Properties of NiTi Shape Memory Alloy Micro-Foils Obtained by Pulsed-Current Sintering of Ni/Ti Foils
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
- A single-step pulsed-current synthesis procedure was formulated and successfully carried out to obtain a 16 μm thick NiTi homogenous micro-foil.
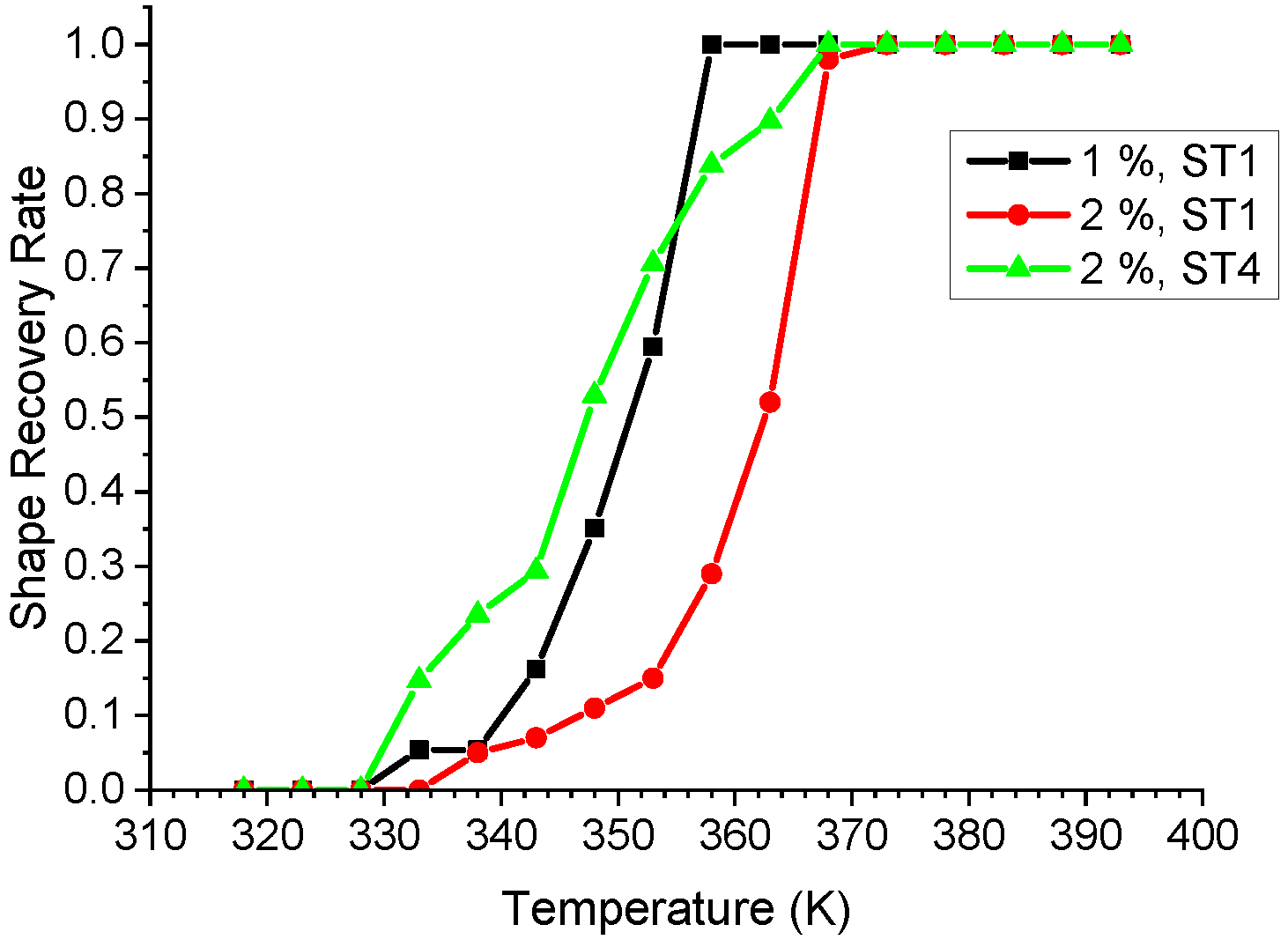
- For the material obtained from Ni/Ti/Ni configuration, a full (100%) shape recovery was obtained in the temperature ranges between room temperature and 388 K, with recovery completing at lower temperatures for a smaller initial strain.
- The Ni/Ti/Ni configuration was found to be superior to those of Ni/Ti and Ti/Ni/Ti, as it allowed for faster diffusion and better homogenization. This occurred possibly due to the Joule heating effect, which was much larger for the Ti layer than for the Ni layer, and more effective in this configuration, leading to homogeneous growth of the NiTi phase.
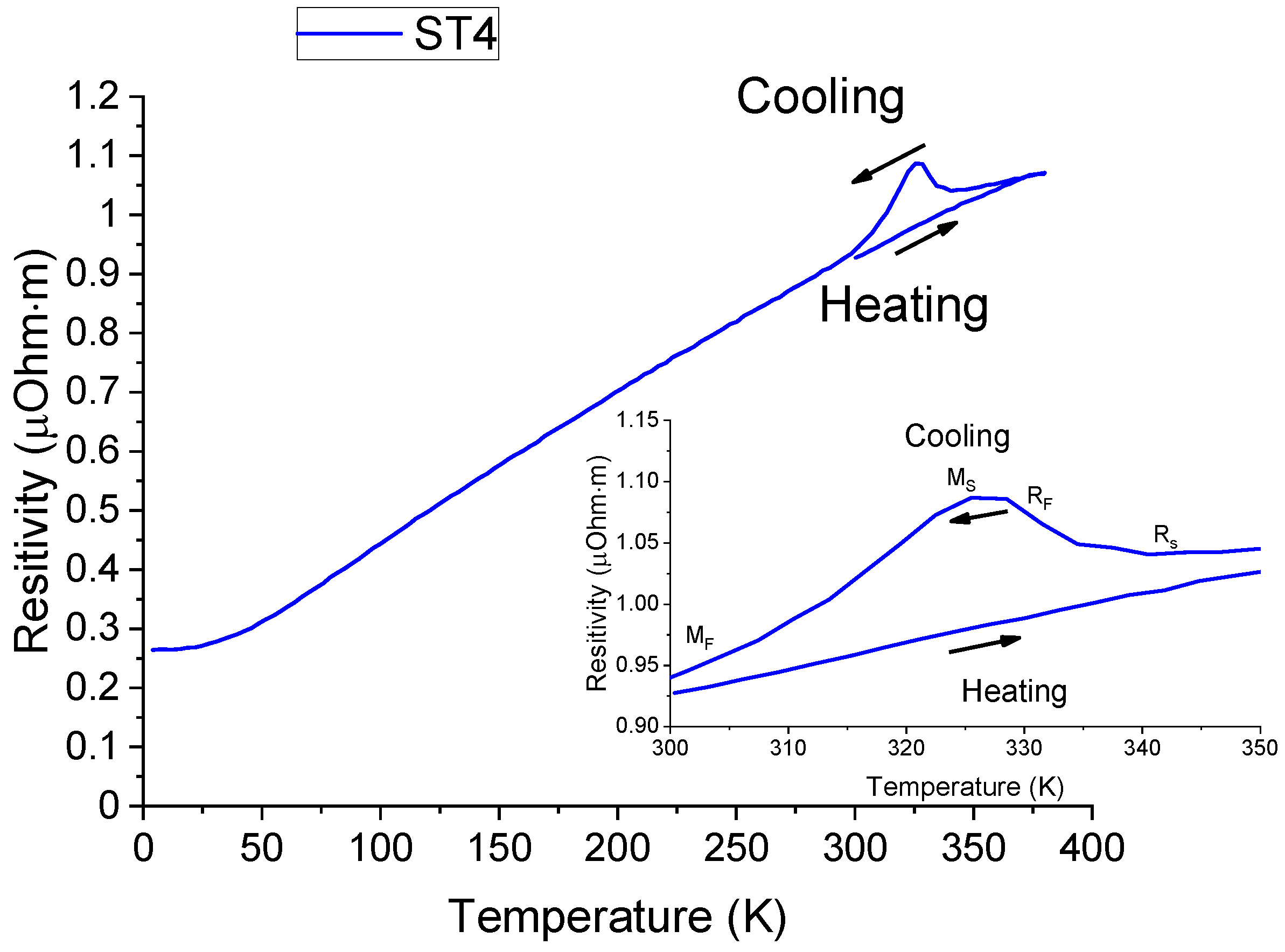
- R-phase participation in the shape change transformation upon the reverse martensitic transition has been confirmed in the temperature dependence of the electrical resistivity and attributed to the presence of rows of Ti-rich particles forming along the direction towards the foil surfaces of the Ni/Ti/Ni configuration.
Author Contributions
Funding
Conflicts of Interest
References
- Petrini, L.; Migliavacca, F. Biomedical Applications of Shape Memory Alloys. J. Metall. 2011, 2011, 501483. [Google Scholar] [CrossRef]
- Kahn, A.; Huff, M.A.; Heuer, A.H. The TiNi shape-memory alloy and its applications for MEMS. J. Micromech. Microeng. 1998, 8, 213–221. [Google Scholar] [CrossRef]
- Predki, W.; Knopik, A.; Bauer, B. Engineering applications of NiTi shape memory alloys. Mater. Sci. Eng. A 2008, 481–482, 598–601. [Google Scholar] [CrossRef]
- Sharifi, E.M.; Kermanpur, A. Superelastic properties of nanocrystalline NiTi shape memory alloy produced by thermomechanical processing. Trans. Nonferrous Met. Soc. China 2018, 28, 515–523. [Google Scholar] [CrossRef]
- Ruiz-Larrea, I.; Breczewski, T.; López, G.A.; Nó, M.L.; López-Echarri, A.; Lopez-Ferreño, I.; San Juan, J.M. The Influence of Thermal History on the Multistage Transformation of NiTi Shape-Memory Alloys. Metals 2018, 8, 246. [Google Scholar] [CrossRef]
- Carl, M.; Smith, J.D.; Doren, B.V.; Young, M.L. Effect of Ni-Content on the Transformation Temperatures in NiTi-20 at. % Zr High Temperature Shape Memory Alloys. Metals 2017, 7, 511. [Google Scholar] [CrossRef]
- Frenzel, J.; George, E.P.; Dlouhy, A.; Somsen, C.; Wagner, M.F.X.; Eggeler, G. Influence of Ni on martensitic phase transformations in NiTi shape memory alloys. Acta Mater. 2010, 5, 3444–3458. [Google Scholar] [CrossRef]
- Loger, K.; Engel, A.; Haupt, J.; Li, Q.; Lima de Miranda, R.; Quandt, E.; Lutter, G.; Selhuber-Unkel, C. Cell adhesion on NiTi thin film sputter-deposited meshes. Mater. Sci. Eng. C 2016, 5, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Ciabattari, F.; Fuso, F.; Arimondo, E. Pulsed laser deposition of NiTi shape memory effect thin films. Appl. Phys. A 1997, 64, 623–627. [Google Scholar] [CrossRef]
- Cha, J.O.; Nam, T.H.; Alghusun, M.; Ahn, J.S. Composition and crystalline properties of TiNi thin films prepared by pulsed laser deposition under vacuum and in ambient Ar gas. Nanoscale Res. Lett. 2012, 7, 37. [Google Scholar] [CrossRef]
- Hou, H.; Hamilton, R.F.; Horn, M.W.; Jin, Y. NiTi thin films prepared by biased target ion beam deposition co-sputtering from elemental Ni and Ti targets. Thin Solid Film 2014, 5, 1–6. [Google Scholar] [CrossRef]
- Fu, Y.; Huang, W.; Du, H.; Huang, X.; Tan, J.; Gao, X. Characterization of TiNi shape-memory alloy thin films for MEMS applications. Surf. Coat. Technol. 2001, 1, 107–112. [Google Scholar] [CrossRef]
- Ho, K.K.; Carman, G.P. Sputter deposition of NiTi thin film shape memory alloy using a heated target. Thin Solid Film 2000, 3, 18–29. [Google Scholar] [CrossRef]
- Antonucci, V.; Faiella, G.; Giordano, M.; Mennella, F.; Nicolais, L. Electrical resistivity study and characterization during NiTi phase transformations. Thermochim. Acta 2007, 4, 64–69. [Google Scholar] [CrossRef]
- Mohri, M.; Nili-Ahmadabadi, M.; Chakravadhanula, V.S.K. Crystallization study of amorphous sputtered NiTi bi-layer thin film. Mater. Charact. 2015, 1, 75–80. [Google Scholar] [CrossRef]
- Tillmann, W.; Momeni, S. Comparison of NiTi thin films sputtered from separate elemental targets and Ti-rich alloy targets. J. Mater. Process. Technol. 2015, 2, 184–190. [Google Scholar] [CrossRef]
- Isalgue, A.; Torra, V.; Seguin, J.-L.; Bendahan, M.; Amigo, J.M.; Esteve-Cano, V. Shape memory NiTi thin films deposited at low temperature. Mater. Sci. Eng. A 1999, 2, 717–721. [Google Scholar] [CrossRef]
- Ramirez, A.G.; Ni, H.; Lee, H.J. Crystallization of amorphous sputtered NiTi thin films. Mater. Sci. Eng. A 2006, 4, 703–709. [Google Scholar] [CrossRef]
- Firstov, G.S.; Vitchev, R.G.; Kumar, H.; Blanpain, B.; van Humbeeck, J. Surface oxidation of NiTi shape memory alloy. Biomaterials 2002, 2, 4863–4871. [Google Scholar] [CrossRef]
- Ou, S.-F.; Peng, B.-Y.; Chen, Y.-C.; Tsai, M.-H. Manufacturing and Characterization of NiTi Alloy with Functional Properties by Selective Laser Melting. Metals 2018, 8, 342. [Google Scholar] [CrossRef]
- Tomus, D.; Tsuchiya, K.; Inuzuka, M.; Sasaki, M.; Imai, D.; Ohmori, T.; Umemoto, M. Fabrication of shape memory TiNi foils via Ti/Ni ultrafine laminates. Scr. Mater. 2003, 4, 489–494. [Google Scholar] [CrossRef]
- Simões, S.; Ramos, A.S.; Viana, F.; Vieira, M.T.; Vieira, M.F. Joining of TiAl to Steel by Diffusion Bonding with Ni/Ti Reactive Multilayers. Metals 2016, 6, 96. [Google Scholar] [CrossRef]
- Shao, X.; Guo, X.; Han, Y.; Lu, W.; Qin, J.; Zhang, D. Characterization of the diffusion bonding behavior of pure Ti and Ni with different surface roughness during hot pressing. Mater. Des. 2015, 6, 1001–1010. [Google Scholar] [CrossRef]
- Chen, G.; Liss, K.-D.; Cao, P. An in situ Study of NiTi Powder Sintering Using Neutron Diffraction. Metals 2015, 5, 530–546. [Google Scholar] [CrossRef]
- Rizvi, S.A.; Khan, T.I. A Novel Fabrication Method for Nitinol Shape Memory Alloys. Key Eng. Mater. 2010, 4, 309–315. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Q.; Sun, D.L.; Han, X.L. Co-effect of heat and direct current on growth of intermetallic layers at the interface of Ti-Ni diffusion couples. J. Alloys Compd. 2011, 5, 1201–1205. [Google Scholar] [CrossRef]
- Hu, L.; Xue, Y.; Shi, F. Intermetallic formation and mechanical properties of Ni-Ti diffusion couples. Mater. Des. 2017, 1, 175–182. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, X.; Cai, H. Fabrication, characterization and tensile property of a novel Ti2Ni/TiNi micro-laminated composite. Mater. Des. 2016, 9, 486–493. [Google Scholar] [CrossRef]
- Garay, J.E.; Anselmi-Tamburini, U.; Munir, Z.A. Enhanced growth of intermetallic phases in the Ni-Ti system by current effects. Acta Mater. 2003, 5, 4487–4495. [Google Scholar] [CrossRef]
- Zhu, R.; Jiang, Y.; Guan, L.; Li, H.; Tang, G. Difference in recrystallization between electropulsing-treated and furnace-treated NiTi alloy. J. Alloys Compd. 2016, 6, 548–554. [Google Scholar] [CrossRef]
- Shao, X.; Guo, X.; Han, Y.; Lin, Z.; Qin, J.; Lu, W.; Zhang, D. Preparation of TiNi films by diffusion technology and the study of the formation sequence of the intermetallics in Ti-Ni systems. J. Mater. Res. 2014, 760, 2707–2716. [Google Scholar] [CrossRef]
- Halani, P.R.; Kaya, I.; Shin, Y.C.; Karaca, H.E. Phase transformation characteristics and mechanical characterization of nitinol synthesized by laser direct deposition. Mater. Sci. Eng. A 2013, 5, 836–843. [Google Scholar] [CrossRef]
- Otubo, J.; Rigo, O.D.; Coelho, A.A.; Neto, C.M.; Mei, P.R. The influence of carbon and oxygen content on the martensitic transformation temperatures and enthalpies of NiTi shape memory alloy. Mater. Sci. Eng. A 2008, 4, 639–642. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, Y.; Zhao, Y.; Liu, S.; Hu, L.; Zhao, C. Influence of Ni4Ti3 precipitates on phase transformation of NiTi shape memory alloy. Trans. Nonferrous Met. Soc. China 2015, 2, 4063–4071. [Google Scholar] [CrossRef]
- Liu, Y.; Blanc, M.; Tan, G.; Kim, J.I.; Miyazaki, S. Effect of ageing on the transformation behaviour of Ti-49.5 at.% Ni. Mater. Sci. Eng. A 2006, 4, 617–621. [Google Scholar] [CrossRef]
- Gyobu, A.; Kawamura, Y.; Horikawa, H.; Saburi, T. Martensitic Transformations in Sputter-Deposited Shape Memory Ti-Ni Films. Mater. Trans. 1996, 3, 697–702. [Google Scholar] [CrossRef]
- Topolski, K.; Wieciński, P.; Szulc, Z.; Gałka, A.; Garbacz, H. Progress in the characterization of explosively joined Ti/Ni bimetals. Mater. Des. 2014, 6, 479–487. [Google Scholar] [CrossRef]
- Bartel, T.; Hackl, K. Multiscale modeling of martensitic phase transformations: On the numerical determination of heterogeneous mesostructures within shape-memory alloys induced by precipitates. Tech. Mech. 2010, 30, 324–342. [Google Scholar]
- Hornbuckle, B.C.; Yu, X.X.; Noebe, R.D.; Martens, R.; Weaver, M.L.; Thompson, G.B. Hardening behavior and phase decomposition in very Ni-rich Nitinol alloys. Mater. Sci. Eng. A 2015, 6, 336–344. [Google Scholar] [CrossRef]
- Maso’od, M.; Ismail, M.H.; Razali, R.; Subuki, I. Effect of sintering temperatures on microstructural changes and phase transformation of nearly equiatomic microporous NiTi alloy produced by metal injection moulding. J. Mech. Eng. 2018, 5, 115–126. [Google Scholar]
- Ke, C.B.; Cao, S.S.; Ma, X.; Zhang, X.P. Modeling of Ni4Ti3 precipitation during stress-free and stress-assisted aging of bi-crystalline NiTi shape memory alloys. Trans. Nonferrous Met. Soc. China 2012, 2, 2578–2585. [Google Scholar] [CrossRef]
- Mokgalaka, M.N.; Pityana, S.L.; Popoola, P.A.I.; Mathebula, T. NiTi intermetallic surface coatings by laser metal deposition for improving wear properties of Ti-6Al-4V substrates. Adv. Mater. Sci. Eng. 2014, 2014, 363917. [Google Scholar] [CrossRef]
- Airoldi, G.; Ranucci, T.; Riva, G. Mechanical and Electrical Properties of A NiTi Shape Memory Alloy. J. Phys. IV 1991, 1, 439–444. [Google Scholar] [CrossRef]
- Kovalev, A.I.; Logunov, A.V.; Petrushin, N.V.; Egorova, L.S. Thermal conductivity and electrical resistivity of NiTi in the temperature interval 90–450 K. J. Eng. Phys. 1977, 3, 183–185. [Google Scholar] [CrossRef]
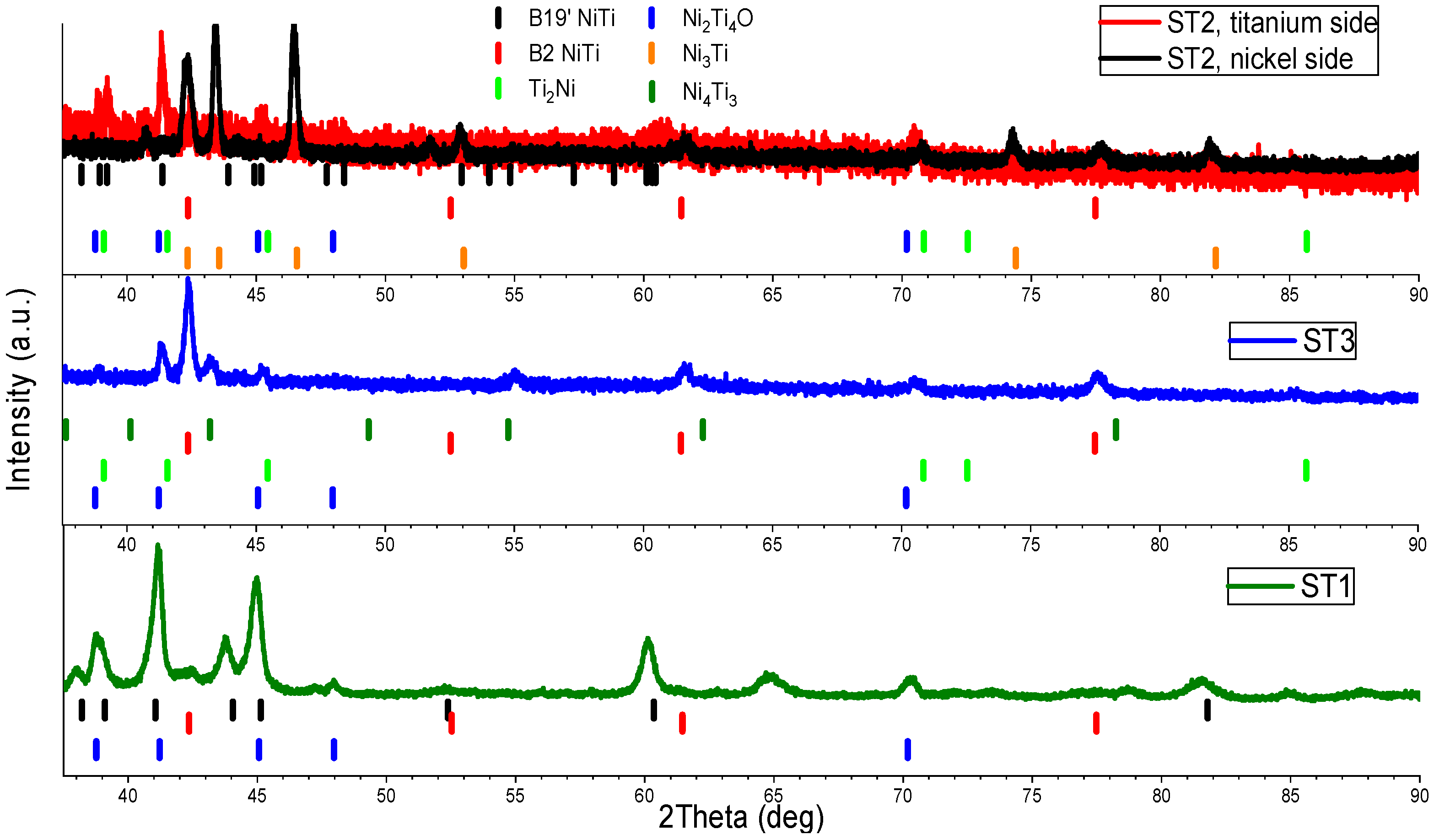
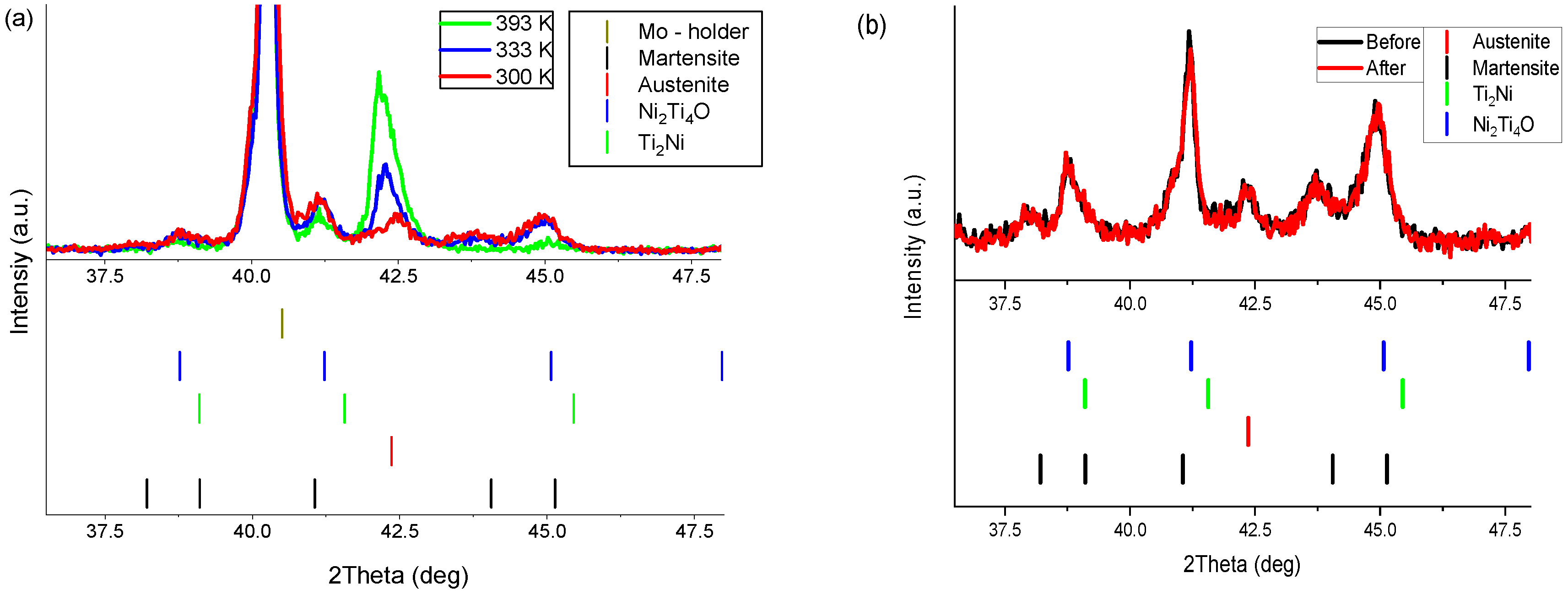
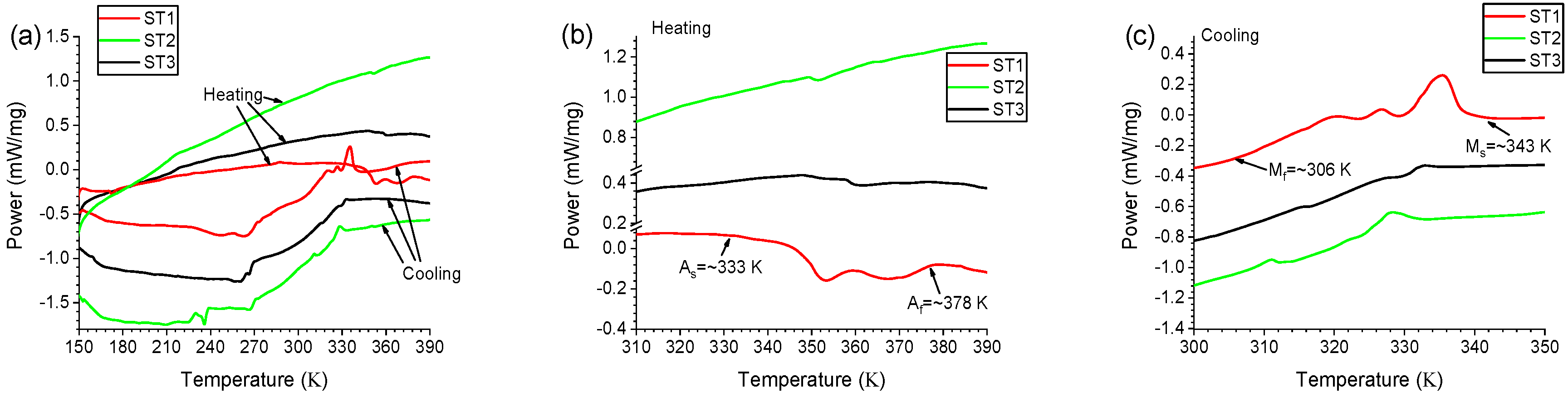
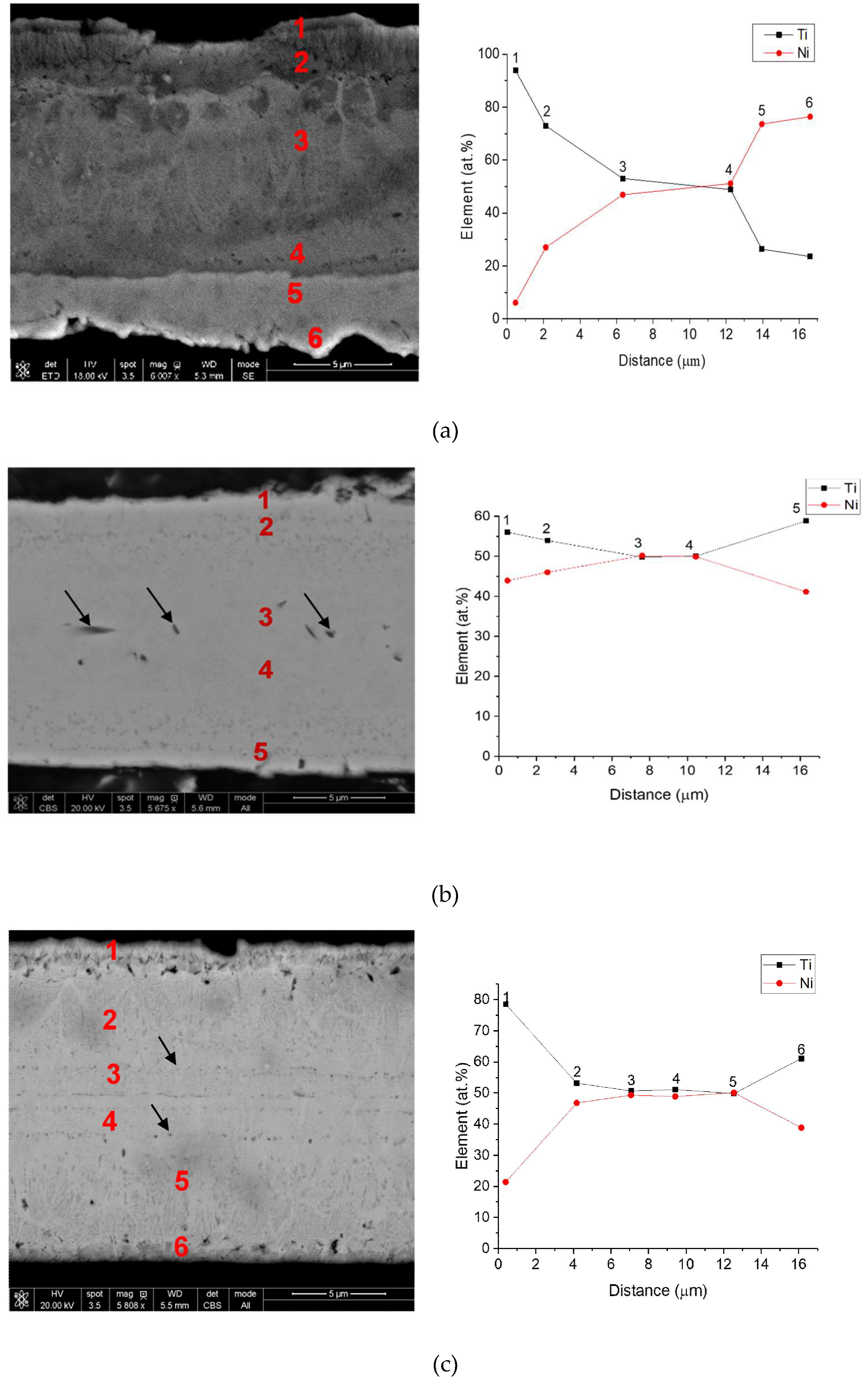

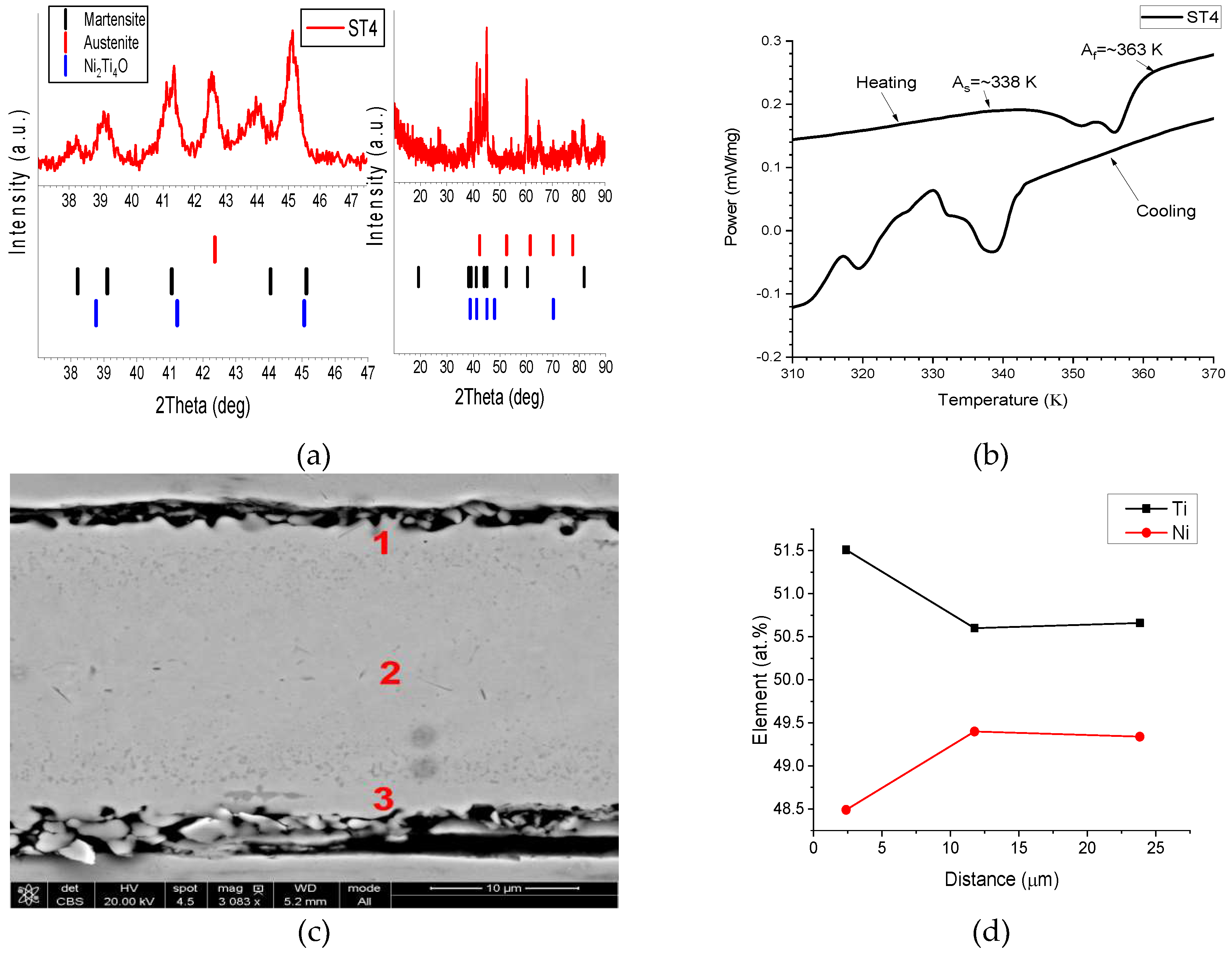
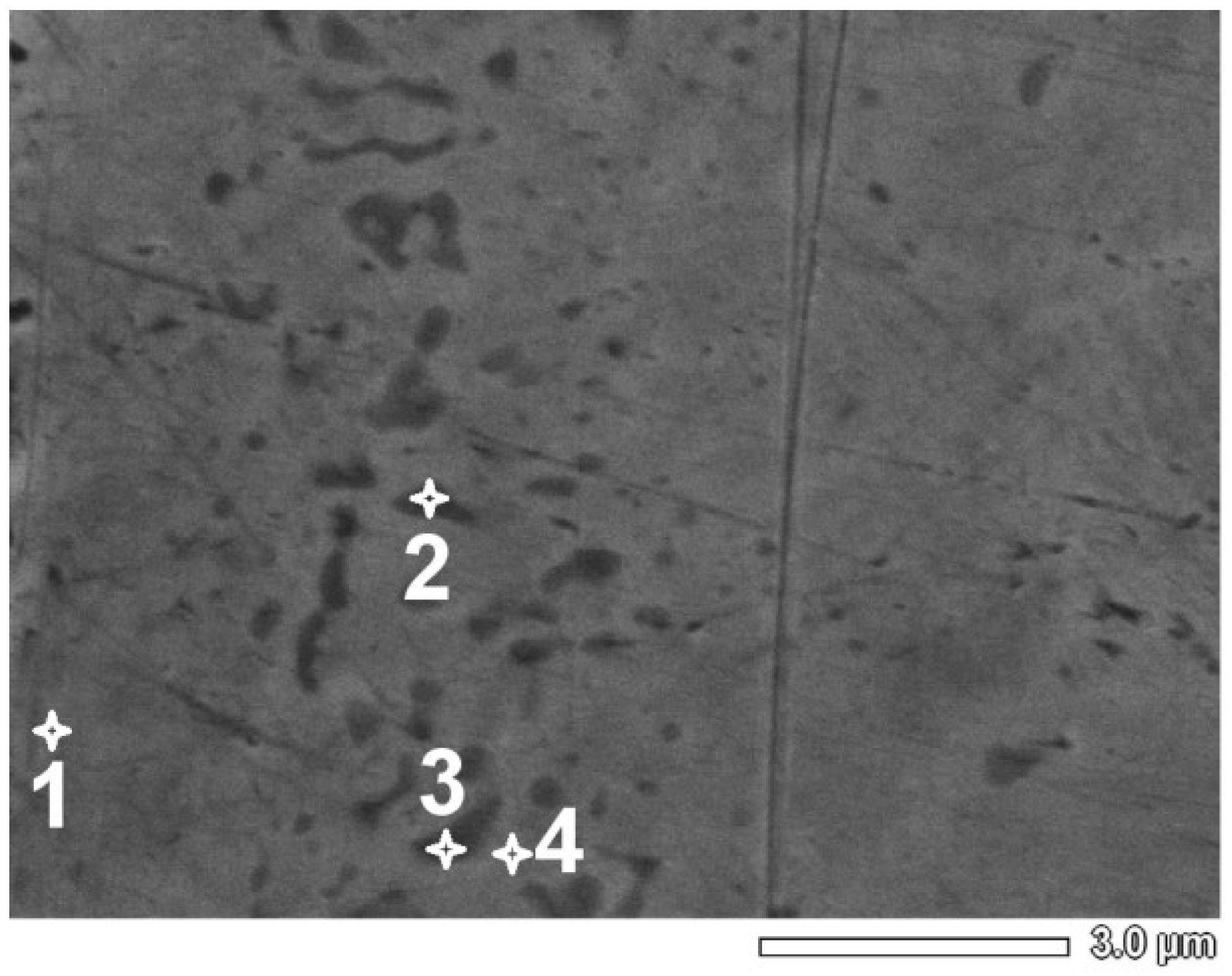
| Point Number | Ni (atom %) | Ti (atom %) |
|---|---|---|
| 1 | 49 | 51 |
| 2 | 42 | 58 |
| 3 | 40 | 60 |
| 4 | 47 | 53 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prendota, W.; Goc, K.; Strączek, T.; Yamada, E.; Takasaki, A.; Przewoźnik, J.; Radziszewska, A.; Uematsu, S.; Kapusta, C. Properties of NiTi Shape Memory Alloy Micro-Foils Obtained by Pulsed-Current Sintering of Ni/Ti Foils. Metals 2019, 9, 323. https://doi.org/10.3390/met9030323
Prendota W, Goc K, Strączek T, Yamada E, Takasaki A, Przewoźnik J, Radziszewska A, Uematsu S, Kapusta C. Properties of NiTi Shape Memory Alloy Micro-Foils Obtained by Pulsed-Current Sintering of Ni/Ti Foils. Metals. 2019; 9(3):323. https://doi.org/10.3390/met9030323
Chicago/Turabian StylePrendota, Witold, Kamil Goc, Tomasz Strączek, Eisuke Yamada, Akito Takasaki, Janusz Przewoźnik, Agnieszka Radziszewska, Susumu Uematsu, and Czesław Kapusta. 2019. "Properties of NiTi Shape Memory Alloy Micro-Foils Obtained by Pulsed-Current Sintering of Ni/Ti Foils" Metals 9, no. 3: 323. https://doi.org/10.3390/met9030323
APA StylePrendota, W., Goc, K., Strączek, T., Yamada, E., Takasaki, A., Przewoźnik, J., Radziszewska, A., Uematsu, S., & Kapusta, C. (2019). Properties of NiTi Shape Memory Alloy Micro-Foils Obtained by Pulsed-Current Sintering of Ni/Ti Foils. Metals, 9(3), 323. https://doi.org/10.3390/met9030323






