Effect of Si Content on the Corrosion Behavior of 420 MPa Weathering Steel
Abstract
:1. Introduction
2. Materials and Experimental
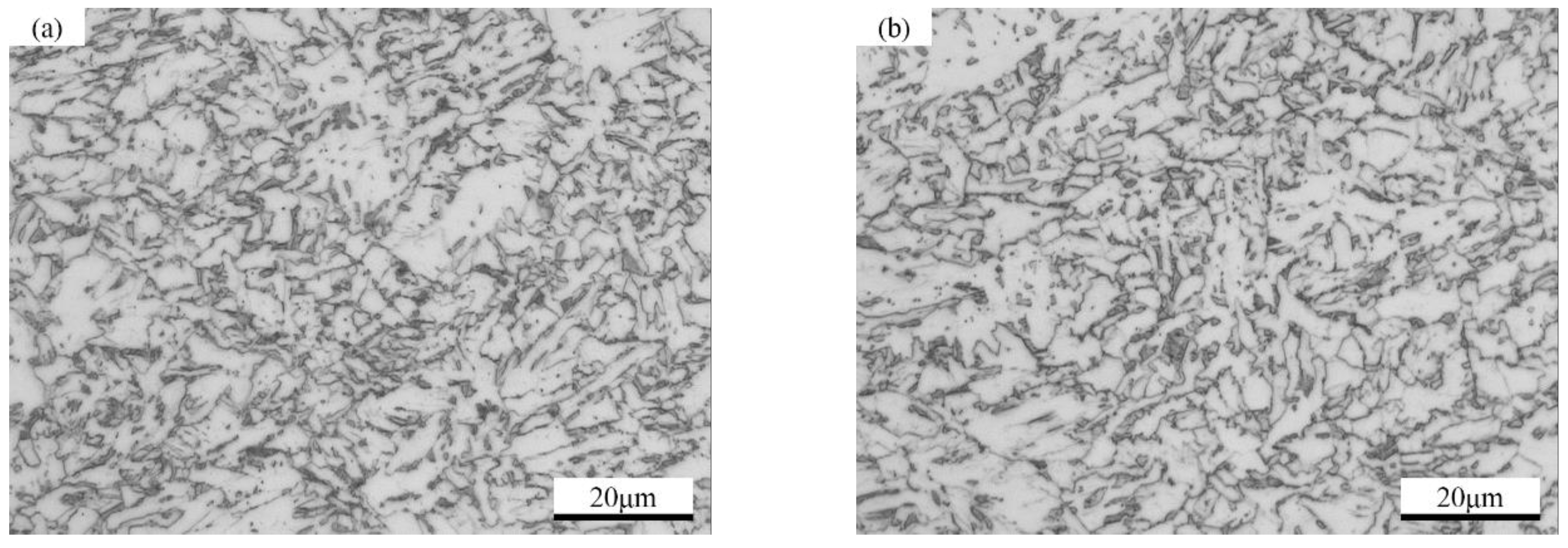
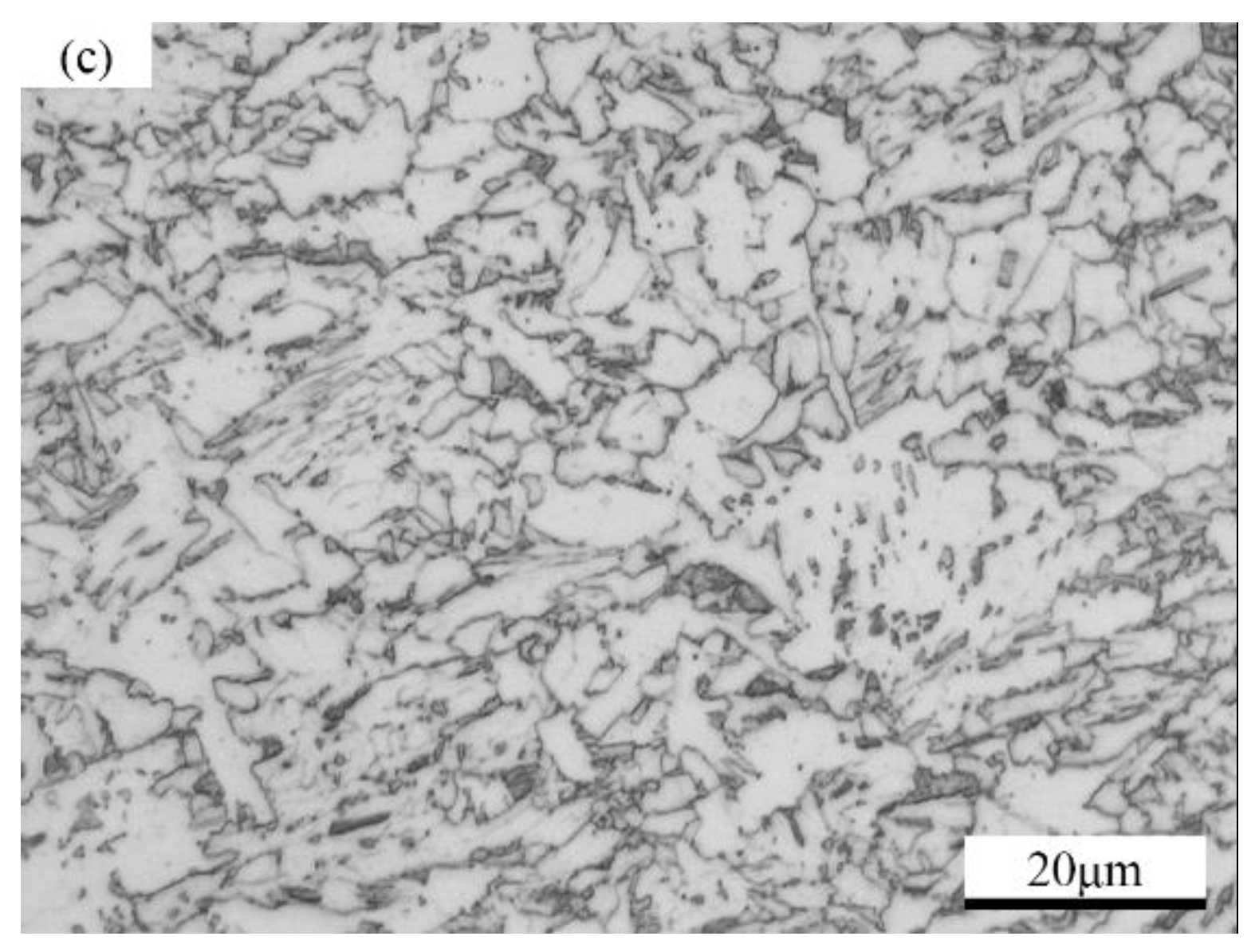
2.1. Materials
2.2. Sample Preparation
2.3. Accelerated Corrosion Test
2.4. Analysis of Corrosion Products
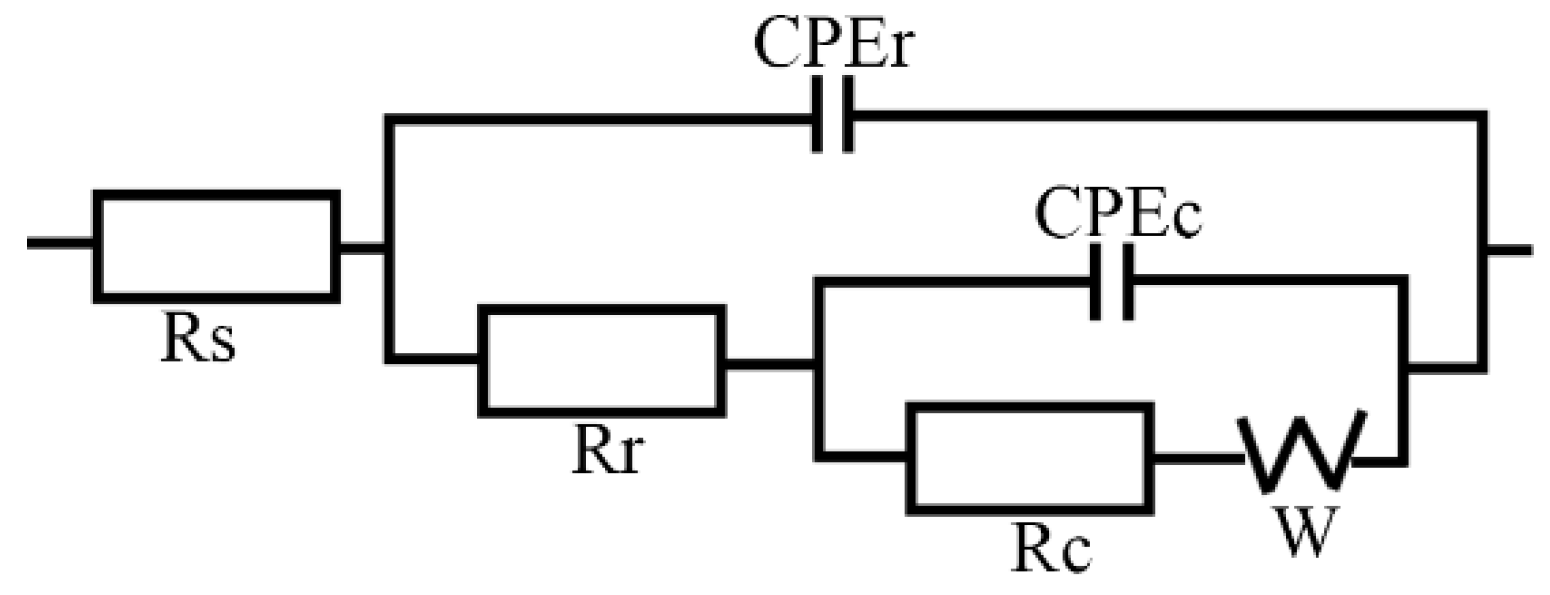
2.5. Electrochemical Experiments
3. Results
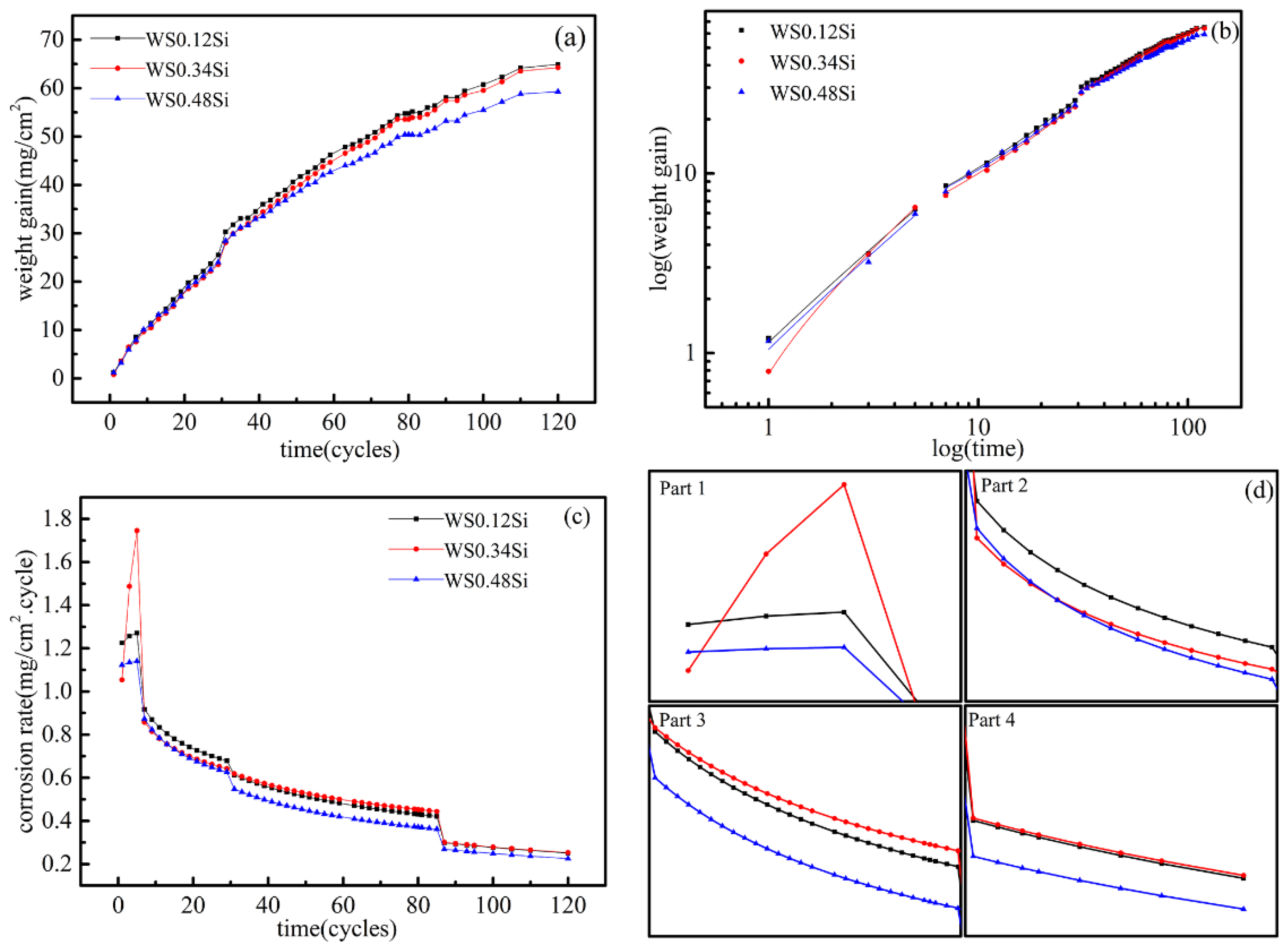
3.1. Corrosion Kinetics
3.2. Phase Composition of the Rust Layer
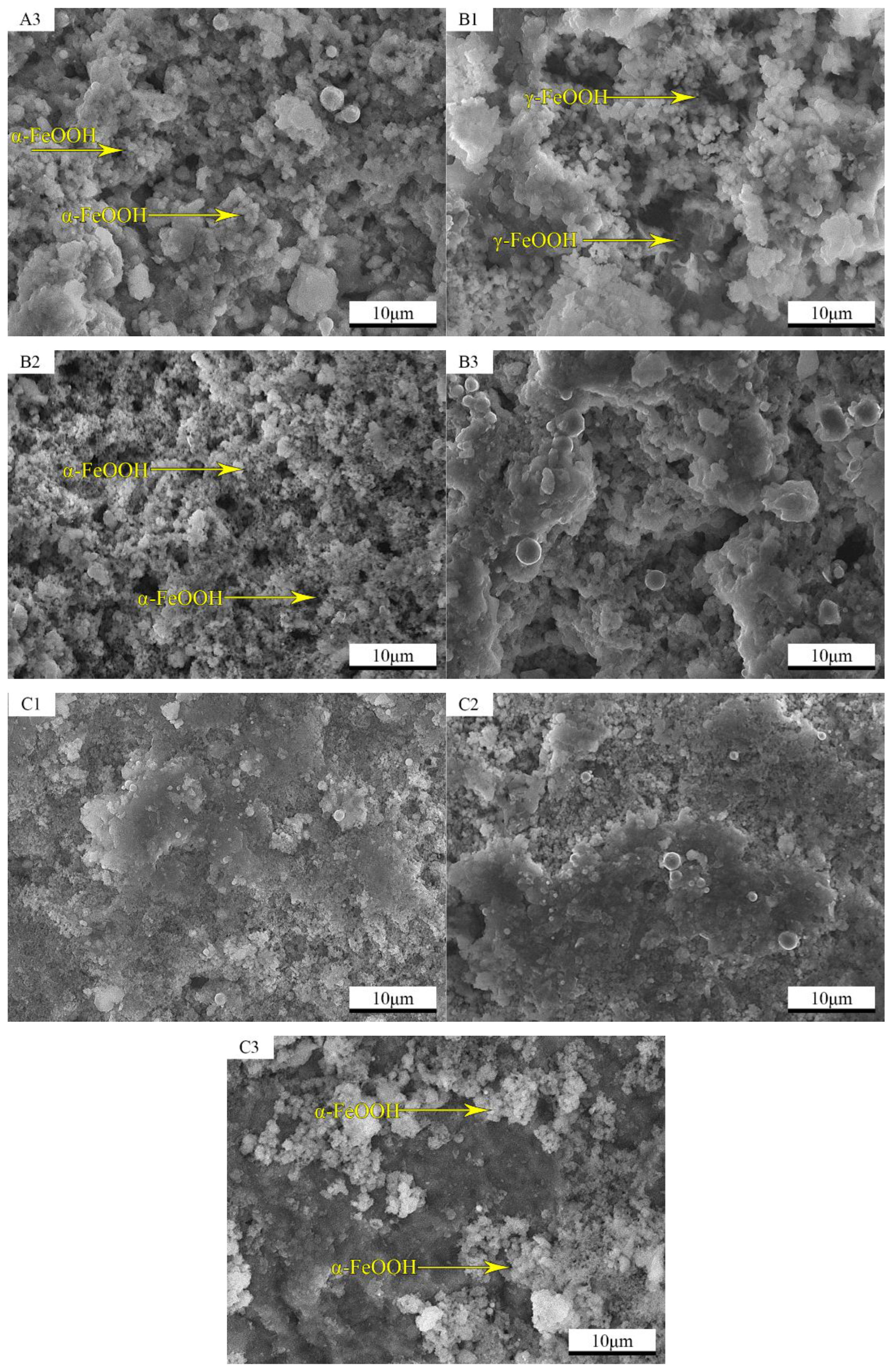
3.3. Microscopic Morphology of the Rust Layer
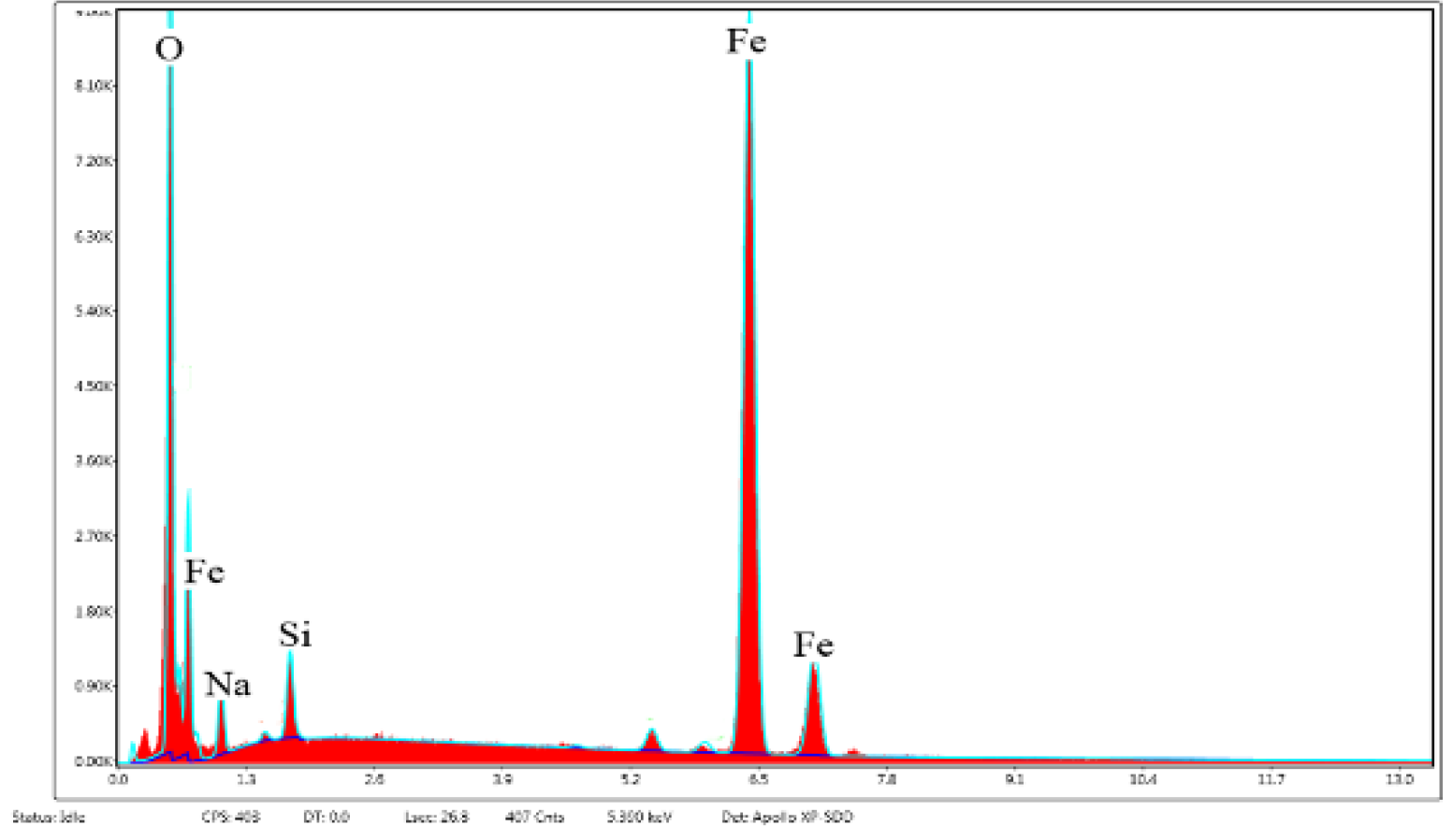
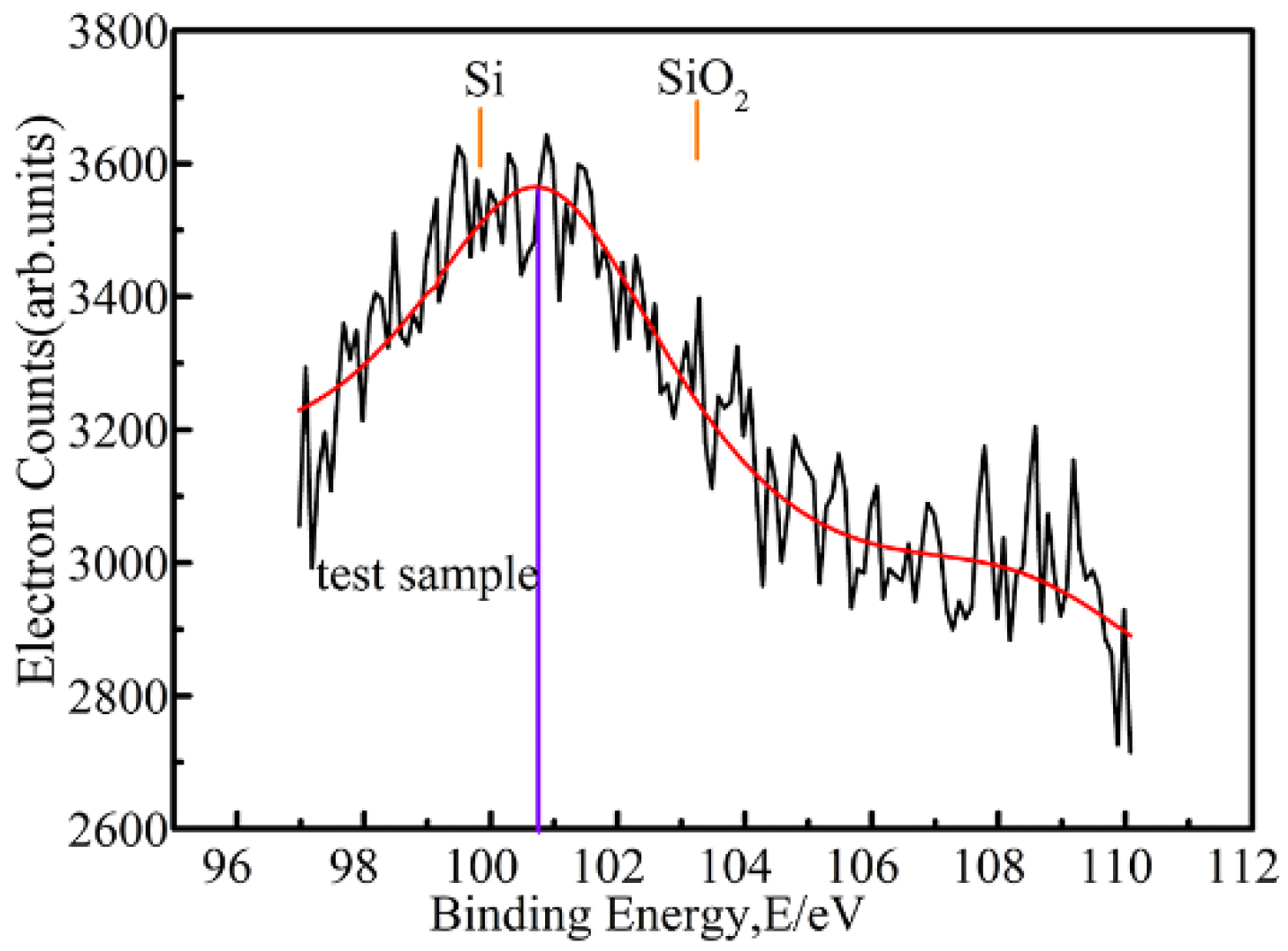
3.4. Elemental Analysis of the Internal Rust Layer
3.5. Electrochemical Properties
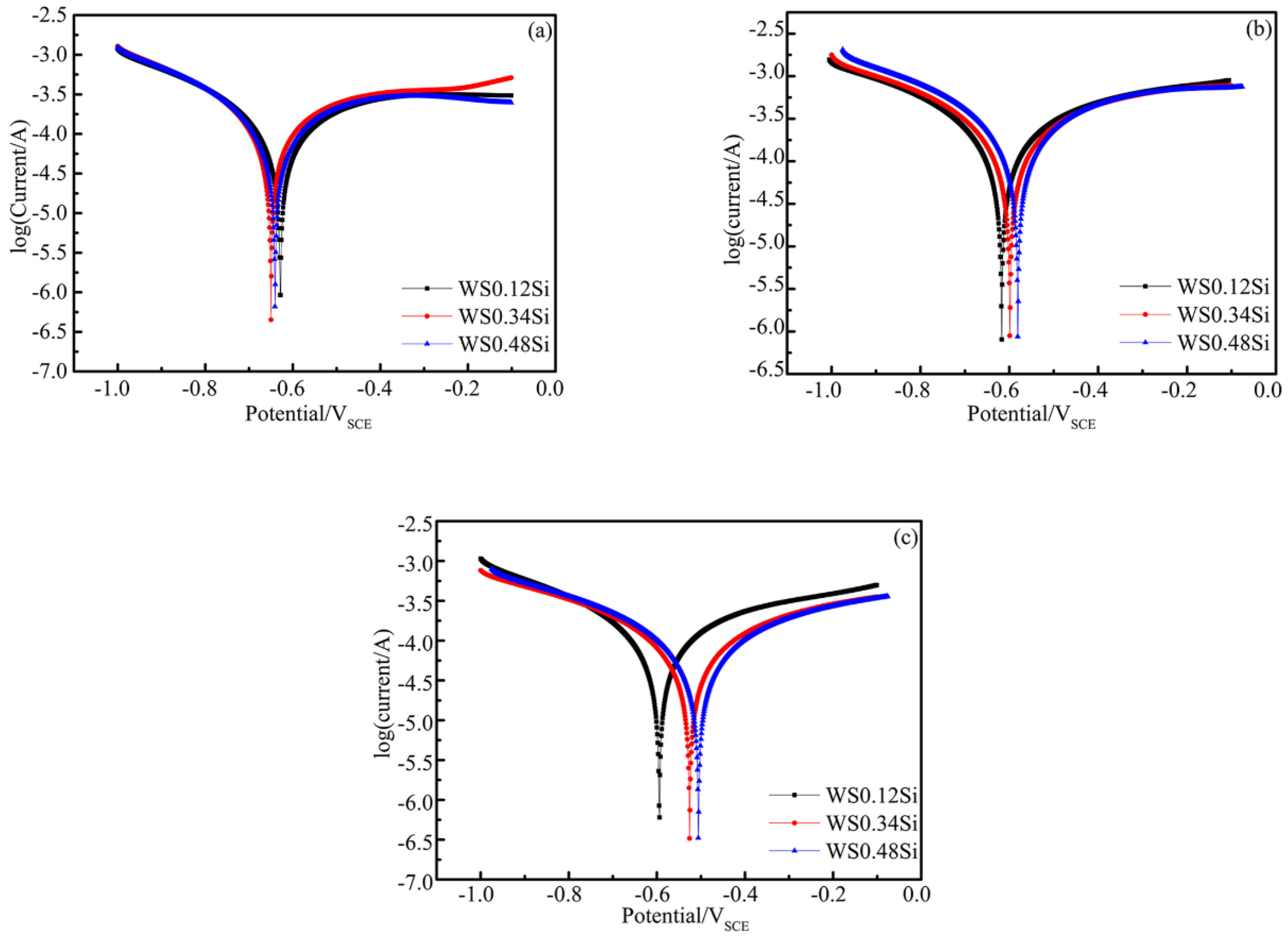
3.5.1. Polarization Curves
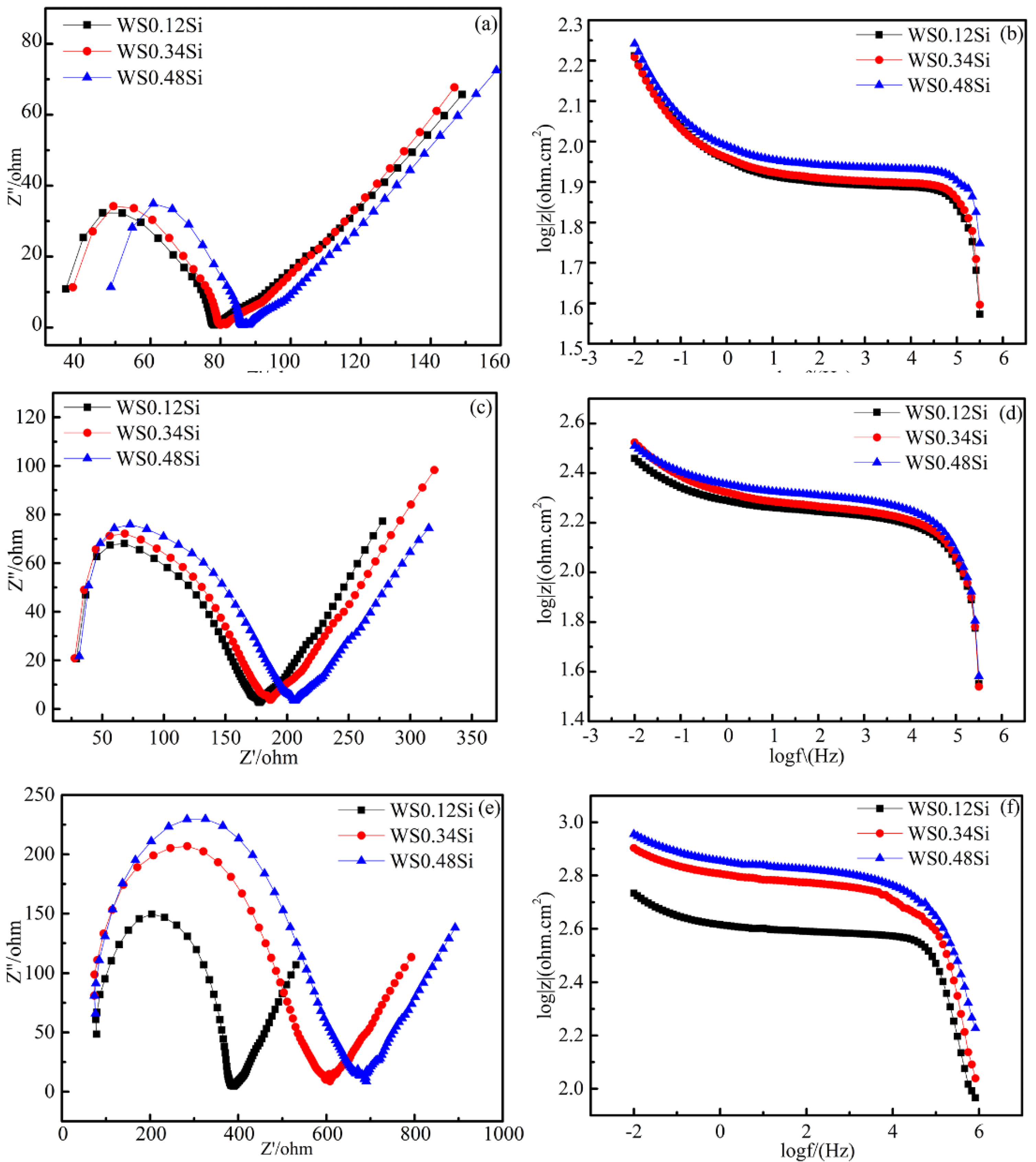
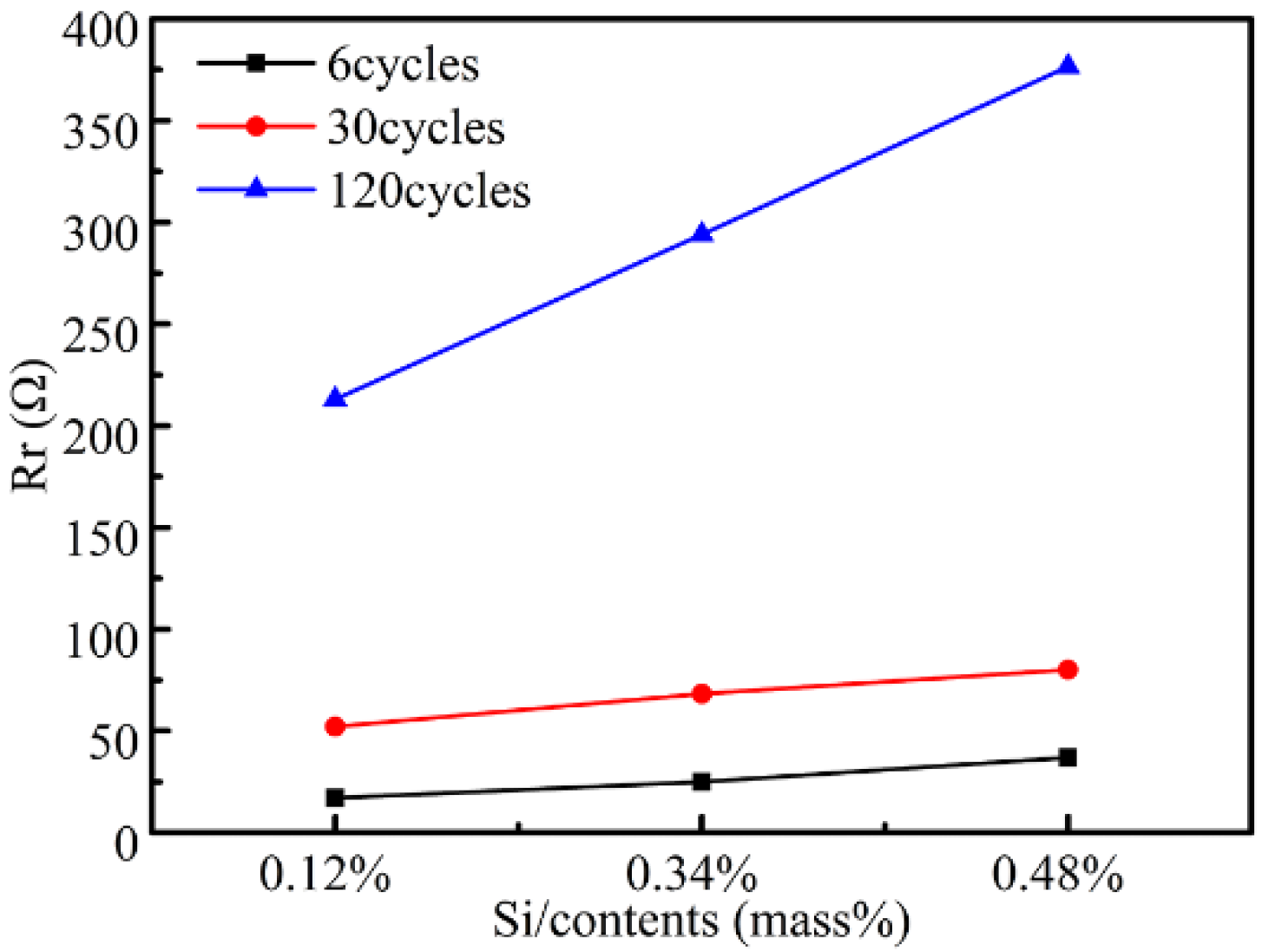
3.5.2. Electrochemical Properties of the Rusted Steel
4. Discussion
4.1. Corrosion Process and Kinetics
4.2. Effect of Silicon Alloying Elements on Corrosion Resistance
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Natesan, M.; Selvaraj, S.; Manickam, T.; Venkatachari, G. Corrosion behavior of metals and alloys in marine-industrial environment. Sci. Technol. Adv. Mater. 2016, 9. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Z.Y.; Ke, W. A study of the evolution of rust on weathering steel submitted to the Qinghai salt lake atmospheric corrosion. Mater. Chem. Phys. 2013, 139, 225–232. [Google Scholar] [CrossRef]
- Hara, S.; Kamimura, T.; Miyuki, H.; Yamashita, M. Taxonomy for protective ability of rust layer using its composition formed on weathering steel bridge. Corros. Sci. 2007, 49, 1131–1142. [Google Scholar] [CrossRef]
- Tewary, N.K.; Kundu, A.; Nandi, R.; Saha, J.K.; Ghosh, S.K. Microstructural characterisation and corrosion performance of old railway girder bridge steel and modern weathering structural steel. Corros. Sci. 2016, 113, 57–63. [Google Scholar] [CrossRef]
- Krivy, V.; Kubzova, M.; Kreislova, K.; Urban, V. Characterization of Corrosion Products on Weathering Steel Bridges Influenced by Chloride Deposition. Metals 2017, 7. [Google Scholar] [CrossRef]
- Yang, J.; Lu, Y.; Guo, Z.; Gu, J.; Gu, C. Corrosion behaviour of a quenched and partitioned medium carbon steel in 3.5 wt.% NaCl solution. Corros. Sci. 2018, 130, 64–75. [Google Scholar] [CrossRef]
- Díaz, I.; Cano, H.; Lopesino, P.; de la Fuente, D.; Chico, B.; Jiménez, J.A.; Medina, S.F.; Morcillo, M. Five-year atmospheric corrosion of Cu, Cr and Ni weathering steels in a wide range of environments. Corros. Sci. 2018, 141, 146–157. [Google Scholar] [CrossRef]
- Tamura, H. The role of rusts in corrosion and corrosion protection of iron and steel. Corros. Sci. 2008, 50, 1872–1883. [Google Scholar] [CrossRef]
- Yamashita, M.; Miyuki, H.; Matsuda, Y.; Nagano, H.; Misawa, T. The long term growth of the protective rust layer formed on weathering steel by atmospheric corrosion during a quarter of a century. Corros. Sci. 1994, 36, 283. [Google Scholar] [CrossRef]
- Soares, C.G.; Garbatov, Y.; Zayed, A.; Wang, G. Influence of environmental factors on corrosion of ship structures in marine atmosphere. Corros. Sci. 2009, 51, 2014–2026. [Google Scholar] [CrossRef]
- Cano, H.; Neff, D.; Morcillo, M.; Dillmann, P.; Diaz, I.; de la Fuente, D. Characterization of corrosion products formed on Ni 2.4 wt%–Cu 0.5 wt%–Cr 0.5 wt% weathering steel exposed in marine atmospheres. Corros. Sci. 2014, 87, 438–451. [Google Scholar] [CrossRef]
- Ma, Y.; Li, Y.; Wang, F. Weatherability of 09CuPCrNi steel in a tropical marine environment. Corros. Sci. 2009, 51, 1725–1732. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, J.; Xu, Y.; Liu, Z. Effects of Cr, Ni and Cu on the Corrosion Behavior of Low Carbon Microalloying Steel in a Cl−Containing Environment. J. Mater. Sci. Technol. 2013, 29, 168–174. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, J.; Wu, L.; Han, R.; Sun, Y. Study of the corrosion behavior of weathering steels in atmospheric environments. Corros. Sci. 2013, 67, 1–10. [Google Scholar] [CrossRef]
- Laird, G.; Powell, G.L.F. Solidification and solid-state transformation mechanisms in Si alloyed high-chromium white cast irons. Metall. Trans. A (Phys. Metall. Mater. Sci.) 1993, 24, 5. [Google Scholar] [CrossRef]
- Ruan, L.H.; Wu, K.M.; Qiu, J.A.; Shirzadi, A.A.; Rodionova, I.G. Effect of Silicon Content on Carbide Precipitation and Low-Temperature Toughness of Pressure Vessel Steels. Met. Sci. Heat Treat. 2017, 59, 97–101. [Google Scholar] [CrossRef]
- Garrison, W.M. The effect of silicon and nickel additions on the sulfide spacing and fracture toughness of a 0.4 carbon low alloy steel. Metall. Trans. A (Phys. Metall. Mater. Sci.) 1986, 17, 8. [Google Scholar] [CrossRef]
- Kim, K.Y.; Hwang, Y.H.; Yoo, J.Y. Effect of Silicon Content on the Corrosion Properties of Calcium-Modified Weathering Steel in a Chloride Environment. Corros. Sci. Sect. 2001, 58, 570. [Google Scholar] [CrossRef]
- Nishimura, T. Electrochemical behaviour and structure of rust formed on Si- and Al-bearing steel after atmospheric exposure. Corros. Sci. 2010, 52, 3609–3614. [Google Scholar] [CrossRef]
- Nishimura, T. Rust formation and corrosion performance of Si- and Al-bearing ultrafine grained weathering steel. Corros. Sci. 2008, 50, 1306–1312. [Google Scholar] [CrossRef]
- Mejía Gómez, J.A.; Antonissen, J.; Palacio, C.A.; De Grave, E. Effects of Si as alloying element on corrosion resistance of weathering steel. Corros. Sci. 2012, 59, 198–203. [Google Scholar] [CrossRef]
- Zhang, Q. Eflect of Si on Corrosion Resistance of Cabon Steel in Atmospheric Conditions. Available online: http://new.oversea.cnki.net/kns/brief/default_result.aspx (accessed on 25 April 2019).
- Chen, W.; Hao, L.; Dong, J.; Ke, W. Effect of sulphur dioxide on the corrosion of a low alloy steel in simulated coastal industrial atmosphere. Corros. Sci. 2014, 83, 155–163. [Google Scholar] [CrossRef]
- Hao, L.; Zhang, S.; Dong, J.; Ke, W. Evolution of atmospheric corrosion of MnCuP weathering steel in a simulated coastal-industrial atmosphere. Corros. Sci. 2012, 59, 270–276. [Google Scholar] [CrossRef]
- Castaño, J.G.; Botero, C.A.; Restrepo, A.H.; Agudelo, E.A.; Correa, E.; Echeverría, F. Atmospheric corrosion of carbon steel in Colombia. Corros. Sci. 2010, 52, 216–223. [Google Scholar] [CrossRef]
- Su, G.; Gao, X. Comparison of Medium Manganese Steel and Q345 Steel on Corrosion Behavior in a 3.5 wt % NaCl Solution. Materials 2017, 10. [Google Scholar] [CrossRef]
- Ma, Y.; Li, Y.; Wang, F. Corrosion of low carbon steel in atmospheric environments of different chloride content. Corros. Sci. 2009, 51, 997–1006. [Google Scholar] [CrossRef]
- Lu, Y.; Dong, J.; Ke, W. Effects of Cl− Ions on the Corrosion Behaviour of Low Alloy Steel in Deaerated Bicarbonate Solutions. J. Mater. Sci. Technol. 2016, 32, 341–348. [Google Scholar] [CrossRef]
- Nishimura, T. Crevice Corrosion Resistance and Structure of Passive Film on Fe-Mn-Si-Cr-Ni Steel. ISIJ Int. 2016, 56, 654–660. [Google Scholar] [CrossRef]
- Nishimura, T. Corrosion Behavior of Silicon-Bearing Steel in a Wet/Dry Environment Containing Chloride Ions. Mater. Trans. 2007, 48, 1438–1443. [Google Scholar] [CrossRef]
- Guo, J.; Yang, S.; Shang, C.; Wang, Y.; He, X. Influence of carbon content and microstructure on corrosion behaviour of low alloy steels in a Cl− containing environment. Corros. Sci. 2009, 51, 242–251. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.Y.; Ke, W. Corrosion behaviour of weathering steel in diluted Qinghai salt lake water in a laboratory accelerated test that involved cyclic wet/dry conditions. Mater. Chem. Phys. 2010, 124, 952–958. [Google Scholar] [CrossRef]
- Xu, S.; Ikpt, M.E.; Dong, J.; Wei, J.; Ke, W.; Chen, N. Effects of Cadmium alloying on the Corrosion and Mechanical Properties of Magnesium. Int. J. Electrochem. Sci. 2012, 7, 4735–4755. [Google Scholar]
- Nishimura, T.; Katayama, H.; Noda, K.; Kodama, T. Effect of Co and Ni on the corrosion behavior of low alloy steels in wet/dry environments. Corros. Sci. 2000, 42, 1611–1621. [Google Scholar] [CrossRef]
- Kamimura, T.; Hara, S.; Miyuki, H.; Yamashita, M.; Uchida, H. Composition and protective ability of rust layer formed on weathering steel exposed to various environments. Corros. Sci. 2006, 48, 2799–2812. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, J.; Zheng, Y.Y. Electrochemical techniques for determining corrosion rate of rusted steel in seawater. Corros. Sci. 2011, 53, 208–216. [Google Scholar] [CrossRef]
- Asami, K.; Kikuchi, M. In-depth distribution of rusts on a plain carbon steel and weathering steels exposed to coastal–industrial atmosphere for 17 years. Corros. Sci. 2003, 45, 2671–2688. [Google Scholar] [CrossRef]
- Murayama, M.; Nishimura, T.; Tsuzaki, K. Nano-scale chemical analysis of rust on a 2% Si-bearing low alloy steel exposed in a coastal environment. Corros. Sci. 2008, 50, 2159–2165. [Google Scholar] [CrossRef]
- Sei, J.; Oha, D.C.; Townsend, H. Atmospheric corrosion of different steels in marine, rural and industrial environments. Corros. Sci. 1999, 41, 1687–1702. [Google Scholar]


| Steel | C | Si | Mn | P | S | Ni | Cr | Mo | Cu | Nb | Ti |
|---|---|---|---|---|---|---|---|---|---|---|---|
| WS0.12Si | 0.053 | 0.12 | 1.25 | 0.007 | 0.002 | 0.31 | 0.51 | 0.05 | 0.26 | 0.028 | 0.013 |
| WS0.34Si | 0.058 | 0.34 | 1.27 | 0.007 | 0.005 | 0.30 | 0.52 | 0.05 | 0.25 | 0.026 | 0.013 |
| WS0.48Si | 0.058 | 0.48 | 1.27 | 0.008 | 0.002 | 0.31 | 0.51 | 0.06 | 0.27 | 0.028 | 0.014 |
| Steel | Part One (1–6) | Part Two (7–29) | Part Three (30–85) | Part Four (86–120) |
|---|---|---|---|---|
| WS0.12Si | ||||
| WS0.34Si | ||||
| WS0.48Si |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, R.; Yu, Q.; Zhang, Y.; Yan, X.; Lu, Y.; Zhang, C.; Wang, Q. Effect of Si Content on the Corrosion Behavior of 420 MPa Weathering Steel. Metals 2019, 9, 486. https://doi.org/10.3390/met9050486
Sun R, Yu Q, Zhang Y, Yan X, Lu Y, Zhang C, Wang Q. Effect of Si Content on the Corrosion Behavior of 420 MPa Weathering Steel. Metals. 2019; 9(5):486. https://doi.org/10.3390/met9050486
Chicago/Turabian StyleSun, Rui, Qiang Yu, Yue Zhang, Xuqiang Yan, Yuchen Lu, Chunling Zhang, and Qingfeng Wang. 2019. "Effect of Si Content on the Corrosion Behavior of 420 MPa Weathering Steel" Metals 9, no. 5: 486. https://doi.org/10.3390/met9050486
APA StyleSun, R., Yu, Q., Zhang, Y., Yan, X., Lu, Y., Zhang, C., & Wang, Q. (2019). Effect of Si Content on the Corrosion Behavior of 420 MPa Weathering Steel. Metals, 9(5), 486. https://doi.org/10.3390/met9050486




