1. Introduction
Nickel-based single crystals have gradually become the preferred material for aero-engine turbine blades because of their excellent mechanical properties at high temperature, especially creep properties, oxidation resistance, corrosion resistance, high toughness, and good processing plasticity [
1]. Good high-temperature mechanical properties were obtained by precipitation strengthening. The molecular structure of Ni
3 (Al, Ti) as precipitation strengthening phase was a primitive cubic crystal structure Pm3m. The macroscopic structure was cubic, and the strengthening phase was surrounded by continuous solid solvent matrix phase, which presented a chessboard-like compact and regular arrangement [
2]. It has been found that the basic characteristics of the strengthened phase, such as shape, size, distribution, and volume fraction, have an important effect on the mechanical properties of superalloys [
3,
4,
5,
6].
Many scholars have done a series of work on the dissolution and precipitation behavior of precipitation phases of Ni-based alloys at high temperature. The microstructural evolution of precipitation phases has been discussed. The morphological changes and distribution of the precipitation of γ phases during continuous cooling after solid solution treatment have been studied. Good results have been obtained in the recovery heat treatment system of the alloys. Wang [
7] mainly studied the effect of solution temperature and holding time on the γ-phase dissolution behavior of IN100 and DS Rene 125 alloys during solution treatment, and explored the dynamic factors of precipitation phase dissolution behavior during solution treatment. The results show that the environmental temperature has a greater influence on the dissolution of the precipitation phase precipitation than the holding time. At the same time, the morphology of the precipitation phase is strongly influenced by the elastic-strain energy in the structure, which is related to the lattice mismatch between the precipitation phase and the γ phase. Grosdidier et al. [
8] studied the precipitation and dissolution of the γ-phase in nickel-based alloys AM1 and CMSX-2 during heat treatment, discussed the microstructural evolution of the precipitation phase just after precipitation, and proposed that the precipitation phase would undergo deformation from spherical to cubic to dendritic from nucleation to growth. Gao et al. [
9] studied the effect of cooling rate on the microstructural evolution and mechanical properties of the Ni-based superalloy Rene 80 during the cooling process after solution treatment. It was found that the higher the cooling rate, the smaller the precipitated precipitation phases during solidification, and the faster the coarsening of the precipitation phases in the subsequent aging process. After many mechanical properties tests on alloys with different cooling rates, it was concluded that air cooling is the most suitable cooling rate for Rene 80 alloy in the cooling stage of heat treatment. Jackson et al. [
10] studied the directionally solidified superalloy (DS) Mar-M200+HF. It was found that a higher solution temperature could dissolve more coarsened γ’ phases and γ-γ’-eutectic structures, and more high-quality gamma-precipitation phases were precipitated during subsequent cooling and aging. Increasing the volume fraction of high-quality precipitation phases could greatly improve the creep properties of the alloy, When the volume fraction of the strengthening phase increases from 30% to 45%, the creep fracture life of the material increases to three times as long as the original lifetime. Li et al. [
11] found in the study of FGH4096 blade materials that the cooling rate after solution treatment determined the morphology and distribution of the precipitated precipitation phases. A faster cooling rate could make the precipitated precipitation phases have better morphology and a narrower channel of matrix phase, which improved the precipitation effect of Orowan [
12,
13,
14], and effectively hindered dislocation movement in matrix phase; thus, the material could be improved. The creep properties were improved at 704 °C/690 MPa. At present, DD6 is widely used in the hot-end components of aero-engines. Besides, plastic deformation caused by load, there is no grain boundary, and the evolution law of the microstructure of the precipitation phase and the matrix phase during creep and after the heat treatment process is different from that of general superalloys. At high temperature, the microstructure morphology of DD6 will change to a certain extent, which will affect the performance of the blades [
15,
16,
17,
18]. The study of the dissolution and precipitation behavior of the γ’ phase at high temperature has important practical engineering significance.
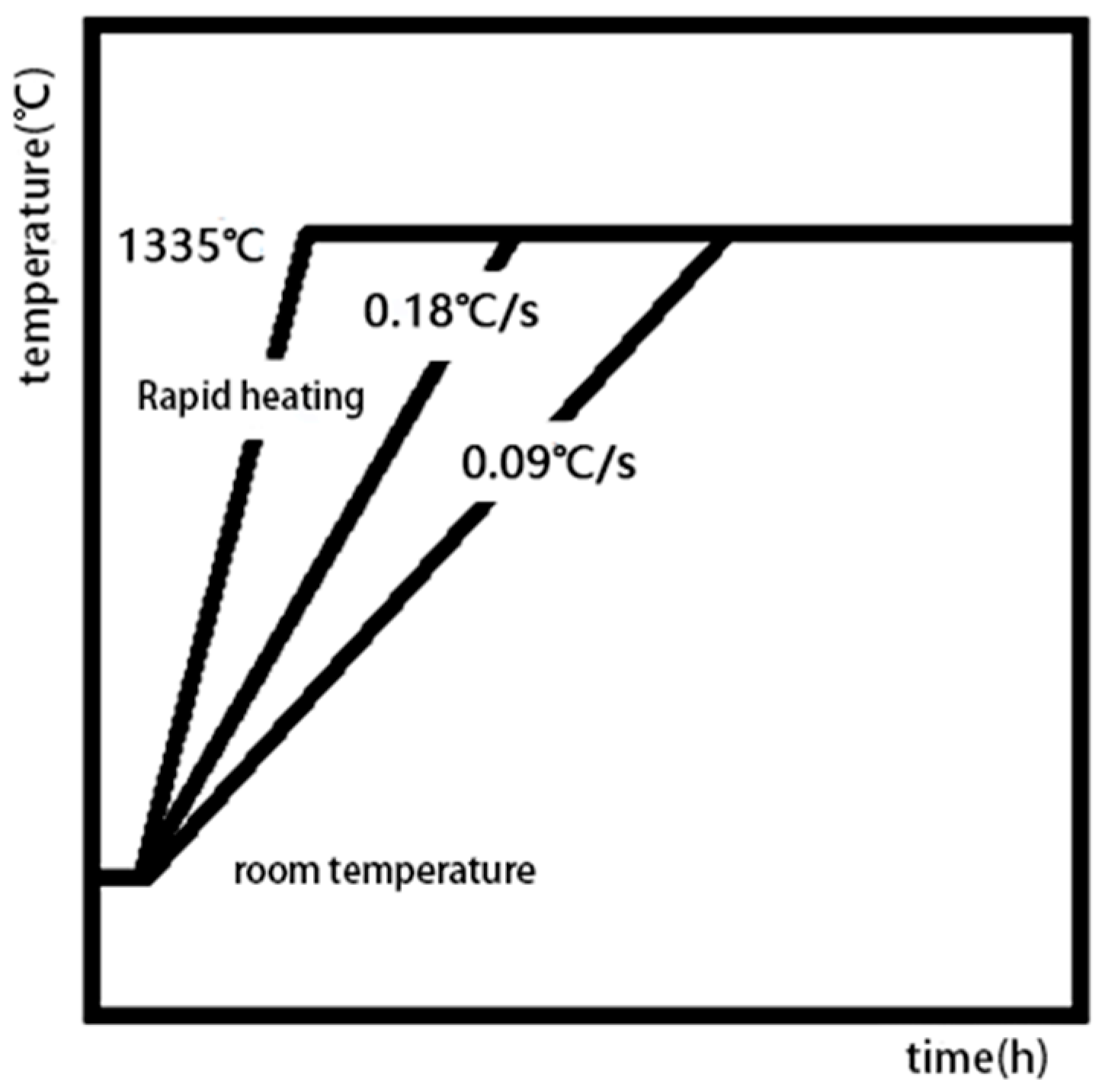
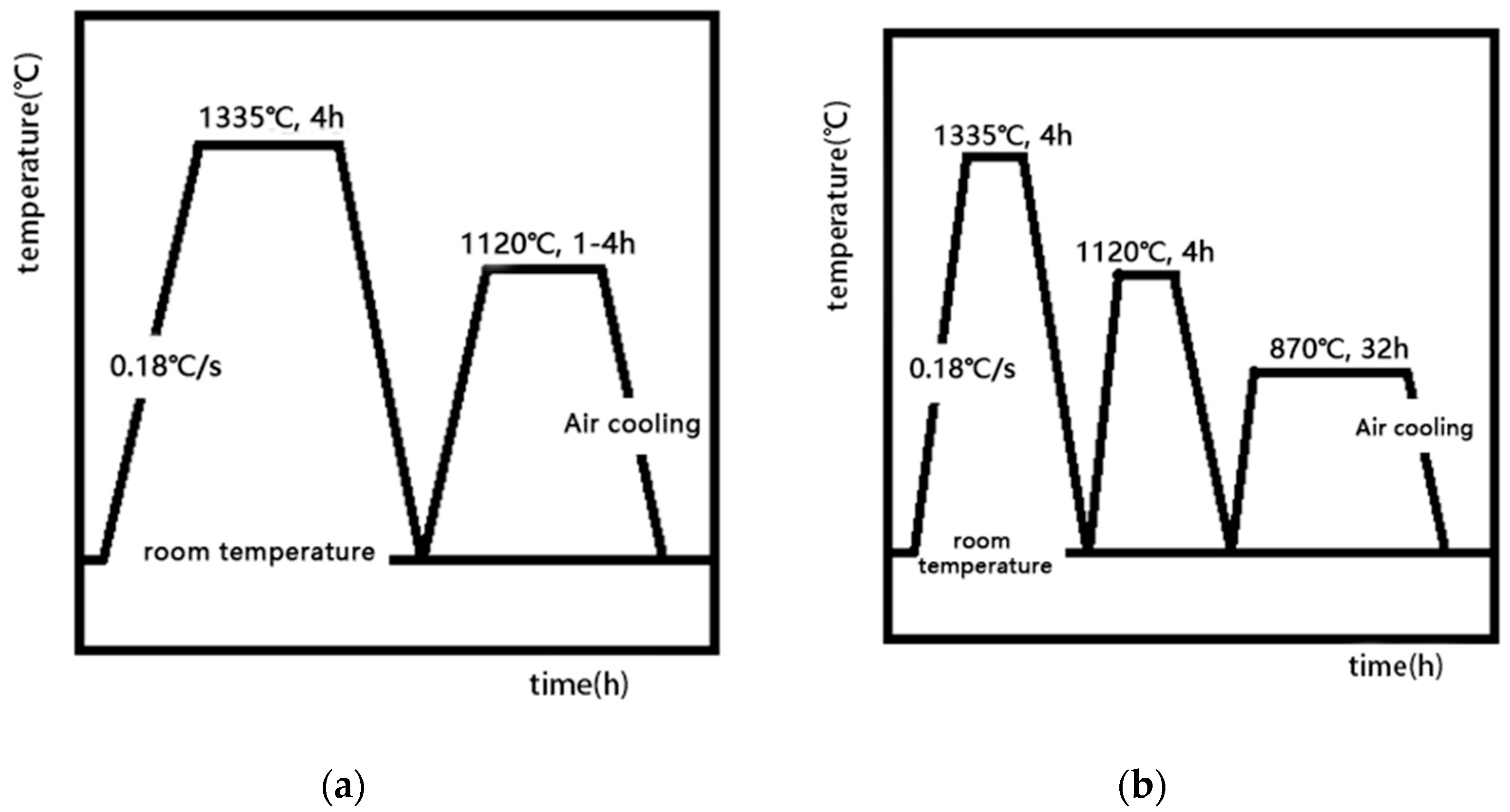
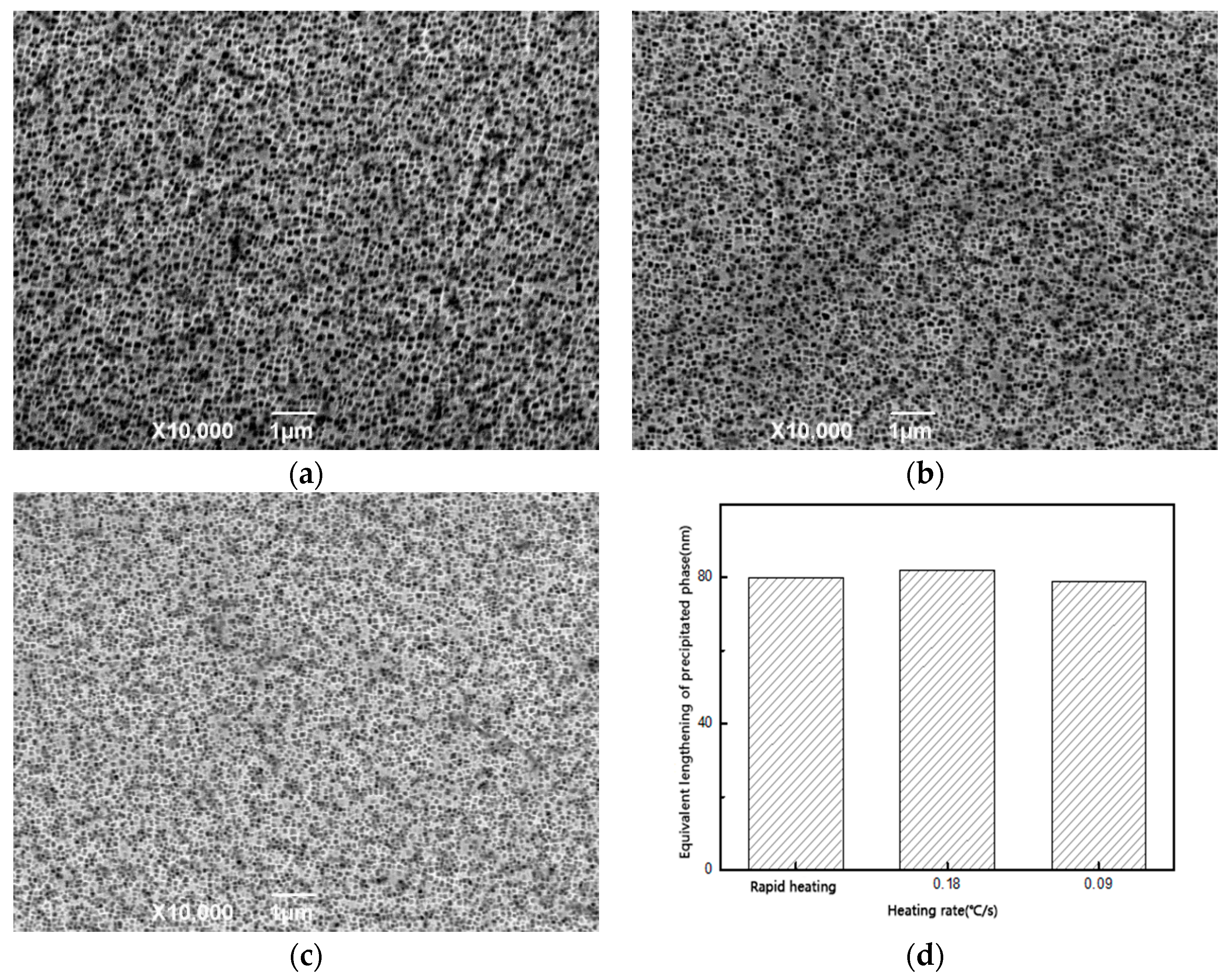
Starting from different stages of the heat treatment system, we focus on many factors, such as the heating rate in the solution treatment stage, the cooling rate in the cooling stage after solution treatment, and the aging temperature and time in the aging stage. By designing different experimental conditions, the effects of various factors (heating rate and cooling rate) on the dissolution and precipitation behavior of the γ’ phase and the number, size, morphology, and distribution of the final precipitates in the heat treatment system were studied.
5. Dissolution and Precipitation Mechanisms
In the dissolution process of the γ’ phase, the elastic field related to the dislocation still exists in the microstructures, although the sample is not affected by external loads, which affects the dissolution behavior of the γ’ phase. Because the channels of cubic reinforcement phase and matrix phase are closely arranged in a chessboard shape, there is elastic interaction between them, thus forming the local stability of the reinforcement phase accumulation group. With the solid solution treatment, the stability of each accumulation group is destroyed, due to the influence of temperature, and the first destroyed accumulation group demonstrates dissolution behavior. Therefore, the overall dissolution behavior of the γ’ phase is not a macroscopic phenomenon that is formed by the simple combination of dissolution of each γ’ phase due, to the influence of temperature. During the solid solution treatment process, some of these γ’ phase stacks connected by elastic interaction become extremely unstable, due to the absorption of heat energy, and they gradually break away from the bonds between them, and they enter the matrix phase. That is to say, the dissolution behavior occurs, and the original stable structure is destroyed after the loss of this part. The structure of the whole precipitated phase group becomes more unstable. The lack of stability that is caused by partial dissolution destroys the integrity of the elastic strain field, and reduces enough of the elastic strain field. The resistance to γ’ phase dissolution is weakened, and the dissolution process becomes smoother and more uniform.
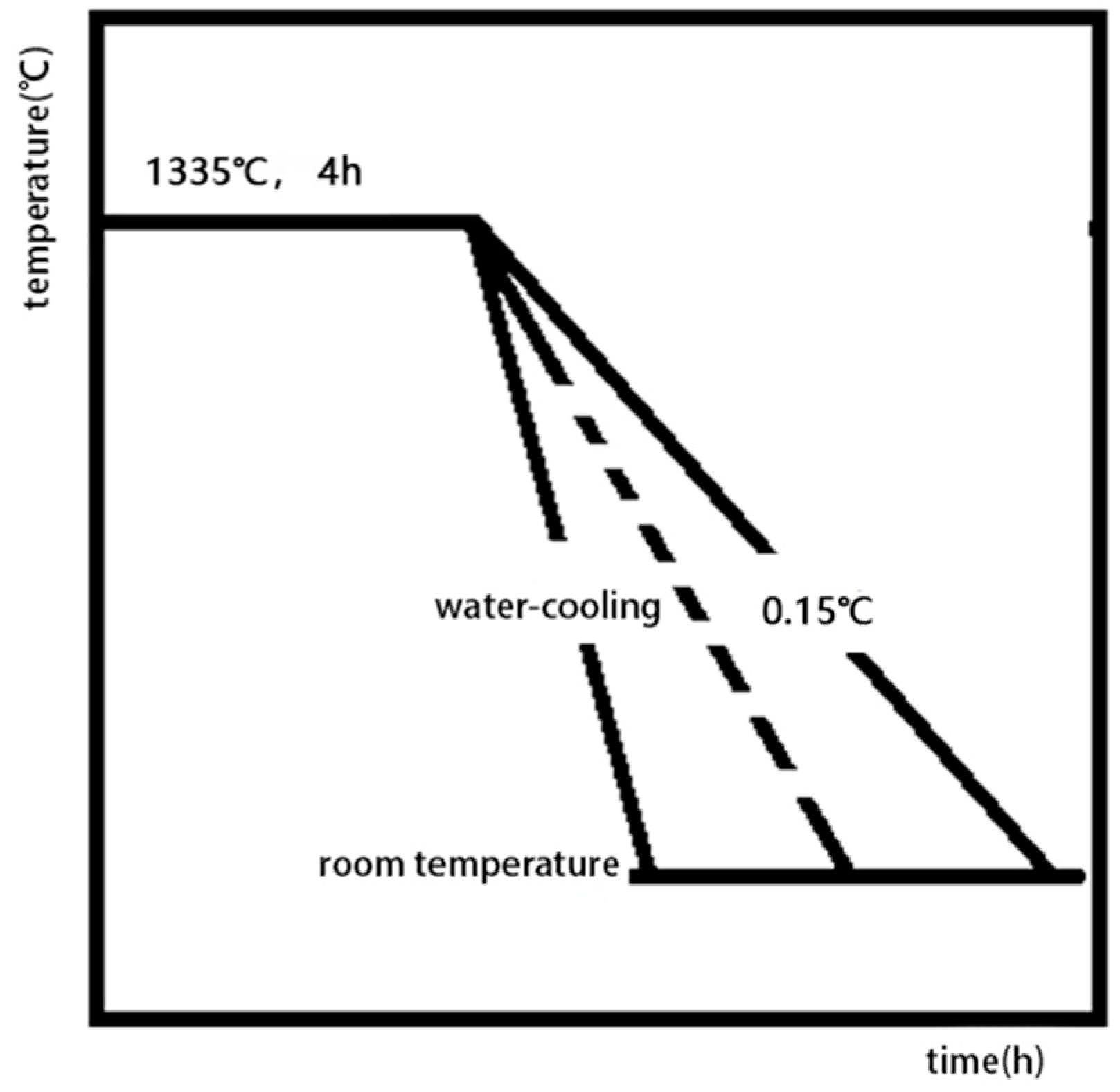
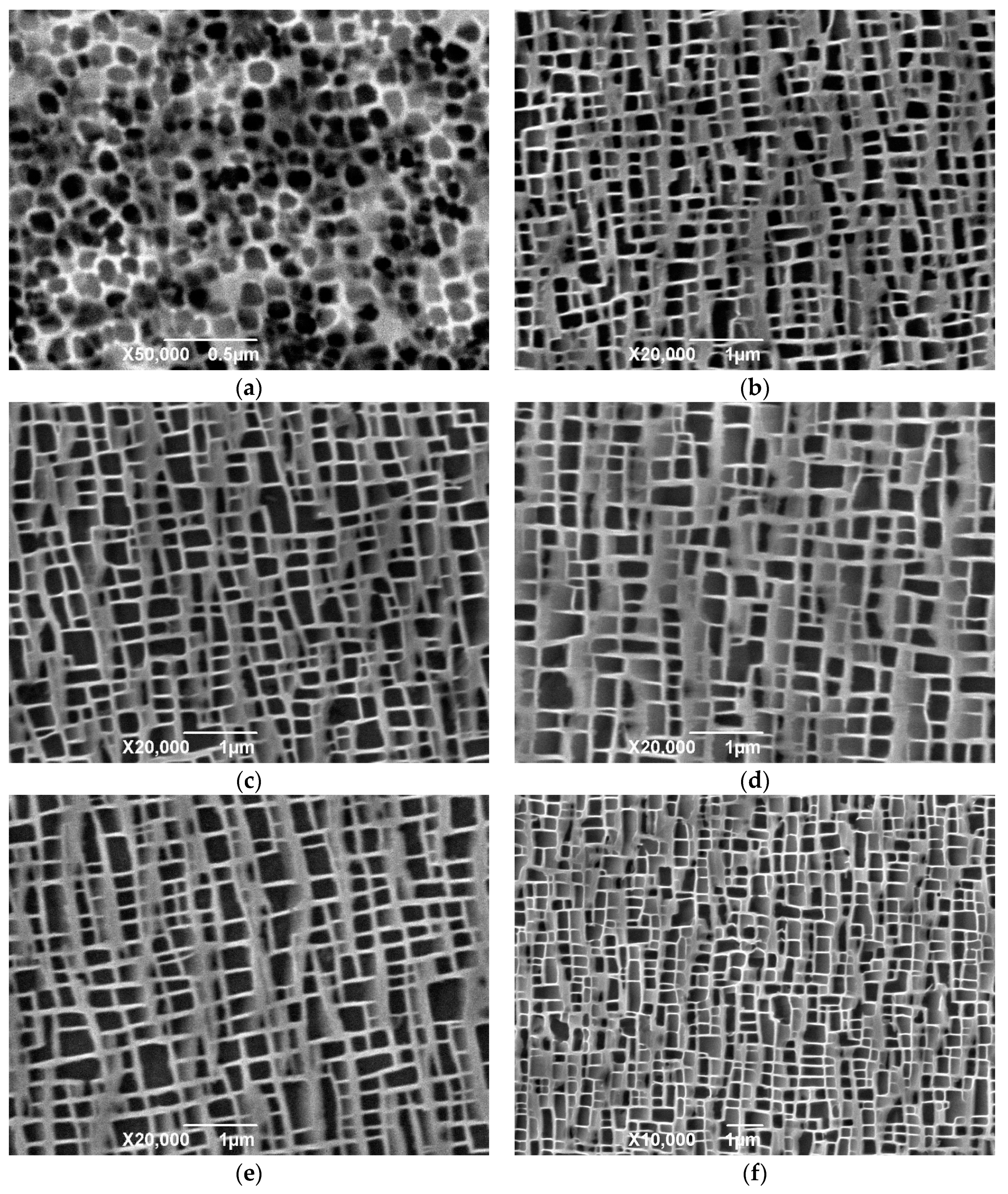
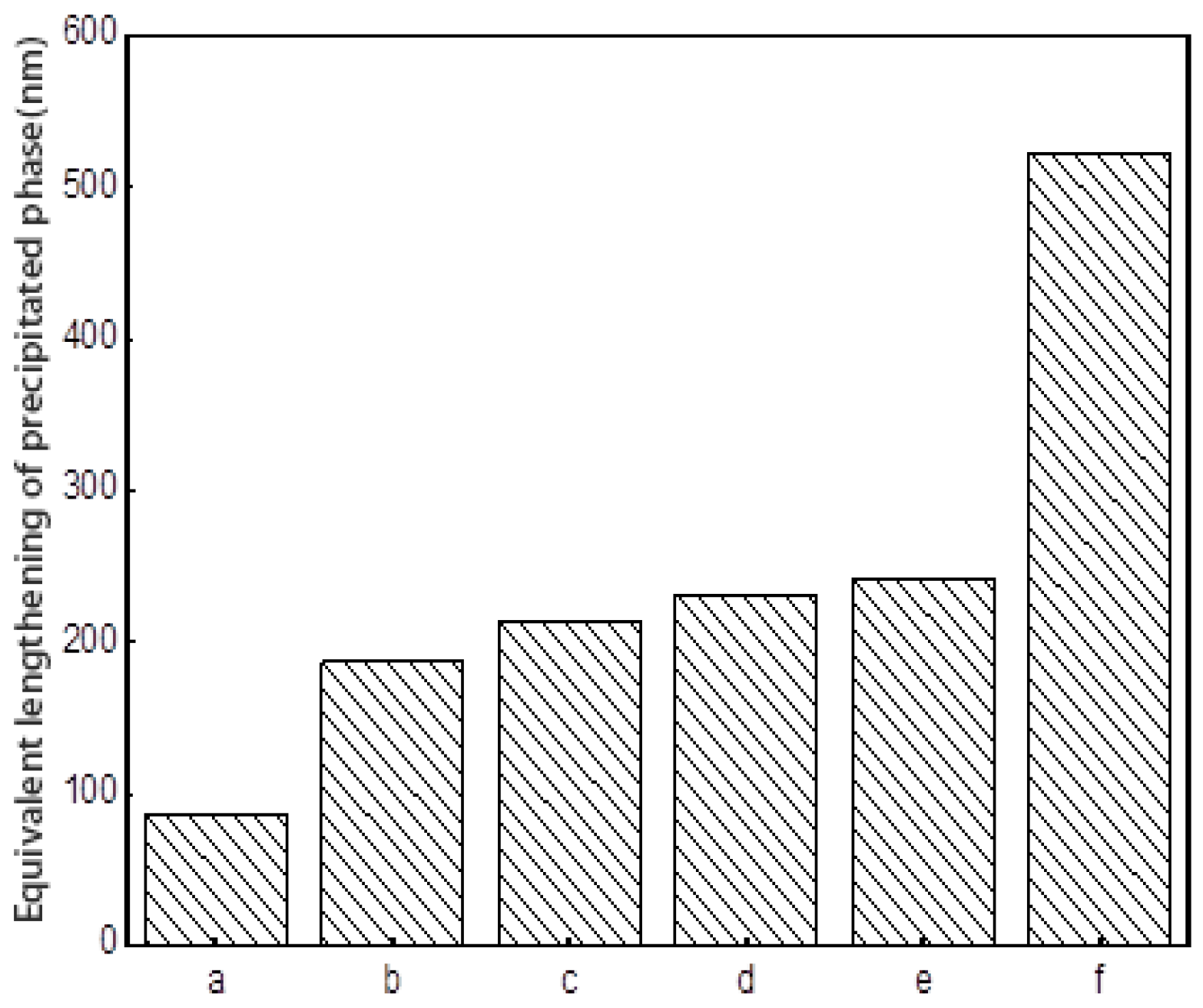
In the cooling stage after solid solution treatment, the solubility of the precipitation phase elements in the matrix phase decrease, due to the decrease of temperature, and then nucleation and precipitation begin to grow. In the process of precipitation, the growth behavior of the phase, including the number of nucleations, the size and shape of the precipitation, the direction of precipitation growth, and the precipitation of the secondary precipitate, are all affected by the cooling rate, which determines the nucleation rate and the kinetics of the precipitate growth. Therefore, the morphology, size, and distribution of the precipitated phases and the overall microstructure characteristics can be controlled and described by different cooling rates during the cooling process. In the cooling process, when the cooling rate is low, as in the case of 0.5 °C/s studied previously, due to the slow drop of temperature, low supersaturation in the nucleation stage and slow nucleation of precipitated phase, the number of primary nucleations is small and the nucleation density is low, which makes the precipitated phase of the primary nucleation have enough time to grow and gradually form dendritic structures. When the cooling rate is accelerated, the precipitate phase is “locked” in the early free-growth process.
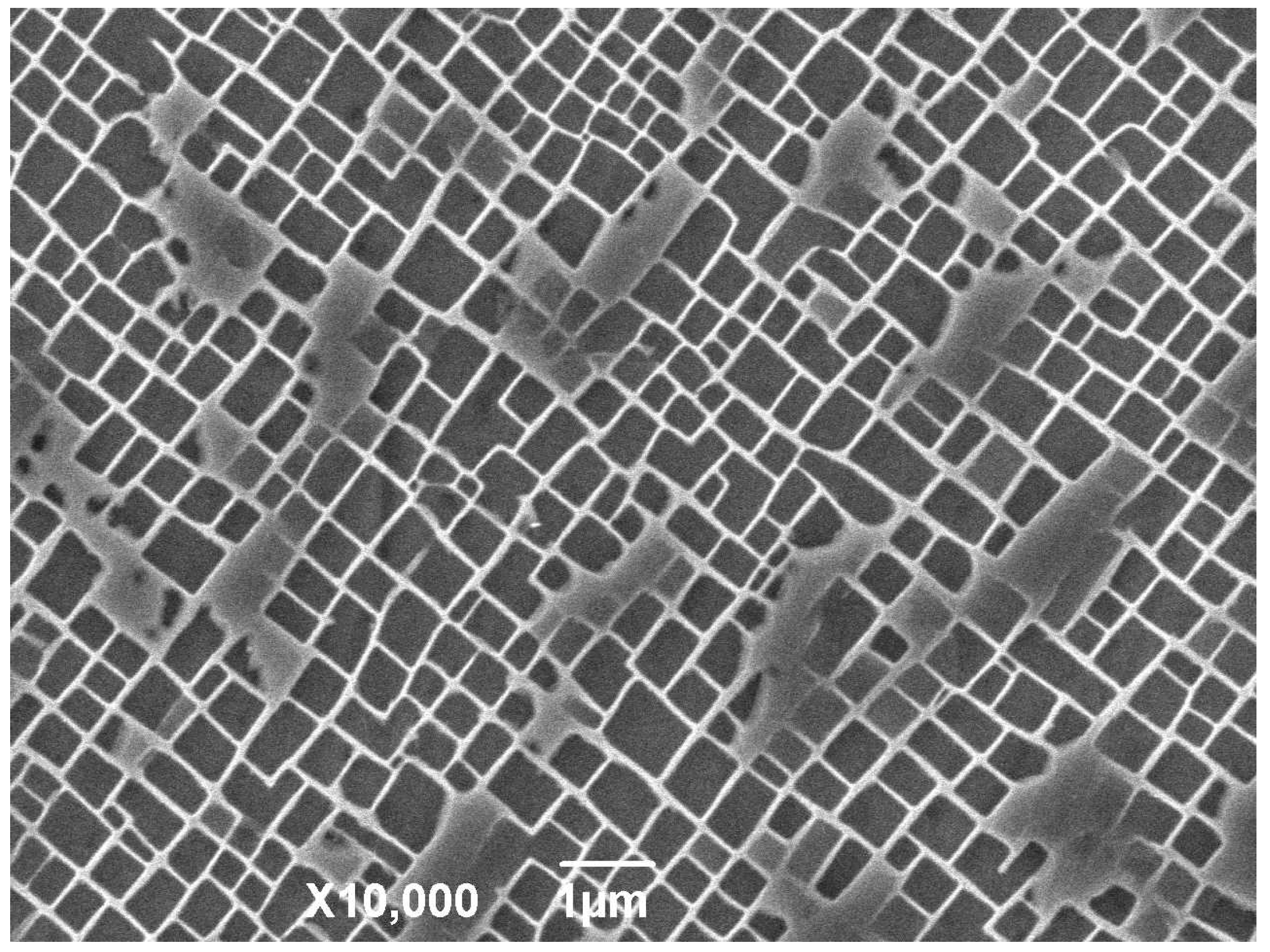
The microstructures of the specimens after solution treatment were studied by using different cooling rates to reduce the temperature from that of the solid solution to room temperature. When the cooling rate is low, most of the precipitates have undergone relatively full free growth. After a long cooling process, the precipitation phases eventually come close to each other in four groups to form butterfly-like structure knots. The volume of the phase is large and the cube structure with sharp edges and corners is tetragonal in cross-section, and the channel of the matrix phase is relatively wide. When the cooling rate increases, the number of phases increases, the volume of the final phase decreases, and the curvature of the corner decreases, and the channel of the matrix phase becomes finer. When air cooling is used, the precipitation phases in the material show irregular spheres, and the volume of the phase decreases obviously compared with the cooling rate at 0.15 °C. From the cross-section, the phase is irregularly circular, a few have the tendency of edge angle, and the channel of the matrix phase is finer, and there are fine secondary precipitation phases that are precipitated. When the cooling rate is faster than that of water cooling, it is difficult to see the microstructure of the structure under the existing micro-electron microscopy. It can be seen that the micro-spheres are piled up in disorderly manner. Because of the rapid cooling rate, the precipitation phase ends its growth, and solidifies over a very short time after precipitation.
It has been pointed out that the exact shape of the precipitation phase in the cooling process after solution treatment is affected by factors that are related to the elastic field and other diffusion dynamics. In the solid solution treatment process, the precipitation phase and the matrix phase fully melt at a high temperature to form a single-phase solid solution. When the temperature decreases, the solubility of the precipitation phase in the matrix phase decreases, and the solid solution is in the supersaturated state. At this time, the surface shape of the precipitated phase presents a depression shape, although from a purely elastic point of view, the phase is not in a balanced state. When the temperature continues to decrease and the precipitation phase continues to precipitate and grow rapidly from the matrix phase, the metastable precipitation phase blocks are formed next to a dendritic structure that is formed along the growth direction. In the subsequent process, the growth of the precipitate phase makes the surface state of the phase closer to the plane state of the equilibrium state. At this time, precipitated phases continue to precipitate and grow gradually in the decreasing channel of matrix phase, resulting in the continuous increase of the volume fraction of the γ’ phase. At the end of the cooling stage, it can be observed that there are fine precipitation phases of different sizes in the channel of γ’ phase, and in the low-saturation region around the primary precipitation phase. This indicates that during the cooling process, new nuclei form and grow, and the existing precipitation phases continuously absorb various elements from the surrounding matrix phase for growth. The system exists at the same time, and there is competition between them.