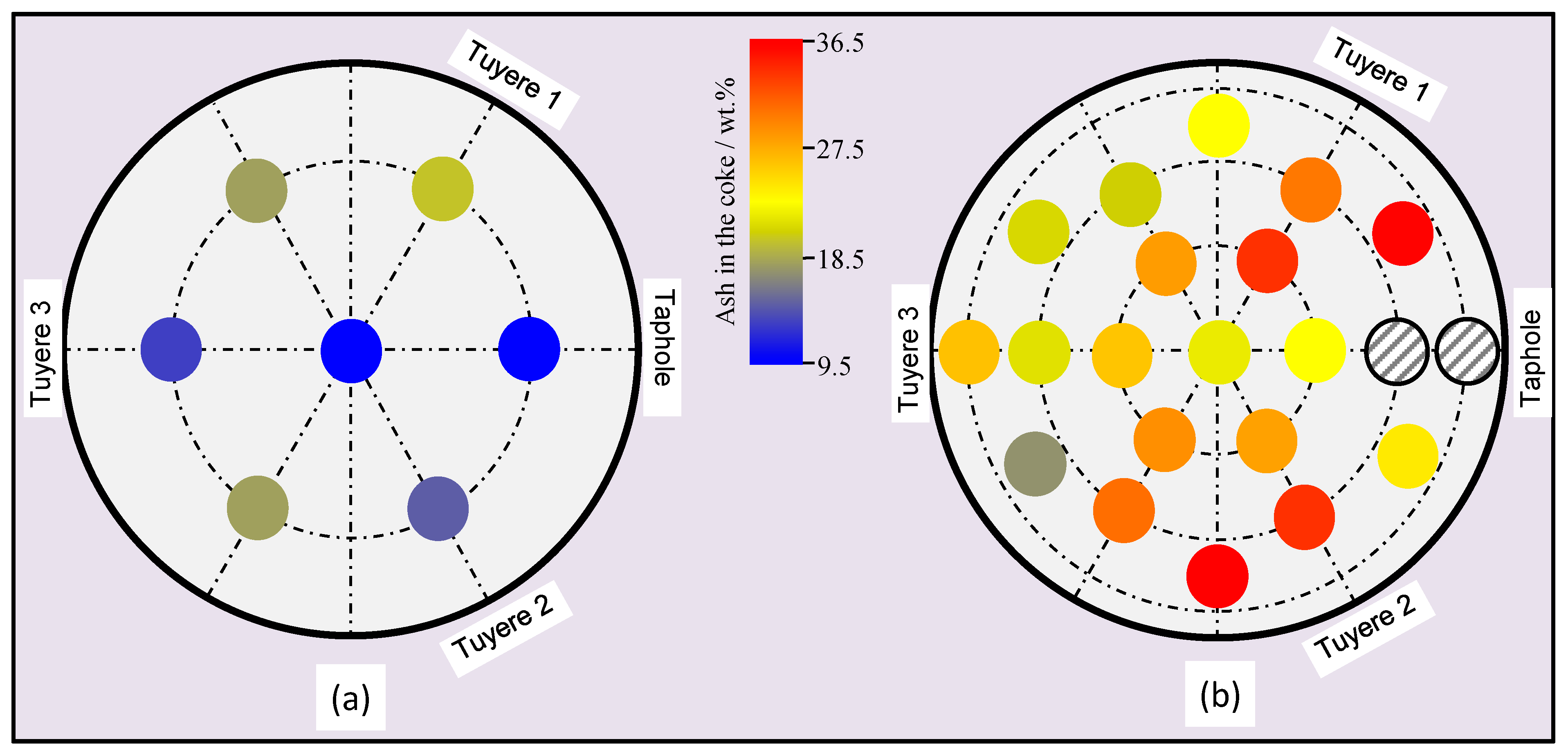
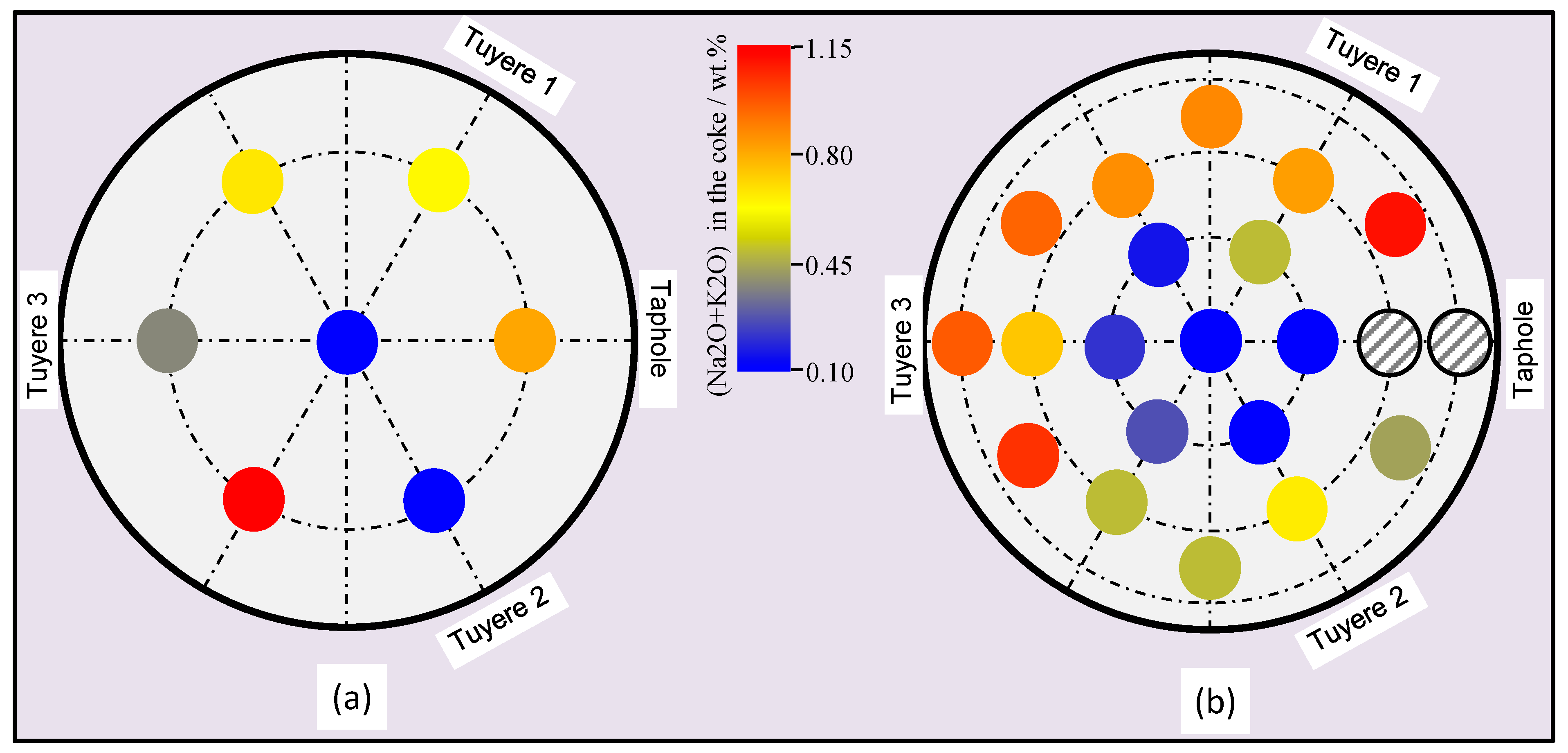
3.1. Properties of the Coke
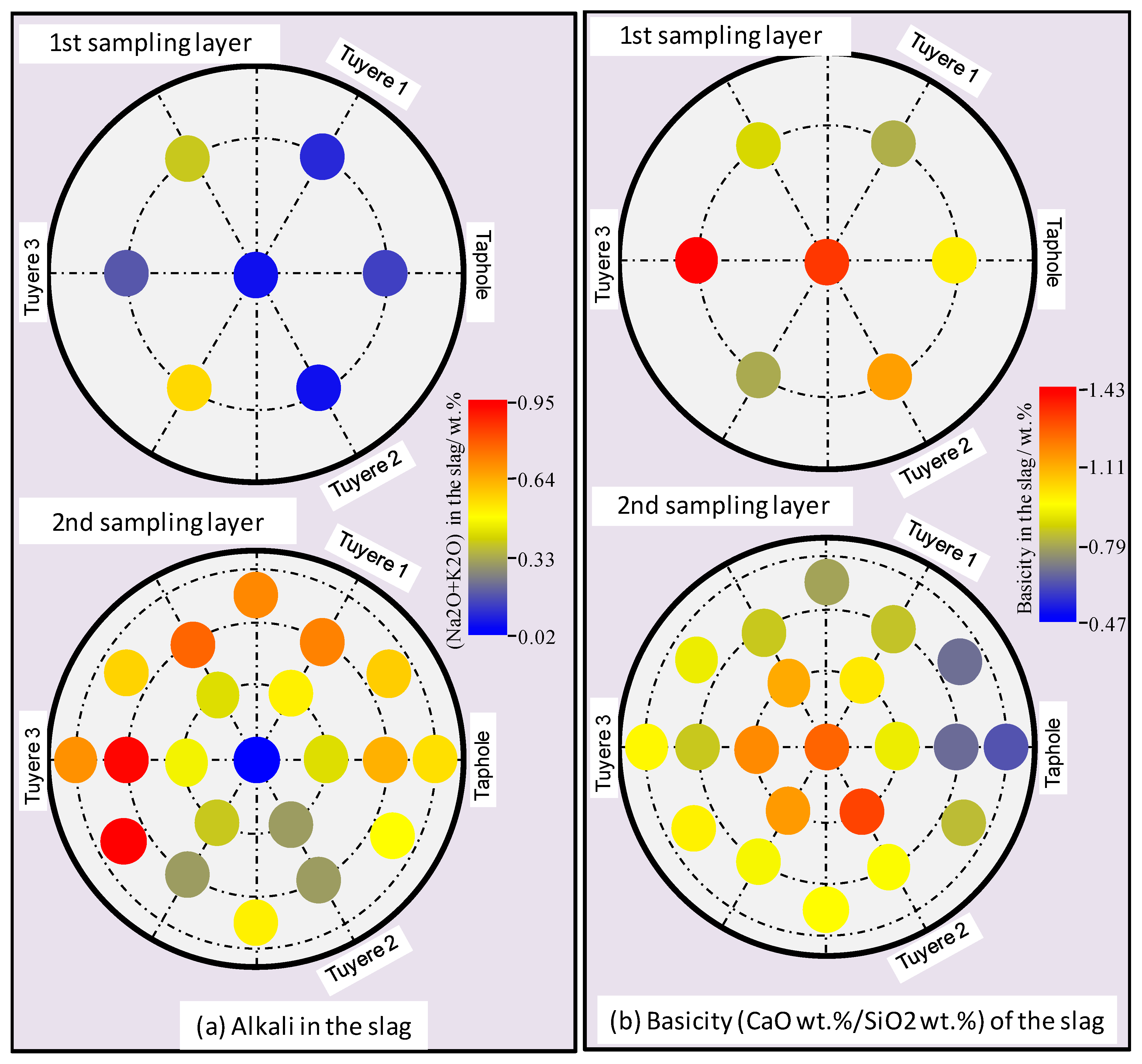
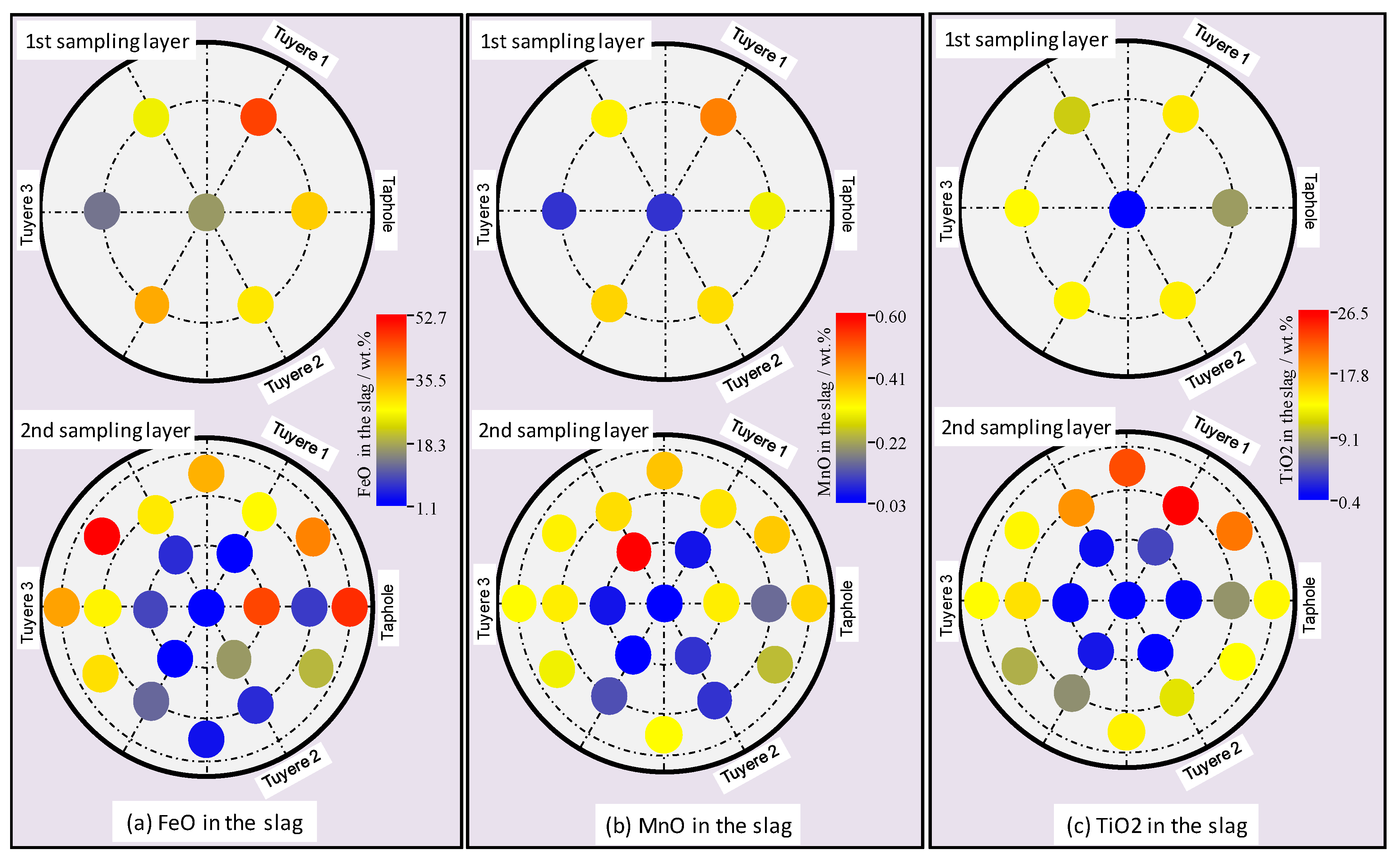
3.2. Properties of the Slag
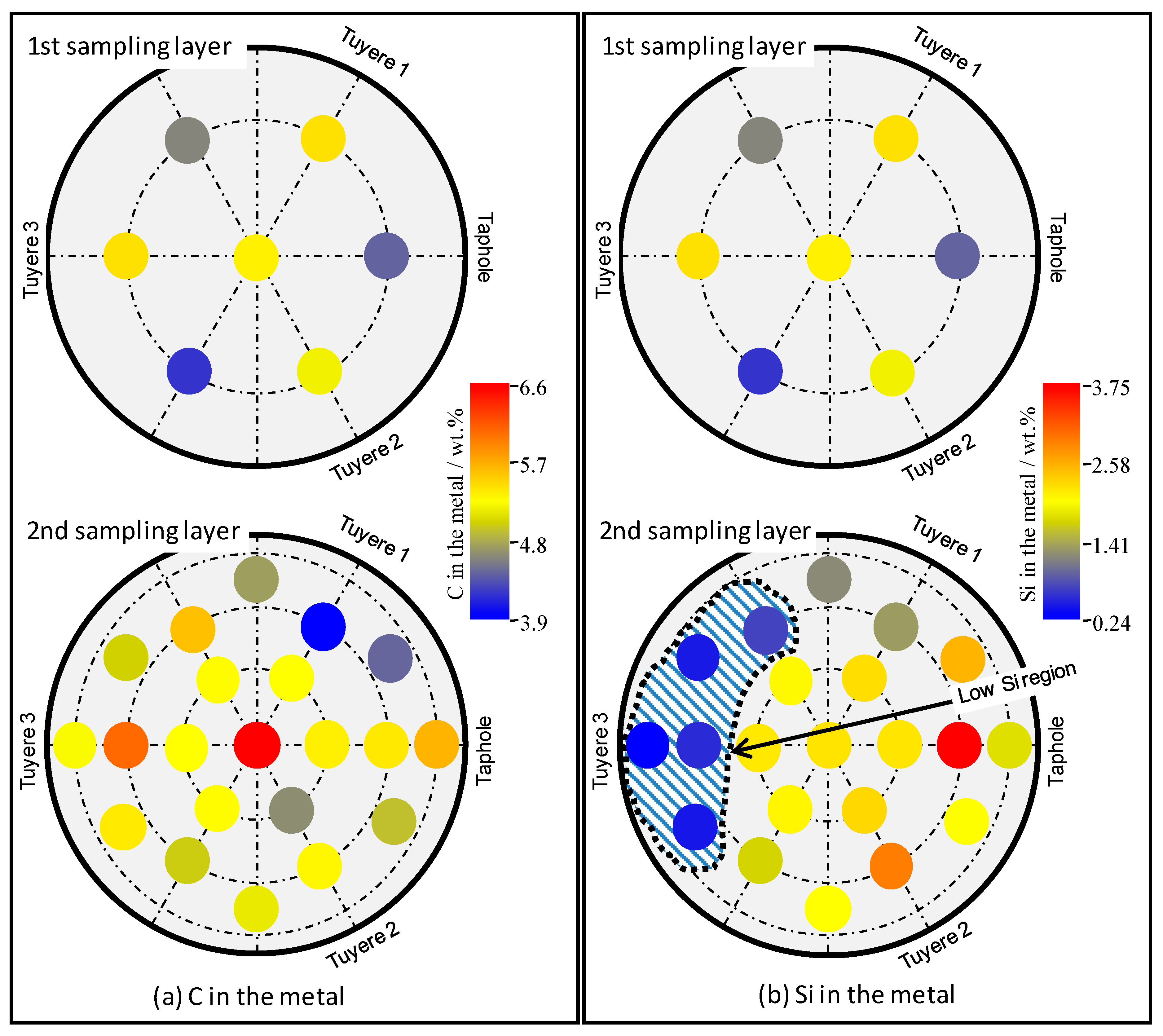
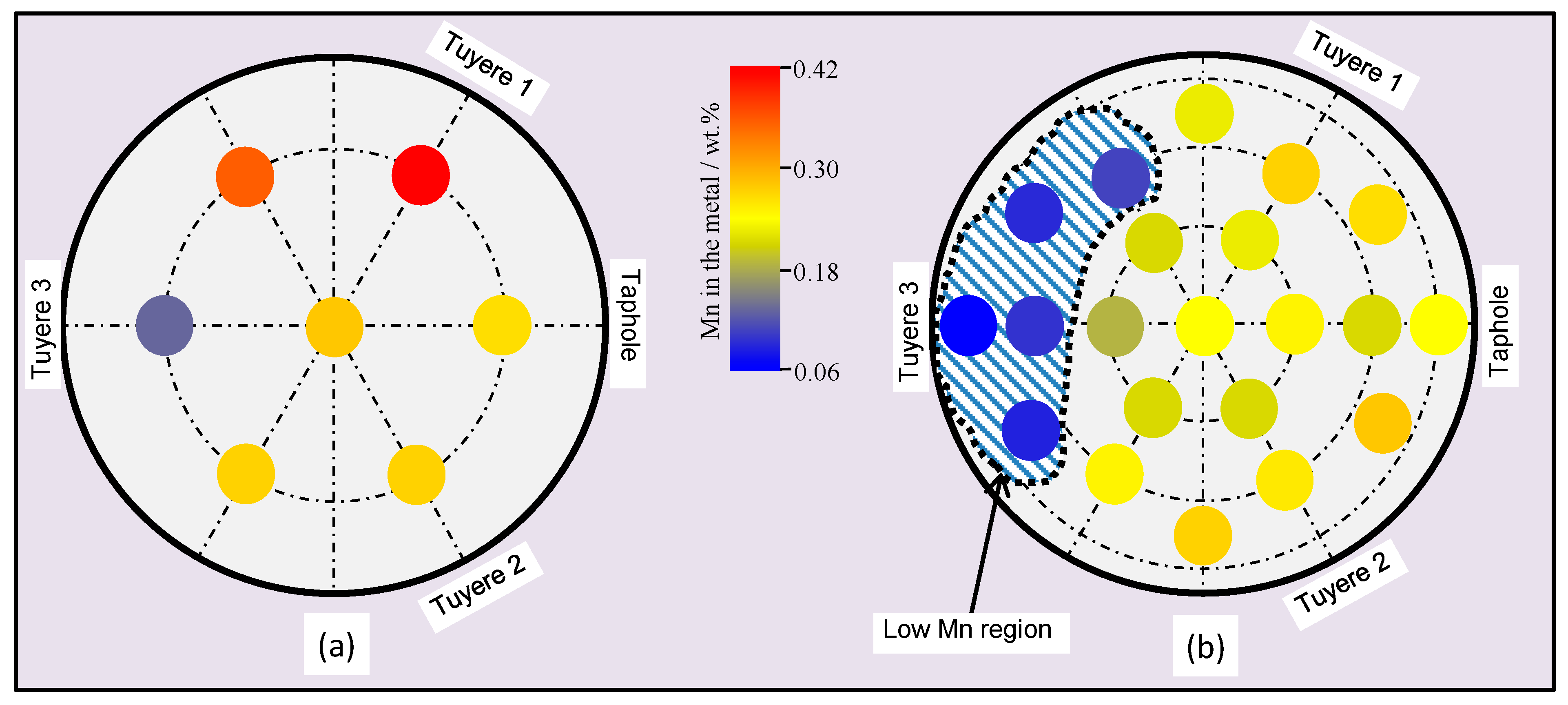
3.3. Properties of the Metal
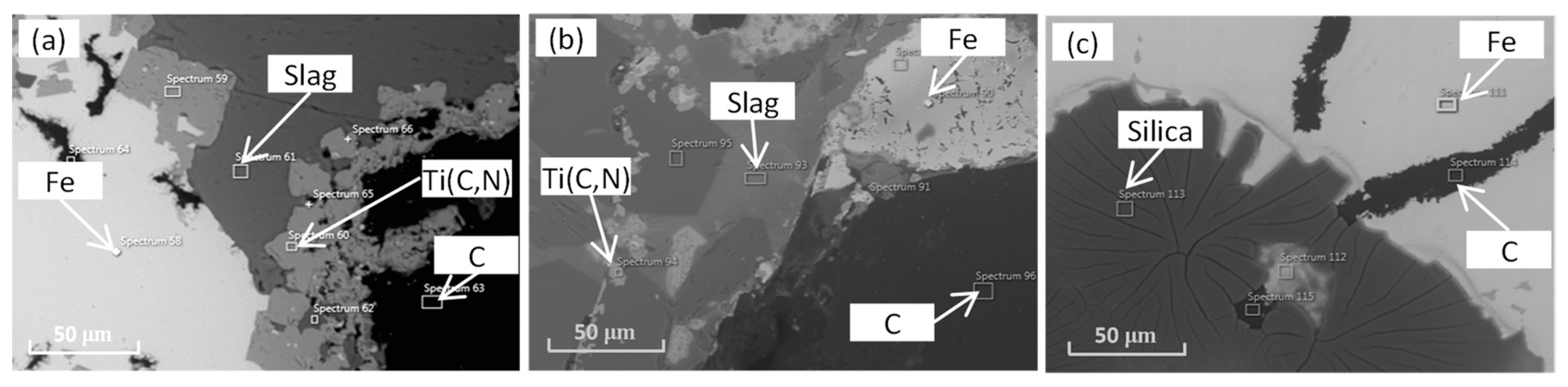
3.4. Properties of the Aggregates
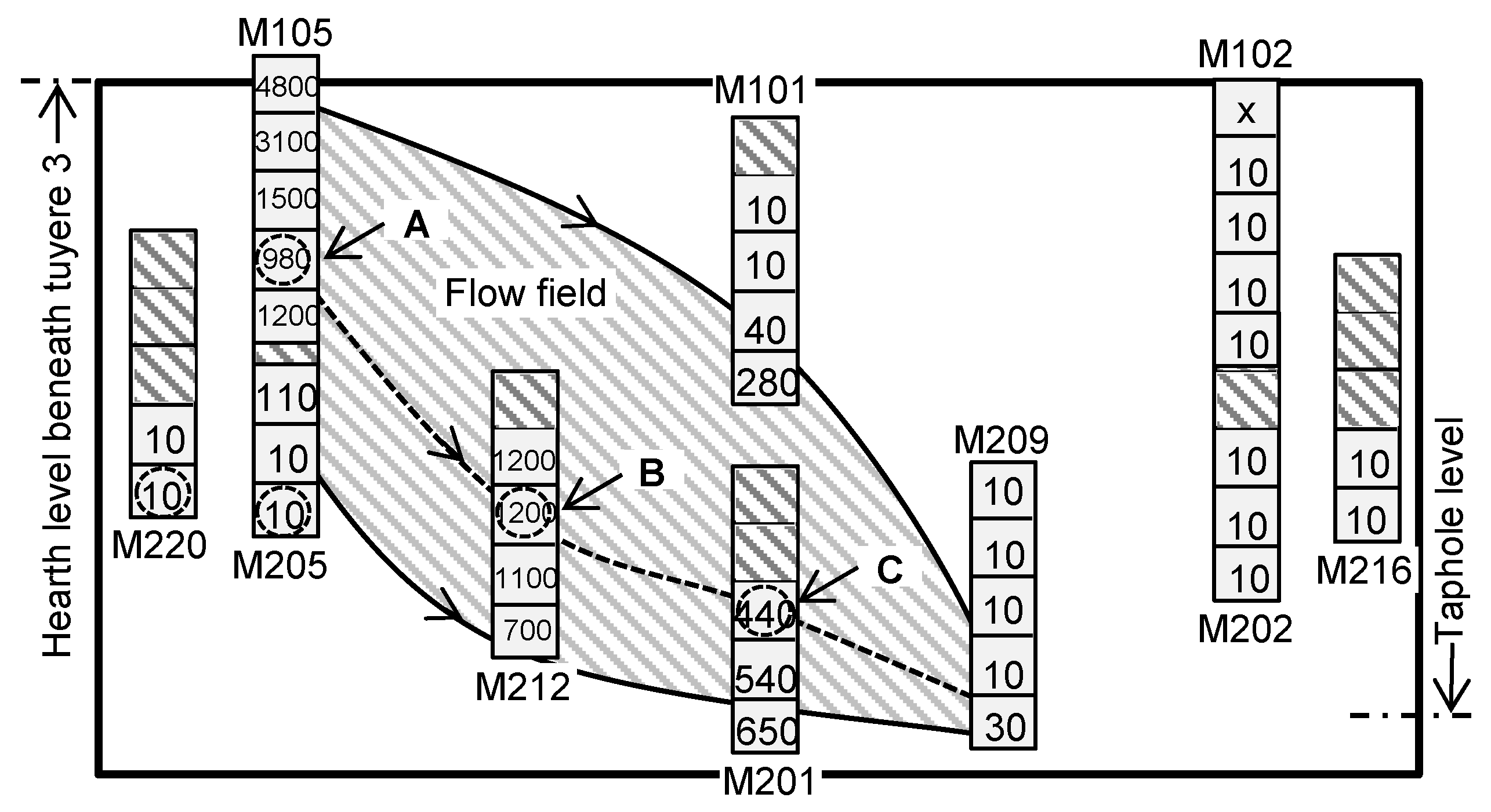
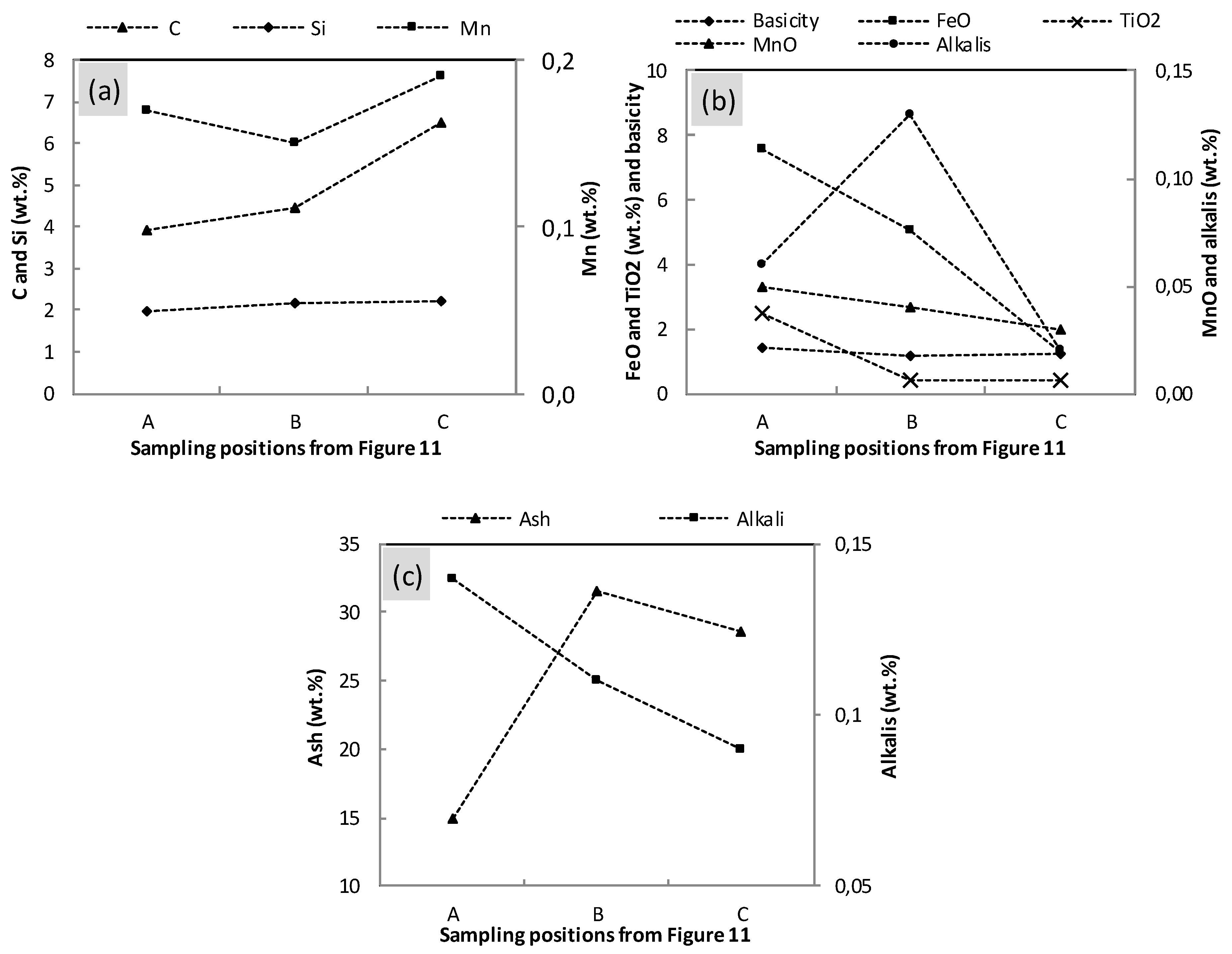
3.5. Properties of the Materials along the Flow Field in the EBF Hearth
3.6. Hearth Conditions before Quenching of the EBF
4. Conclusions
- Alkali in the coke and slag, ash contents in the coke and basicity of the slag show vertical and radial variations in the hearth; higher alkali in the coke and slag, higher ash content in the coke and lower basicity (wt.%CaO/wt.%SiO2) in the slag were found in the close-to-wall region and the lower part of the hearth.
- The chemistries of the hot metal, slag, and coke were found to vary slightly along the liquid flow field.
- There exists an area beneath and in front of tuyere 3, where the flow resistance of the liquid is high.
- The flow resistance contributed to the formation of a cold zone in the close-to-wall region and at the bottom of the EBF hearth. The division of the cold zone and hot zone has significant impacts on the chemical properties of the materials in the EBF hearth, as well as on the radial and vertical distributions of certain elements/components in the hearth materials.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nishoka, K.; Maeda, T.; Shimizu, M. Effect of Various In-furnace Conditions on Blast Furnace Hearth Drainage. ISIJ Int. 2005, 45, 1496–1505. [Google Scholar] [CrossRef]
- Nouchi, T.; Yasui, M.; Takeda, K. Effects of Particle Free Space on Hearth Drainage Efficiency. ISIJ Int. 2003, 43, 175–180. [Google Scholar] [CrossRef]
- Torrkulla, J.; Brännbacka, J.; Saxén, H.; Waller, M. Indicators of the Internal State of the Blast Furnace Hearth. ISIJ Int. 2002, 42, 504–511. [Google Scholar] [CrossRef]
- Raipala, K. Deadman and hearth phenomena in the blast furnace. Scand. J. Metall. 2000, 29, 39–46. [Google Scholar] [CrossRef]
- Kumar, S. Heat Transfer Analysis and Estimation of Refractory Wear in an Iron Blast Furnace Hearth Using FInite Element Method. ISIJ Int. 2005, 45, 1122–1128. [Google Scholar] [CrossRef]
- Geerdes, M.; Chaigneau, R.; Kurunov, I.; Lingiardi, O.; Ricketts, J. Modern Blast Furnace Ironmaking: An Introduction, 3rd ed.; IOS Press BV: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Sun, H.; Chew, S.J. Slag Reduction in the Coke Bed of a Blast Furnace. ISIJ Int. 2006, 46, 1108–1110. [Google Scholar] [CrossRef]
- Ichida, M.; Orimoto, T.; Tanaka, T.; Sakatani, M.; Ueno, H. Behaviour of Pulverized Coal Ash and Physical Property of Dripping Slag under High Pulverized Coal Injection Operation. In Proceedings of the International Blast Furnace Lower Zone Symposium, Wollongong, Australia, 25–27 November 2002. [Google Scholar]
- Hooey, L.; Sterneland, J.; Hallin, M. Evaluation of Operational Data from the LKAB Experimental Blast Furnace. In Proceedings of the 60th Ironmaking Conference Proceedings, Baltimore, MD, USA, 25–28 March 2001. [Google Scholar]
- Hu, X.; Sundqvist-Ökvist, L.; Åström, E.; Forsberg, F.; Checchia, P.; Bonomi, G.; Calliari, I.; Calvini, P.; Donzella, A.; Faraci, E.; et al. Exploring the Capability of Muon Scattering Tomography for Imaging the Components in the Blast Furnace. ISIJ Int. 2018, 58, 35–42. [Google Scholar] [CrossRef]
- Sundqvist-Ökvist, L.; Eklund, N.; Wikström, J.-O. Characterization of the State of LKAB Experimental BF Hearth. In Proceedings of the AISTech 2010: Iron and Steel Technology Conference Proceedings, Pittsburgh, PA, USA, 3–6 May 2010. [Google Scholar]
- Sundqvist-Ökvist, L.; Lundgren, M.; Wikström, J.-O.; Eklund, N. Raceway and Hearth Conditions Relative Process Conditions in the LKAB Experimental BF. In Proceedings of the European Coke and Ironmaking Congress, Düsseldorf, Germany, 27 June–1 July 2011. [Google Scholar]
- Jiao, K.; Zhang, J.; Liu, Z.; Kuang, S.; Liu, Y. Dissection Investigation of Ti(C,N) Behavior in Blast Furnace Hearth during Vanadium Titano-magnetite Smelting. ISIJ Int. 2017, 57, 48–54. [Google Scholar] [CrossRef]
- Jiao, K.; Zhang, J.; Liu, Z.; Xu, M.; Liu, F. Formation Mechanism of the Protective Layer in a Blast Furnace Hearth. Int. J. Min. Met. Mater. 2015, 22, 1017–1024. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Schenk, J.; Rantitsch, G.; Thaler, C.; Stocker, H. Effect of Alkaline Elements on the Reactivity, Strength and Structural Properties of Blast Furnace Cokes. Metalurgija 2015, 54, 503–506. [Google Scholar]
- Jeong, I.-H.; Kim, H.-S.; Sasaki, Y. Trickle Flow Behaviors of Liquid Iron and Molten Slag in the Lower Part of Blast Furnace. ISIJ Int. 2013, 53, 2090–2098. [Google Scholar] [CrossRef]
- Kang, T.W.; Gupta, S.; Saha-Chaudhury, N.; Sahajwalla, V. Wetting and Interfacial Reaction Investigations of coke/slag Systems and associated Liquid Permeability of Blast Furnaces. ISIJ Int. 2005, 45, 1526–1535. [Google Scholar] [CrossRef]

| MPBO Pellets | Pulverized Coal | Coke | Quartzite | BOF Slag (from SSAB) | Limestone |
|---|---|---|---|---|---|
| 1380 | 130 | 420 | 18-22 | 40-45 | 40 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, X.; Sundqvist Ökvist, L.; Ölund, M. Materials Properties and Liquid Flow in the Hearth of the Experimental Blast Furnace. Metals 2019, 9, 572. https://doi.org/10.3390/met9050572
Hu X, Sundqvist Ökvist L, Ölund M. Materials Properties and Liquid Flow in the Hearth of the Experimental Blast Furnace. Metals. 2019; 9(5):572. https://doi.org/10.3390/met9050572
Chicago/Turabian StyleHu, Xianfeng, Lena Sundqvist Ökvist, and Martin Ölund. 2019. "Materials Properties and Liquid Flow in the Hearth of the Experimental Blast Furnace" Metals 9, no. 5: 572. https://doi.org/10.3390/met9050572
APA StyleHu, X., Sundqvist Ökvist, L., & Ölund, M. (2019). Materials Properties and Liquid Flow in the Hearth of the Experimental Blast Furnace. Metals, 9(5), 572. https://doi.org/10.3390/met9050572




