Microstructural Characterization and Formation Mechanism of Nitrided Layers on Aluminum Substrates by Thermal Plasma Nitriding
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Microstructural Characteristics of the Nitrided Layer
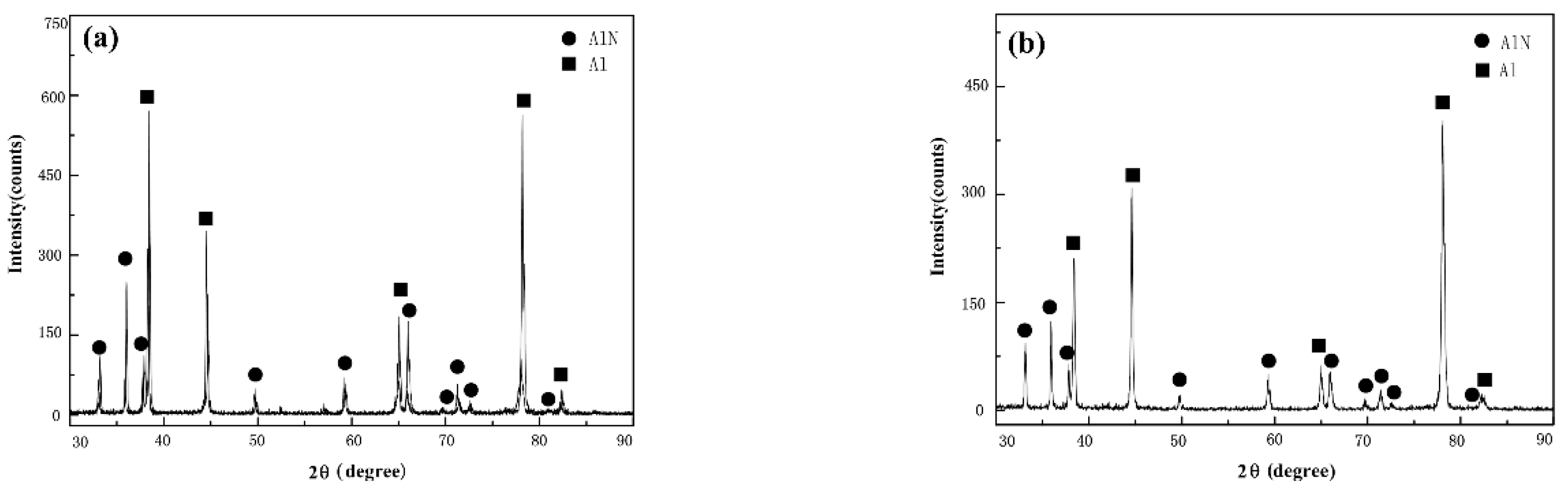
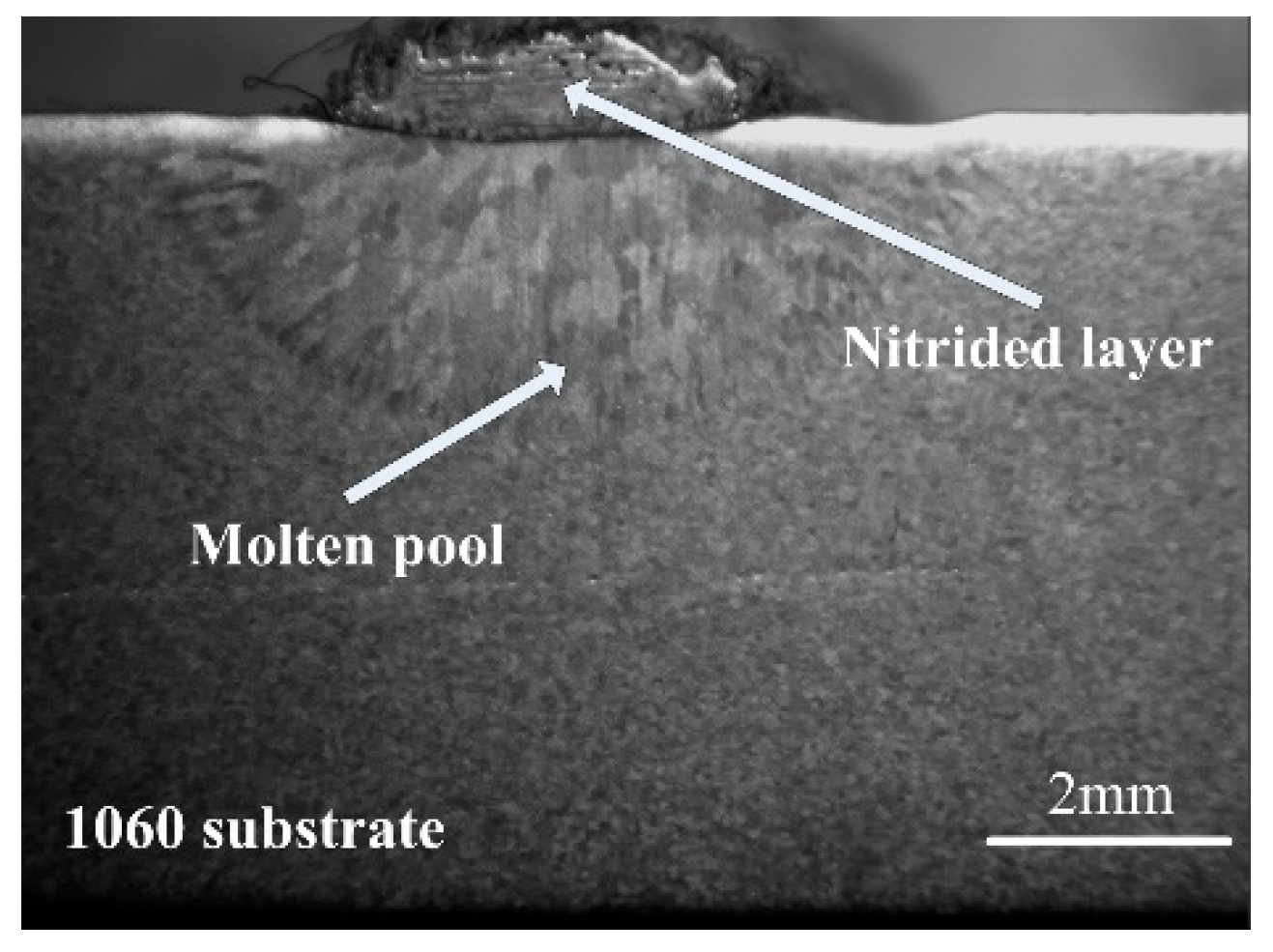
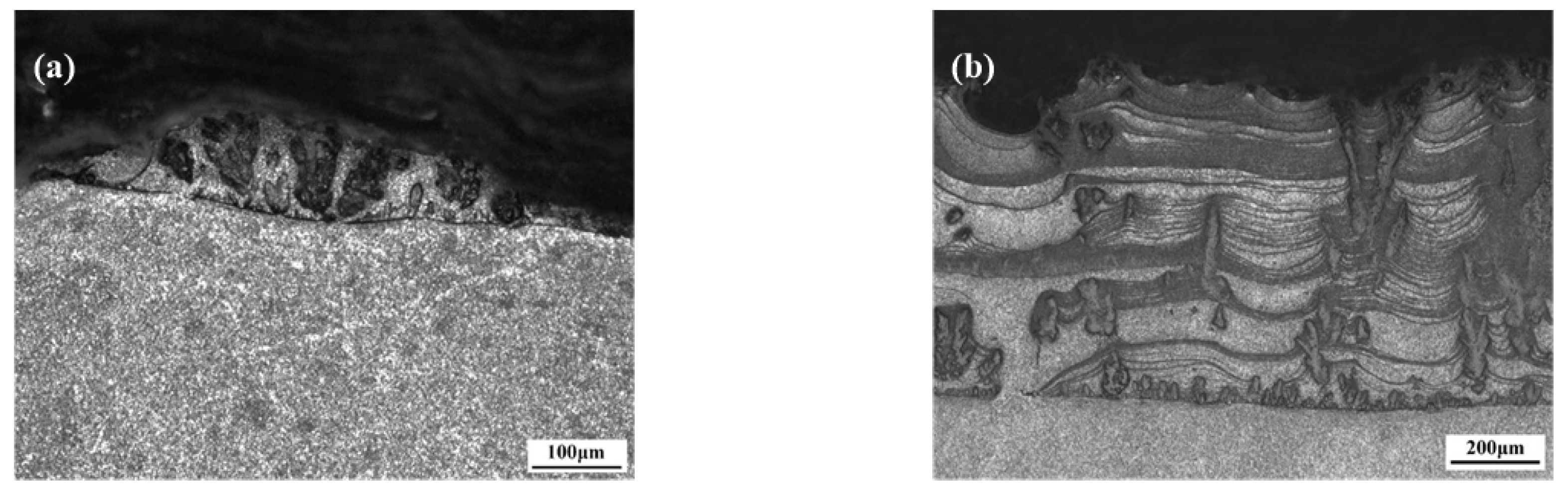
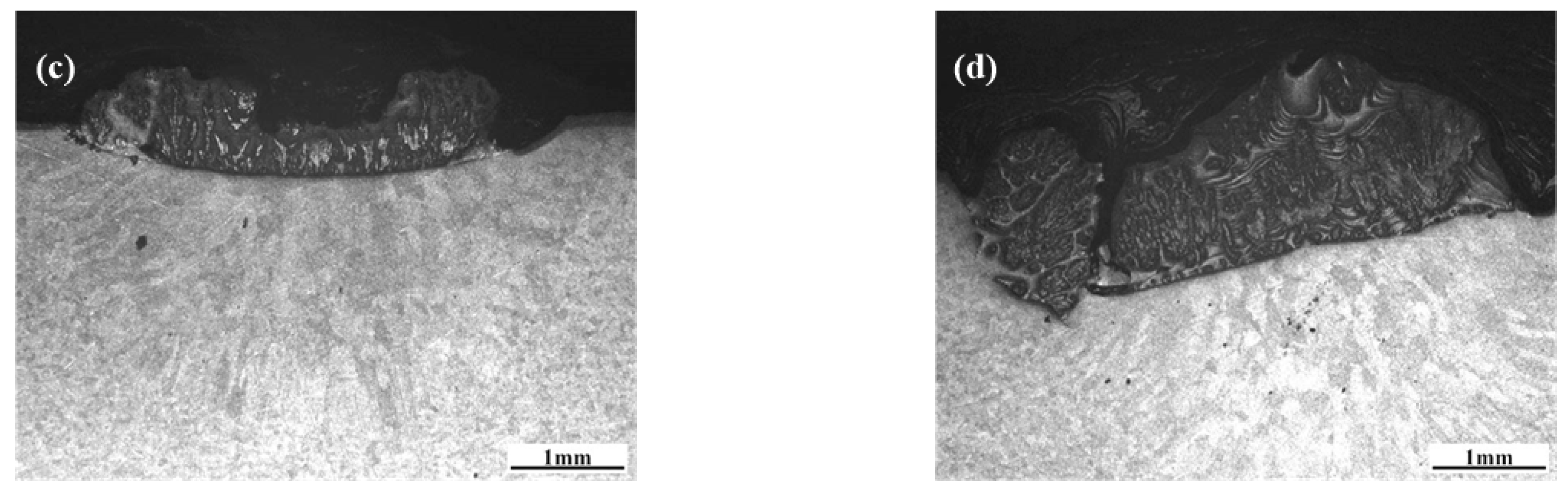
3.1.1. Morphological Characteristics and Phase Compositions of the Nitrided Layer
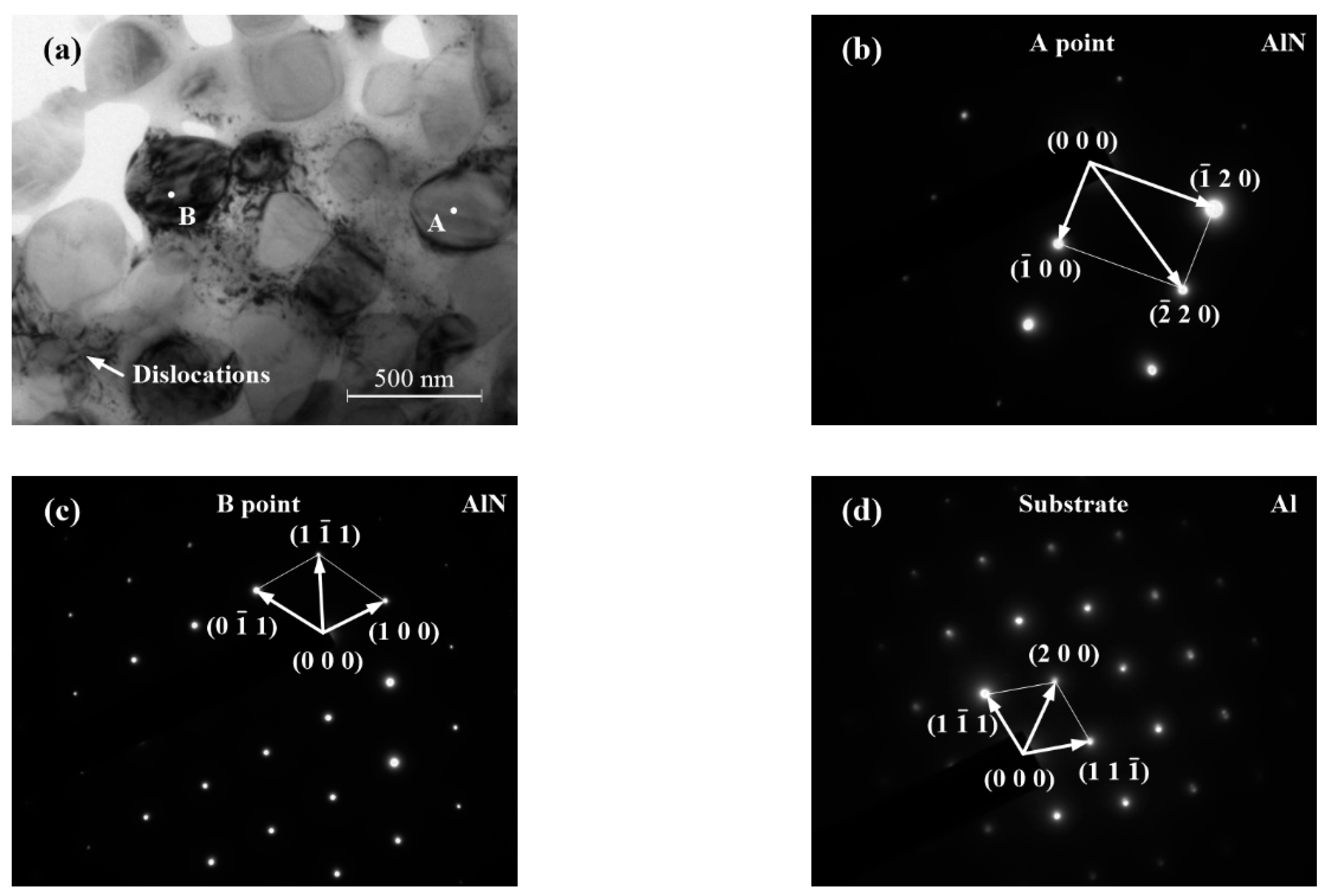
3.1.2. Microstructures of the Transition Region
3.1.3. Microstructures of the Dendrite Region
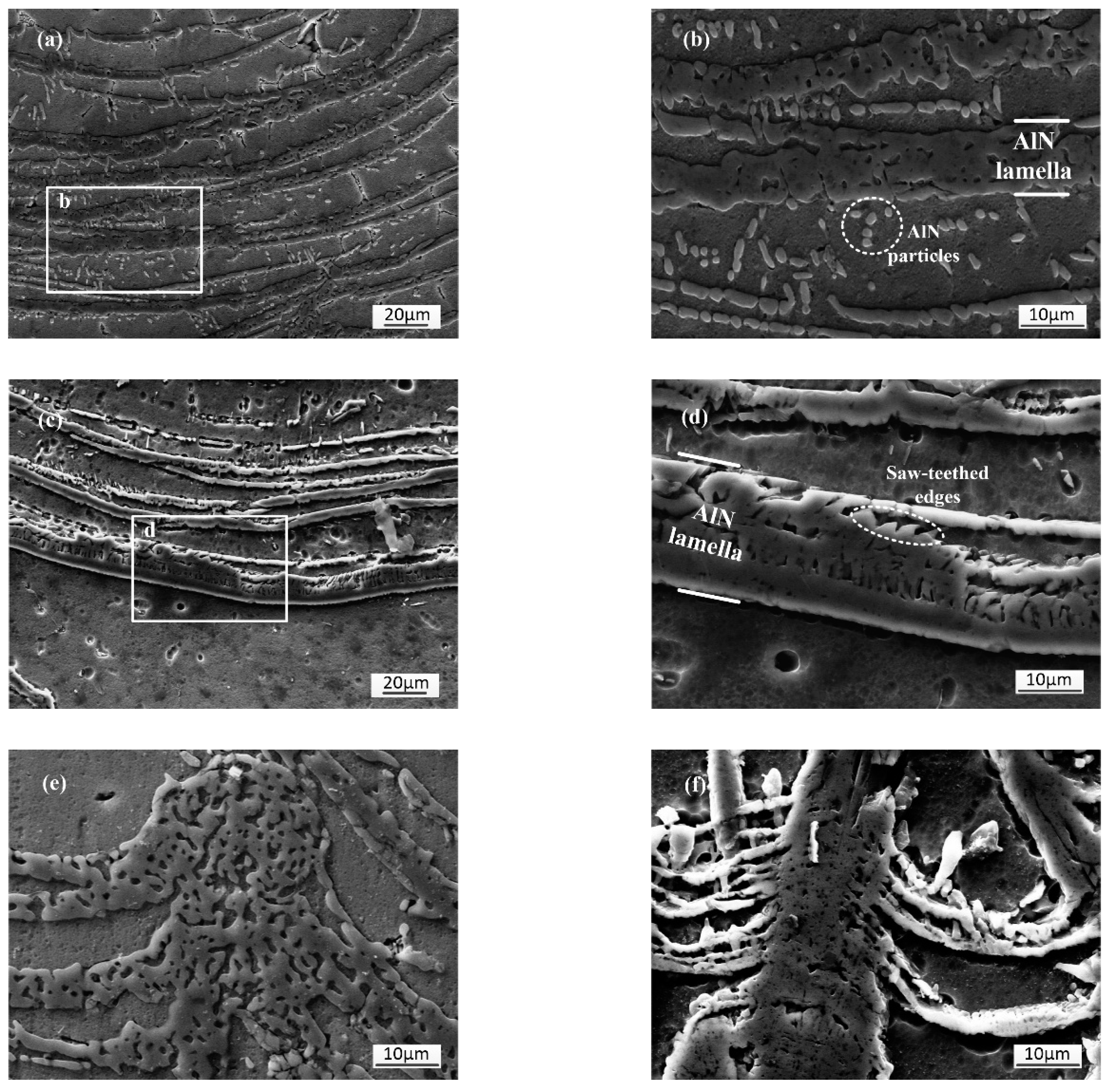
3.1.4. Microstructures of the Lamella Region
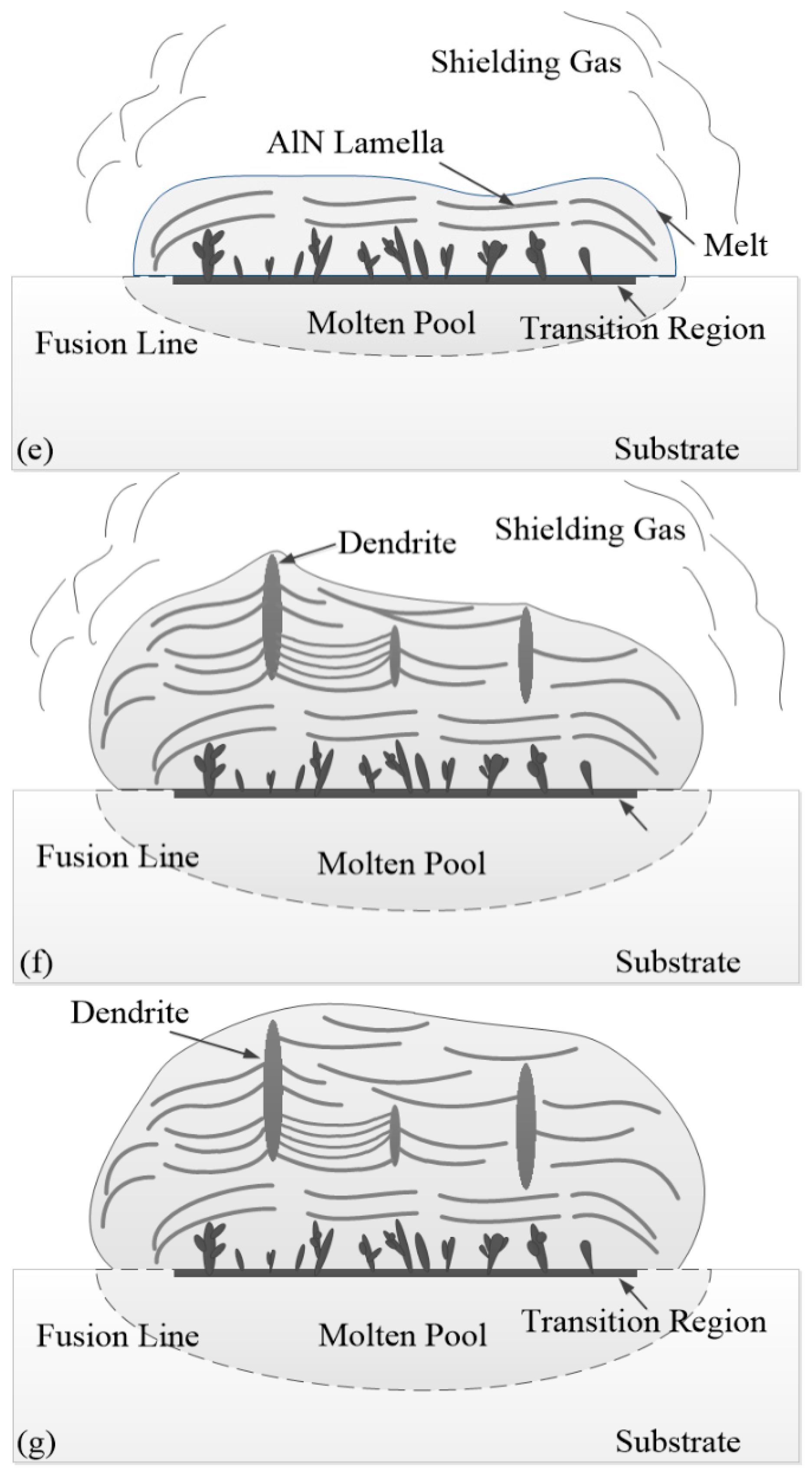
3.2. Formation Mechanisms and Processes of the Nitrided Layer
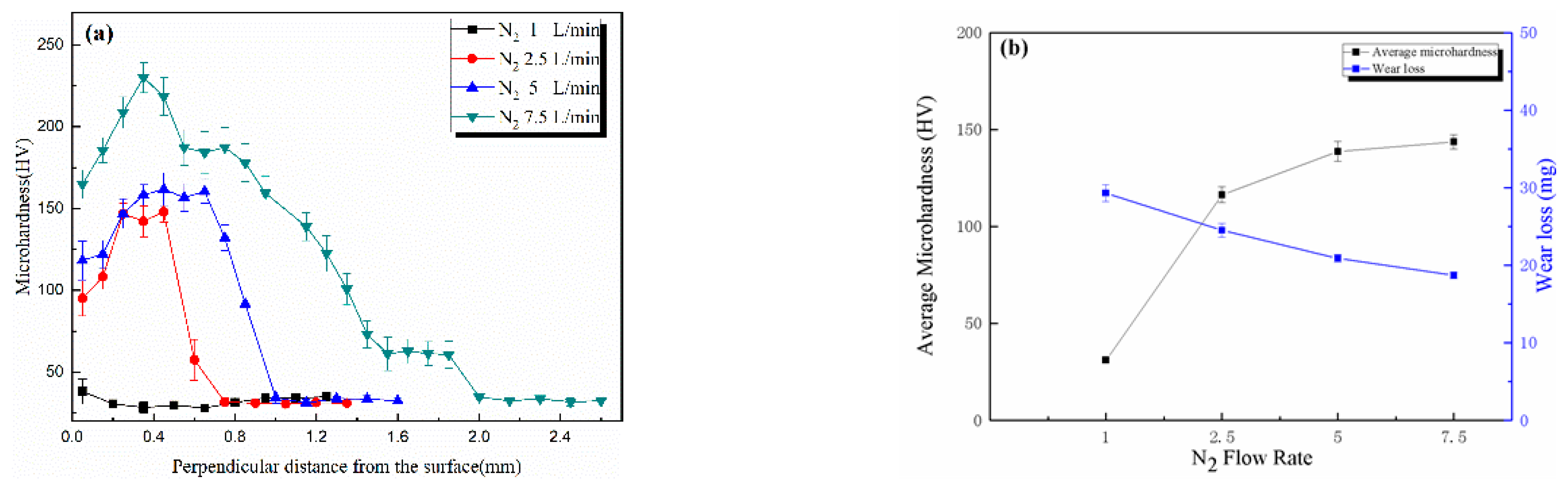
3.3. Effects of N2 Flow Rate upon Nitrided Layers on 6082 Substrates
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jagielski, J.; Piatkowska, A.; Aubert, P.; Legrand-Buscema, C.; Le Paven, C.; Gawlik, G.; Piekoszewski, J.; Werner, Z. Effects of high dose nitrogen implantation into aluminum. Vacuum 2003, 70, 147–152. [Google Scholar] [CrossRef]
- Bashir, M.I.; Shafiq, M.; Naeem, M.; Zaka-ul-Islam, M.; Díaz-Guillén, J.C.; Lopez-Badillo, C.M.; Zakaullah, M. Enhanced surface properties of aluminum by PVD-TiN coating combined with cathodic cage plasma nitriding. Surf. Coat. Technol. 2017, 327, 59–65. [Google Scholar] [CrossRef]
- Huashun, Y.; Kim, J.D.; Kang, S.B. The formation of AlN and TiN particles during nitrogen bearing gas injection into Al-Mg-Ti melt. Mater. Sci. Eng. A 2004, 386, 318–325. [Google Scholar] [CrossRef]
- Abreu, C.M.; Cristóbal, M.J.; Figueroa, R.; Pena, G. Wear and corrosion performance of two different tempers (T6 and T73) of AA7075 aluminium alloy after nitrogen implantation. Appl. Surf. Sci. 2015, 327, 51–61. [Google Scholar] [CrossRef]
- Czerwiec, T.; Michel, H.; Bergmann, E. Low-pressure-high density plasma nitriding: Mechanisms, technology and results. Surf. Coat. Technol. 1998, 108–109, 182–190. [Google Scholar] [CrossRef]
- Minto, T.A.; De Oliveira, V.M.C.A.; Voorwald, H.J.C. Plasma immersion ion implantation: Influence on the rotating bending fatigue strength of AA 7050-T7451 aluminum alloy. Int. J. Fatigue 2017, 103, 17–27. [Google Scholar] [CrossRef]
- Ebisawa, T.; Saikudo, R. Formation of aluminum nitride on aluminum surfaces by ECR nitrogen plasmas. Surf. Coat. Technol. 1996, 86–87, 622–627. [Google Scholar] [CrossRef]
- Thibault, S.; Hug, E. Corrosion and wear mechanisms of aluminum alloys surface reinforced by multicharged N-implantation. Appl. Surf. Sci. 2014, 310, 311–316. [Google Scholar] [CrossRef]
- Manova, D.; Mändl, S.; Rauschenbach, B. Evolution of surface morphology during ion nitriding of aluminium. Surf. Coat. Technol. 2004, 180–181, 118–121. [Google Scholar] [CrossRef]
- Tran, A.T.; Pandraud, G.; Schellevis, H.; Sarro, P.M. Enhancement of AlN slender piezoelectric cantilevers actuation by PECVD Silicon Nitride coating. Procedia Eng. 2012, 47, 104–107. [Google Scholar] [CrossRef]
- Figueroa, U.; Salas, O.; Oseguera, J. Production of AlN films ion nitriding versus PVD coating. Thin Solid Film. 2004, 469–470, 295–303. [Google Scholar] [CrossRef]
- Renevier, N.; Czerwiec, T.; Billard, A.; Von Stebut, J.; Michel, H. A way to decrease the nitriding temperature of aluminium the low-pressure arc-assisted nitriding process. Surf. Coat. Technol. 1999, 116–119, 380–385. [Google Scholar] [CrossRef]
- Möller, W.; Parascandola, S.; Telbizova, T.; Günzel, R.; Richter, E. Surface processes and diffusion mechanisms of ion nitriding of stainless steel and aluminum. Surf. Coat. Technol. 2001, 136, 73–79. [Google Scholar] [CrossRef]
- Siang-Chung, L. Phase stability of the Al–N system. J. Mater. Sci. Lett. 1997, 16, 759–760. [Google Scholar] [CrossRef]
- LeHuy, H.; Dallarie, S. Effects of Si and Mg dopants on the kinetics of aluminum alloys nitridation. Proc. Ceram. Met. Matrix Compos. 1989, 302–311. [Google Scholar] [CrossRef]
- Jin, H.B.; Chen, K.X.; Zhou, H.P.; Wang, Y.D.; Zou, Z.S.; Wang, W.W. Synthesis of aluminum nitride powder by in situ reaction. Acta Metall. Sin. (China) 2000, 36, 775–779. [Google Scholar]
- Newkirk, M.S.; Urquhart, A.W.; Zwicker, H.R.; Breval, E. Formation of Lanxide TM ceramic composite materials. J. Mater. Res. 1986, 1, 81–89. [Google Scholar] [CrossRef]
- Antolin, S.; Nagelberg, A.S.; Creber, D.K. Formation of Al2O3/Metal Composites by the Directed Oxidation of Molten Aluminum—Magnesium—Silicon Alloys: Part I, Microstructural Development. J. Am. Ceram. Soc. 1992, 75, 447–454. [Google Scholar] [CrossRef]
- Kagawa, Y.; Khatri, S.C.; Koczak, M.J. Directed nitridation of liquid aluminum alloy: Growth process and modeling. In Proceedings of the 17th Annual Conference on Composites and Advanced Ceramic Materials: Ceramic Engineering and Science Proceedings, Hoboken, NJ, USA, 1 January 1993; pp. 776–785. [Google Scholar]
- Cao, J.H.; Liu, Y.; Ning, X.S. Influence of AlN(0001) Surface Reconstructions on the Wettability of an Al/AlN System: A First-Principle Study. Materials 2018, 11, 775. [Google Scholar] [CrossRef] [PubMed]

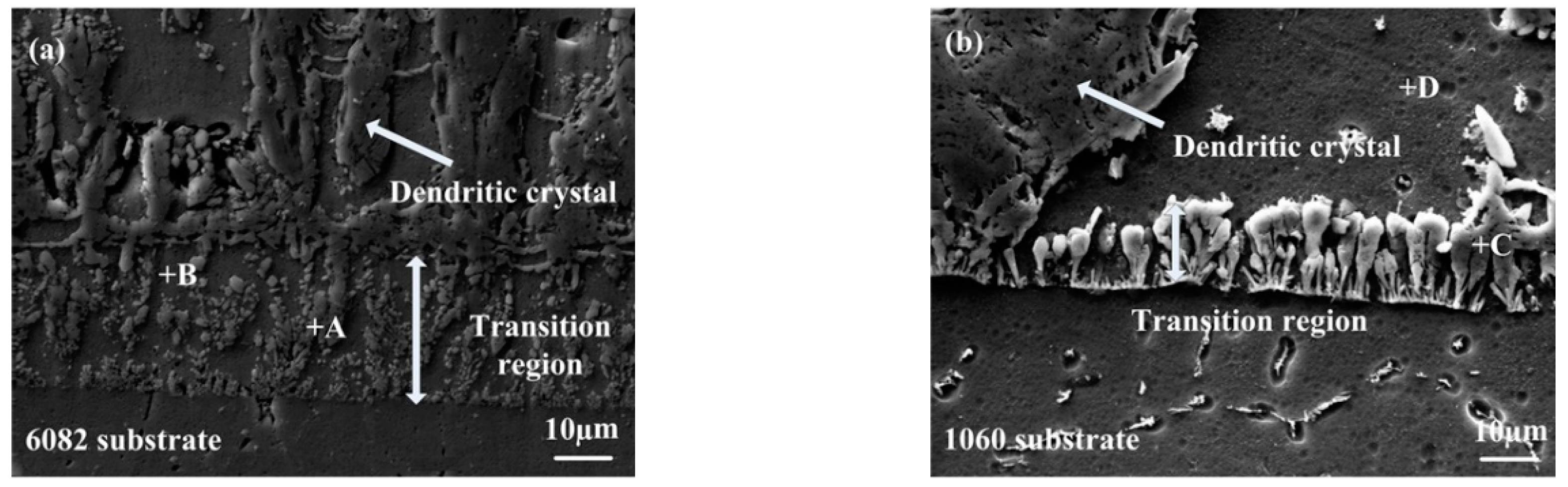
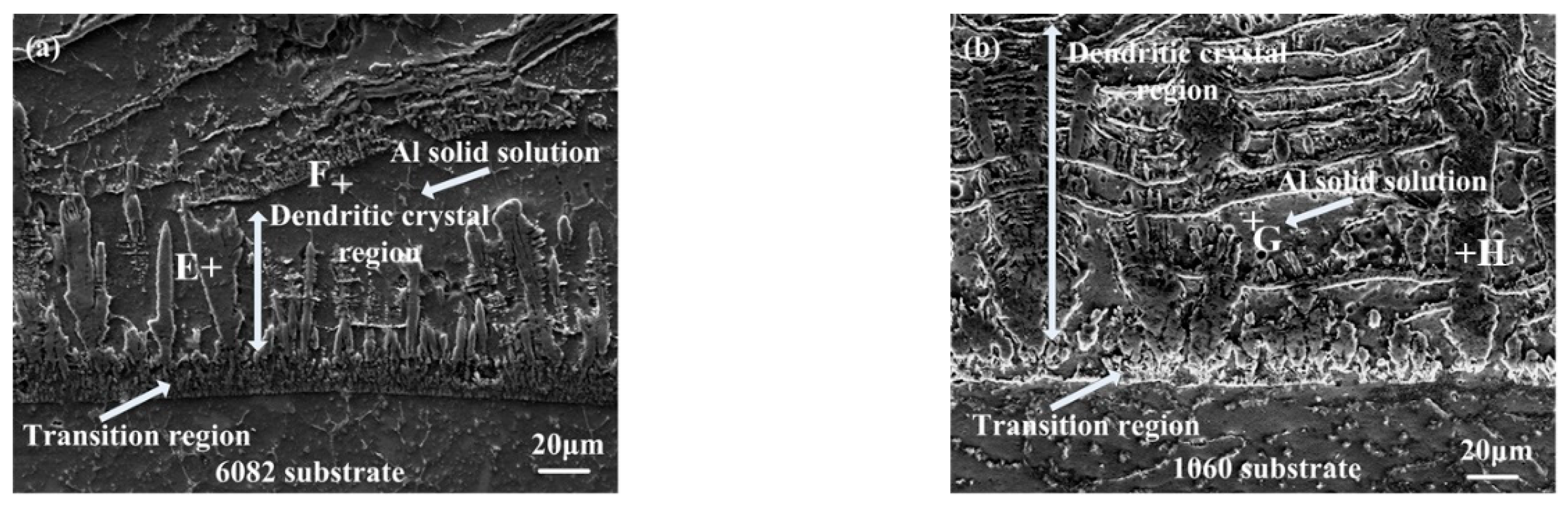


| Element | A | B | C | D |
|---|---|---|---|---|
| N | 45.5 | - | 50.8 | - |
| Al | 53.6 | 98.8 | 49.2 | 100 |
| Mg | 0.5 | 0.5 | - | - |
| Si | 0.4 | 0.7 | - | - |
| Phase | AlN | Al | AlN | Al |
| Element | E | F | G | H |
|---|---|---|---|---|
| N | 59.7 | - | 49.8 | - |
| Al | 48.8 | 99.0 | 50.2 | 100 |
| Mg | - | 0.5 | - | - |
| Si | 0.5 | 0.5 | - | - |
| Phase | AlN | Al | AlN | Al |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Xin, W.; Zheng, X.; Ren, Z.; Sun, D.; Lu, W. Microstructural Characterization and Formation Mechanism of Nitrided Layers on Aluminum Substrates by Thermal Plasma Nitriding. Metals 2019, 9, 523. https://doi.org/10.3390/met9050523
Li X, Xin W, Zheng X, Ren Z, Sun D, Lu W. Microstructural Characterization and Formation Mechanism of Nitrided Layers on Aluminum Substrates by Thermal Plasma Nitriding. Metals. 2019; 9(5):523. https://doi.org/10.3390/met9050523
Chicago/Turabian StyleLi, Xin, Weida Xin, Xiaoyi Zheng, Zhen’an Ren, Daqian Sun, and Wanli Lu. 2019. "Microstructural Characterization and Formation Mechanism of Nitrided Layers on Aluminum Substrates by Thermal Plasma Nitriding" Metals 9, no. 5: 523. https://doi.org/10.3390/met9050523
APA StyleLi, X., Xin, W., Zheng, X., Ren, Z., Sun, D., & Lu, W. (2019). Microstructural Characterization and Formation Mechanism of Nitrided Layers on Aluminum Substrates by Thermal Plasma Nitriding. Metals, 9(5), 523. https://doi.org/10.3390/met9050523




