Nanoporous Quasi-High-Entropy Alloy Microspheres
Abstract
:1. Introduction
2. Materials and Methods
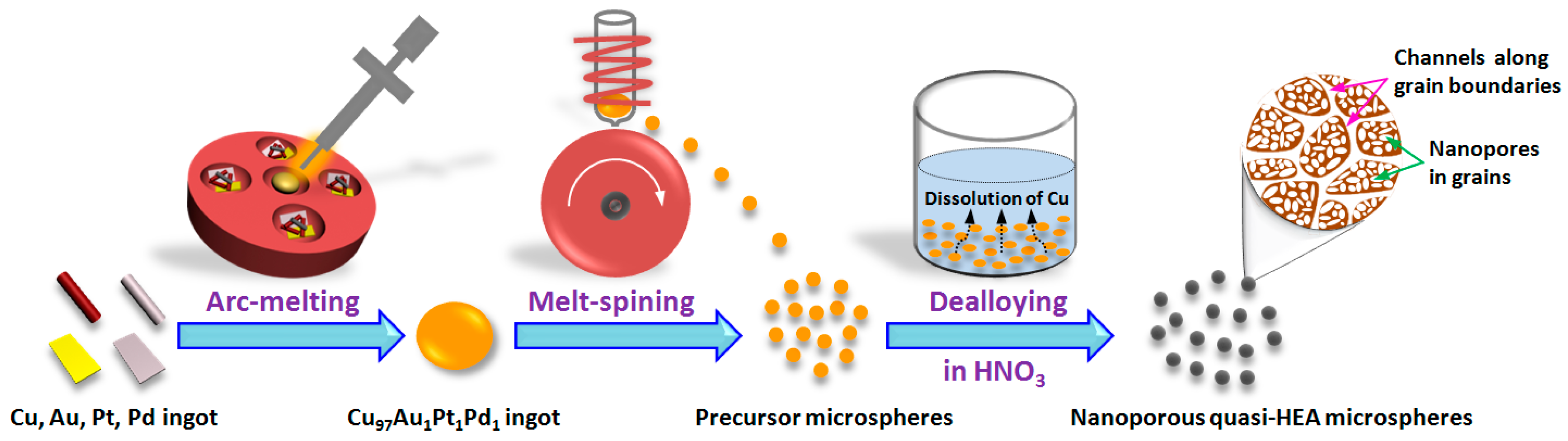
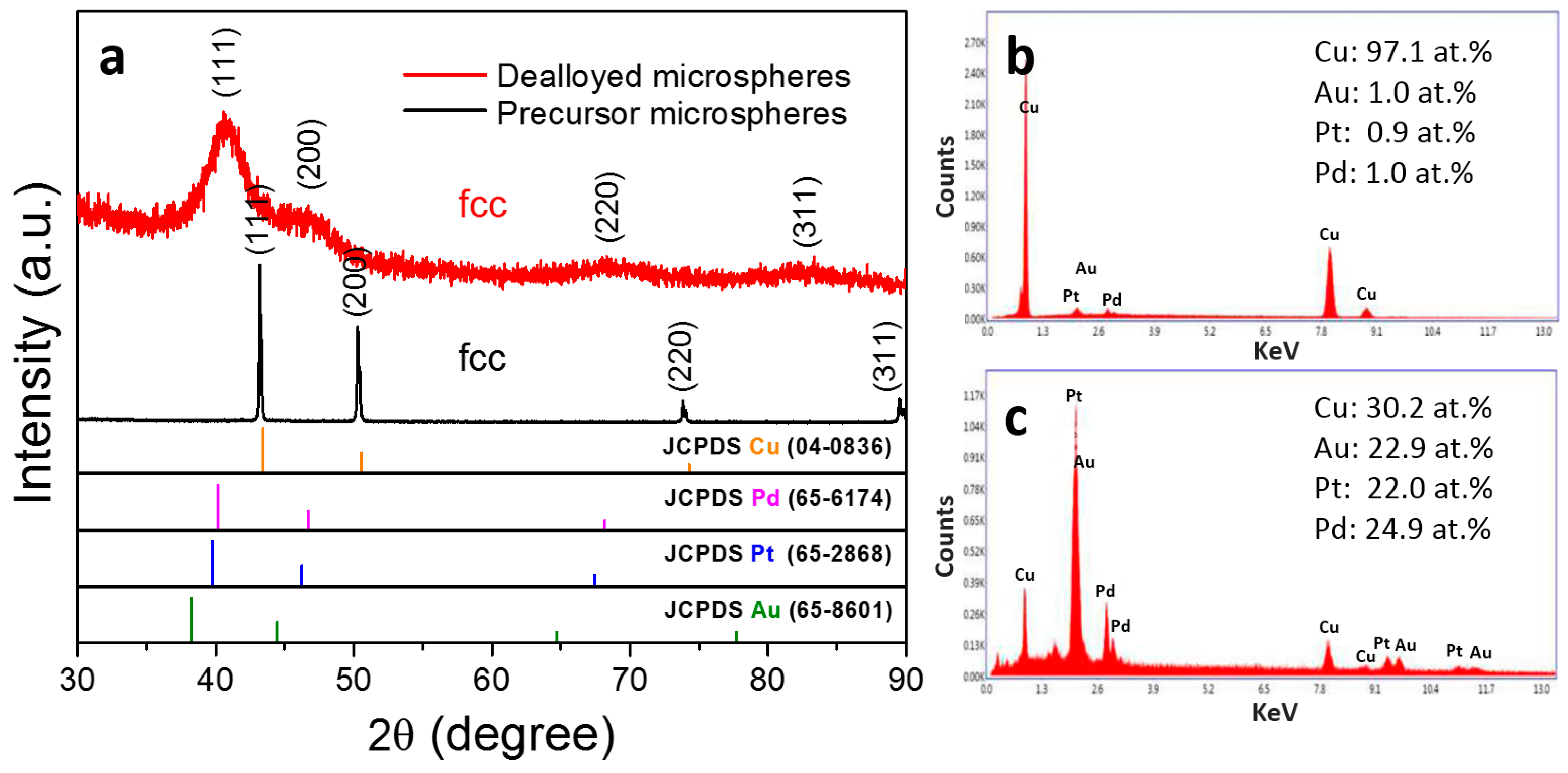
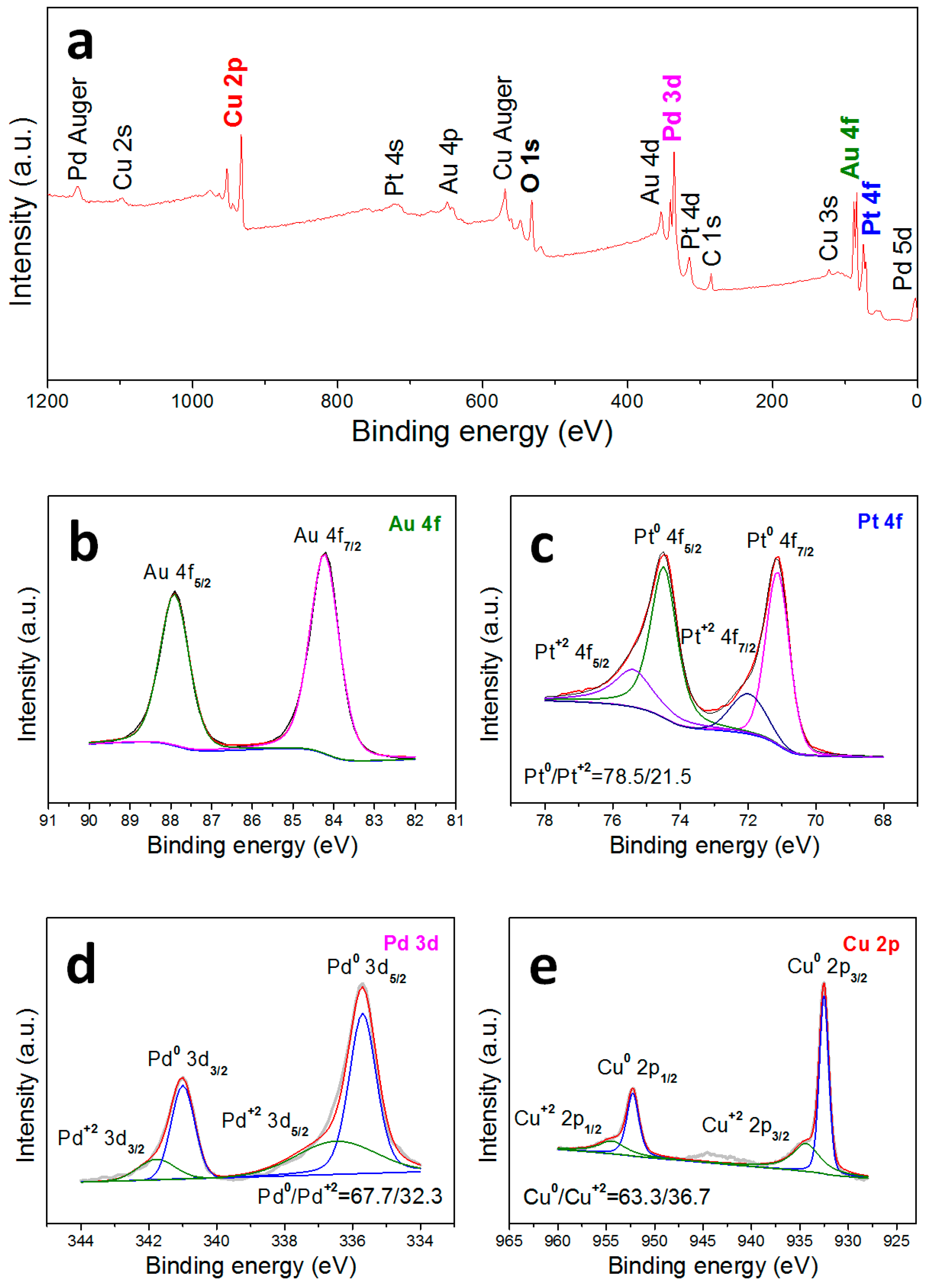
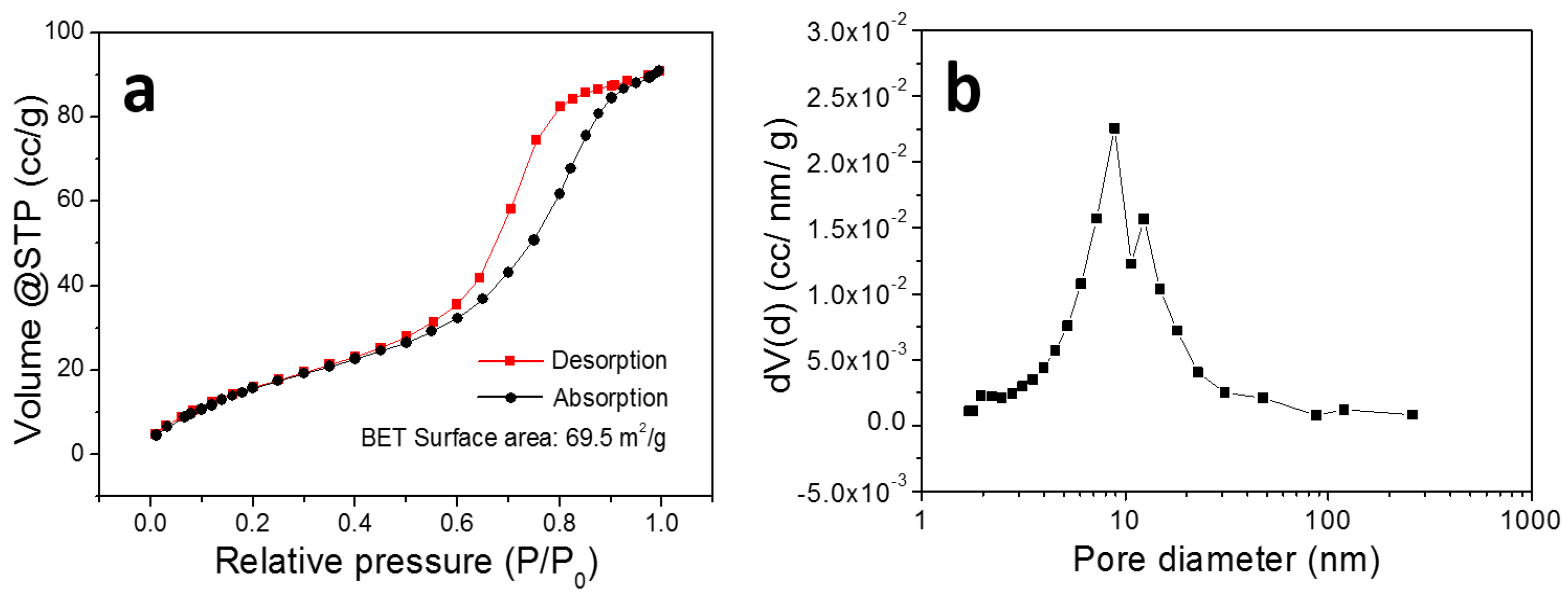
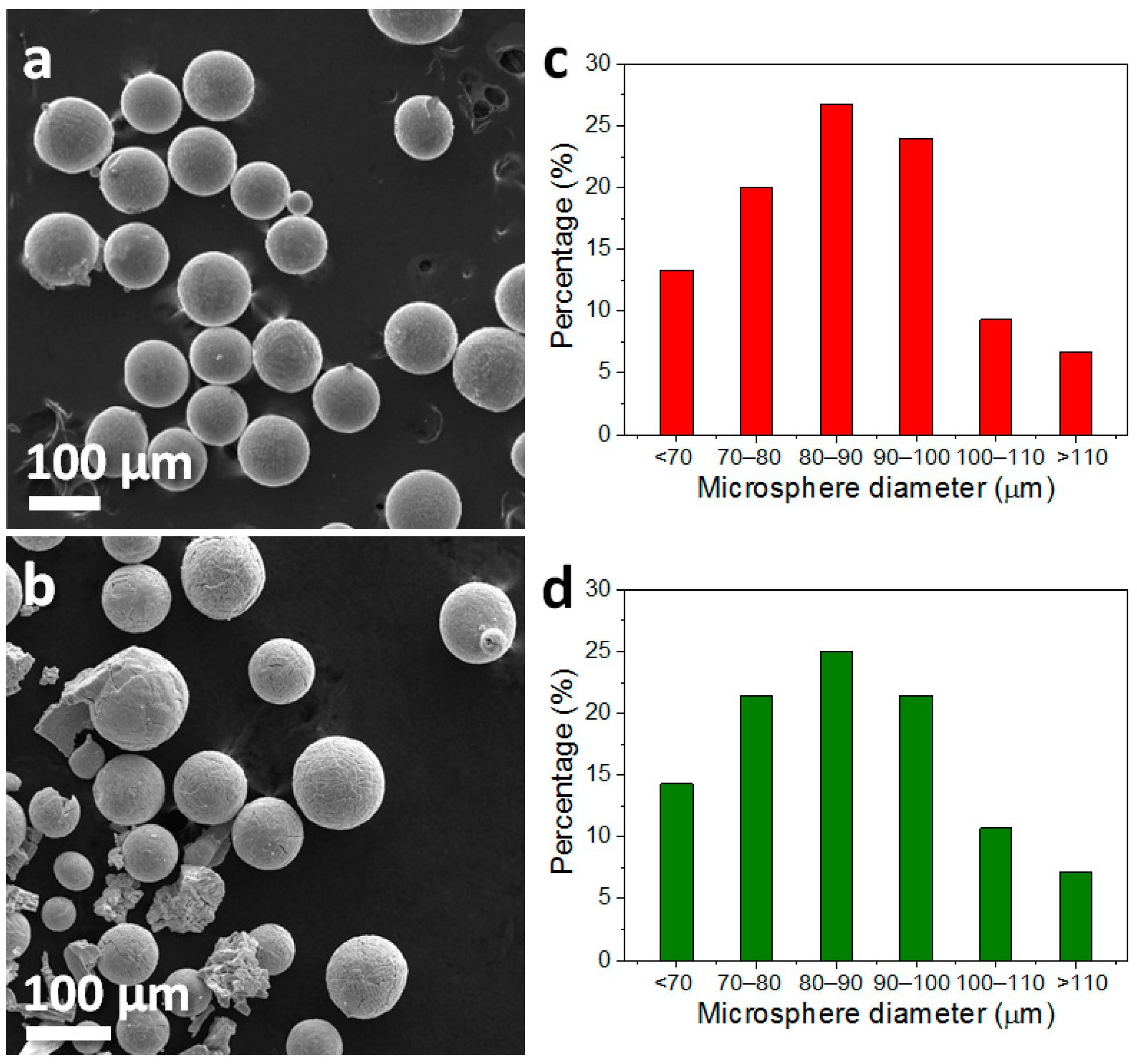
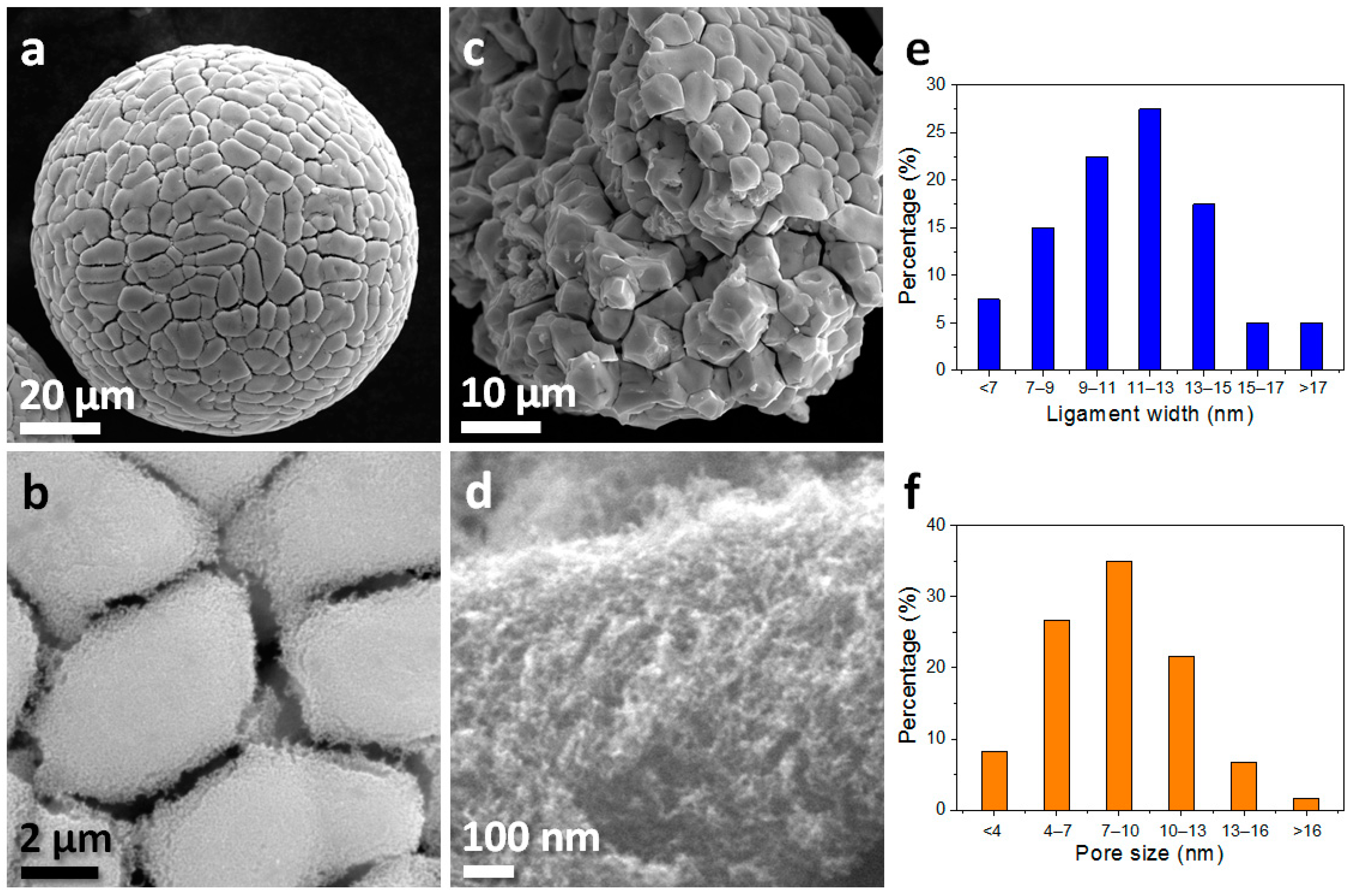
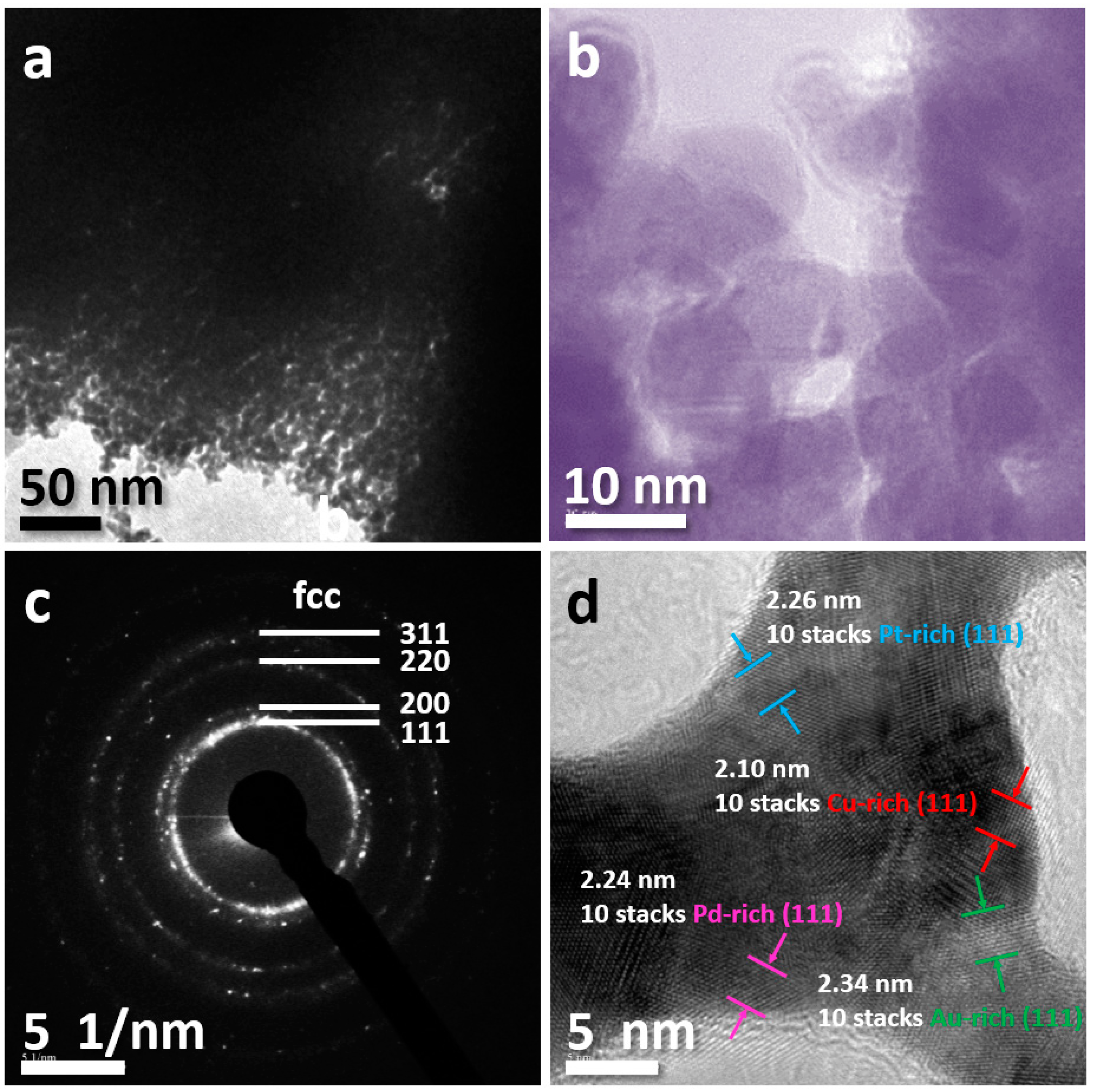
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zou, Y.; Ma, H.; Spolenak, R. Ultrastrong ductile and stable high-entropy alloys at small scales. Nat. Commun. 2015, 6, 7748. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.R.; Liaw, P.K.; Zhang, Y. Science and technology in high-entropy alloys. Sci. China Mater. 2018, 61, 2–22. [Google Scholar] [CrossRef] [Green Version]
- Gludovatz, B.; Hohenwarter, A.; Catoor, D.; Chang, E.H.; George, E.P.; Ritchie, R.O. A fracture-resistant high-entropy alloy for cryogenic applications. Science 2014, 345, 1153–1158. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.M.; Pradeep, K.G.; Deng, Y.; Raabe, D.; Tasan, C.C. Metastable high-entropy dual-phase alloys overcome the strength-ductility trade-off. Nature 2016, 534, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Tracy, C.L.; Park, S.; Rittman, D.R.; Zinkle, S.J.; Bei, H.B.; Lang, M.; Ewing, R.C.; Mao, W.L. High pressure synthesis of a hexagonal close-packed phase of the high-entropy alloy CrMnFeCoNi. Nat. Commun. 2017, 8, 15634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, C.N.; LaRosa, C.R.; Miao, J.S.; Mills, M.J.; Ghazisaeidi, M. Magnetically-driven phase transformation strengthening in high entropy alloys. Nat. Commun. 2018, 9, 1363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jo, Y.H.; Jung, S.; Choi, W.M.; Sohn, S.S.; Kim, H.S.; Lee, B.J.; Kim, N.J.; Lee, S. Cryogenic strength improvement by utilizing room-temperature deformation twinning in a partially recrystallized VCrMnFeCoNi high-entropy alloy. Nat. Commun. 2017, 8, 15719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, Y.G.; Huang, Z.N.; Xie, P.F.; Lacey, S.D.; Jacob, R.J.; Xie, H.; Chen, F.J.; Nie, A.M.; Pu, T.C.; Rehwoldt, M.; et al. Carbothermal shock synthesis of high-entropy-alloy nanoparticles. Science 2018, 359, 1489–1494. [Google Scholar] [CrossRef] [PubMed]
- Dan, Z.H.; Qu, J.H.; Yang, Y.L.; Qin, F.X.; Chang, H. Evolution of nanoporous surface layers on gas-atomized Ti60Cu39Au1 powders during dealloying. Nanomaterials 2018, 8, 581. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.F.; Liu, J.Y.; Qin, C.L.; Yu, H.; Xia, X.C.; Wang, C.Y.; Zhang, Y.S.; Hu, Q.F.; Zhao, W.M. Dealloying of Cu-based metallic glasses in acidic solutions: Products and energy storage applications. Nanomaterials 2015, 5, 697–721. [Google Scholar] [CrossRef]
- Qiu, H.J.; Gao, J.J.; Chiang, F.K.; Wen, Y.R.; Yao, A.Y.; Du, P.; Fang, G.; Wang, J.Q.; Liu, X.J. A general and scalable approach to produce nanoporous alloy nanowires with rugged ligaments for enhanced electrocatalysis. J Mater. Chem. A 2018, 6, 12541–12550. [Google Scholar] [CrossRef]
- Zheng, D.H.; Zhao, F.; Li, Y.Y.; Qin, C.L.; Zhu, J.S.; Hu, Q.F.; Wang, Z.F.; Inoue, A. Flexible NiO micro-rods/nanoporous Ni/metallic glass electrode with sandwich structure for high performance supercapacitors. Electrochim. Acta 2019, 297, 767–777. [Google Scholar] [CrossRef]
- Liu, S.Y.; Pang, F.J.; Zhang, Q.W.; Guo, R.J.; Wang, Z.F.; Wang, Y.C.; Zhang, W.Q.; Ou, J.Z. Stable nanoporous Sn/SnO2 composites for efficient electroreduction of CO2 to formate over wide potential range. Appl. Mater. Today 2018, 13, 135–143. [Google Scholar] [CrossRef]
- Zhao, W.M.; Fei, P.Y.; Zhang, X.M.; Zhang, Y.G.; Qin, C.L.; Wang, Z.F. Porous TiO2/Fe2O3 nanoplate composites prepared by de-alloying method for Li-ion batteries. Mater. Lett. 2018, 211, 254–257. [Google Scholar] [CrossRef]
- Wang, Z.F.; Zhang, X.M.; Yan, Y.H.; Zhang, Y.G.; Wang, Y.C.; Qin, C.L.; Bakenov, Z. Nanoporous GeO2/Cu/Cu2O network synthesized by dealloying method for stable Li-ion storage. Electrochim. Acta 2019, 300, 363–372. [Google Scholar] [CrossRef]
- Qin, C.L.; Zhang, Y.S.; Wang, Z.F.; Xiong, H.Q.; Yu, H.; Zhao, W.M. One-step synthesis of CuO@brass foil by dealloying method for low-cost flexible supercapacitor electrodes. J. Mater. Sci. Mater. Electron. 2016, 27, 9206–9215. [Google Scholar] [CrossRef]
- Zhang, Q.; Kusada, K.; Wu, D.S.; Yamamoto, T.; Toriyama, T.; Matsumura, S.; Kawaguchi, S.; Kubota, Y.; Kitagawa, H. Selective control of fcc and hcp crystal structures in Au–Ru solid-solution alloy nanoparticles. Nat. Commun. 2018, 9, 510. [Google Scholar] [CrossRef] [Green Version]
- Sarker, M.S.I.; Nakamura, T.; Sato, S. All-proportional solid-solution Rh–Pd–Pt alloy nanoparticles by femtosecond laser irradiation of aqueous solution with surfactant. J. Nanopart. Res. 2015, 17, 259. [Google Scholar] [CrossRef]
- Yew, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar]
- Ma, D.C.; Yao, M.J.; Pradeep, K.G.; Tasan, C.C.; Springer, H.; Raabe, D. Phase stability of non-equiatomic CoCrFeMnNi high entropy alloys. Acta. Mater. 2015, 98, 288–296. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta. Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef] [Green Version]
- Kao, Y.F.; Chen, T.J.; Chen, S.K.; Yeh, J.W. Microstructure and mechanical property of as-cast, -homogenized, and -deformed AlxCoCrFeNi (0 ≤ x ≤ 2) high-entropy alloys. J. Alloys Compd. 2009, 488, 57–64. [Google Scholar] [CrossRef]
- Wu, Z.; Bei, H.; Otto, F.; Pharr, G.M.; George, E.P. Recovery, recrystallization, grain growth and phase stability of a family of FCC-structured multi-component equiatomic solid solution alloys. Intermetallics 2014, 46, 131–140. [Google Scholar] [CrossRef]
- Sohn, S.W.; Liu, Y.H.; Liu, J.B.; Gong, P.; Prades-Rodel, S.; Blatter, A.; Scanley, B.E.; Broadbridge, C.C.; Schroers, J. Noble metal high entropy alloys. Scr. Mater. 2017, 126, 29–32. [Google Scholar] [CrossRef] [Green Version]
- Ge, X.B.; Chen, L.Y.; Zhang, L.; Wen, Y.R.; Hirata, A.; Chen, M.W. Nanoporous metal enhanced catalytic activities of amorphous molybdenum sulfide for high-efficiency hydrogen production. Adv. Mater. 2014, 26, 3100–3104. [Google Scholar] [CrossRef]
- Wang, R.Y.; Liu, J.G.; Liu, P.; Bi, X.X.; Yan, X.L.; Wang, W.X.; Ge, X.B.; Chen, M.W.; Ding, Y. Dispersing Pt atoms onto nanoporous gold for high performance direct formic acid fuel cells. Chem. Sci. 2014, 5, 403–409. [Google Scholar] [CrossRef]
- Tressaud, A.; Khairoun, H.; Touhara, H.; Watanabe, N. X-ray photoelectron spectroscopy of palladium fluorides. Z. Anorg. Allg. Chem. 1986, 540, 291–299. [Google Scholar] [CrossRef]
- Du, Y.X.; Ni, K.; Zhai, Q.X.; Yun, Y.P.; Xu, Y.J.; Sheng, H.T.; Zhu, Y.W.; Zhu, M.Z. Facile air oxidative induced dealloying of hierarchical branched PtCu nanodendrites with enhanced activity for hydrogen evolution. Appl. Catal. A General 2018, 557, 72–78. [Google Scholar] [CrossRef]
- Chen, L.Y.; Guo, H.; Fujita, T.; Hirata, A.; Zhang, W.; Inoue, A.; Chen, M.W. Nanoporous PdNi bimetallic catalyst with enhanced electrocatalytic performances for electro-oxidation and oxygen reduction reactions. Adv. Funct. Mater. 2011, 21, 4364–4370. [Google Scholar] [CrossRef]
- Xu, C.X.; Wang, R.Y.; Chen, M.W.; Zhang, Y.; Ding, Y. Dealloying to nanoporous Au/Pt alloys and their structure sensitive electrocatalytic properties. Phys. Chem. Chem. Phys. 2010, 12, 239–246. [Google Scholar] [CrossRef]
- Dong, C.Q.; Kou, T.Y.; Gao, H.; Peng, Z.Q.; Zhang, Z.H. Eutectic-derived mesoporous Ni-Fe-O nanowire network catalyzing oxygen evolution and overall water splitting. Adv. Eng. Mater. 2018, 8, 1701347. [Google Scholar] [CrossRef]
- Liu, X.; Du, J.; Shao, Y.; Zhao, S.F.; Yao, K.F. One-pot preparation of nanoporous Ag-Cu@Ag core-shell alloy with enhanced oxidative stability and robust antibacterial activity. Sci. Rep. 2017, 7, 10249. [Google Scholar] [CrossRef]
- Gong, M.X.; Fu, G.T.; Chen, Y.; Tang, Y.W.; Lu, T.H. Autocatalysis and selective oxidative etching induced synthesis of platinum-copper bimetallic alloy nanodendrites electrocatalysts. ACS Appl. Mater. Inter. 2014, 6, 7301–7308. [Google Scholar] [CrossRef]
- Xing, Y.L.; Wang, S.B.; Fang, B.Z.; Feng, Y.F.; Zhang, S.C. Three-dimensional nanoporous Cu65Sn5/Cu composite from dealloying as anode for lithium ion batteries. Micropor. Mesopor. Mat. 2018, 261, 237–243. [Google Scholar] [CrossRef]
- Hong, J.W.; Kang, S.W.; Choi, B.S.; Kim, D.; Lee, S.B.; Han, S.W. Controlled synthesis of Pd-Pt alloy hollow nanostructures with enhanced catalytic activities for oxygen reduction. ACS Nano 2012, 6, 2410–2419. [Google Scholar] [CrossRef]
- Liu, K.; Bai, Y.C.; Zhang, L.; Yang, Z.B.; Fan, Q.K.; Zheng, H.Q.; Yin, Y.D.; Gao, C.B. Porous Au-Ag nanospheres with high-density and highly accessible hotspots for SERS analysis. Nano Lett. 2016, 16, 3675–3681. [Google Scholar] [CrossRef]
- Zhou, Q.X.; Qi, L.; Yang, H.X.; Xu, C.X. Hierarchical nanoporous platinum-copper alloy nanoflowers as highly active catalysts for the hydrolytic dehydrogenation of ammonia borane. J. Colloid Inerf. Sci. 2018, 513, 258–265. [Google Scholar] [CrossRef]
- Xu, Y.; Yiu, P.M.; Shan, G.C.; Shibayama, T.; Watanabe, S.; Ohnuma, M.; Huang, W.; Shek, C.H. 3D nanoporous gold with very low parting limit derived from Au-based metallic glass and enhanced methanol electro-oxidation catalytic peiformance induced by metal migration. ChemNanoMat 2018, 4, 88–97. [Google Scholar] [CrossRef]
- Shi, S.; Markmann, J.; Weissmuller, J. Actuation by hydrogen electrosorption in hierarchical nanoporous palladium. Philos. Mag. 2017, 97, 1571–1587. [Google Scholar] [CrossRef]
- Hou, Y.; Chen, L.Y.; Liu, P.; Kang, J.L.; Fujita, T.; Chen, M.W. Nanoporous metal based flexible asymmetric pseudocapacitors. J. Mater. Chem. A 2014, 2, 10910–10916. [Google Scholar] [CrossRef]
- Zhang, W.Q.; He, J.; Liu, S.Y.; Niu, W.X.; Liu, P.; Zhao, Y.; Pang, F.J.; Xi, W.; Chen, M.W.; Zhang, W.; et al. Atomic origins of high electrochemical CO2 reduction efficiency on nanoporous gold. Nanoscale 2018, 10, 8372–8376. [Google Scholar] [CrossRef]
- Pang, F.J.; Wang, Z.F.; Zhang, K.; He, J.; Zhang, W.Q.; Guo, C.X.; Ding, Y. Bimodal nanoporous Pd3Cu1 alloy with restrained hydrogen evolution for stable and high yield electrochemical nitrogen reduction. Nano Energy 2019, 58, 834–841. [Google Scholar] [CrossRef]
- Liu, P.; Guan, P.F.; Hirata, A.; Zhang, L.; Chen, L.Y.; Wen, Y.R.; Ding, Y.; Fujita, T.; Erlebacher, J.; Chen, M.W. Visualizing under-coordinated surface atoms on 3D nanoporous gold catalysts. Adv. Mater. 2016, 28, 1753–1759. [Google Scholar] [CrossRef]
- Li, J.; Yin, H.M.; Li, X.B.; Okunishi, E.; Shen, Y.L.; He, J.; Tang, Z.K.; Wang, W.X.; Yücelen, E.; Li, C.; et al. Surface evolution of a Pt–Pd–Au electrocatalyst for stable oxygen reduction. Nat. Energy 2017, 2, 17111. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Li, Y.; Wang, Z.; Zhao, W.; Qin, C. Nanoporous Quasi-High-Entropy Alloy Microspheres. Metals 2019, 9, 345. https://doi.org/10.3390/met9030345
Yang L, Li Y, Wang Z, Zhao W, Qin C. Nanoporous Quasi-High-Entropy Alloy Microspheres. Metals. 2019; 9(3):345. https://doi.org/10.3390/met9030345
Chicago/Turabian StyleYang, Lianzan, Yongyan Li, Zhifeng Wang, Weimin Zhao, and Chunling Qin. 2019. "Nanoporous Quasi-High-Entropy Alloy Microspheres" Metals 9, no. 3: 345. https://doi.org/10.3390/met9030345
APA StyleYang, L., Li, Y., Wang, Z., Zhao, W., & Qin, C. (2019). Nanoporous Quasi-High-Entropy Alloy Microspheres. Metals, 9(3), 345. https://doi.org/10.3390/met9030345






