Enhanced Performance of Bimetallic Co-Pd Catalysts Prepared by Mechanical Alloying
Abstract
1. Introduction
2. Materials and Methods
2.1. Catalyst Preparation
2.2. Carbon Nanofiber Deposition
2.3. Characaterization
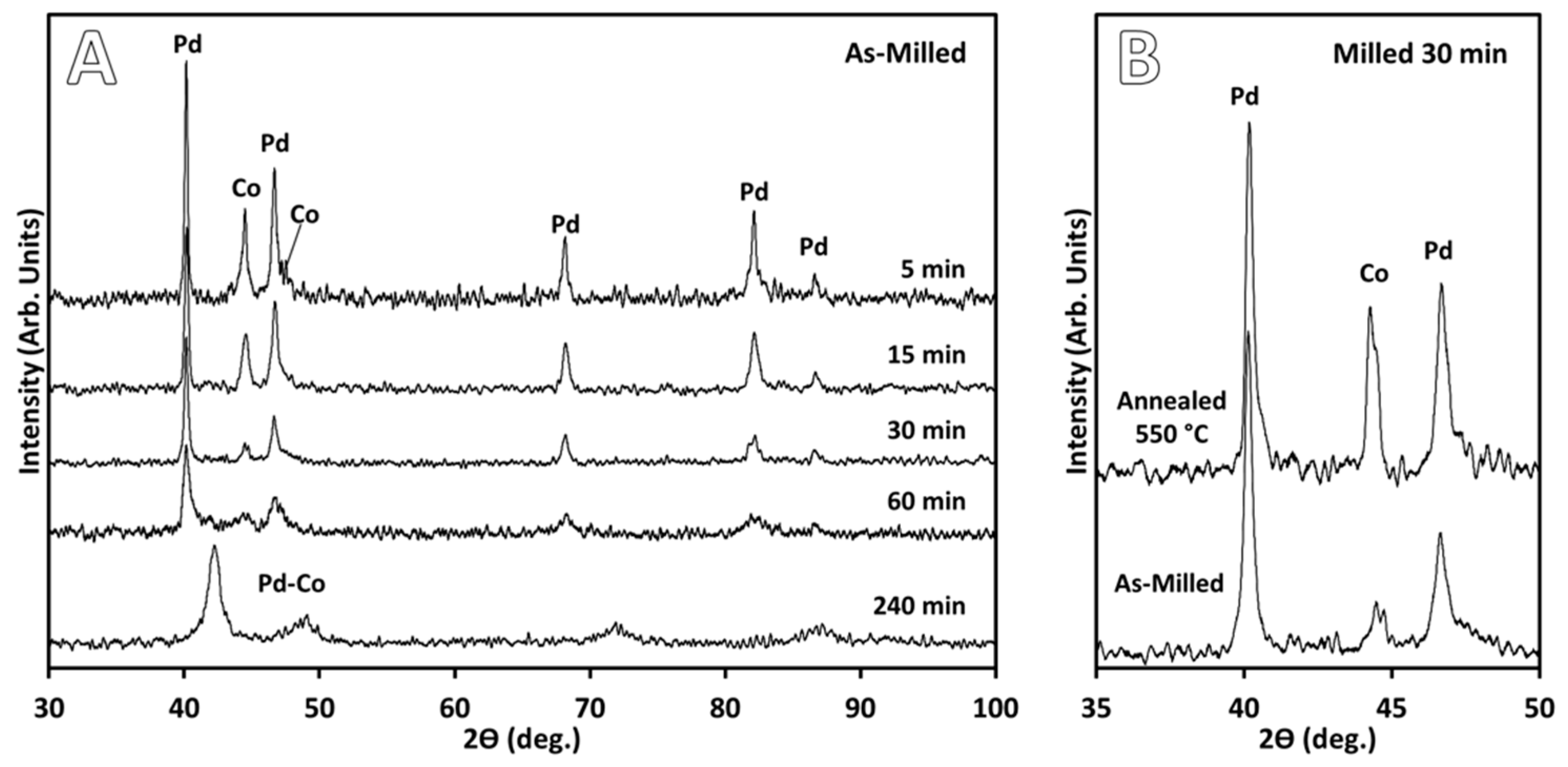
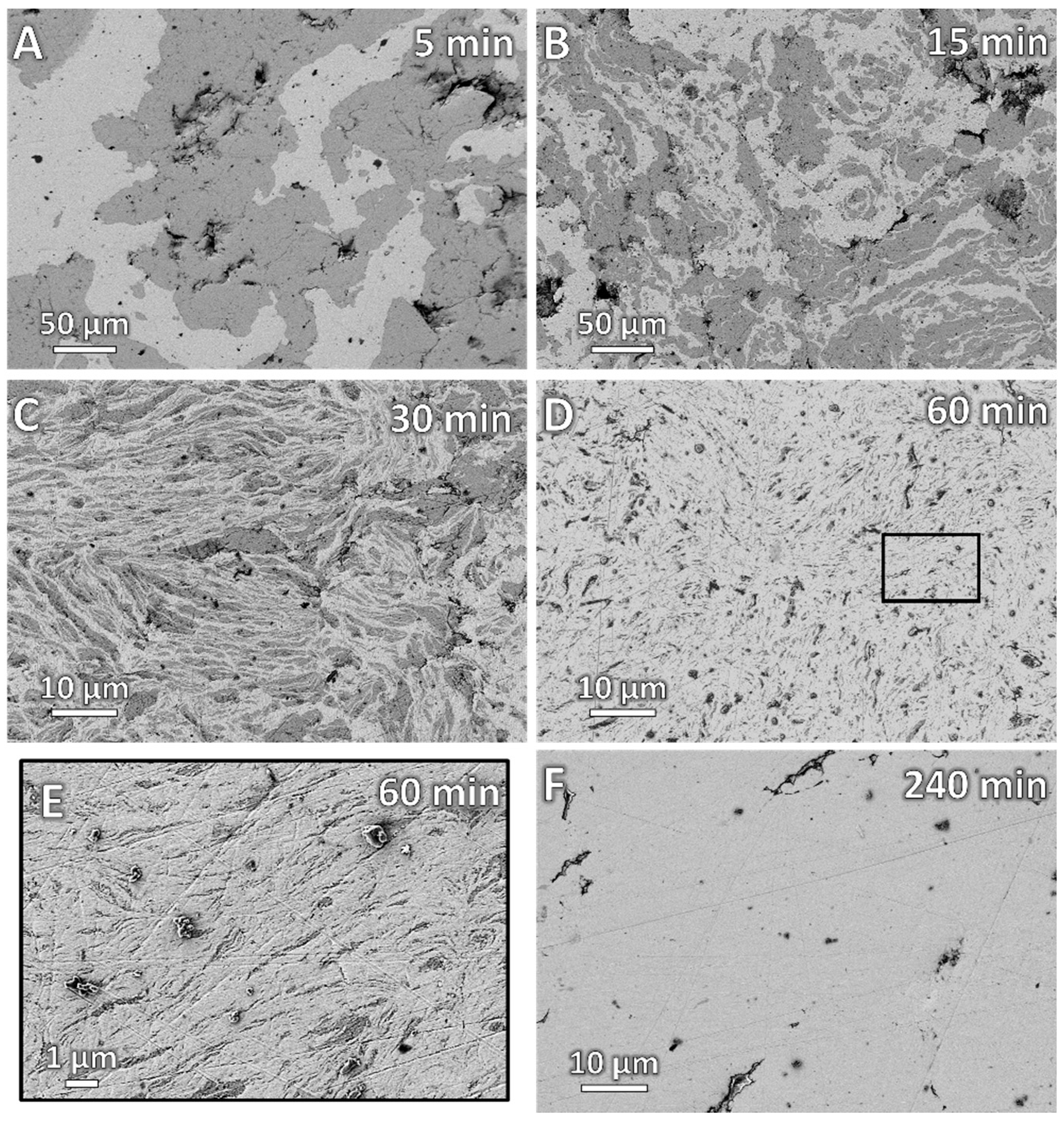
3. Results
3.1. Catalyst Preparation
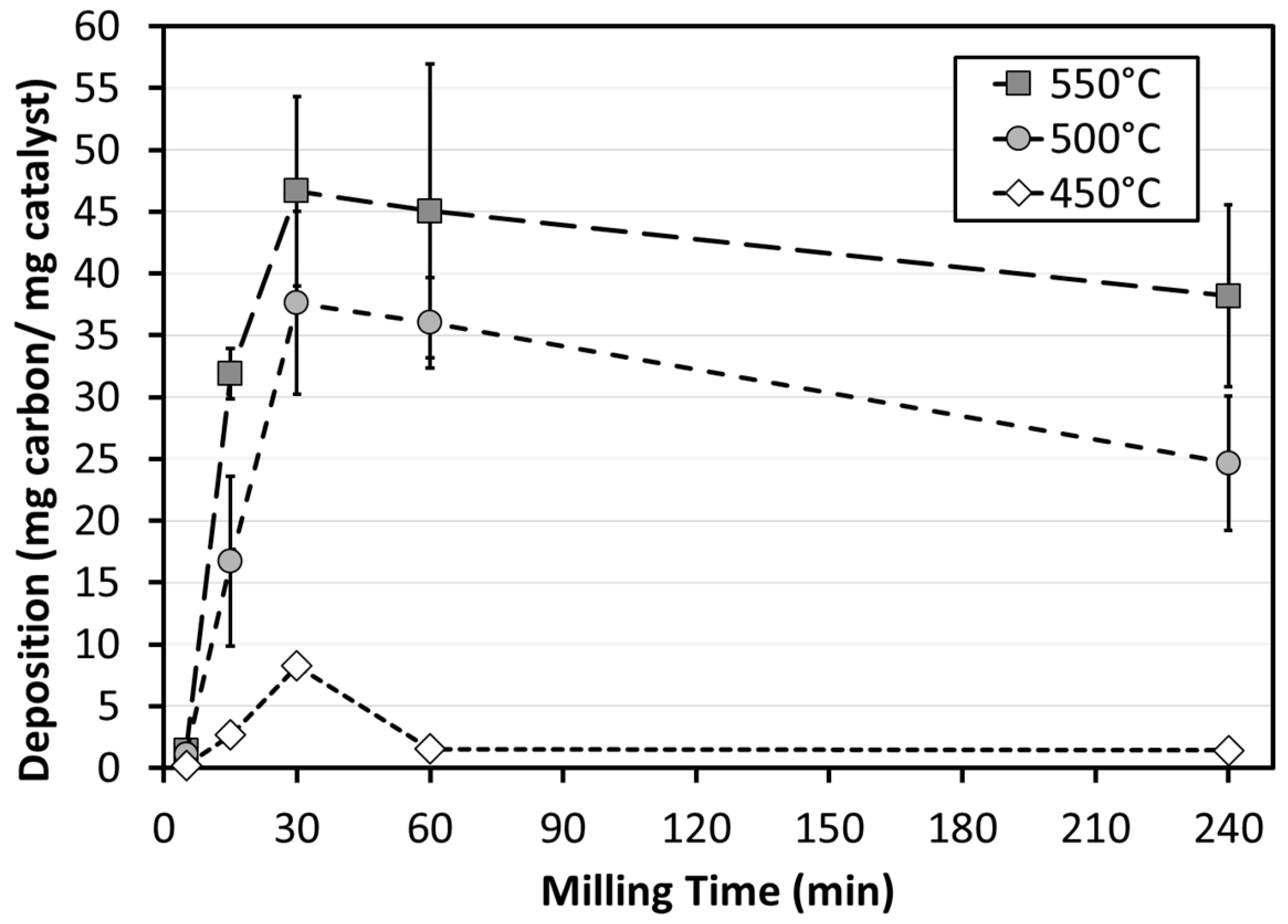
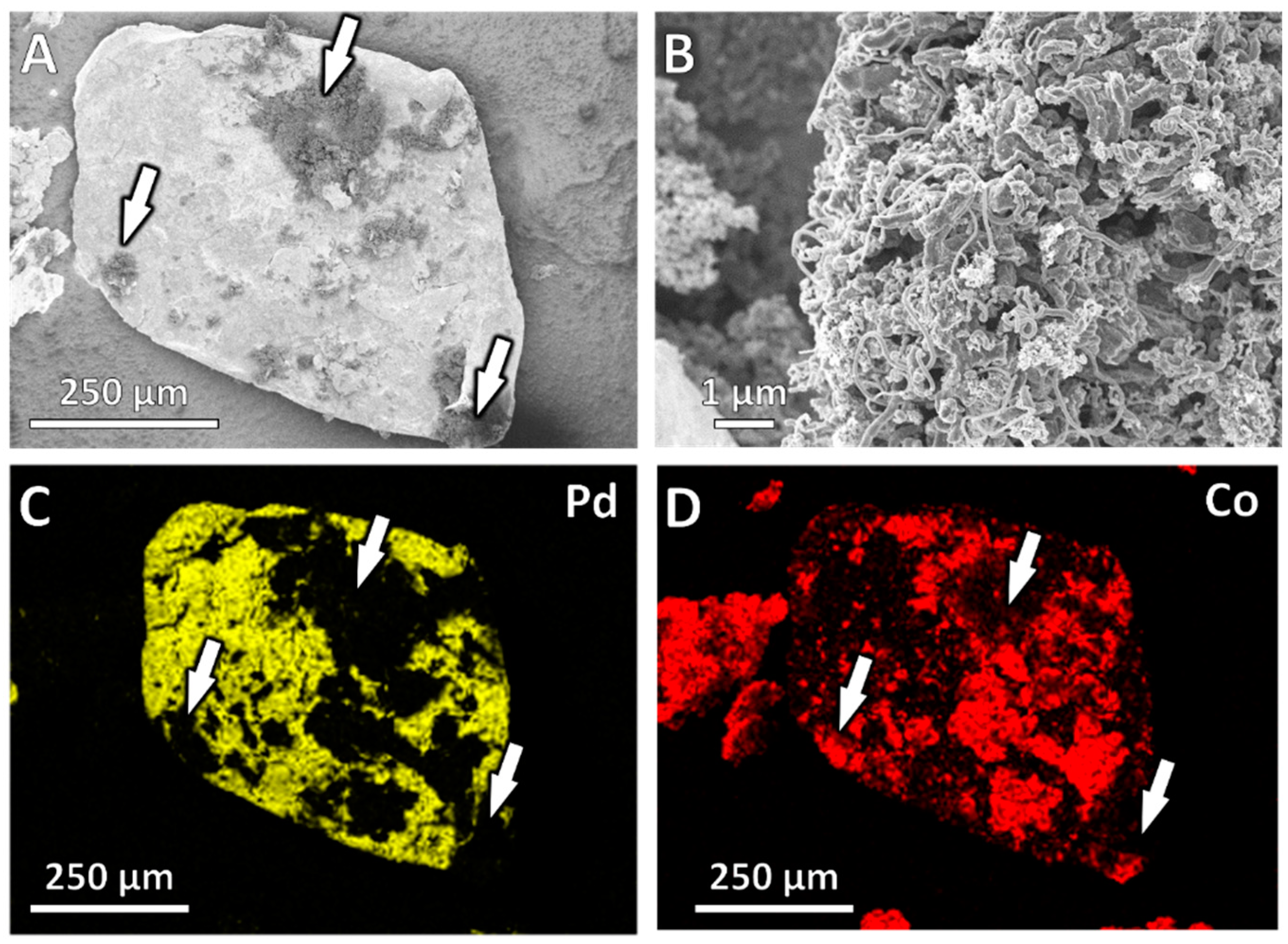
3.2. Catalytic Performance
4. Discussion
4.1. Catalyst Preparation
4.2. Catalytic Performance
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ponec, V. Selectivity in catalysis by alloys. Catal. Rev. Sci. Eng. 1975, 11, 41–70. [Google Scholar] [CrossRef]
- Rizzi, M.; Furlan, S.; Peressi, M.; Baldereschi, A.; Dri, C.; Peronio, A.; Africh, C.; Lacovig, P.; Vesselli, E.; Comelli, G. Tailoring bimetallic alloy surface properties by kinetic control of self-diffusion processes at the nanoscale. J. Am. Chem. Soc. 2012, 134, 16827–16833. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, N.M.; Kim, M.S.; Baker, R.T.K. Deactivation of copper-nickel catalysts due to changes in surface composition. J. Catal. 1993, 140, 16–29. [Google Scholar] [CrossRef]
- Klein, K.L.; Melechko, A.V.; Rack, P.D.; Fowlkes, J.D.; Meyer, H.M.; Simpson, M.L. Cu–Ni composition gradient for the catalytic synthesis of vertically aligned carbon nanofibers. Carbon 2005, 43, 1857–1863. [Google Scholar] [CrossRef]
- Park, C.; Baker, R.T.K. Carbon deposition on iron-nickel during interaction with ethylene-hydrogen mixtures. J. Catal. 1998, 179, 361–374. [Google Scholar] [CrossRef]
- Carneiro, O.C.; Rodriguez, N.M.; Baker, R.T.K. Growth of carbon nanofibers from the iron–copper catalyzed decomposition of Co/C2H4/H2 mixtures. Carbon 2005, 43, 2389–2396. [Google Scholar] [CrossRef]
- Rodriguez, N.M.; Chambers, A.; Baker, R.T.K. Catalytic engineering of carbon nanostructures. Langmuir 1995, 11, 3862–3866. [Google Scholar] [CrossRef]
- Chambers, A.; Rodriguez, N.M.; Baker, R.T.K. Influence of silver addition on the catalytic behavior of cobalt. J. Phys. Chem. 1996, 100, 4229–4236. [Google Scholar] [CrossRef]
- Chambers, A.; Baker, R.T.K. Influence of the nature of the catalyst precursor on the carbon deposition characteristics during ethylene decomposition over copper–cobalt. J. Catal. 1996, 158, 356–360. [Google Scholar] [CrossRef]
- Chambers, A.; Rodriguez, N.M.; Baker, R.T.K. Modification of the catalytic behavior of cobalt by the addition of copper. J. Phys. Chem. 1995, 99, 10581–10589. [Google Scholar] [CrossRef]
- Chambers, A.; Rodriguez, N.M.; Baker, R.T.K. Influence of copper on the structural characteristics of carbon nanofibers produced from the cobalt-catalyzed decomposition of ethylene. J. Mater. Res. 1996, 11, 430–438. [Google Scholar] [CrossRef]
- Kim, M.S.; Rodriguez, N.M.; Baker, R.T.K. The interplay between sulfur adsorption and carbon deposition on cobalt catalysts. J. Catal. 1993, 143, 449–463. [Google Scholar] [CrossRef]
- Atwater, M.A.; Phillips, J.; Leseman, Z.C. Accelerated growth of carbon nanofibers using physical mixtures and alloys of Pd and Co in an ethylene–hydrogen environment. Carbon 2011, 49, 1058–1066. [Google Scholar] [CrossRef]
- Noronha, F.B.; Schmal, M.; Nicot, C.; Moraweck, B.; Frety, R. Characterization of graphite-supported palladium–cobalt catalysts by temperature-programmed reduction and magnetic measurements. J. Catal. 1997, 168, 42–50. [Google Scholar] [CrossRef]
- Koch, C.C. Materials synthesis by mechanical alloying. Annu. Rev. Mater. Sci. 1989, 19, 121–143. [Google Scholar] [CrossRef]
- Bolz, F. Advanced Materials in Catalysis; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Zhou, X.; Wadley, H.; Johnson, R.A.; Larson, D.; Tabat, N.; Cerezo, A.; Petford-Long, A.; Smith, G.; Clifton, P.; Martens, R. Atomic scale structure of sputtered metal multilayers. Acta Mater. 2001, 49, 4005–4015. [Google Scholar] [CrossRef]
- Choi, S.W.-K.; Puddephatt, R.J. Cobalt−palladium and cobalt−platinum bilayer films formed by chemical vapor deposition. Chem. Mater. 1997, 9, 1191–1195. [Google Scholar] [CrossRef]
- Tayyebi, M.; Eghbali, B. Study on the microstructure and mechanical properties of multilayer cu/ni composite processed by accumulative roll bonding. Mater. Sci. Eng. A 2013, 559, 759–764. [Google Scholar] [CrossRef]
- Carpenter, J.; Vogel, S.; LeDonne, J.; Hammon, D.; Beyerlein, I.; Mara, N. Bulk texture evolution of Cu–Nb nanolamellar composites during accumulative roll bonding. Acta Mater. 2012, 60, 1576–1586. [Google Scholar] [CrossRef]
- Cullity, B.D.; Stock, S.R. Elements of X-ray Diffraction, 3rd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2001; p. 170. [Google Scholar]
- Abenojar, J.; Velasco, F.; Mota, J.; Martınez, M. Preparation of Fe/B powders by mechanical alloying. J. Solid State Chem. 2004, 177, 382–388. [Google Scholar] [CrossRef]
- Koch, C. Amorphization reactions during mechanical alloying/milling of metallic powders. React. Solids 1990, 8, 283–297. [Google Scholar] [CrossRef]
- Ishida, K.; Nishizawa, T. The Co-Pd (cobalt-palladium) system. J. Phase Equilib. 1991, 12, 83–87. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Nouri, A.; Wen, C. Surfactants in mechanical alloying/milling: A catch-22 situation. Cr. Rev. Sol. State 2014, 39, 81–108. [Google Scholar] [CrossRef]
- Koch, C. Synthesis of nanostructured materials by mechanical milling: Problems and opportunities. Nanostruct. Mater. 1997, 9, 13–22. [Google Scholar] [CrossRef]
- Atwater, M.A.; Roy, D.; Darling, K.A.; Butler, B.G.; Scattergood, R.O.; Koch, C.C. The thermal stability of nanocrystalline copper cryogenically milled with tungsten. Mater. Sci. Eng. A 2012, 558, 226–233. [Google Scholar] [CrossRef]
- Darling, K.A.; Roberts, A.J.; Mishin, Y.; Mathaudhu, S.N.; Kecskes, L.J. Grain size stabilization of nanocrystalline copper at high temperatures by alloying with tantalum. J. Alloys Compd. 2013, 573, 142–150. [Google Scholar] [CrossRef]
- Xu, J.; He, J.; Ma, E. Effect of milling temperature on mechanical alloying in the immiscible Cu-Ta system. Metall. Mater. Trans. A 1997, 28, 1569–1580. [Google Scholar] [CrossRef]
- Mula, S.; Bahmanpour, H.; Mala, S.; Kang, P.C.; Atwater, M.; Jian, W.; Scattergood, R.O.; Koch, C.C. Thermodynamic feasibility of solid solubility extension of Nb in Cu and their thermal stability. Mater. Sci. Eng. A 2012, 539, 330–336. [Google Scholar] [CrossRef]
- Xi, S.; Zuo, K.; Li, X.; Ran, G.; Zhou, J. Study on the solid solubility extension of mo in cu by mechanical alloying Cu with amorphous Cr (Mo). Acta Mater. 2008, 56, 6050–6060. [Google Scholar] [CrossRef]
- Benjamin, J.S. Mechanical alloying. Sci. Am. 1976, 234, 40–49. [Google Scholar] [CrossRef]
- Guevara, L.; Welsh, R.; Atwater, M. Parametric effects of mechanical alloying on carbon nanofiber catalyst production in the Ni-Cu system. Metals 2018, 8, 286. [Google Scholar] [CrossRef]
- Gale, W.F.; Totemeier, T.C. Smithells Metals Reference Book; Elsevier: Amsterdam, The Netherlands, 2003. [Google Scholar]
- Noronha, F.B.; Schmal, M.; Frety, R.; Bergeret, G.; Moraweck, B. Evidence of alloy formation during the activation of graphite-supported palladium-cobalt catalysts. J. Catal. 1999, 186, 20–30. [Google Scholar] [CrossRef]
- Bowker, M.; Morgan, C.; Perkins, N.; Holroyd, R.; Fourre, E.; Grillo, F.; MacDowall, A. Ethene adsorption, dehydrogenation and reaction with Pd (110): Pd as a carbon ‘sponge’. J. Phys. Chem. B 2005, 109, 2377–2386. [Google Scholar] [CrossRef] [PubMed]
- Moulijn, J.A.; Van Diepen, A.; Kapteijn, F. Catalyst deactivation: Is it predictable?: What to do? Appl. Catal. A-Gen. 2001, 212, 3–16. [Google Scholar] [CrossRef]
- Kim, M.S.; Rodriguez, N.M.; Baker, R.T.K. The interaction of hydrocarbons with copper-nickel and nickel in the formation of carbon filaments. J. Catal. 1991, 131, 60–73. [Google Scholar] [CrossRef]
- Rodriguez, N.M. A review of catlytically grown carbon nanofibers. J. Mater. Res. 1993, 8, 3233–3250. [Google Scholar] [CrossRef]
- Miniach, E.; Śliwak, A.; Moyseowicz, A.; Gryglewicz, G. Growth of carbon nanofibers from methane on a hydroxyapatite-supported nickel catalyst. J. Mater. Sci. 2016, 51, 5367–5376. [Google Scholar] [CrossRef]
- Atwater, M.A.; Phillips, J.; Leseman, Z.C. Formation of carbon nanofibers and thin films catalyzed by palladium in ethylene-hydrogen mixtures. J. Phys. Chem. C 2010, 114, 5804–5810. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knauss, S.J.; Guevara, L.N.; Atwater, M.A. Enhanced Performance of Bimetallic Co-Pd Catalysts Prepared by Mechanical Alloying. Metals 2019, 9, 335. https://doi.org/10.3390/met9030335
Knauss SJ, Guevara LN, Atwater MA. Enhanced Performance of Bimetallic Co-Pd Catalysts Prepared by Mechanical Alloying. Metals. 2019; 9(3):335. https://doi.org/10.3390/met9030335
Chicago/Turabian StyleKnauss, Steven J., Laura N. Guevara, and Mark A. Atwater. 2019. "Enhanced Performance of Bimetallic Co-Pd Catalysts Prepared by Mechanical Alloying" Metals 9, no. 3: 335. https://doi.org/10.3390/met9030335
APA StyleKnauss, S. J., Guevara, L. N., & Atwater, M. A. (2019). Enhanced Performance of Bimetallic Co-Pd Catalysts Prepared by Mechanical Alloying. Metals, 9(3), 335. https://doi.org/10.3390/met9030335



