The Influence of Mg-Based Inclusions on the Grain Boundary Mobility of Austenite in SS400 Steel
Abstract
1. Introduction
2. Materials and Experiments
3. Results and Discussion
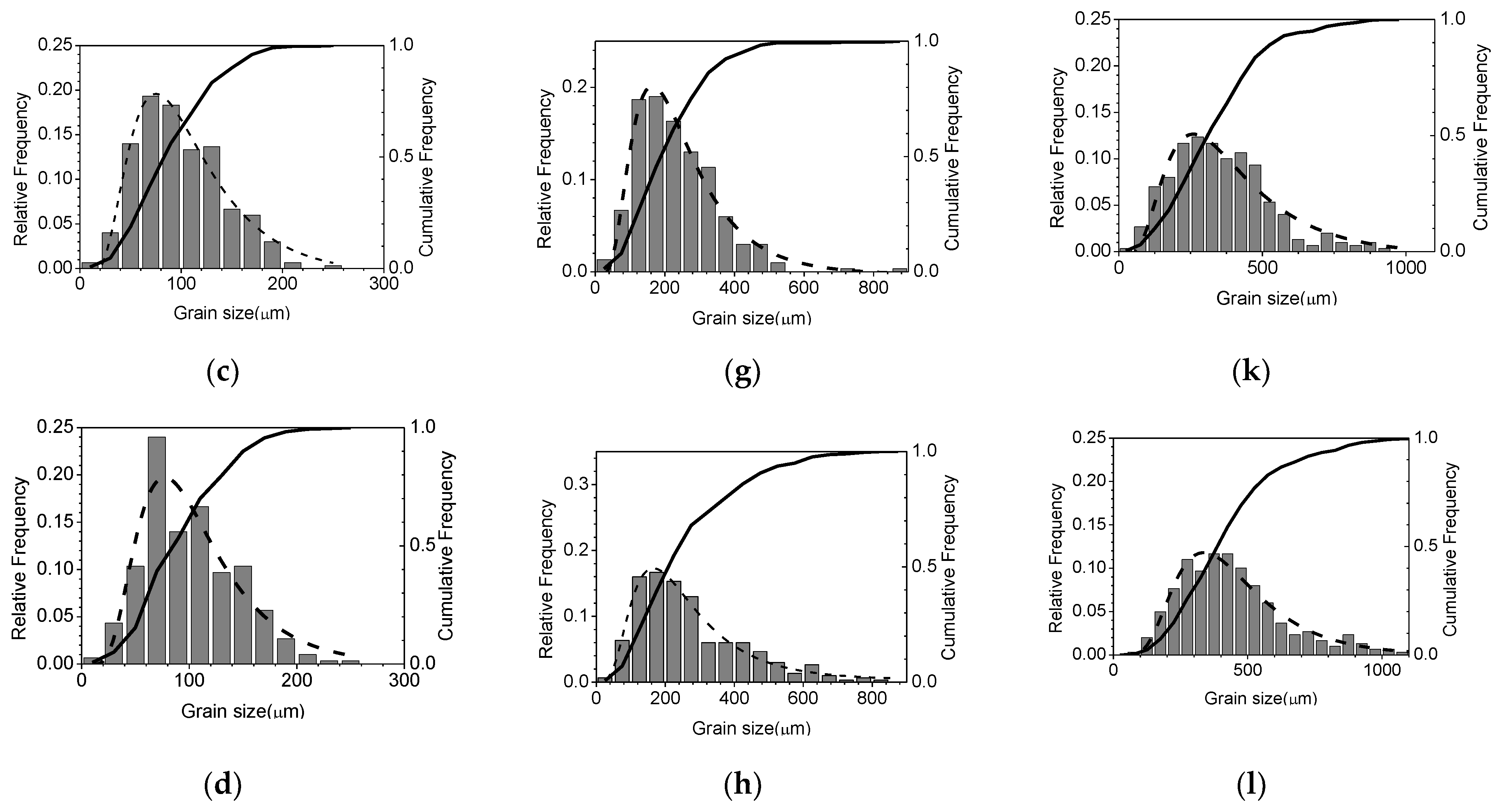
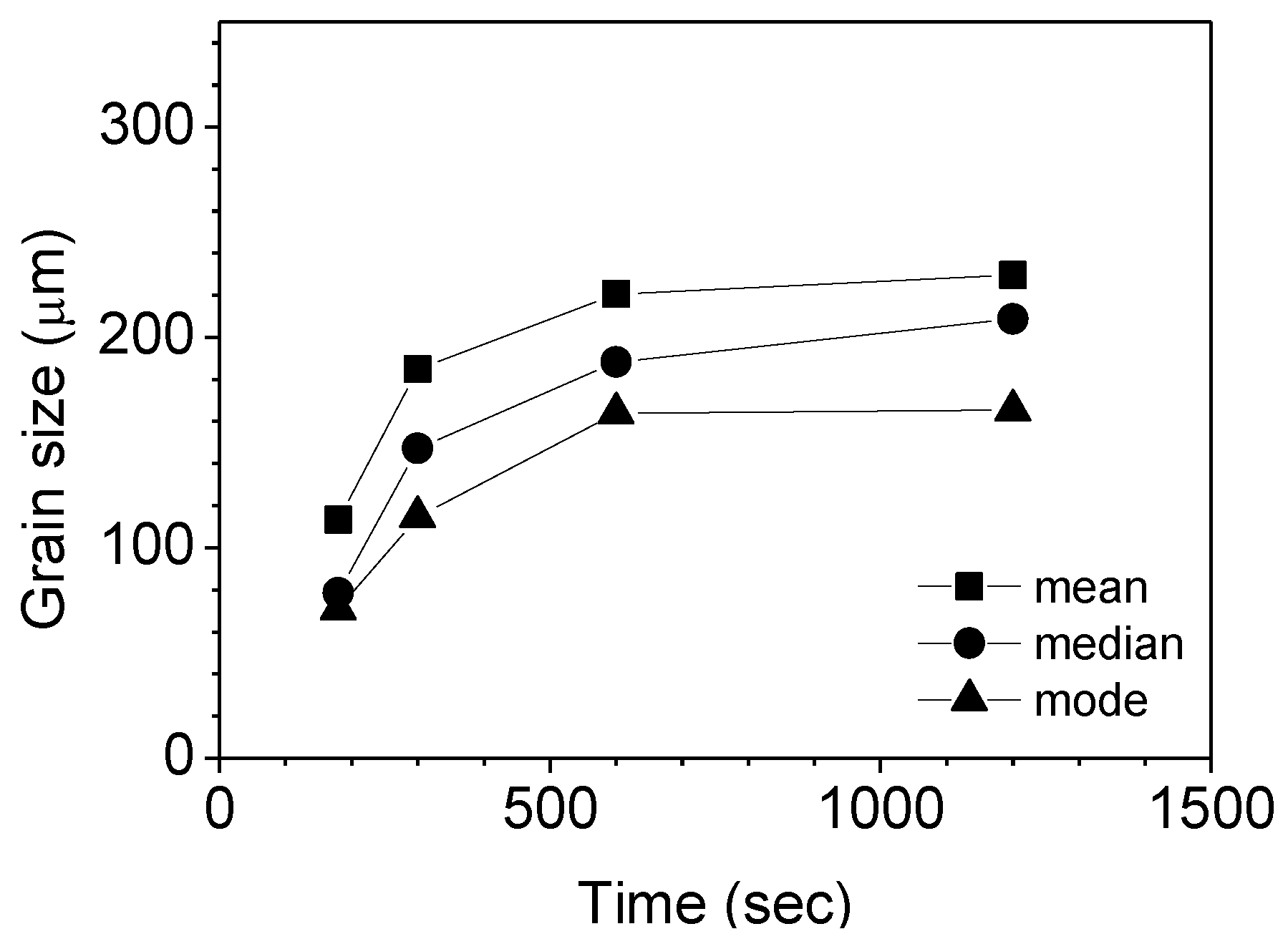
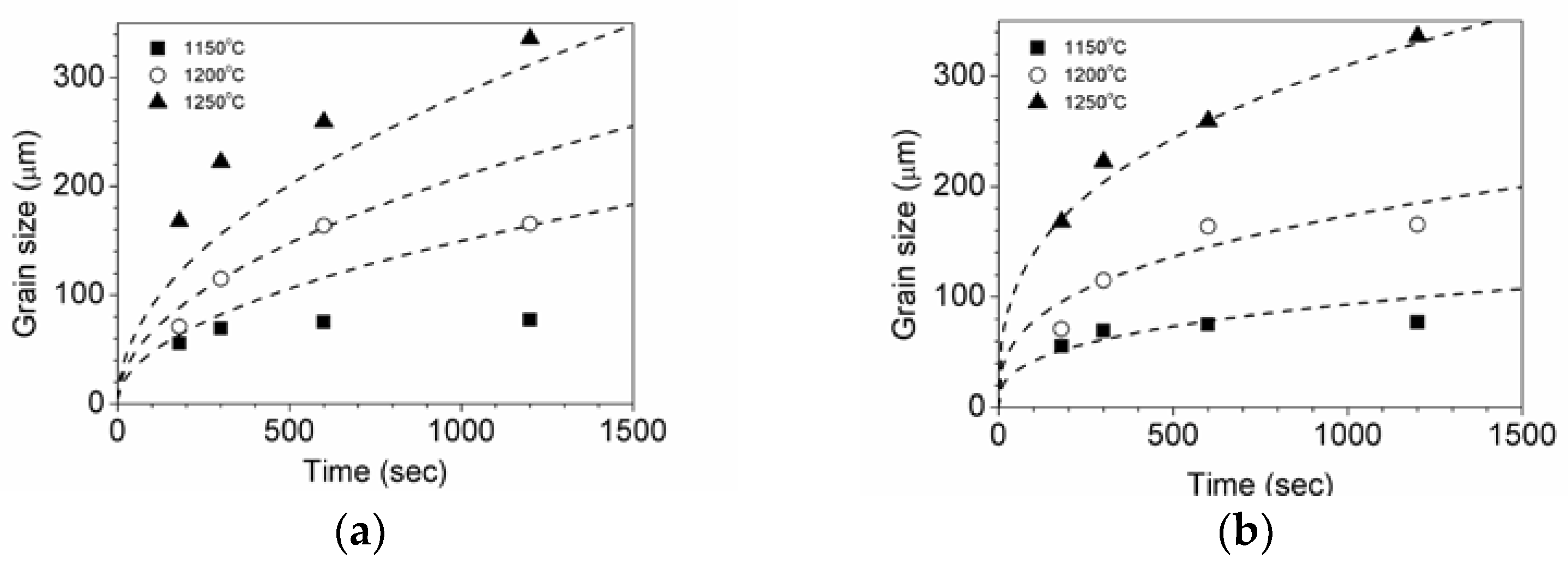
3.1. Parameters of Grain Growth Equation
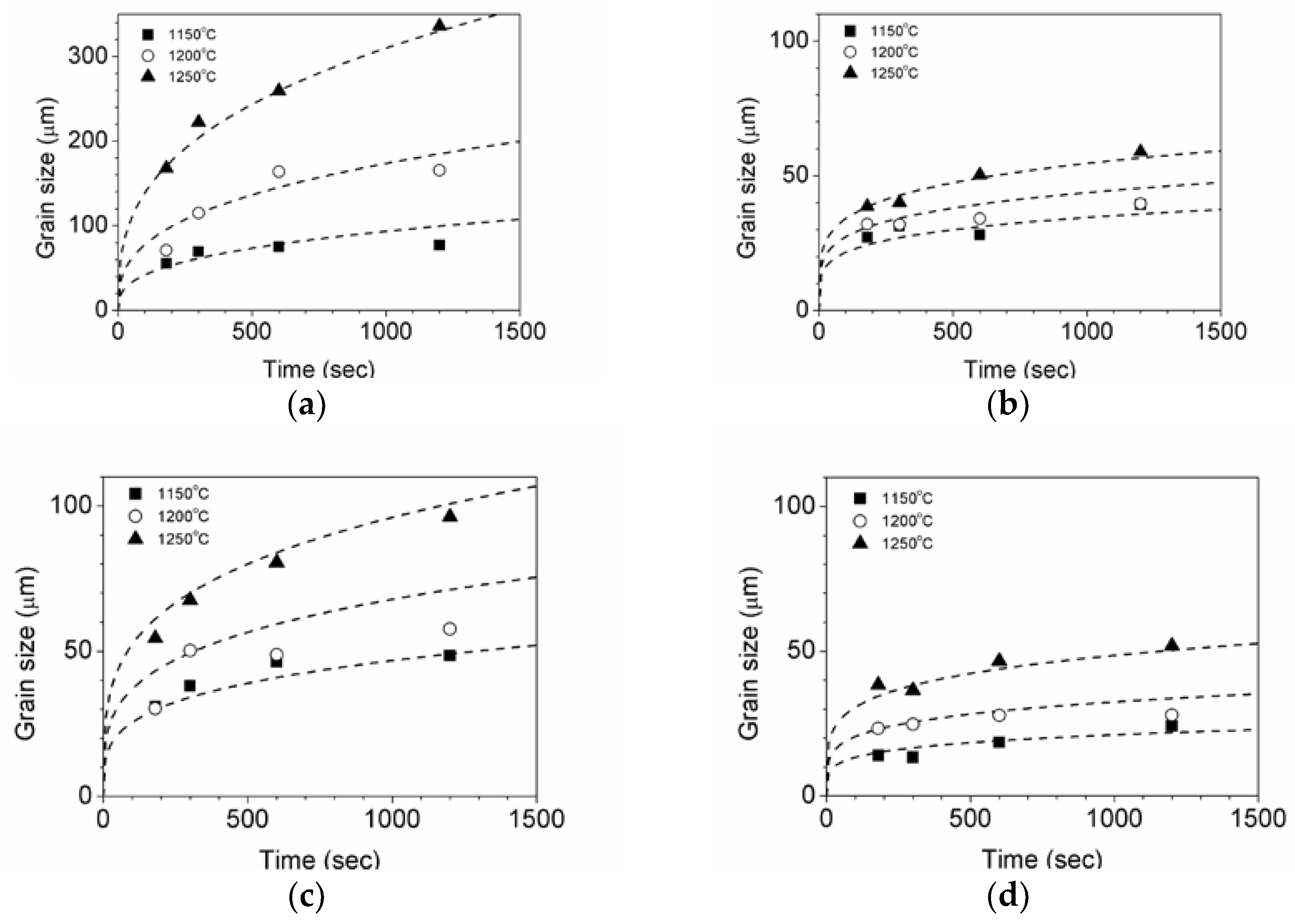
3.2. Effects of Temperature and Mg-Content on Austenite Grain Growth
3.3. Zener Pinning Effect of Inclusions on the Austenite Grain Mobility
3.4. Effect of Solute Drag on the Grain Mobility of Austenite
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thewlis, G. Transformation Kinetics of Ferrous Weld Metals. Mater. Sci. Technol. 1994, 10, 110–125. [Google Scholar] [CrossRef]
- Bramfitt, B.L. The effect of carbide and nitride additions on the heterogeneous nucleation behavior of liquid iron. Metall. Trans. 1970, 1, 1987–1995. [Google Scholar] [CrossRef]
- Zhang, S.H.; Hattori, N.; Enomoto, M.; Tarui, T. Ferrite Nucleation at Ceramic/Austentie Interfaces. ISIJ Int. 1996, 36, 1301–1309. [Google Scholar] [CrossRef]
- Jin, H.H.; Shim, J.H.; Chao, Y.W.; Lee, H.C. Formation of Intragranular Acicular Ferrite Grains in a Ti-containing Low Carbon Steel. ISIJ Int. 2003, 43, 1111–1113. [Google Scholar] [CrossRef]
- Kojima, A.; Kiyose, A.; Uemori, R.; Minagawa, M.; Hoshino, M.; Nakashima, T.; Ishida, K.; Yasui, H. Super High HAZ Toughness Techbology with Fine Microstructure Imoarted by Fine Particles. Nippon Steel Tech. Rep. 2004, 380, 2–5. [Google Scholar]
- Chen, Z.; Loretto, M.H.; Cochrane, R.C. Nature of Large Precipitates in Titanium-containing HSLA Steels. Mater. Sci. Technol. 1987, 3, 836–844. [Google Scholar] [CrossRef]
- Prikryl, M.; Kroupa, A.; Weatherly, G.C.; Subramanian, S.V. Precipitation behavior in a Medium Carbon, Ti-V-N Microalloyed Steel. Metall. Mater. Trans. A 1996, 27, 1149–1165. [Google Scholar] [CrossRef]
- Yan, W.; Shan, Y.Y.; Yang, K. Effect of TiN Inclusions on the Impact Toughness of Low-carbon Microalloyed Steels. Metall. Mater. Trans. A 2006, 37, 2147–2158. [Google Scholar] [CrossRef]
- Baker, T.N. Role of Zicronium in Microalloyed Steels: A Review. Mater. Sci. Technol. 2015, 31, 265–294. [Google Scholar] [CrossRef]
- Mizoguchi, S.; Takamura, J. Roles of Oxides in Steels Performance-Metallurgy of Oxides in Steels. In Proceedings of the 6th International Iron Steel Congress, Nagoya, Japan, 21–26 October 1990; Volume 1, pp. 591–597. [Google Scholar]
- Grong, O.; Matlock, D.K. Microstructural Development in Mild and Low-alloy Steel Weld Metals. Int. Mater. Rev. 1986, 31, 27–48. [Google Scholar] [CrossRef]
- Shim, J.H.; Oh, Y.J.; Suh, J.Y.; Cho, Y.W.; Shim, J.D.; Byun, J.S.; Lee, D.N. Ferrite Nucleation Potency of Non-metallic Inclusions in Mediun Carbon Steels. Acta Mater. 2001, 49, 2115–2122. [Google Scholar] [CrossRef]
- Byun, J.S.; Shim, J.H.; Cho, Y.W.; Lee, D.N. Non-metallic inclusion and intragranular nucleation of ferrite in Ti-killed C-Mn Steel. Acta Mater. 2003, 51, 1593–1606. [Google Scholar] [CrossRef]
- Barbaro, F.J.; Krauklis, P.; Easterling, K.E. Formation of Acicular Ferrite at Oxide Particles in Steels. Mater. Sci. Technol. 1989, 5, 1057–1068. [Google Scholar] [CrossRef]
- Mills, A.R.; Thewlis, G.; Whitemen, J.A. Nature of Inclusions in Steel Weld Metals and their Influence on Formation of Acicular Ferrite. Mater. Sci. Technol. 1987, 3, 1051–1061. [Google Scholar] [CrossRef]
- Mundra, K.; DebRoy, T.; Babu, S.S.; David, S.A. Towards Predicting Weld Metal Microstructure from Fundamentals of Transport Phenomena; Department of Energy: Washington, DC, USA, 1995.
- Goto, H.; Miyazawa, K.; Amada, W.Y.; Tanaka, K. Effect of Cooling Rate on Composition of Oxides Precipitated during Solidification of Steels. ISIJ Int. 1995, 35, 708–714. [Google Scholar] [CrossRef]
- Chao, Y.W.; Byun, J.S.; Shim, J.H. Effect of Ti Addition on Mixed Microstructure of Allotriomorphic and Bainitic Ferrite in Wrought C-Mn Steels. Mater. Sci. Forum 2003, 426–432, 1511–1516. [Google Scholar] [CrossRef]
- Byun, J.S.; Shim, J.H.; Cho, Y.W. Influence of Mn on Microstructural Evolution in Ti-Killed C-Mn Steel. Scr. Mater. 2003, 48, 449–454. [Google Scholar] [CrossRef]
- Kim, H.S.; Chang, C.H.; Lee, H.G. Evolution of Inclusions and Resultant Microstructural change with Mg Addition in Mn/Si/Ti Deoxidized Steels. Scr. Mater. 2005, 53, 1253–1258. [Google Scholar] [CrossRef]
- Smith, C. Zener pining. Met. Technol. Trans. Metall. Soc. 1948, 175, 15–51. [Google Scholar]
- Nes, E.; Ryum, N.; Hunderi, O. On the Zener Drag. Acta Metall. 1985, 1, 11–22. [Google Scholar] [CrossRef]
- Schwarze, C.; Kamachali, R.D.; Steinbach, I. Phase-field study of Zener Drag and Pinning Cylindrical Particles in Polycrystalline Materials. Acta Mater. 2016, 106, 59–65. [Google Scholar] [CrossRef]
- Sakata, K.; Suito, H. Grain-growth-inhibiting Effects of Primary Inclusion Particles of ZrO2 and MgO in Fe-10 mass pct Ni Alloy. Metall. Mater. Trans. A 2000, 31, 1213–1223. [Google Scholar] [CrossRef]
- Ohta, H.; Suito, H. Effect of Nonrandomly Dispersed Particles on Austenite Grain Growth in Fe-10 mass% Ni and Fe-0.20 mass% C-0.02 mass% P Alloys. ISIJ Int. 2006, 46, 832–839. [Google Scholar] [CrossRef]
- Karasev, A.V.; Suito, H. Effect of Particle Size Distribution on Austenite Grain Growth in Fe–0.05mass%C Alloy Deoxidized with Mn–Si, Ti, Mg, Zr and Ce. ISIJ Int. 2006, 46, 718–727. [Google Scholar] [CrossRef]
- Karasev, A.V.; Suito, H. Effects of Oxide Particles and Solute Elements on Austenite Grain Growth in Fe–0.05mass%C and Fe–10mass%Ni Alloys. ISIJ Int. 2008, 48, 658–666. [Google Scholar] [CrossRef]
- Zhu, K.; Yang, J.; Wang, R.Z.; Yang, Z.G. Effect of Mg Addition on Inhibiting Austenite Grain Growth in Heat Affected Zones of Ti-bearing Low Carbon Steels. J. Iron Steel Res. Int. 2011, 18, 60–64. [Google Scholar] [CrossRef]
- Zhu, K.; Yang, Z.G. Effect of Magnesium on Austenite Grain Growth of the Heat-affected Zone in Low-carbon High-strength Steels. Metall. Mater. Trans. A 2011, 42, 2207–2213. [Google Scholar] [CrossRef]
- Zhu, K.; Yang, Z.G. Effect of Mg Addition on the Ferrite Grain Boundaries Misorientation in HAZ of Low Carbon Steels. J. Mater. Sci. Technol. 2011, 27, 252–256. [Google Scholar] [CrossRef]
- Sarma, D.S.; Karasev, A.V.; Jonsson, P.G. On the Role of Non-metallic Inclusions in the Nucleation of Acicular Ferrite in Steels. ISIJ Int. 2009, 49, 1063–1074. [Google Scholar] [CrossRef]
- Beck, P.A. Annealing of cold worded metals. Adv. Phys. 1954, 3, 245–324. [Google Scholar] [CrossRef]
- Sellars, C.M.; Whiteman, J.A. Recrystallization and Grain Growth in Hot Rolling. Met. Sci. 1979, 13, 187–194. [Google Scholar] [CrossRef]
- Sellars, C.M.; Davies, G.J. Hot Working and Forming Process; The Society: London, UK, 1990; pp. 3–15. [Google Scholar]
- Sellars, C.M. Modelling of Structural Evolution during Hot Working Processes; Risol National Laboratory: Roskilde, Denmark, 1986; pp. 167–187. [Google Scholar]
- Hodgson, P.D.; Gibbs, R.K. A Mathematical Model to Predict the Mechanical Properties of Hot Rolled C-Mn and Microalloyed Steels. ISIJ Int. 1992, 32, 1329–1338. [Google Scholar] [CrossRef]
- Anelli, E. Application of Mathematical Modelling to Hot Rolling and Controlled Cooling of Wire Rods and Bars. ISIJ Int. 1992, 32, 440–449. [Google Scholar] [CrossRef]
- Yoshie, A.; Fujioka, M.; Watanabe, Y.; Nishioka, K.; Morikawa, H. Modelling of Microstructural Evolution and Mechanical Properties of Steel Plates Produced by Thermo-Mechanical Control Process. ISIJ Int. 1992, 32, 395–404. [Google Scholar] [CrossRef]
- Nishizawa, T. Grain Growth in Single- and Dual-Phase Steels. Tetsu-to-hagané 1984, 70, 1984–1992. [Google Scholar] [CrossRef]
- Namba, S.; Kitamura, M.; Shimada, M.; Katsumata, M.; Inoue, T.; Imamura, H.; Maeda, Y.; Hattori, S. Prediction of Microstructure Distribution in the Through-thickness Direction during and after Hot Rolling in Carbon Steels. ISIJ Int. 1992, 32, 377–386. [Google Scholar] [CrossRef]
- Senuma, T.; Yada, H. Microstructural Evolution of Plain Carbon Steels in Multiple Hot Working; Risol Natl Lab: Roskidle, Demark, 1986; pp. 547–552. [Google Scholar]
- Atkinson, H.V. Overview no. 65: Theories of Normal Grain Growth in Pure Single Phase Systems. Acta Metall. 1988, 36, 469–491. [Google Scholar] [CrossRef]
- Burke, J.; Turnbull, D. Recrystallization and Grain Growth. Process Met. Phys. 1952, 3, 220–292. [Google Scholar] [CrossRef]
- Vynokur, B.B. Influence of Alloying on the Free Energy of Austenitic Grain Boundaries in Steel. Mater. Sci. 1996, 32, 448–455. [Google Scholar] [CrossRef]
- Luo, S.J.; Su, Y.H.; Lu, M.J.; Kuo, J.C. EBSD Analysis of Magnesium Addition on Inclusion Formation in SS400 Structural Steel. Mater. Charct. 2013, 82, 103–112. [Google Scholar] [CrossRef]
- Masayo, S. An Analysis of the Solute Drag Effect of Nb on Recrystallization of Ultra Low Carbon Steel. ISIJ Int. 1998, 38, 547–552. [Google Scholar]
- Enomoto, M. Influence of Solute Drag on the Growth of Proeutectoid Ferrite in Fe-C-Mn Alloy. Acta Mater. 1999, 47, 3533–3540. [Google Scholar] [CrossRef]
- Suehiro, M.; Liu, Z.K.; Agren, J. Effect of Niobium on Massive Transformation in Ultra Low Carbon Steels: A Solute Drag Treatment. Acta Mater. 1996, 44, 4241–4251. [Google Scholar] [CrossRef]
- Kim, S.G.; Park, Y.B. Grain Boundary Segregation, Solute Drag and Abnormal Grain Growth. Acta Mater. 2008, 56, 3739. [Google Scholar] [CrossRef]
- Yu, Q.; Sun, Y. Abnormal Growth of Austenite Grain of Low-carbon Stele. Mater. Sci. Eng. A 2006, 420, 34–38. [Google Scholar] [CrossRef]
- Cahn, J.W. The Impurity-drag Effect in Grain Boundary Motion. Acta Metall. 1962, 10, 789–798. [Google Scholar] [CrossRef]
- Kang, K.B.; Kwon, O.; Lee, W.B.; Park, C.G. Effect of Precipitation on the Recrystallization Behavior of a Nb Containing Steel. Scr. Mater. 1997, 36, 1303–1308. [Google Scholar] [CrossRef]
- Sinclair, C.W.; Hutchinson, C.R.; Brechet, Y. The Effect of Nb on the Recrystallization and Grain Growth of Ultra-High-Purity α-Fe: A Combinatorial Approach. Metal. Mater. Trans. A 2007, 38, 821–830. [Google Scholar] [CrossRef]
- Amram, D.; Schuh, C.A. Interplay between Thermodynamic and Kinetic Stabilization Mechanisms in Nanocrystalline Fe-Mg Alloys. Acta Mater. 2018, 144, 447–458. [Google Scholar] [CrossRef]
- Bornstein, L. Numerical Data and Functional Relationships in Science and Technology; Springer: Berlin, Germany, 1987. [Google Scholar]
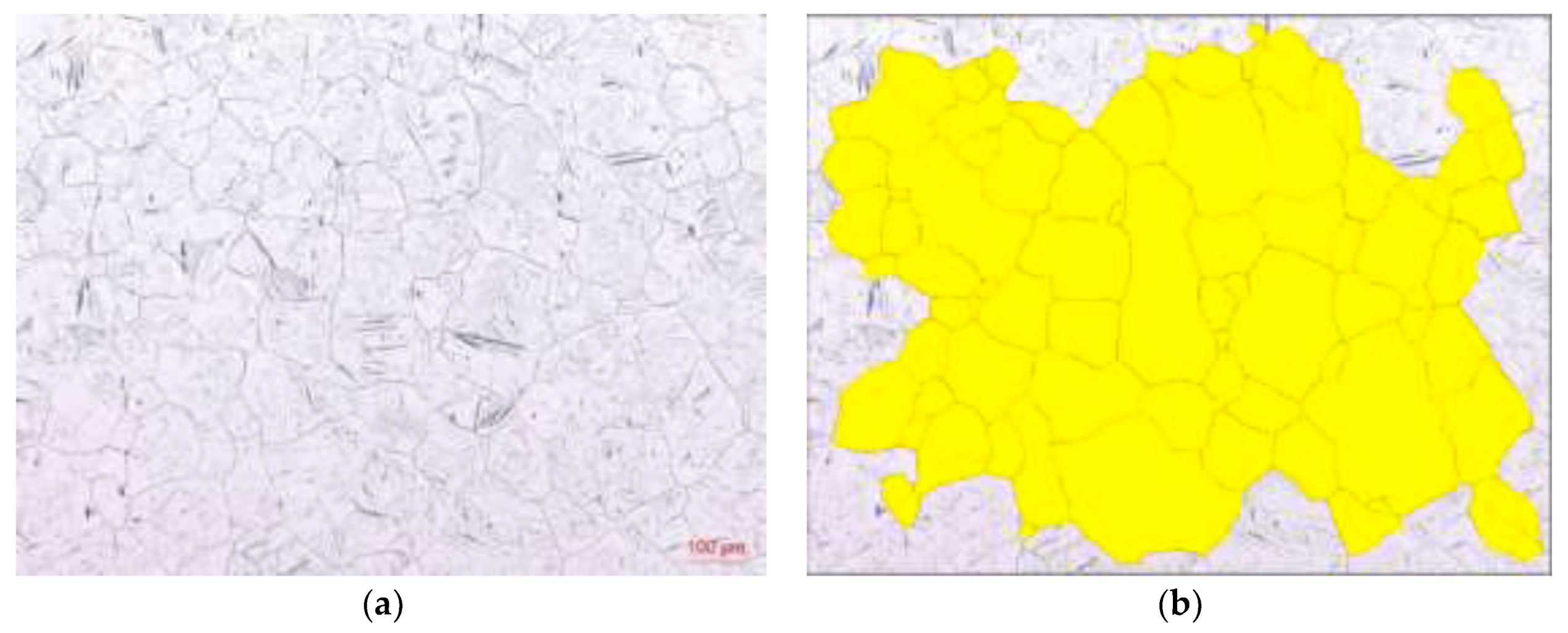
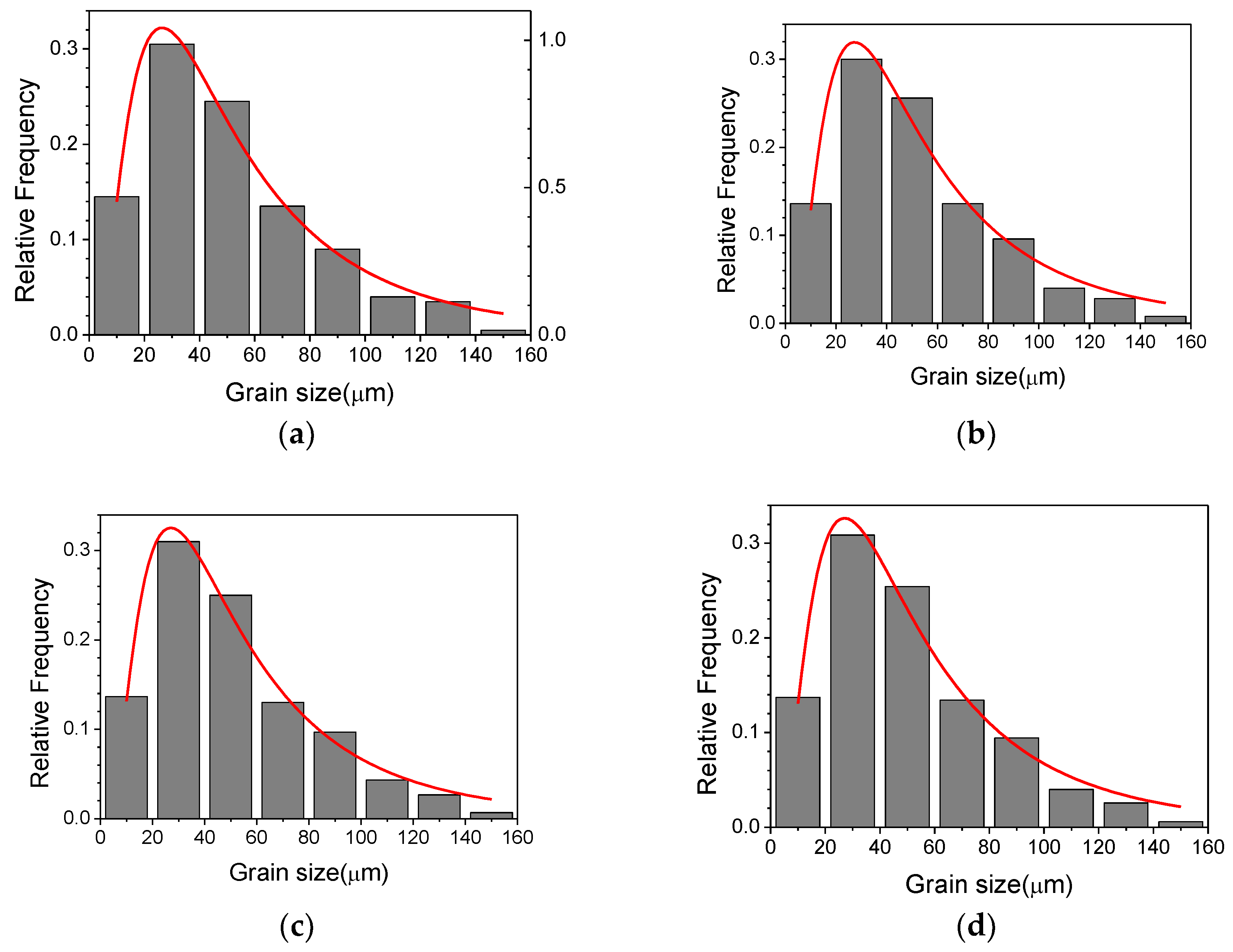
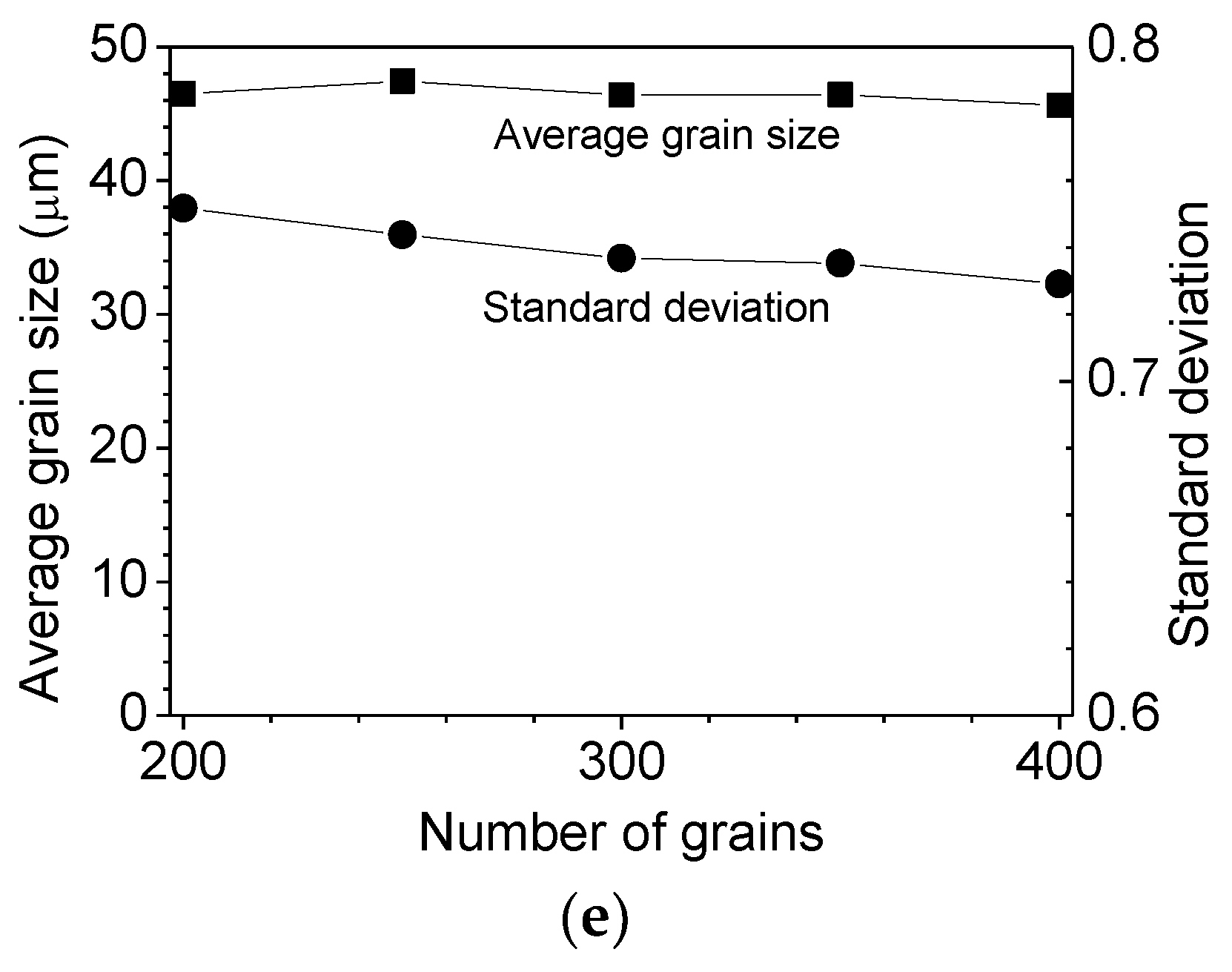
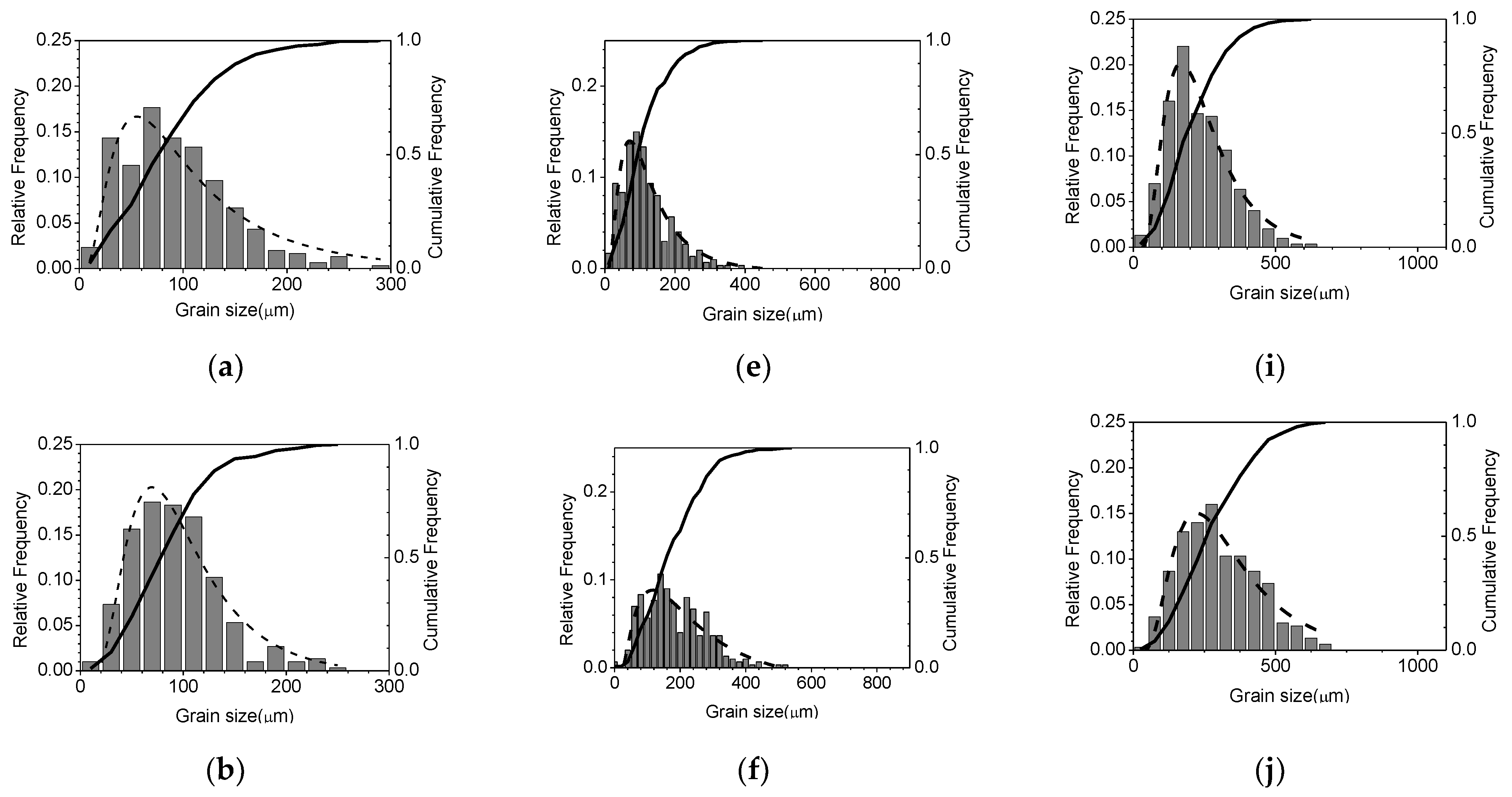
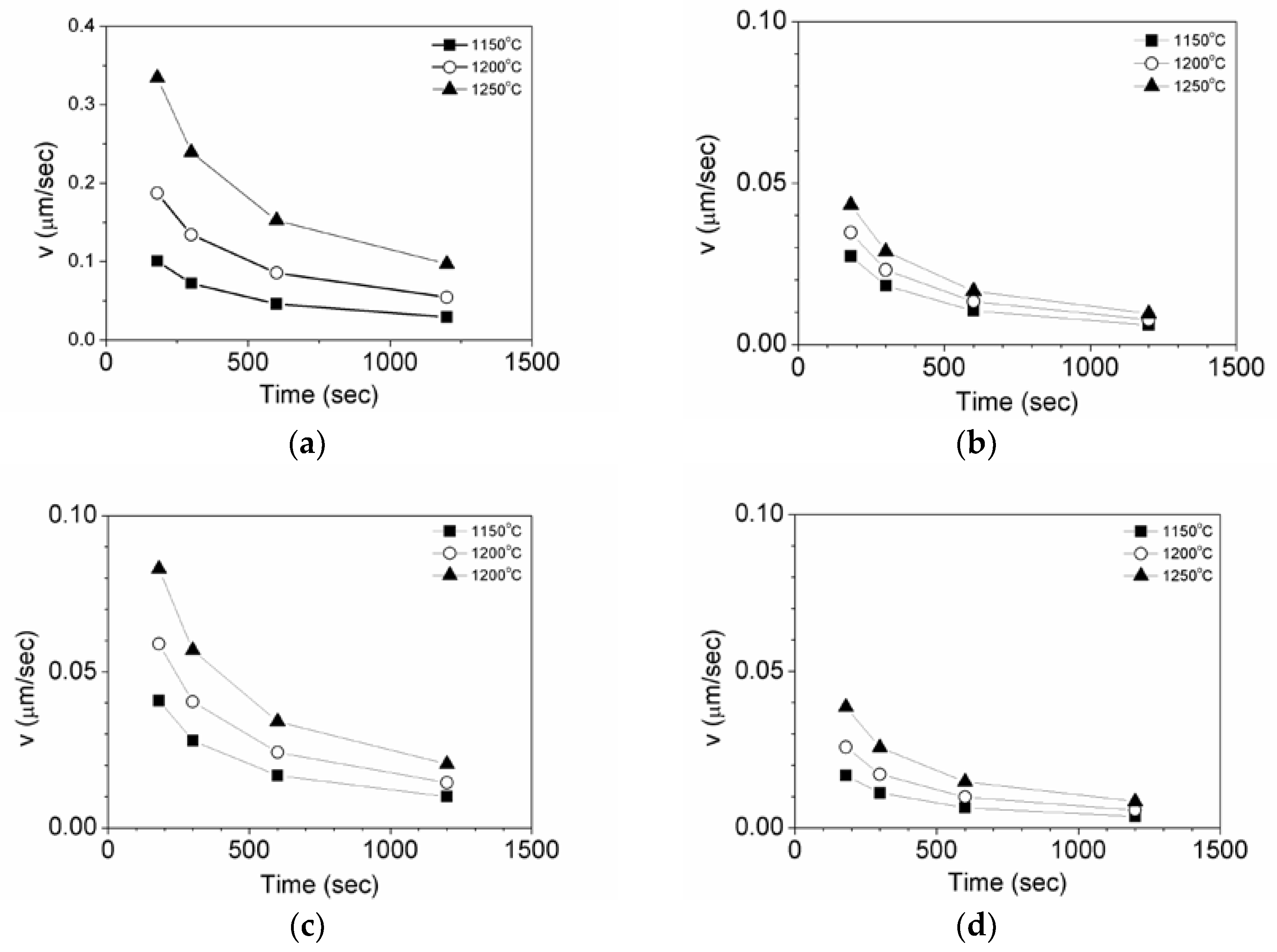
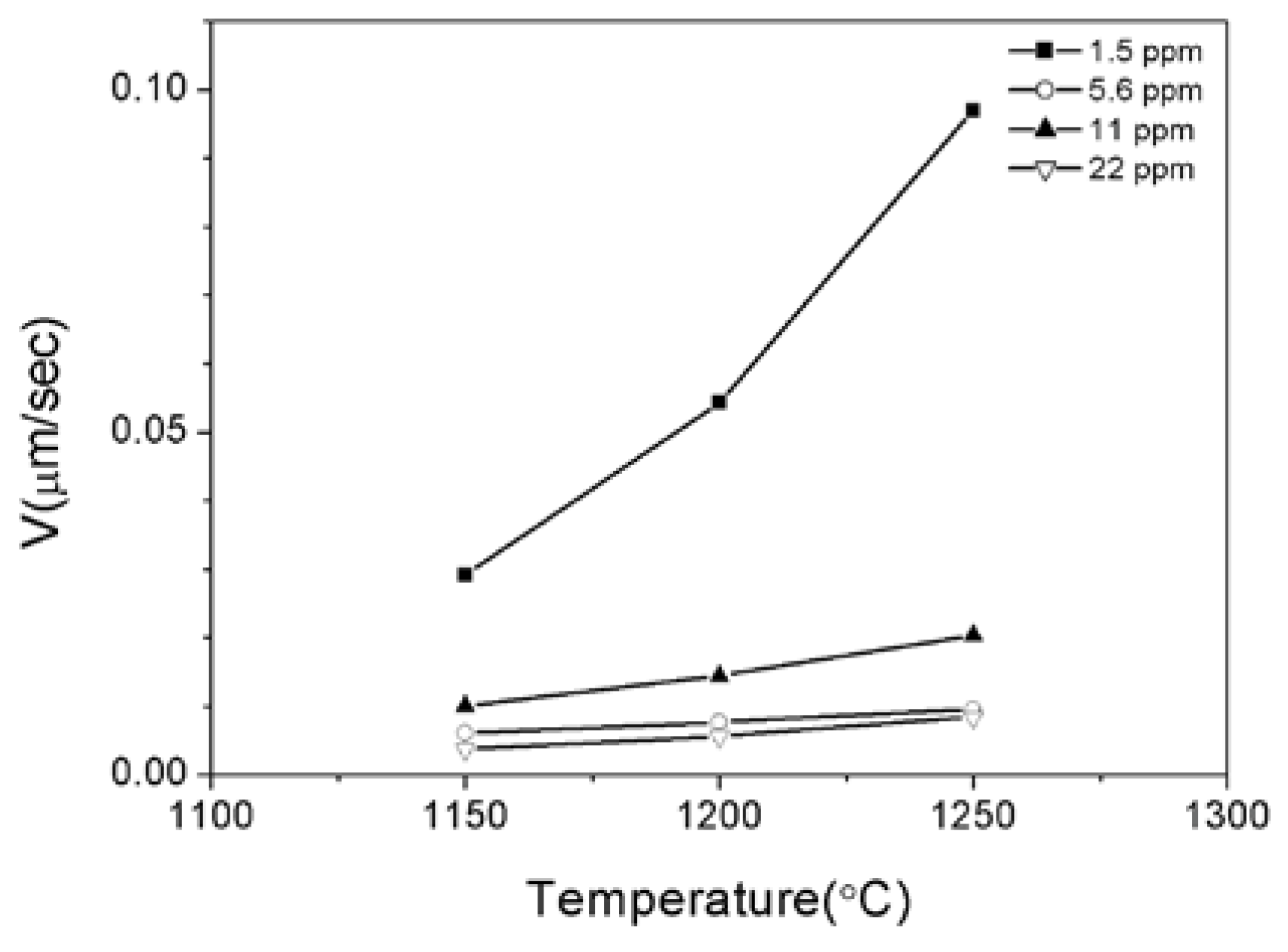
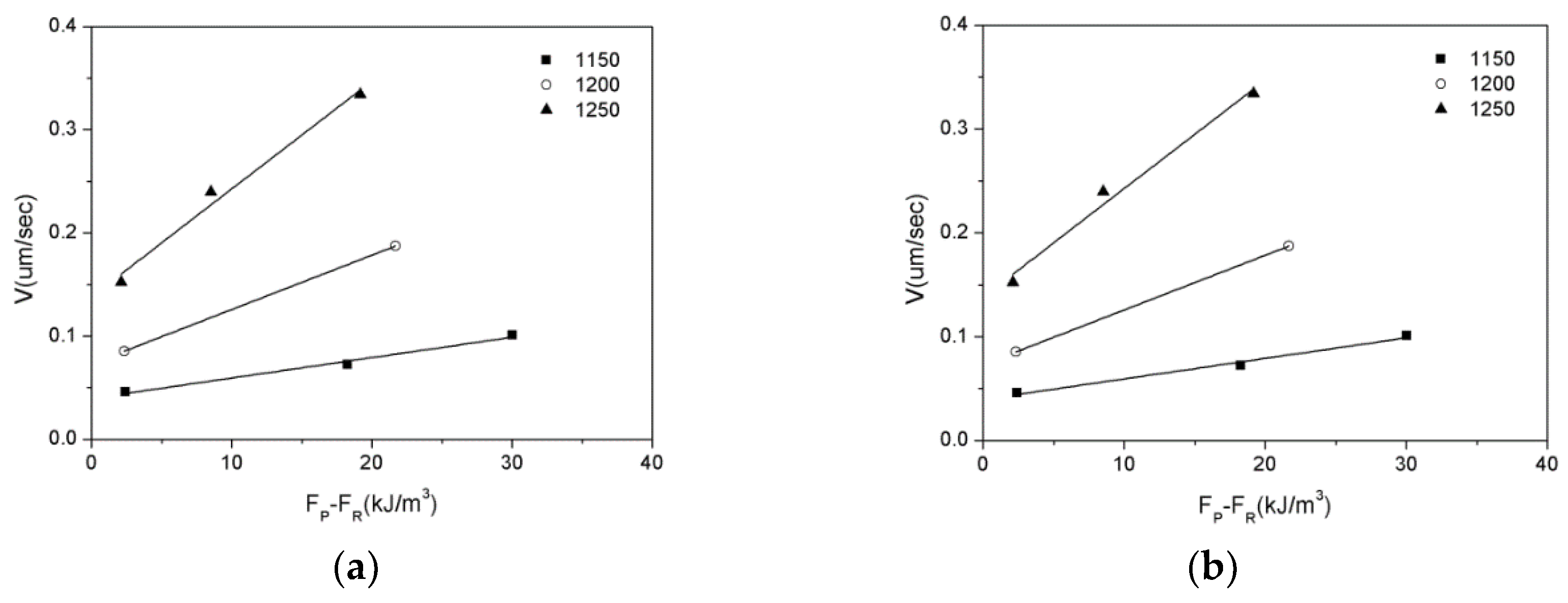
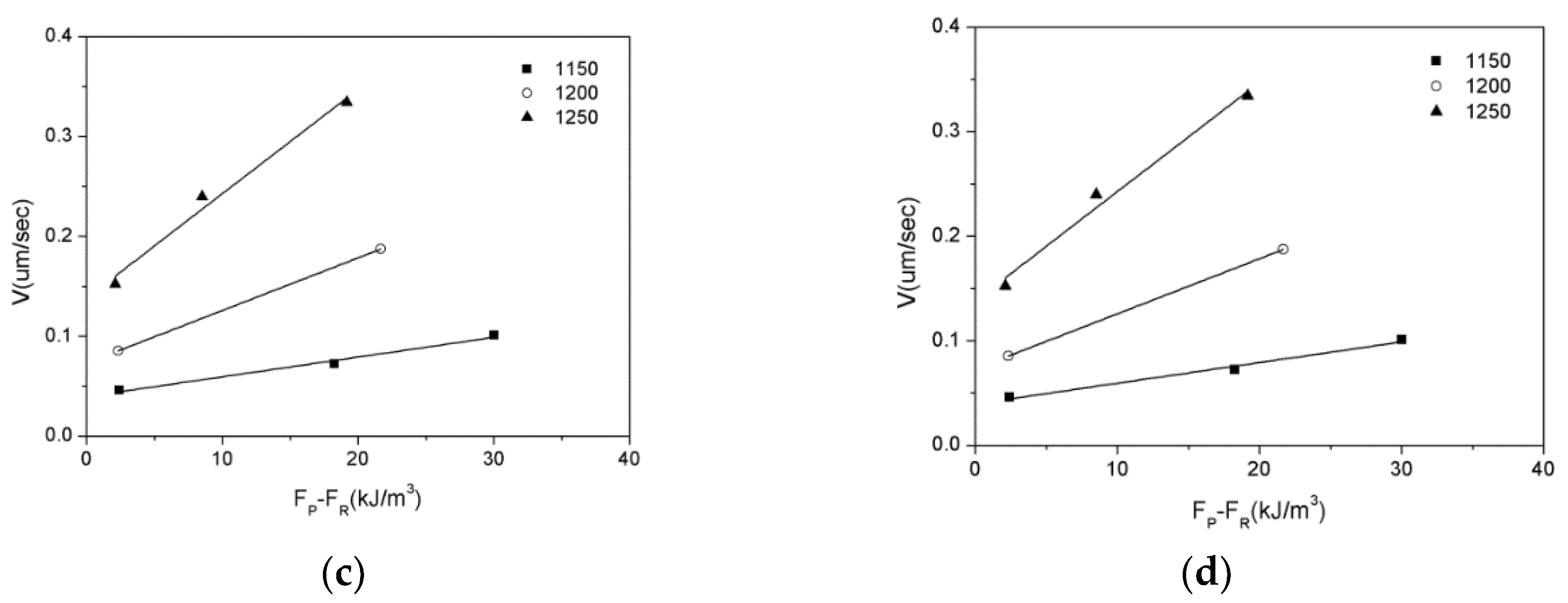
| Mg | C | Si | Mn | P | S | Al | N | O |
|---|---|---|---|---|---|---|---|---|
| 1.5 ppm | 0.120 | 0.17 | 1.18 | 0.0071 | 0.0006 | 0.028 | 0.0053 | 0.0041 |
| 5.6 ppm | 0.118 | 0.17 | 0.74 | 0.0102 | 0.0035 | 0.033 | 0.0054 | 0.0082 |
| 11.0 ppm | 0.136 | 0.28 | 0.94 | 0.0075 | 0.0012 | 0.020 | 0.0043 | 0.0027 |
| 22.0 ppm | 0.127 | 0.28 | 0.87 | 0.0104 | 0.0021 | 0.018 | 0.0052 | 0.0016 |
| Fitting equation | d0 (μm) | m | Q (kJ/mole) | k0 (μm/s) |
|---|---|---|---|---|
| Equation (1) | 4.3 | 2 | 232 | 7.2109 |
| Equation (2) | 0 | 2.9 | 622 | 3.21025 |
| Mg (ppm) | m | Q (kJ/mole) | k0 (μm/s) |
|---|---|---|---|
| 1.5 | 2.9 | 622 | 3.2 × 1025 |
| 5.6 | 4.9 | 405 | 2.5 × 1019 |
| 11.0 | 3.8 | 493 | 2.8 × 1021 |
| 22.0 | 6 | 752 | 1.7 × 1031 |
| Steels | m | Q (kJ/mol) | k0 (μm/s) | References |
|---|---|---|---|---|
| Low C-Mn | 2 | 67 | 4.3 × 1012 | [40] |
| Low C-Mn | 5.6 | 126 | 9.4 × 1038 | [37] |
| C-Mn | 2 | 64 | 1.4 × 1012 | [41] |
| C-Mn | 1 | 400 (T>1273) | 3.9 × 1032 | [36] |
| C-Mn | 1 | 914 (T<1273) | 5.0 × 1053 | [36] |
| C-Mn-V | 7 | 400 | 1.5 × 1027 | [36] |
| C-Mn-Ti | 10 | 437 | 2.6 × 1028 | [33] |
| C-Mn-Nb | 4.5 | 435 | 4.1 × 1023 | [33] |
| High C-Mn | 5.3 | 366 | 9.1 × 1025 | [37] |
| High C-Mn | 0.12 | 1170 | 4.1 x 1063 | [31] |
| Mg (ppm) | Inclusion | A (m2) | D (m) | fA (10−6 × %) | fV (10−4 × %) | FR (kJ/m3) | FR (kJ/m3) |
|---|---|---|---|---|---|---|---|
| 1.5 | MgO-MnS | 87.6 | 1.9 | 2.5 | 15.9 | 2.9 | 11.7 |
| MnS | 67.9 | 1.4 | 2.0 | 14.0 | 3.5 | ||
| MgAl2O4 | 693 | 2.9 | 19.9 | 44.6 | 5.3 | ||
| 5.6 | MgO-MnS | 26.8 | 1.9 | 0.8 | 8.8 | 1.6 | 8.5 |
| MnS | 26.9 | 2.1 | 0.8 | 8.8 | 1.5 | ||
| MgAl2O4 | 834 | 3.1 | 23.9 | 48.9 | 5.4 | ||
| 11 | MgO | 10.4 | 1.7 | 0.2 | 4.7 | 1.0 | 20.3 |
| MgO-MnS | 46.6 | 1.9 | 1.0 | 10.0 | 1.8 | ||
| MnS | 861 | 3.4 | 18.5 | 43.0 | 4.3 | ||
| MgAl2O4 | 2030 | 3.8 | 43.7 | 66.1 | 6.0 | ||
| Mg-Al-MnS | 2790 | 3.8 | 60.0 | 77.5 | 7.1 | ||
| 22 | MgO | 108 | 2.7 | 3.1 | 17.6 | 2.2 | 17.1 |
| MgO-MnS | 224 | 4.4 | 6.4 | 25.3 | 2.0 | ||
| MnS | 310 | 2.5 | 8.9 | 29.8 | 4.2 | ||
| MgAl2O4 | 235 | 2.8 | 6.7 | 26.0 | 3.2 | ||
| Mg-Al-MnS | 759 | 3.0 | 21.8 | 46.6 | 5.5 |
| Mg (ppm) | Temperature (oC) | ||
|---|---|---|---|
| 1150 | 1200 | 1250 | |
| 1.5 | 19.8 | 52.6 | 105 |
| 5.6 | 8.0 | 5.9 | 7.5 |
| 11.0 | 7.7 | 14.2 | 21.6 |
| 22.0 | 1.3 | 1.7 | 3.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, C.-T.; Lai, H.-H.; Su, Y.-H.F.; Huang, F.-Y.; Lin, C.-K.; Kuo, J.-C.; Lee, H.-T. The Influence of Mg-Based Inclusions on the Grain Boundary Mobility of Austenite in SS400 Steel. Metals 2019, 9, 370. https://doi.org/10.3390/met9030370
Lai C-T, Lai H-H, Su Y-HF, Huang F-Y, Lin C-K, Kuo J-C, Lee H-T. The Influence of Mg-Based Inclusions on the Grain Boundary Mobility of Austenite in SS400 Steel. Metals. 2019; 9(3):370. https://doi.org/10.3390/met9030370
Chicago/Turabian StyleLai, Chih-Ting, Hsuan-Hao Lai, Yen-Hao Frank Su, Fei-Ya Huang, Chi-Kang Lin, Jui-Chao Kuo, and Hwa-Teng Lee. 2019. "The Influence of Mg-Based Inclusions on the Grain Boundary Mobility of Austenite in SS400 Steel" Metals 9, no. 3: 370. https://doi.org/10.3390/met9030370
APA StyleLai, C.-T., Lai, H.-H., Su, Y.-H. F., Huang, F.-Y., Lin, C.-K., Kuo, J.-C., & Lee, H.-T. (2019). The Influence of Mg-Based Inclusions on the Grain Boundary Mobility of Austenite in SS400 Steel. Metals, 9(3), 370. https://doi.org/10.3390/met9030370





