1. Introduction
Due to their high specific strength, aluminum structural alloys are widely used in the aviation industry, automotive industry, power production, and the construction industry [
1,
2]. However, the parts from aluminum alloys are characterized by a short service life due to their low abrasive wear resistance and a tendency to point capture in the process of friction interaction with other materials. To increase their serviceability, the hard chrome plating is galvanically applied to the working surfaces of the parts [
3,
4,
5,
6,
7]. However, the process of galvanic chrome plating is environmentally dangerous and, therefore, is gradually withdrawn from technological processes. The synthesis of oxide layers on aluminum alloys by the method of plasma electrolytic oxidation (PEO) in weakly alkaline electrolytes can be an alternative to galvanic chrome plating. Oxide layers synthesized on aluminum alloys have a high hardness (1200 HV 0.2–2000 HV 0.2) and wear and corrosion resistance [
7,
8,
9,
10,
11,
12,
13,
14,
15,
16]. In addition, PEO layers synthesized on lightweight alloys have significant advantages over other coatings used in friction units. However, the insufficient knowledge about the behavior of oxide layers in the conditions of tribological tests hinders the introduction of this technology into production.
To use light alloys in friction units, their surface layers are modified. The most promising is PEO synthesis technology. This technology provides for the transformation of light alloy surfaces into composite oxide-ceramic layers with high wear resistance. Such PEO layers are an ideal material for use in friction units of internal combustion engines, turbine components, slide bearings, and the like.
Aluminum alloys are often exploited under conditions of boundary lubrication in friction pairs “PEO layer–steel”. In the process of frictional interaction of these friction pairs, hydrogen is formed due to the destruction of technological media (oils or aqueous solutions), causing the degradation of the tribological characteristics of these friction pairs.
Permanent cyclic deformation and destruction of the protrusions formed on surfaces of contacting friction pairs occur during their tribological interaction. As a result, juvenile (freshly formed) surfaces are formed during friction process, and conditions are created for synthesizing the structurally new (secondary) compounds with high wear resistance that provide for the low wear of metal on these local sites. The intensity of these processes depends on the properties of the lubricating medium used, the load applied to a friction pair, the composition and structural condition of materials of the contacting surfaces [
16].
The degradation of the lubricating medium in the friction zone occurs more easily due to the high local temperatures in the points of maximum contact pressure of the friction surfaces. Atomic hydrogen formed in these local contact zones acts as a catalyst of the tribochemical reactions [
17]. There are several features that describe the interaction between hydrogen and metals (in particular, steels) caused by the existence of diffusive and residual hydrogen [
18,
19,
20]. In the atomic form, hydrogen can be “diffusion active” and move freely in the crystal lattice of the metal. Alternatively, it can be captured by low-energy crystal traps (vacancies, dislocations, grain boundaries, and sub-boundaries). The “residual” hydrogen can also be present in the metal. In the atomic form, hydrogen will be bound in high-energy traps of the crystal lattice. In molecular form, it will be located in bulk defects (pores and microcracks) [
21]. Such hydrogen is difficult to remove from the crystal lattice of the metal. However, during frictional interaction, when the temperature of the surface layers between the friction pairs exceeds 450 °C, hydrogen in atomic form can be released from the crystal lattice near the friction surface.
The purpose of this research is to determine the effect of hydrogen on the tribological properties of friction pairs consisting of conductive steel elements and dielectric PEO layers synthesized on aluminum alloys.
2. Materials and Methods
The behavior of friction pairs of “steel–PEO layers synthesized on aluminum alloys of two widely used groups” was investigated. The first group is Al-Mg alloys (Mg 2…6 wt.%), which cannot be hardened by heat treatment. The second group is Al-Cu alloys (Cu 3…6 wt. %), which can be strengthened by heat treatment. PEO layers with dielectric properties were synthesized on the light alloys AMg-6 and D16T. Steel 45 and steel U8 with pronounced conductor properties were used as tribological pairs during wear tests. The chemical compositions of the materials used are presented in
Table 1.
The disc-shaped specimens with a thickness of 10.0 mm and a diameter of 42.0 mm were made of alloys AMg-6 and D16T. The high hardness layers were synthesized by PEO methods on the cylindrical surfaces of these specimens in an electrolyte with 3.0 g/l of KOH + 2 g/l of Na2SiO3 using the cathode-anode mode of the pulsating current with a frequency of 50 Hz. The ratio of the cathode and anode currents was Ic/Ia = 1.0 at a current density of 20.0 A/dm2. The thickness of the synthesized layer was 250–300 μm. The microhardness of the PEO layer synthesized on the AMg-6 alloy was 1200–1400 HV 0.2, and that of the PEO layer synthesized on the D16T alloy was 1400–1005 HV 0.2. Before the tribological tests, all specimens with PEO layers synthesized on their cylindrical surfaces were polished by diamond grinding wheel to obtain the outer diameter d = 42.0 ± 0.02 mm at a surface roughness Ra = 0.6 μm.
Steel specimens were previously annealed in vacuum at 800 °C for 1.0 h. The hardness value (HV0,2) of steel 45 steel was about 180 НВ, and that of steel U8 was about 190 НВ. After annealing, the specimens were electrolytically hydrogenated in the 1N H2SO4 solution with the addition of 10 mg/L of As2O3 as a stimulator of the hydrogenation process. The steel specimens were hydrogenated for 1.0 h at a density of the cathode current of 1.0, 2.0, and 3.0 A/dm2, or at a density of 2.0 A/dm2 for 1.0, 2.0 and 3.0 h. This electrolyte solution was used at room temperature together with the platinum grid as an anode. The release of hydrogen from the acid solution can be represented by a total reaction: 2Н3О + 2е−→ Н2 + 2Н2O. The effect of diffusion hydrogen on the tribological properties of both steels was estimated after holding hydrogenated specimens in air for 15 min, whereas the effect of the residual hydrogen on these properties was determined after holding the hydrogenated specimens for 24.0 h.
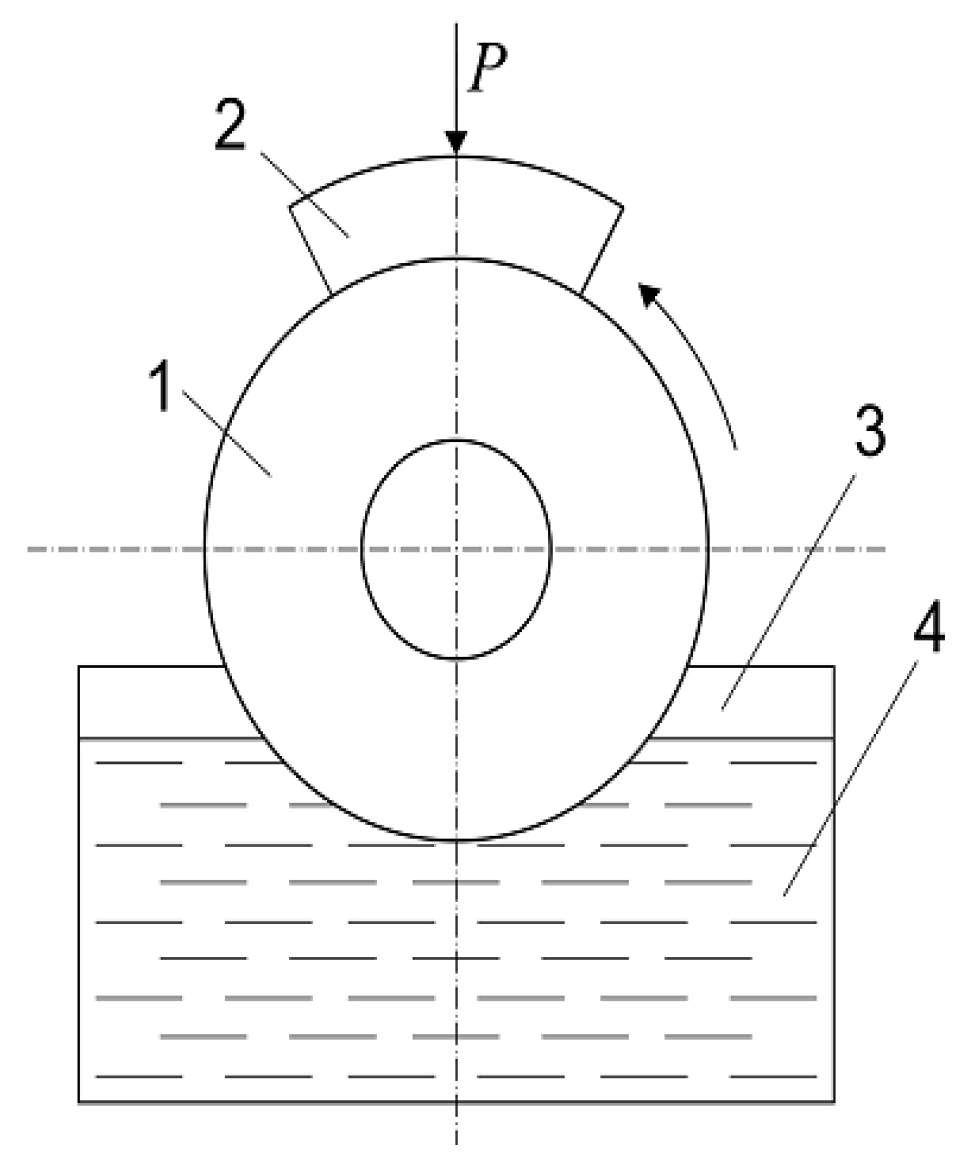
The tribological properties of both steels in contact with the obtained PEO layers were estimated on the testing machine SMC-2 (Tochmashpribor, Ivanovo, Russia). The schematic diagram of these friction tests is shown in
Figure 1. It was believed that this test pattern was most relevant for simulating the frictional behavior of crankshafts under boundary lubrication conditions. The “disk-block” friction scheme that describes the contact between disc-shaped specimens from light alloys and cylindrical surfaces of the steel specimens in the form of disc segments was used. Their diameters in the contact zone corresponded to one another (42.0 ± 0.02 mm). The ratio between the friction surfaces of both elements was 0.125. During the tribological tests, the fixed element of the friction pair in the form of disk-segment (namely, the block) was clamped by means of a self-adjusting device that guaranteed the stability of pressing of the surfaces in the zone of contact, and the stable mutual position of the contacting surfaces during the long-term wear resistance tests.
This made it possible to correctly assess the changes in the tribological characteristics of the materials studied. During all test, the changes of two parameters—the frictional moment and heating temperature—were recorded simultaneously in the friction zone. The frictional moment was estimated by an inductive sensor located on the rotating shaft of the testing machine with the investigated disk fixed on it. The heating temperature in the contact zone of the friction pair was determined using the chromel-alumel thermocouple fixed in the holes made on steel specimens at a distance of 0.5 mm from the surface of their contact. The sliding rate was 0.67 m/s, the specific load in the friction zone between both tribo-elements was 4.0 MPa, the test duration was 4.0 h. These specific load levels in friction testing (electrical signals in millivolts from the corresponding strain gauges) were controlled and recorded in the computer memory with increments of 0.25 sec, and were converted into friction coefficient values. Tribological characteristics (mass loss and coefficient of friction) were determined as the arithmetic mean value of 5 measurements. The maximum deviation of the current measurements of the weight loss by each specimen investigated did not exceed 5.0% of the mean arithmetic value for 5 measurements. Since the variance of this parameter was very small, its in-depth statistical analysis was not performed.
The environmentally friendly additives, in particular, aqueous solutions of glycerine, which are well combined with traditional mineral oils, have been used in recent years to increase the performance of friction pairs. Such mixtures have better lubricating properties than pure mineral oil. If they are used, the coefficient of friction and wear of metal pairs contacting at conditions of the boundary lubrication can be reduced by an order of magnitude [
22,
23].
Mineral oil of the I-20 type (GOST 20799-88, Moscow, SU) was used as a base lubricant in the friction test. To reveal the effect of glycerine on the tribo-characteristics of steel specimens, a 1.0% aqueous solution of glycerine was added to this oil. As a result, the contents of glycerine and water in one liter of the final composition of the lubricating medium were 0.25 mL and 10 mL, respectively. To reveal the effect of water on the friction properties of both steels, 0.5 vol. % of distilled water was also added to the base oil composition.
Wear resistance of the steel specimens was estimated using the data on their weight loss by the gravimetric method using the Radwag WAA 160 analytical balance (Radwag Balances and Scales, Radom, Poland) with a measurement error ±104 g. The phase analysis of the PEO layers was carried out on a diffractometer DRON-3M (Bourevestnik JSC, Saint-Petersburg, Russia) using the Cu-Kα radiation. The secondary structures formed on the surfaces of the steel specimens due to its friction with PEO layers was analyzed using the Co-Kα radiation.
The features of the surfaces formed after the friction tests were studied using the Zeiss Stemi-2000c microscope (Carl Zeiss Mikroskopie, Jena, Germany). The microstructure and elemental analysis were obtained using the EVO-40XVP (Carl Zeiss Mikroskopie, Jena, Germany) scanning electron microscope with the INCA-Energy system for the X-ray microanalysis.
4. Phase Analysis of the Layer Formed on the Friction Surface of the Steel
The phase analysis of tribo-contact layers has shown that this film was represented by a complex iron oxide of spinel type (FeAl
2O
4). Components such as iron, oxygen and a small amount of aluminum, magnesium, and silicon dioxide were found in the composition of this film (
Figure 7). The thickness of this film can be increased by preliminary electrolytic hydrogenation of steel specimens or by using mineral oil with water or an aqueous solution of glycerine added during wear tests.
Consequently, the spinel-like oxide layer (hercynite (Fe
0,816·Al
0,184)·(Al
1,816·Fe
0,184·O
4) type) was synthesized on the specimen surfaces of steel 45 during the friction process. As it is known, such oxide protects the steel surfaces against damage under the hydrogen effect. In the absence of a mechanical effect, hercynite is synthesized under various conditions. It is usually synthesized at temperatures exceeding 1500 °C in the electric arc furnaces as a result of the direct reaction between FeO and Al
2O
3. It is often represented by the formula Fe(Fe
0.5·Al
1.5)·O
4. Hercynite can be synthesized at temperatures of 490–600 °C under the combined action of nitrogen and hydrogen [
24,
25]. In this research, it was found that under the influence of the mechanical factor upon tribocontact between the PEO layer synthesized on the AMg-6 alloy and carbon steels, hercynite can be formed on the friction surfaces at a temperature of about 120 °C in the contact zone. A hercynite layer synthesized on the surface of hydrogenated steel specimens before friction tests prevents the wear of the steel substantially.
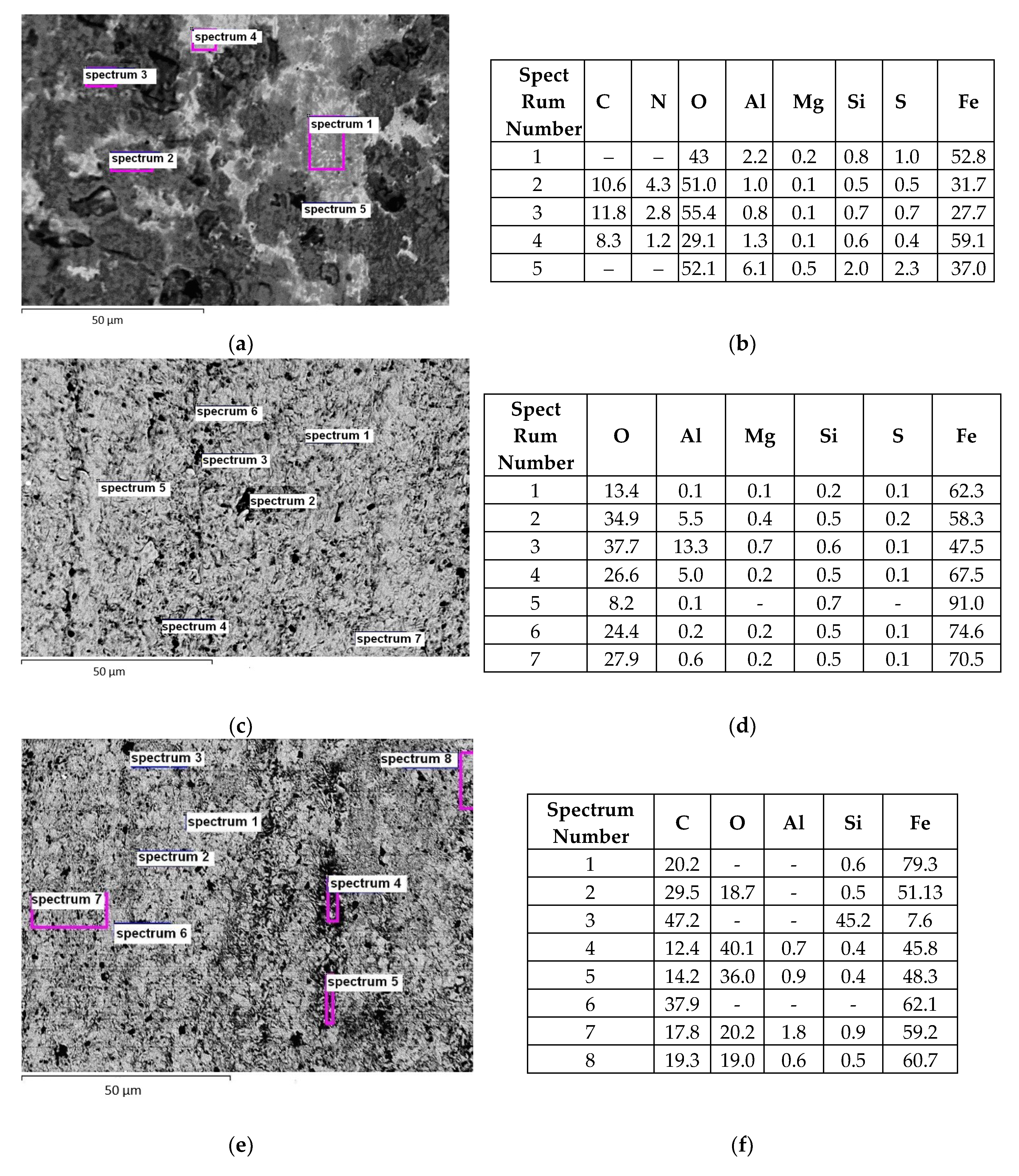
Tribo-layers formed on the friction surfaces of steel specimens were also examined using the optical and electron microscopes. Studies using an optical microscope revealed that, at first glance, the morphology of the friction surfaces does not change after the wear tests. However, the elemental composition of the formed films has shown some differences between them. Moreover, a cluster structure is characteristic of the formed tribo-layers. The local areas with practically white, light-, dark-grey and practically black color were identified in the structure of this layer. White areas contained more iron, less aluminum, and oxygen (
Figure 8a, spectrum 4). On the contrary, a dark gray area contained more aluminum, magnesium, and oxygen (
Figure 8a, spectrum 2 and 3). Mainly iron and a small amount of oxygen were revealed in light grey areas (
Figure 8a spectrum 1). Apparently, not only oxides but also compounds doped with oxygen were formed locally. Black areas contained impurities of aluminum, magnesium and a significant amount of oxygen (
Figure 8a spectrum 5).
In two cases, a predominantly light grey film was formed on the friction surfaces of the specimens from steel U8 upon tribo-contact with the PEO layer synthesized on the AMg-6 alloy during testing in the I-20 oil (
Figure 8c–f). In the first case, it is formed after the electrolytical hydrogenation of steel specimens before friction test (
Figure 8c). And in the second case, it is formed after the addition of the water-glycerine solution to friction lubricant (
Figure 8e). It means that in both cases, iron oxides with an insignificant admixture of aluminum oxides predominate on the friction surfaces. An insignificant amount of black areas was revealed on tribo surfaces, too.
The effect of the diffusive hydrogen (coming into the zones of tribocontact from a pre-hydrogenated steel specimen used as counter bodies) and atomic one (formed as a result of lubricant destruction in the tribocontact zones) on the friction process was explained as follows.
The following mechanism is proposed for the formation of spinel on the surfaces of steel specimens as a result of friction. Atomic hydrogen released on the surface of hydrogenated steel specimens or formed as a result of the destruction of water and oil in the contact zone of friction surfaces cannot contribute to the formation of an oxide film on the steel surface. However, it can be assumed that atomic hydrogen interacts with the molecular oxygen dissolved in the oil with the formation of water. During friction, juvenile surfaces are formed due to local deformation and damage with flashes of temperature. This temperature can be higher than 400 °C [
26]. It is known that 1–3 µm thick oxide films are formed on the steel surface in the water vapor environment at these temperatures. This film is similar to the one formed on the steel surfaces during their bluing.
An oxide film layer (FeAl
2O
4) is not formed on the steel surface when the steel wears in a tribo-pair with a PEO layer synthesized on the surface of the alloy-D16T. It was explained by the appearance of nanoscale copper inclusions in the structure of the PEO layer. These copper particles were actually found on the surface of steel specimens upon completion of the wear test. In this case, the phenomenon of selective transfer is realized [
17]. The copper ions from the surface layer with nanoscale copper particles in the PEO structure are selectively transferred to the surface of the steel specimen (as a friction pair) under the influence of hydrogen ions H
+. This phenomenon is similar to the destruction of steel under the influence of hydrogen. An abnormally low coefficient of friction (0.006) (
Figure 6b, columns 5) is due to the formation of a glycerate film on the steel surface.
Thus, in the process of tribological destruction of glycerol added to the lubricant, the oxides of copper and iron are reduced under the action of hydronium ion H
3O
+, and friction polymers are created in the form of copper and iron glycerates [
17]. These tribosynthetic compounds protect steel surfaces from the destructive action of hydrogen.
However, friction polymers arose only in the case of triboelectron emission. Oxide-coated surfaces (oxide ceramics or PEO layers synthesized on aluminum alloys) are the strongest sources of triboelectrons emission [
17]. High local (flash) temperatures on contacted micro-areas caused by their interaction at friction can activate the emission of triboelectrons, which leads to tribochemical reactions in accordance with the concepts of NIRAM (the negative-ion-radical-action mechanism), due to which the reactivity of surfaces increases [
27,
28,
29,
30,
31].
Author Contributions
Conceptualization, M.S.; formal analysis, P.M. and V.H.; investigation, M.S., V.H., V.D., P.M., V.P., O.S., and I.K.; methodology, M.S. and P.M.; project administration, V.H.; validation, M.S., V.H., V.D., P.M., V.P., O.S., and I.K.; writing—original draft, M.S., O.S., P.M.; writing—review and editing, V.H.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ductile Aluminum Alloys for Automotive Structural Applications. Available online: http://rheinfelden-alloys.eu/wp-content/uploads/2015/04/Handbook-Alloys-for-Structural-Applications_RHEINFELDEN-ALLOYS_2017_EN.pdf (accessed on 26 February 2019).
- Kissell, J.R.; Ferry, R.L. Aluminum Structures: A Guide to Their Specifications and Design, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2002; p. 544. [Google Scholar]
- Bolelli, G.; Giovanardi, R.; Lusvarghi, L.; Manfredini, T. Corrosion resistance of HVOF-sprayed coatings for hard chrome replacement. Corros. Sci. 2006, 48, 3375–3397. [Google Scholar] [CrossRef]
- Bozyaz, E.; Urgen, M.; Cakr, A.F. Comparison of reciprocating wear behaviour of electrolytic hard chrome and arc-PVD CrN coatings. Wear 2014, 256, 832–839. [Google Scholar] [CrossRef]
- Espallargas, N.; Berget, J.; Guilemany, J.M.; Benedetti, A.V.; Suegama, P.H. Cr3C2-NiCr and WC-Ni thermal spray coatings as alternatives to hard chromium for erosion-corrosion resistance. Surf. Coat. Technol. 2008, 202, 1405–1417. [Google Scholar] [CrossRef]
- Picas, J.A.; Forn, A.; Matthaus, G. HVOF coatings as an alternative to hard chrome for pistons and valves. Wear 2006, 261, 477–484. [Google Scholar] [CrossRef]
- Dehnavi, V. Surface Modification of Aluminum Alloys by Plasma Electrolytic Oxidation. Ph.D. Thesis, University of Western Ontario, London, ON, Canada, September 2014; p. 216. [Google Scholar]
- Dehnavi, V.; Liu, X.Y.; Luan, B.L.; Shoesmith, D.W.; Rohani, S. Phase transformation in plasma electrolytic oxidation coatings on 6061 aluminum alloy. Surf. Coat. Technol. 2014, 251, 106–114. [Google Scholar] [CrossRef]
- Tran, Q.P.; Chin, T.S. Plasma electrolytic oxation coating on 6061 Al alloy using an electrolyte without alkali ions. J. Sci. Technol. 2016, 54, 151–158. [Google Scholar] [CrossRef]
- Liu, Y.J.; Xu, J.Y.; Lin, W.; Gao, C.; Zhang, J.C.; Chen, X.H. Effects of different electrolyte systems on the formation of micro-arc oxidation ceramic coatings of 6061 aluminum alloy. Rev. Adv. Mater. Sci. 2013, 33, 126–130. [Google Scholar]
- Xue, W.; Wang, C.; Deng, Z.; Chen, R.; Lai, Y.; Zhang, T. Evaluation of the mechanical properties of microarc oxidation coatings and 2024 aluminium alloy substrate. J. Phys. Condens. Matter. 2002, 14, 10947–10952. [Google Scholar] [CrossRef]
- Sah, S.P.; Tsuji, E.; Aoki, Y.; Habazaki, H. Cathodic pulse breakdown of anodic films on aluminium in alkaline silicate electrolyte understanding the role of cathodic half-cycle in ac plasma electrolytic oxidation. Corros. Sci. 2012, 55, 90–96. [Google Scholar] [CrossRef]
- Student, M.M.; Dovhunyk, V.M.; Klapkiv, M.D.; Shmyrko, V.V.; Kytsya, А.Р. Tribologycal properties of combined metal-oxide ceramic layers on light alloys. Mater. Sci. 2012, 48, 180–190. [Google Scholar] [CrossRef]
- Pokhmurskii, V.; Nykyforchyn, H.; Student, M.; Klapkiv, M.; Pokhmurska, H.; Wielage, B.; Grund, T.; Wank, A. Plasma electrolytic oxidation of arc-sprayed aluminum coatings. J. Therm. Spray Tech. 2007, 16, 998–1004. [Google Scholar] [CrossRef]
- Student, M.M.; Dovhunyk, V.M.; Posuvailo, V.M.; Koval’chuk, I.V.; Hvozdets’kyi, V.M. Friction behavior of iron-carbon alloys in couples with plasma-electrolytic oxide-ceramic layers synthesized on D16T alloy. Mater. Sci. 2017, 53, 359–367. [Google Scholar] [CrossRef]
- Posuvailo, V.M.; Klapkiv, M.D.; Student, M.M.; Sirak, Y.Y.; Pokhmurska, H.V. Gibbs energy calculation of electrolytic plasma channel with inclusions of copper and copper oxide with Al-base. Mater. Sci. Eng. 2017, 181, 012045. [Google Scholar] [CrossRef]
- Kostetsky, B.I. The structural-energetic concept in the theory of friction and wear (synergism and self-organization). Wear 1992, 159, 1–15. [Google Scholar] [CrossRef]
- Andreikiv, O.E.; Gembara, O.V. Destructive effect of hydrogen on the strength of materials in nonstationary temperature fields. Mater. Sci. 1996, 32, 411–416. [Google Scholar] [CrossRef]
- Iasnii, V.; Maruschak, P.; Yasniy, O.; Lapusta, Y. Hydrogen crack growth resistance of thermal power plant material collector. Procedia Mater. Sci. 2014, 3, 1400–1405. [Google Scholar] [CrossRef]
- Pokhmurskii, V.; Khoma, M.; Vynar, V.; Vasyliv, Ch.; Ratska, N.; Voronyak, T.; Stasyshyn, I. The influence of hydrogen desorption on micromechanical properties and tribological behavior of iron and carbon steels. Procedia Struct. Integr. 2018, 13, 2190–2195. [Google Scholar] [CrossRef]
- Yasniy, P.V.; Okipnyi, I.B.; Maruschak, P.O.; Panin, S.V.; Konovalenko, I.V. Crack tip strain localisation on mechanics of fracture of heat resistant steel after hydrogenation. Theor. Appl. Fract. Mech. 2013, 63–64, 63–68. [Google Scholar] [CrossRef]
- Kuznetsov, G.V.; Vershinina, K.Y.; Valiullin, T.R.; Strizhak, P.A. Differences in ignition and combustion characteristics of waste-derived oil-water emulsions and coal-water slurries containing petrochemicals. Fuel Process. Technol. 2018, 179, 407–421. [Google Scholar] [CrossRef]
- Audibert, F. Waste Engine Oils: Rerefining and Energy Recovery, 1st ed.; Elsevier Science: Amsterdam, The Netherlands, 2006; p. 340. [Google Scholar]
- Smiyan, O.D. Hydrogen in a metal as a boson fluid. Physics Chem. Solids 2004, 5, 750–757. (In Ukrainian) [Google Scholar]
- Stoch, P.; Jablonska, A.; Szczerba, J.; Guzdek, P.; Suwalski, J. Synthesis of hercynite FeAl2O4 studied by mossbsuer spectroscopy; Annual report for Solid State Physics. 2005. Available online: https://inis.iaea.org/collection/NCLCollectionStore/_Public/37/103/37103138.pdf (accessed on 26 February 2019).
- Yang, L.; Ito, A.; Negishi, H. A valve train friction and lubrication analysis model and its application in a cam/tappet wear study. SAE 1996, 962030. [Google Scholar] [CrossRef]
- Molina, G.J.; Furey, M.J.; Ritter, A.L.; Kajdas, C. Triboemission phenomena: electronic and photonic outputs from surface modification and its use as novel probes for the dynamics of surface processes. Wear 2001, 249, 214–219. [Google Scholar] [CrossRef]
- Brezinová, J.; Viňáš, J.; Maruschak, P.O.; Guzanová, A.; Draganovská, D.; Vrabeľ, M. Sustainable Renovation within Metallurgical Production; RAM-Verlag: Lüdenscheid, Germany, 2017; p. 215. [Google Scholar]
- Brezinová, J.; Guzanová, A.; Draganovská, D.; Maruschak, P.; Landová, M. Study of selected properties of thermally sprayed coatings containing WC and WB hard particles. Acta Mech. Autom. 2016, 10, 296–299. [Google Scholar] [CrossRef]
- Guzanová, A.; Brezinová, J.; Bronček, J.; Maruschak, P.; Landová, M. Study of selected properties of coatings devoted to extreme tribo-corrosive conditions. Mater. Sci. Forum 2015, 818, 32–36. [Google Scholar] [CrossRef]
- Panin, S.; Vlasov, I.; Dudina, D.; Ulyanitsky, V.; Stankevich, R.; Batraev, I.; Maruschak, P.; Landová, M. Deposition of titanium based coatings by reactive detonation spraying. Koroze a Ochrana Materiálu 2018, 62, 6–13. [Google Scholar] [CrossRef]
Figure 1.
Schematic diagram of the friction tests: 1—rotating specimen in the form of a disk from the D16T or AMg16 light alloys with plasma electrolytic oxidation (PEO) layers synthesized on their outer cylindrical surface; 2—blocking segment-like specimen from the 45 or U8 steels; 3—reservoir with lubricant, 4—lubricating medium; P—pressing force.
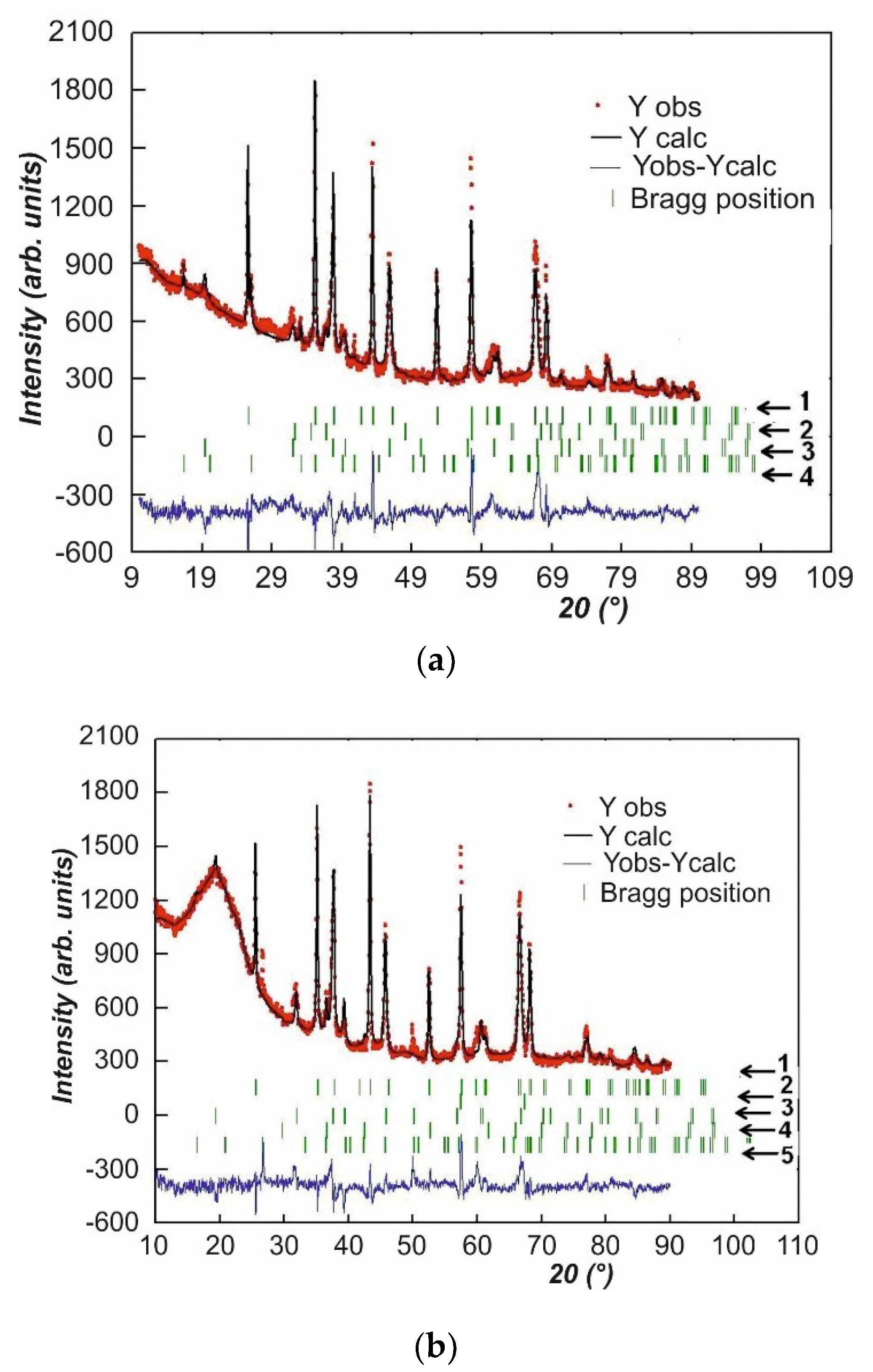
Figure 2.
X-ray diffraction patterns of the PEO layers synthesized on AMg-6 (a) and D 16T (b) alloys. The designations of revealed phases: (a) 1—α-Al2O3, 2—Mg. 3—γ-Al2O3, 4—MgAl2Si3O10; (b) 1—α-Al2O3, 2—Cu, 3—γ-Al2O3, 4—Cu2O, 5—SiO2.
Figure 3.
Microstructure of PEO layers synthesized on the alloys AMg-6 (a), D 16T (b).
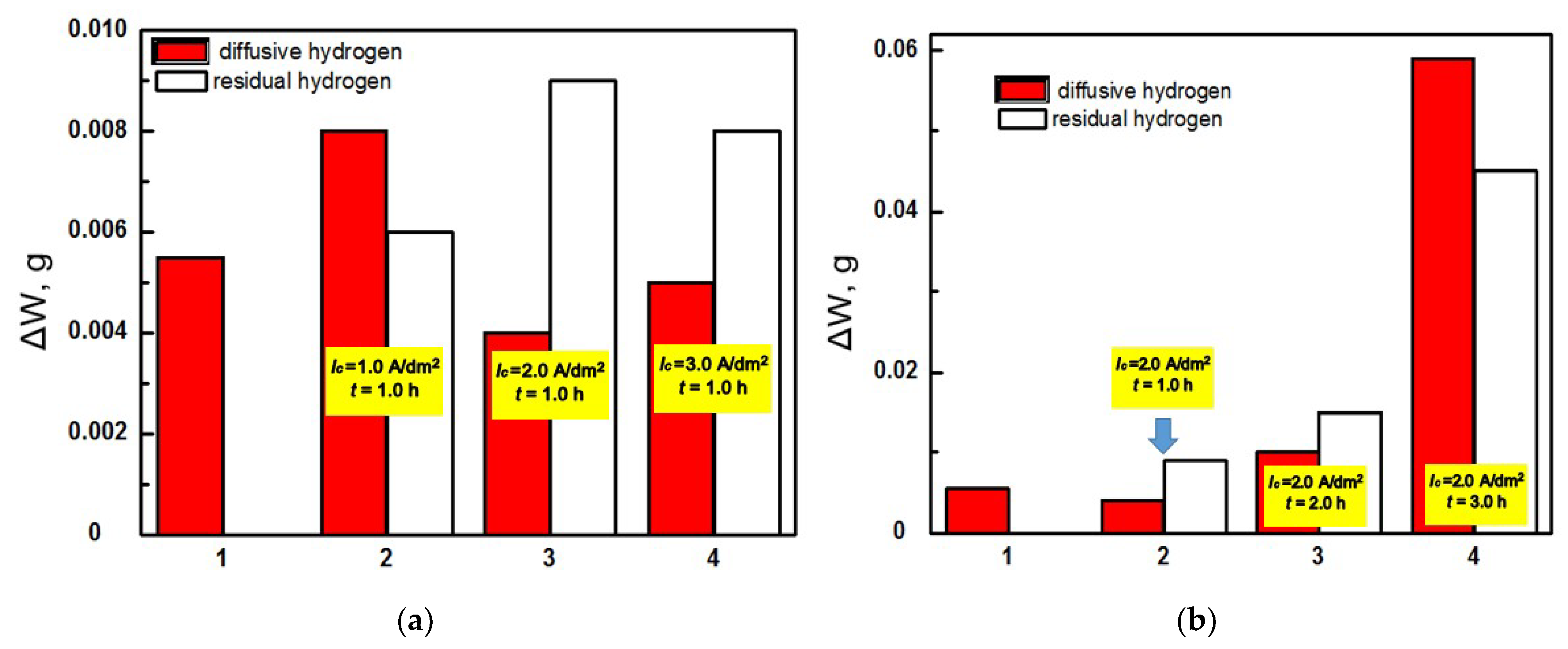
Figure 4.
Weight losses of specimens from steel 45, taking into account preliminary hydrogenation at a current density of 1.0 A/dm2 (a) and 2.0 A/dm2 (b) during their tribocontact with the PEO layer synthesized on the AMg-6 alloy, determined by testing in mineral oil I-20 by contact load 4.0 MPa; 1—without preliminary hydrogenation; 2–4—taking into account hydrogenation (hydrogenаtion parameters are shown on columns).
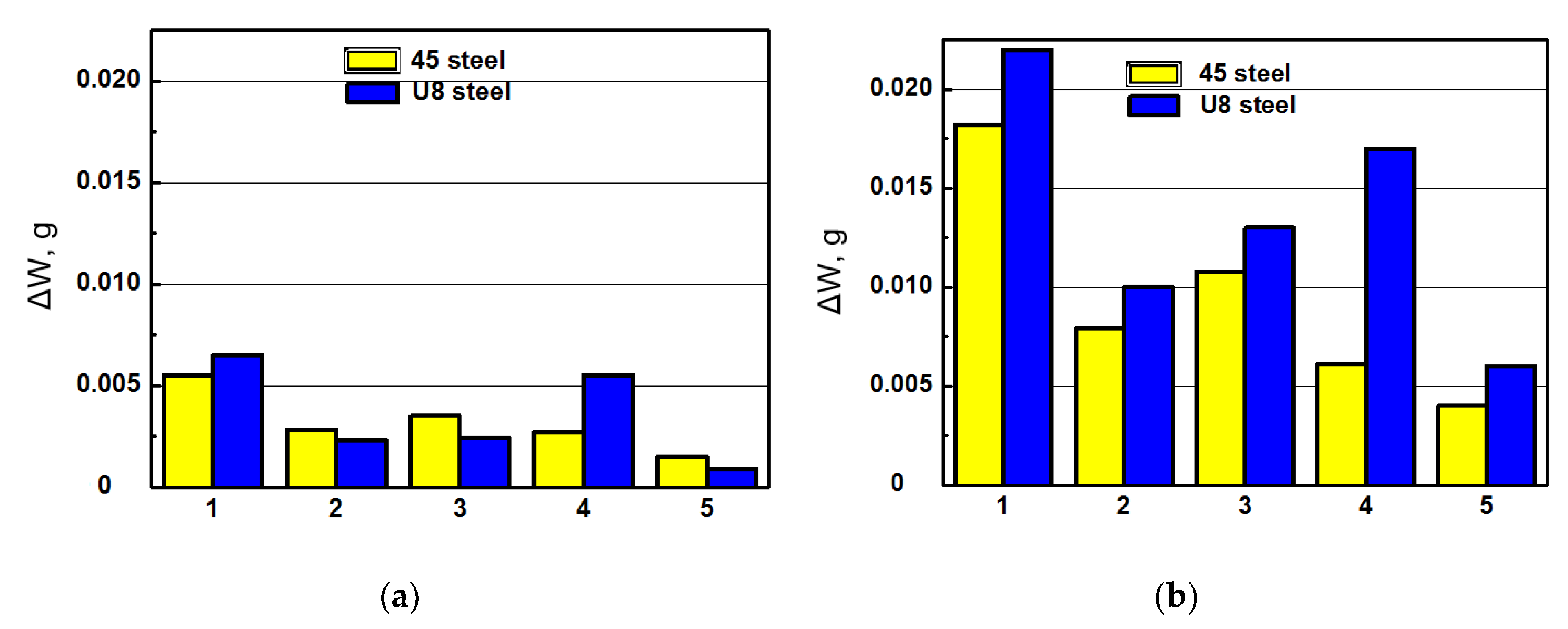
Figure 5.
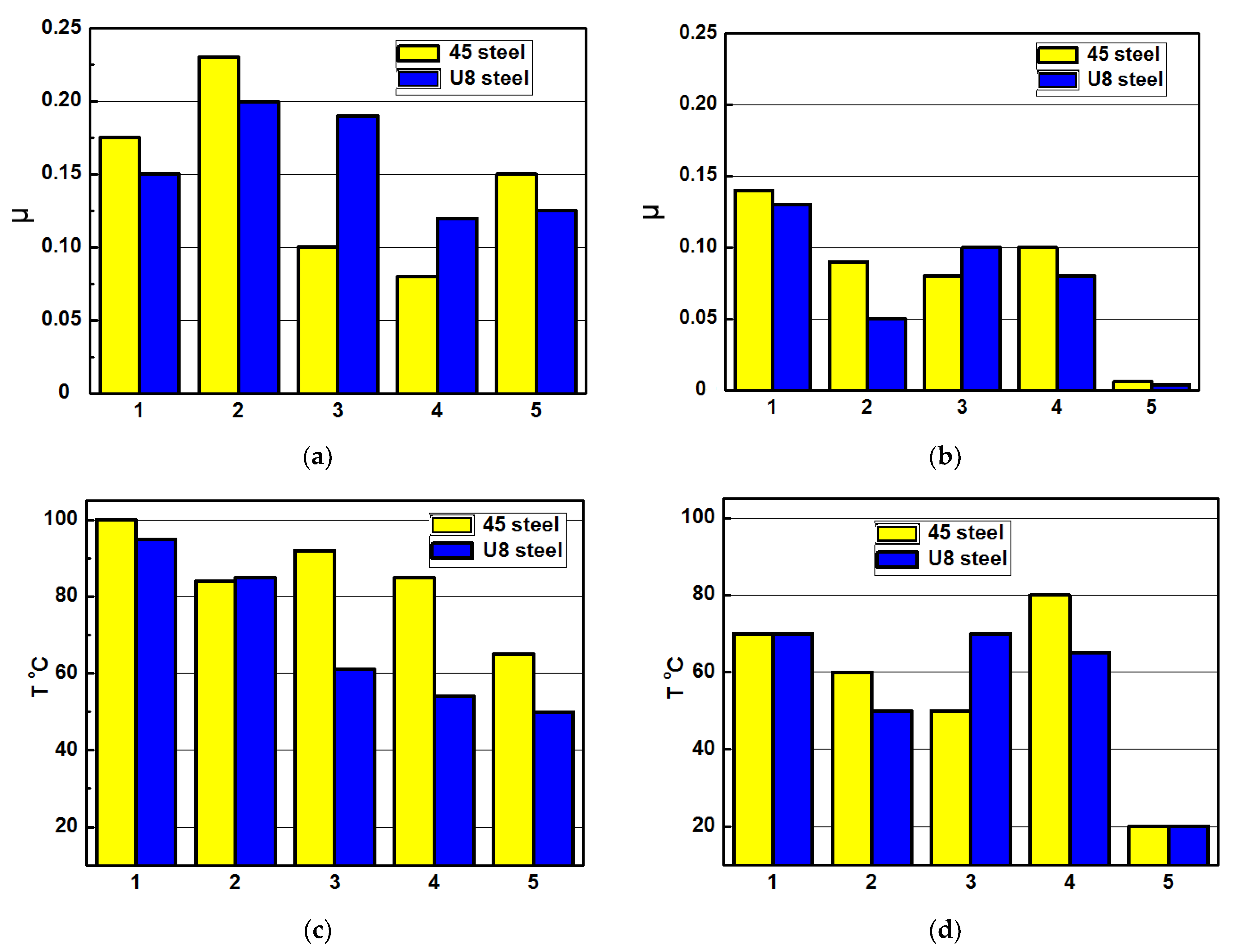
The mass loss of the 45 and U8 steels after their tribocontact with PEO layers synthesized on the AMg-6 (a) and D16T (b) alloys estimated by the weight losses ΔW of specimens tested at unit load level of 4 MPa within 4 h: in the pure mineral oil of the I-20 type (1); after their hydrogenation within 1 h at current density 2.0 A/dm2 with following friction in the mineral oil of the I-20 type with the addition of 0.5 vol. % distilled water (2); after hydrogenation of specimens followed by their holding in air within 15 min (3) and 24.0 h (4) to provide the diffusive or residual hydrogen in the metal, correspondently; in the mineral oil of the I-20 type with the addition of 1.0 vol. % 2.5% aqueous solution of glycerin (5).
Figure 6.
Averaged values of the friction coefficients (a,b) and the heating temperatures in the vicinity of the tribocontact zones (c,d) between the PEO layers synthesized on the AMg-6 (a,c) and D16T (b,d) alloys and specimens from the 45 and U8 steels without hydrogenation (1), after their preliminary hydrogenation with the diffusive (3) or residual hydrogen (4) under tribo-testing in the pure mineral oil of the I-20 type, or without hydrogenation of steel specimens and with their tribo-testing in mineral oil of the I-20 type with the addition of the 0.5 vol. % distilled water (2), or of the 1.0 vol. % aqueous solution of glycerin (5). Current density during electrolytic hydrogenation of steel specimens was 2.0 A/dm2 and the time of hydrogenation was 1.0 h. The specific load in the friction zone was 4.0 MPa. Testing time was 4.0 h.
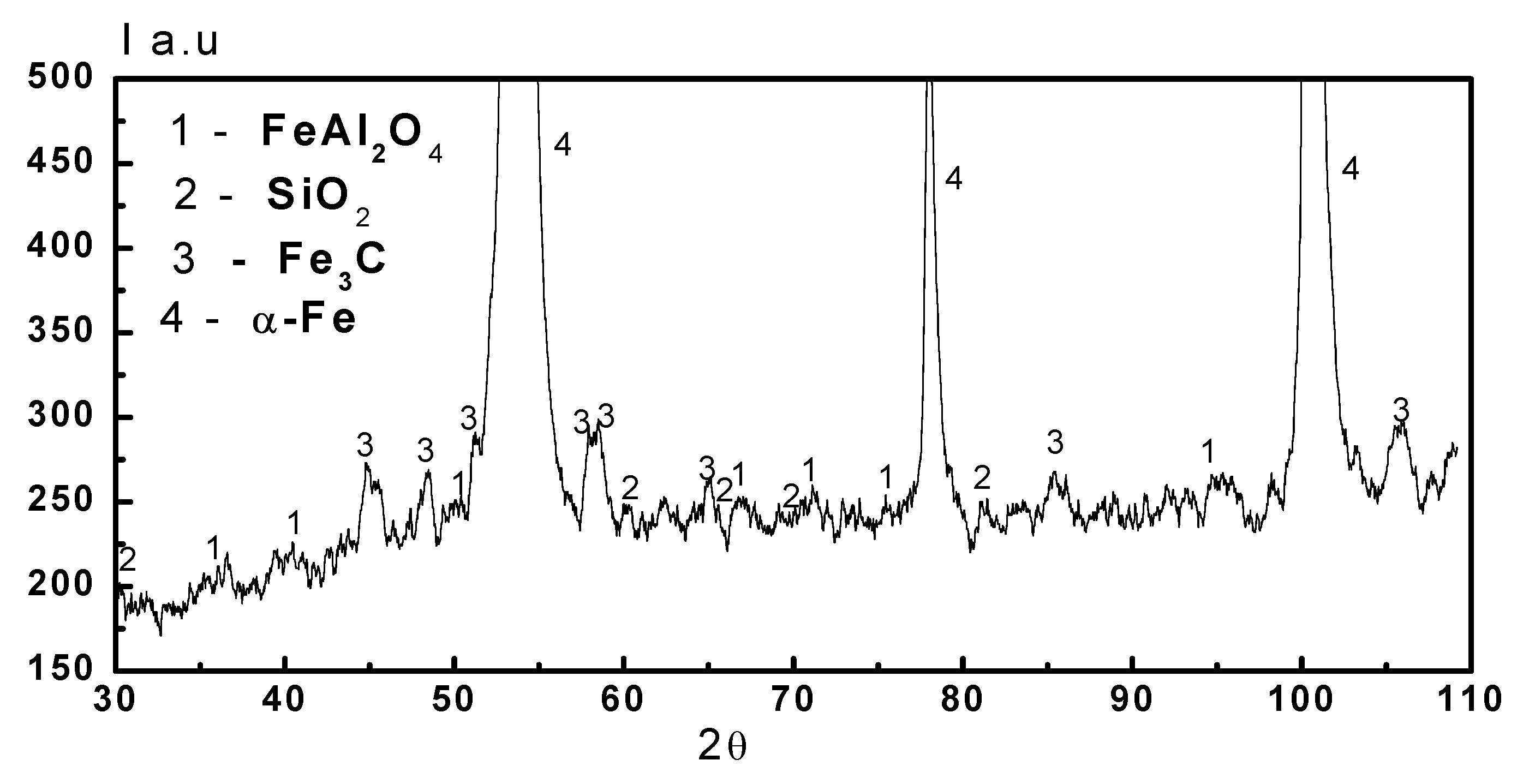
Figure 7.
XRD pattern of the layer formed on the friction surface of the 45 steel after the friction testing in pair with the PEO layer synthesized on the AMg-6 alloy in mineral oil of the I-20 type with the addition of 1.0 vol. % aqueous solution of glycerin. The specific load in the friction zone was 4.0 MPa. The designations of revealed phases: 1—FeAl2O4; 2—SiO2; 3—Fe3C; 4—α-Fe.
Figure 8.
The structure of the friction surfaces (a,c,e) and the results of their microspectral analysis (b,d,f) with elemental composition (at. %) of the various regions of tribo-layers formed on the surfaces of specimens from the 45 (a,b) and U8 (c–f) steels after their electrolytic pre-hydrogenation (hydrogen of diffusion type) and after their subsequent wear test in tribo-pair with PEO layer synthesized on the AMg-6 in the pure mineral oil of the I-20 type (a,b) and with the addition of 0.5 vol. % water (c,d) or of 1.0 vol. % of an aqueous solution of glycerine (e,f) at the specific load in the friction zones of 4.0 MPa.
Table 1.
The chemical compositions of the investigated materials, wt. %.
| Elements | AMG-6 | D16T | Steel 45 | Steel U8 |
|---|
| С | - | - | 0.43 | 0.8 |
| Al | 91.5 | 92.3 | - | - |
| Mg | 6.2 | 1.5 | - | - |
| Cu | 0.1 | 4.1 | 0.06 | 0.05 |
| Mn | 0.6 | 0.6 | 0.62 | 0.19 |
| Fe | 0.2 | 0.2 | - | - |
| Si | 0.3 | 0.4 | 0.23 | 0.21 |
| Zn | 0.1 | 0.03 | - | - |
| Cr | - | 0.07 | 0.08 | 0.09 |
| Ti | - | 0.012 | - | - |
| Ве | - | - | - | - |
| Ni | - | - | 0.1 | 0.12 |
| S | - | - | 0.04 | 0.028 |
| P | - | - | 0.035 | 0.03 |
Table 2.
Phase composition of the PEO layers synthesized on AMg-6 and D16T alloys.
| PEO Layer Formed on the AMg-6 Alloy | PEO Layer Formed on the D16T Alloy |
|---|
| Components | SG * | mass. % | Components | SG * | mass. % |
| α-Al2O3 | R -3 C | 54.72 | α-Al2O3 | R -3 C | 56.26 |
| Mg | P63/mmc | 2.69 | Cu | F M 3 M | 2.45 |
| γ-Al2O3 | F D -3 M | 34.56 | γ-Al2O3 | F D -3 M | 37.08 |
| MgAl2Si3O10 ** | P 6 2 2 | 8.03 | Cu2O | P N 3 M | 2.93 |
| - | - | - | SiO2 | P 3 2 | 1.28 |
Table 3.
Element composition of the PEO layers synthesized on АMg-6 and D16T alloys.
| PEO Layer |
|---|
| Element | AMg-6 | D16T |
|---|
| mass.% | at.% | mass.% | at.% |
|---|
| Na | 0.1 | 0.1 | 0.2 | 0.3 |
| Mg | 1.8 | 0.8 | 1.1 | 0.6 |
| Al | 49.4 | 37.4 | 42.6 | 33.0 |
| Si | 1.2 | 0.7 | 4.2 | 3.2 |
| K | 0.5 | 0.4 | 2.1 | 1.0 |
| Ca | - | - | 0.3 | 0.2 |
| Cu | - | - | 3.1 | 1.1 |
| O | 50.0 | 59.8 | 46.4 | 60.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).