Abstract
Nano-magnesia is the intermediate product during the growth of magnesia inclusion in Mg-deoxidized steel. Understanding the thermodynamics on nano-magnesia is important to explore the relationship between magnesia product size and deoxidation reaction in molten steel. In this work, a thermodynamic modeling is developed to study the Mg-deoxidation reaction between nano-magnesia inclusions and liquid iron. The thermodynamic results based on the first principle method show that the Gibbs free energy change for the forming magnesia product decrease gradually with the increasing nano-magnesia size in liquid iron. The published experimental data about Mg-deoxidation equilibria in liquid iron are scattered across the region between the thermodynamic curves of 2 nm magnesia and bulk-magnesia. It is suggested that these scattered experimental data of Mg-deoxidized liquid iron are in different thermodynamic states. Some of these experiments are in equilibrium with bulk-magnesia, while most of these experiments do not reach the equilibrium state between bulk magnesia and liquid iron, but in quasi-equilibria between nano-magnesia and liquid iron. This is the reason that different researchers gave different equilibrium constants. Furthermore, the behavior of the metastable magnesia is one of the most important reasons for the supersaturation ratio or the excess oxygen for MgO formation in liquid iron.
1. Introduction
As the stress raisers and the source of cracks, non-metallic inclusion seriously restricts the quality of steel products because of its different properties from steel. Controlling the chemical composition, sizes, and quantities of inclusion is the decisive issue for the development of super-clean production. However, it is very difficult to eliminate all inclusions during the steelmaking process. Usually, the steelmakers use magnesium or magnesium alloy to reduce the harm of inclusion to steel products. For example, the sharp and large size alumina inclusion can be turned into the dispersive and small curved MgO·Al2O3 spinel inclusion by Mg-treatment in molten steel [1,2,3,4,5]. On the other hand, the small, globular, and uniformly distributed inclusion is practically harmless to fatigue and other properties of the steel [1]. Lowe and Mitchell [6] indicated that the inclusion particle is not harmful to the mechanical properties of steel, if its size is less than 1 μm, while the distance between particles is greater than 10 μm in the steel matrix. In addition, the inclusion with nano-size can be utilized as heterogeneous nucleation sites for phase transformation and play a positive role on the nucleation of acicular ferrite [7,8]. Therefore, refining the inclusion size is one of the effective strategies to improve the steel performance. In order to accomplish this objective, it is necessary to have a deep insight into the thermodynamics on the growth of inclusion in nanoscale during metal-deoxidization for molten steel.
Magnesium is one of the most important deoxidizers and inclusion modifier for its strong affinity to oxygen in the iron melt and has drawn great interest and attention recently. The chemical equation of Mg-deoxidation reaction for molten steel can be written as
Since the activity of MgO(s) can be regarded as unity; the equilibrium constant can be expressed as
where is the solubility product; ai and fi are the activity of element i in the molten iron and its activity coefficient relative to an infinitely dilute solution on a mass percentage basis, respectively. The fi can be expressed as [9,10]
where and are the first-order interaction coefficient. , and are the second-order interaction coefficients.
[Mg] + [O] = MgO(s)
Table 1 lists the measured various equilibrium constants and interaction coefficients among magnesium and oxygen in liquid iron at 1873 K [10,11,12,13,14,15,16,17,18,19,20,21,22]. It can be seen from Table 1, only the first-order interaction coefficients were considered by Gorobetz et al. [11], Kulikov et al. [12], Inoue et al. [17], and Han et al. [18] during the calculation for the equilibrium constant KMg. Due to the strong interaction between Mg and O in liquid iron, Itoh et al. [19], Ohta and Suito [20], Seo and Kim [21], and Seo et al. [22] obtained their equilibrium constant based on a second-order interaction coefficients according to their own experiment data. However, there are large differences among the equilibrium constants and the interaction coefficients proposed by different researchers. The first interaction coefficient ranges from −370 to −106, and the second interaction coefficient ranges from −40,000 to 59,000. The parameter logK by thermodynamic calculation ranges from 7.74 to 9.24, but the parameter logK by experiment ranges from 5.12 to 7.8. It should be note that the equilibrium constants and the interaction coefficients obtained by various researchers are different from each other and even vary widely. Consequently, it is not easy to select the suitable equilibrium constant and the interaction coefficients. Such a strange phenomenon puzzled the researchers for years.

Table 1.
Equilibrium constants and interaction coefficients of Mg-O system in liquid iron at 1873 K.
Why the equilibrium constants obtained by various researchers are different to each other? Some researchers suggested that the thermodynamic properties of inclusions have a close relationship with its size [23,24,25,26,27]. Wasai et al. [24] indicated that the interfacial free energy between nano-inclusions and liquid iron increased with the increasing of the inclusion size, and the Gibbs free energy change of deoxidation reaction has a close relationship with the size change of inclusion. Wang et al. [25] found that the thermodynamics on Al-deoxidation reaction in liquid iron had a close relationship with the size of alumina inclusion. Therefore, the thermodynamics of nano-magnesia is very important to reveal the mechanism on Mg-deoxidation equilibrium in liquid iron. Moreover, nano-magnesia is the intermediate product of the crystallization for bulk magnesia inclusion during Mg-deoxidation process. Understanding the thermodynamics of nanoscale inclusion in liquid iron is vital to explore the relationship between the sizes of inclusion and deoxidation reaction, and is also extremely useful in the inclusions size controlling.
However, there is hardly any research known about the thermodynamic properties of nano-magnesia in liquid iron. In order to understand the thermodynamics of nano-magnesia growth in liquid iron, a new thermodynamic modeling for nano-magnesia was developed to investigate the relationship between the thermodynamics of nano-magnesia and their size in Fe-O-Mg melt.
2. Theoretical Modeling for Nano-MgO in Liquid Iron
2.1. Thermodynamic Modeling
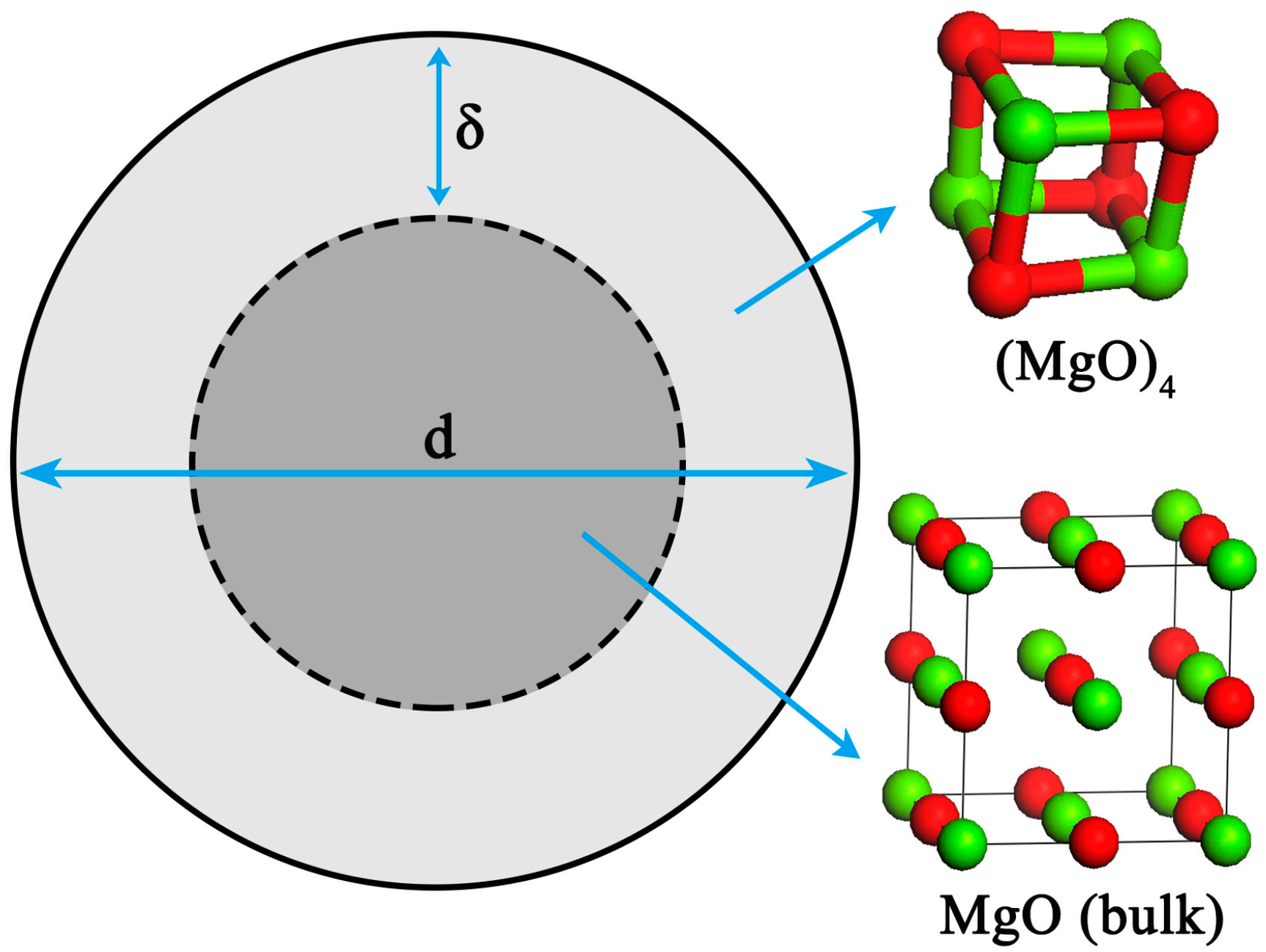
The nano-particle consists of two parts [27,28,29,30]: An internal part (atoms located in the lattice of crystallites) and an external part (atoms situated in the particle surface). In this work, the nano-magnesia, which is described as a sphere particle with diameter d, contains a shell with thickness δ and a core with diameter (d − 2δ), as schematically shown in Figure 1.
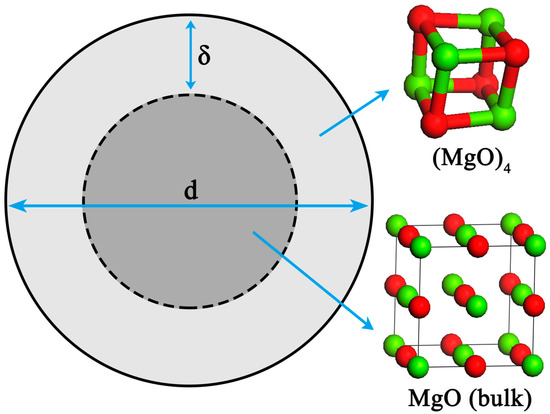
Figure 1.
Structure of nano-MgO.
Some investigations [30,31] of surface structure reported that there is a liquid-like structure layer on the surface of nano-particle. Thomas et al. [32] proved that the nanocrystalline interface is short-range ordered structure by high-resolution transmission electron microscopy. Therefore, the surface structure of the nano-magnesia is a short-range ordered structure and is similar to the structure of (MgO)n clusters. It was reported that the thickness of grain boundary is generally about 2.5–3.5 times of the lattice parameter [33]. The bond length of Mg-O for MgO (bulk) crystal is 0.21 nm. Therefore, the thickness of the nano-MgO surface δ was taken as 0.6 nm in calculation. In addition, the surface of nano-particle usually contains two or three atom layers [34]. In order to simplify our modeling, the (MgO)4 cluster, which contains two-atom layers [35,36,37,38,39], was used to describe the particle surface structure, and the MgO (bulk) crystal was used to describe the structure of particle internal part. Thus, the thermodynamic properties of the internal part of nano-magnesia is the same as the thermodynamic properties of MgO (bulk) crystal, and the thermodynamic properties of the external part of nano-magnesia is the same as the thermodynamic properties of (1/4)(MgO)4.
The total thermodynamic properties of nano-magnesia can be obtained as
where An is total thermodynamic properties of nano-magnesia with size of n nm, As and Ai are the thermodynamic properties of the external and internal part of nano-magnesia, xs is the atomic fraction in the shell of nano-magnesia. The atomic fraction in the shell of nano-magnesia xs can be expressed as
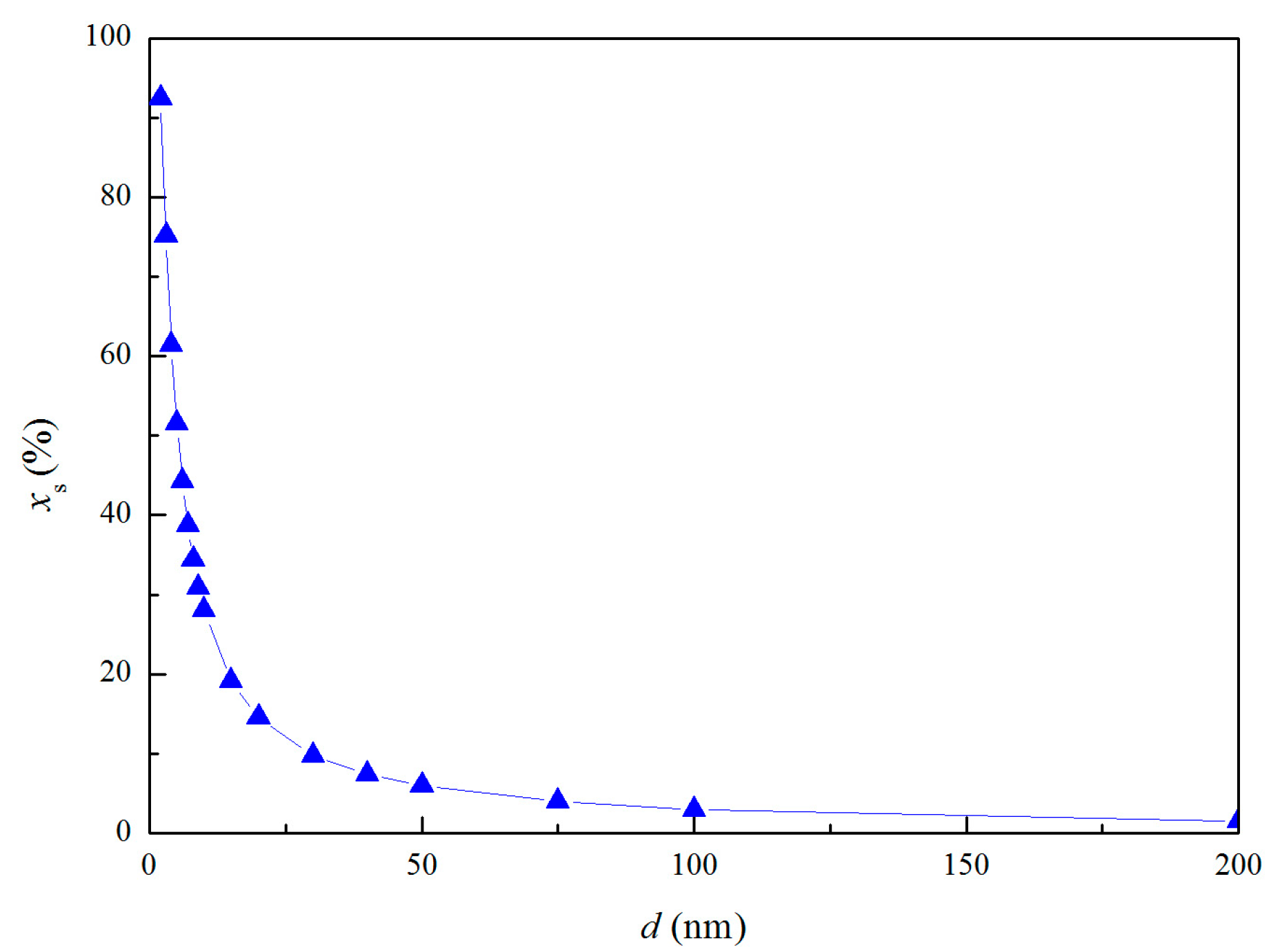
where Ni and Ns are the atom numbers at the inner and surface, respectively. ρi is the atomic densities of internal part. ρs is the atomic densities of surface part. Experiments indicated that the atomic density at particle surface is lower than that of the perfect crystal by 10–30% [28,29]. Thus, the value of ρi/ρs was taken as 1.2 in the calculation of thermodynamic properties of nano-magnesia. The atomic fraction in the shell of nano-magnesia xs are shown in Figure 2.
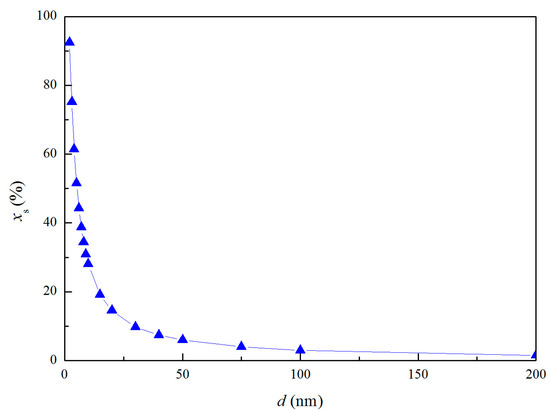
Figure 2.
Relationship between the atomic fraction and the diameter of the MgO nano-particle.
2.2. Calculation Method
In this work, the energy and thermodynamic properties of (MgO)4 clusters, and MgO (bulk) crystal were calculated by Dmol3 module of Materials Studio 7.0 (Accelrys, San Diego, CA, USA), a molecular orbital theory computational program, which was based on density functional theory. The framework of the generalized gradient approximation (GGA) of Perdew, Burke, and Ernzerhof (PBE) [40] is applied in the calculations. The geometry optimization convergence criteria are energy ≤2.7 × 10−4 eV, maximum force ≤ 0.054 eV/Å, and maximum displacement ≤ 0.005 Å. Electrons outside the atomic nucleus are handled by the all-electron method. The atomic orbital basis set of DNP 3.5 is used in the calculations, and the orbital cutoff radius of the DNP basis set equals 5.2 Å. The self-consistent field (SCF) method is used to control the electronic minimization, and the precision of the total energy and charge density equals 2.7 × 10−6 eV.
The thermodynamic properties of (MgO)4 clusters and MgO (bulk) crystal are calculated by using the atomic harmonic vibrational frequency. The heat capacity at constant pressure (CP), enthalpy (H) and entropy (S) are obtained by the vibrational analysis or Hessian evaluation as functions of temperature. The heat capacity CP is computed as [41]
The enthalpy H is calculated as [42]
The entropy S is given by [42]
where the subscripts (trans, rot, vib) at C, H and S stand for translation, rotation and vibration, respectively. R is the ideal gas constant; k is the Boltzmann constant; h is Planck’s constant; T is the absolute temperature; vi is the vibrational frequency. w is the molecular mass; p is the pressure; σ is the symmetry number; x is the molar concentration of the molecules; and Ia(b,c) is the moment of inertia.
3. Results and Discussions
3.1. Thermodynamic Properties of Nano-MgO
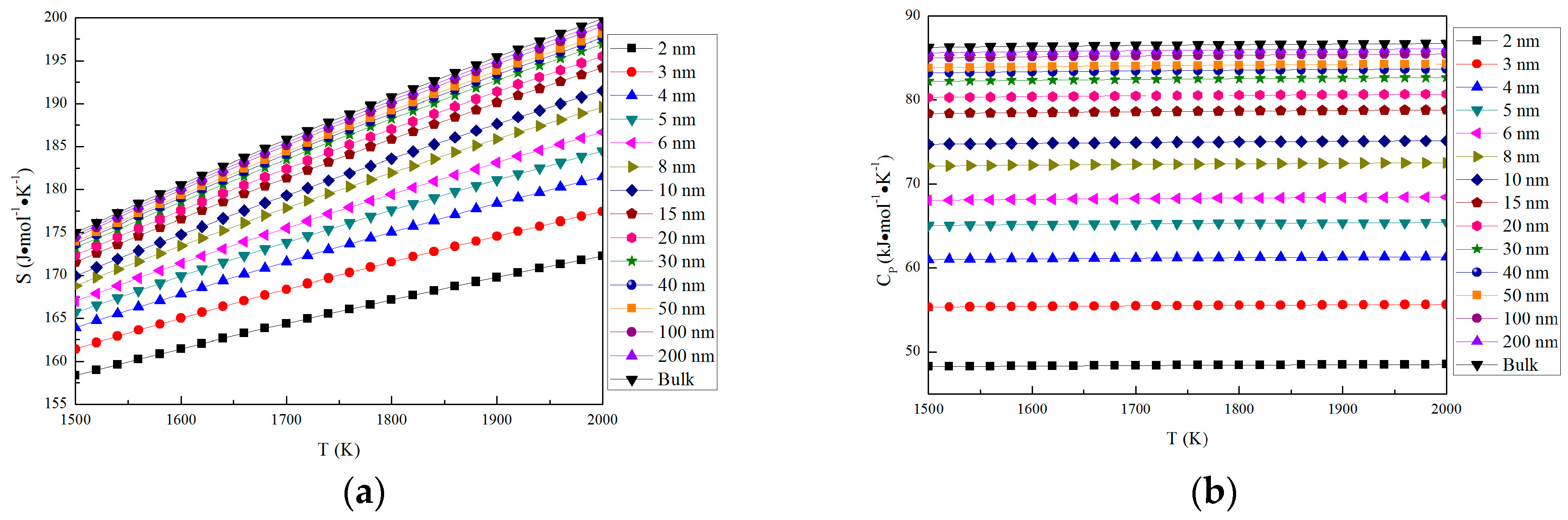
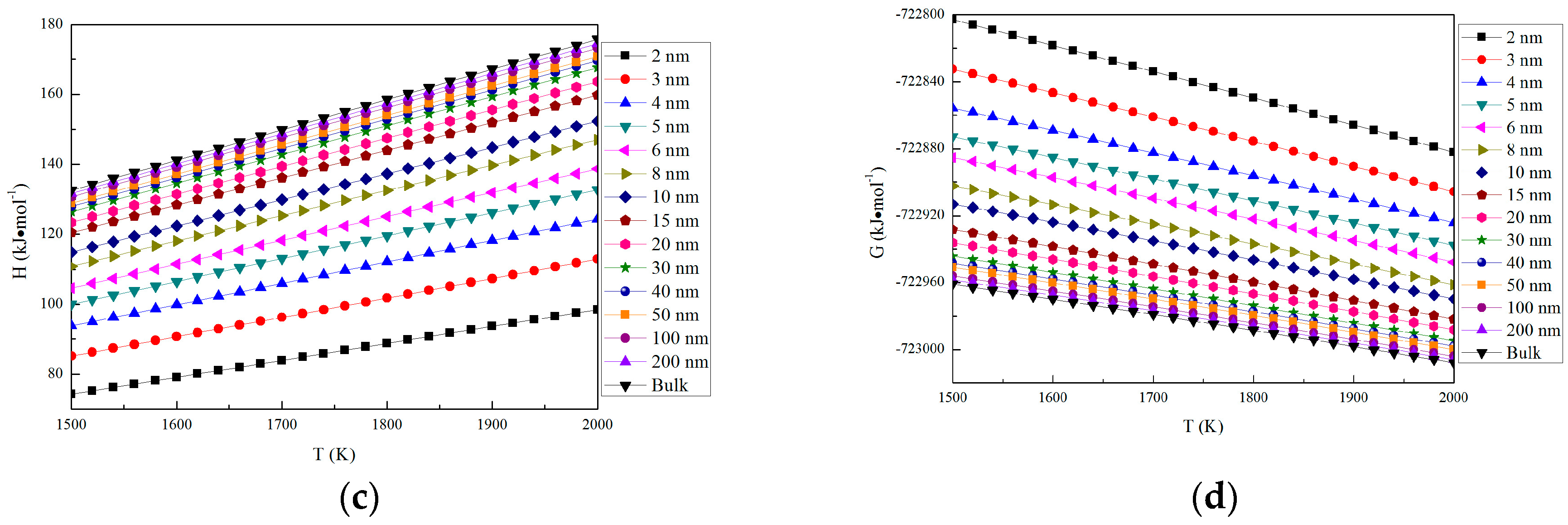
The thermodynamic properties of nano-MgO in the temperature range from 1500 K to 2000 K are shown in Figure 3. The parameters of nano-MgO (H, S and CP) increase with the increasing particle size. In addition, H and S increase with the increasing temperature, while CP is almost a constant in the same particle size at the range from 1500 K to 2000 K. The Gibbs free energy for nano-MgO was calculated by G = E(0 K) + GV, where E (0 K) is the total energy at 0 K and GV is the vibrational free energy. The vibrational free energy was calculated as GV = H − TS. The Gibbs free energy of nano-MgO decrease with the increasing temperature. This result indicates that the stability of MgO increases with the increasing temperature, and the crystals (bulk) is more stable than nano-crystal. The Gibbs free energy of nano-MgO decrease with the increasing particle size. Therefore, the nano-MgO tends to grow up into MgO (bulk).
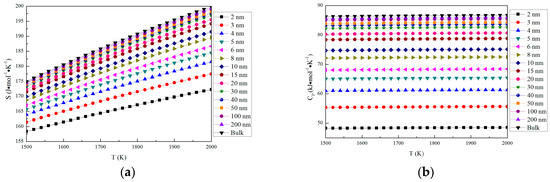
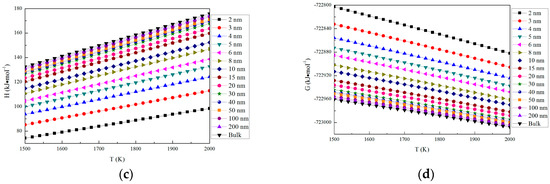
Figure 3.
Thermodynamic properties of nano-MgO and MgO (bulk). (a) S; (b) CP; (c) H; and (d) G.
3.2. Nucleation and Excess Oxygen for Mg-Deoxidation Reaction in Fe-O-Mg Melt
The Gibbs free energy change (∆G) in one mole of the liquid Fe-O-Mg system, when n0 nuclei with radius r are formed, is written as [24]:
where n0 is the number of nuclei in one mole of the liquid Fe-O-Mg system, ∆gM is the Gibbs free energy change of parent liquid iron before and after nucleation per nucleus, ∆gR is the Gibbs free energy change of the magnesia formation reaction of a nucleus, ∆gI is the interface free energy change of a nucleus formation,
In Equation (10), ∆GM can be expressed as
where ai is the activity of element i in Fe-O-Mg melt, the superscripts 1, and 2 show the parent iron phase before nucleation, after nucleation, and in equilibrium with magnesia, respectively, and xi is the initial molar fraction of i. m is equal to (4/3)πr3/VA. Wasai et al. [24] reported that the Gibbs free energy change of parent liquid iron before and after nucleation were almost zero in the small-radius region. Therefore, the ∆GM is neglected in our calculation. ∆GR is written as
where the superscript e shows the parent iron phase in equilibrium with magnesia, Y is the supersaturation ratio of [Mg] and [O] in Fe-O-Mg melt. The value of log K = 7.24, which was reported by Seo et al. [22], was used in the calculation of Y. In Table 2, the Y was calculated in the initial oxygen contents of 0.01, 0.005, 0.0025, and 0.001 mass pct, and initial magnesium contents of 0.01, 0.001, 0.0005, and 0.0001 mass pct. The interfacial free energy between magnesia and liquid iron is calculated as [43]
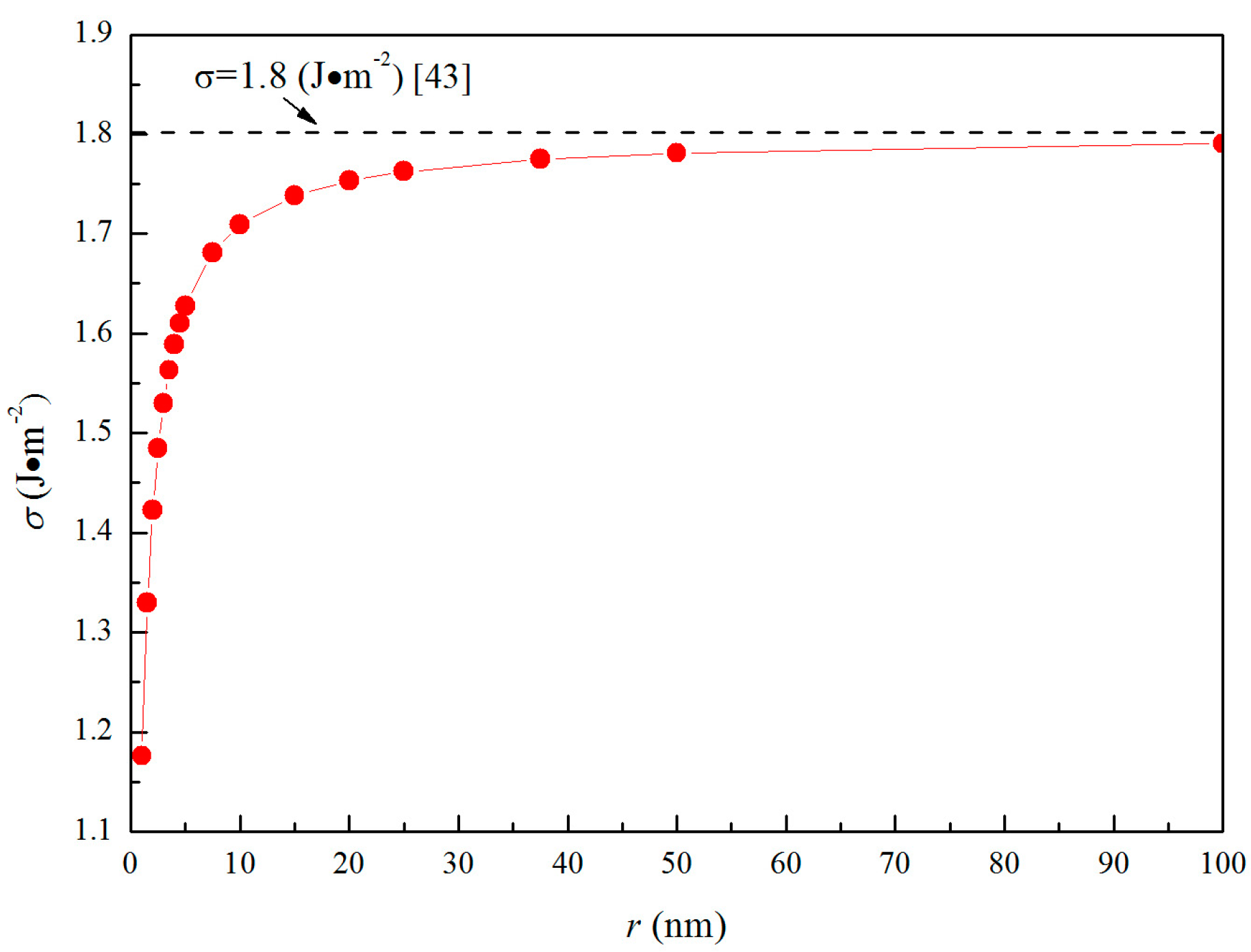
where σI is the surface free energy of magnesia, σM is the surface free energy of molten steel, θIM is the contact angle between magnesia and Fe-O-Mg melt. The value of σ reported by Nakajima et al. [43] is 1.8 J/m2. However, this interfacial free energy is that between magnesia, having zero curvature, and Fe-O-Mg melt. The interfacial free energy of fine inclusion, having a large curvature, should be smaller than the interfacial free energy of large bulk phase such as a plane, having zero curvature. According to Defay and Prigogine [44], the surface free energy between liquid and gas is written as
where σ0 is the surface free energy of a liquid droplet with radius of r, σ is the surface free energy of zero curvature, VA is the molar volume of magnesia, and Г is the surface excess. Here, Г is assumed to be Г = N−1/3VA−2/3 [24], where N is Avogadro’s number. Equation (14) is applicable only to liquid droplets surrounded by the gas phase. However, the relationship between gas and liquid droplets is similar to that between liquid iron and magnesia inclusion because [Mg] and [O] in liquid iron are very low and the bond energy of magnesia is considerably larger than that of liquid iron. Therefore, Equation (14) was used in the estimation of the interfacial free energy between magnesia particle and liquid iron. Figure 4 shows the dependence of σ0 on r. In this way, the interfacial free energy changes ∆GI can be written as

Table 2.
Degree of supersaturation for various contents of oxygen and magnesium.
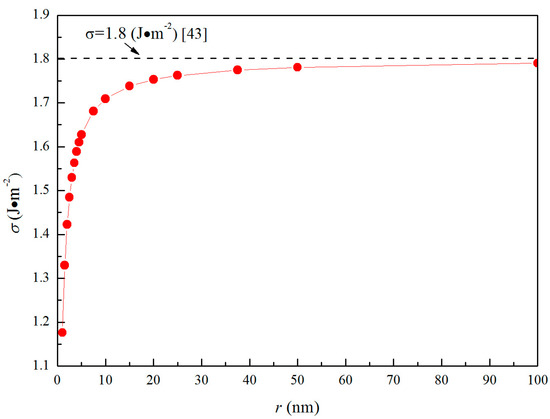
Figure 4.
Interfacial free energies between Fe-O-Mg melt and magnesia.
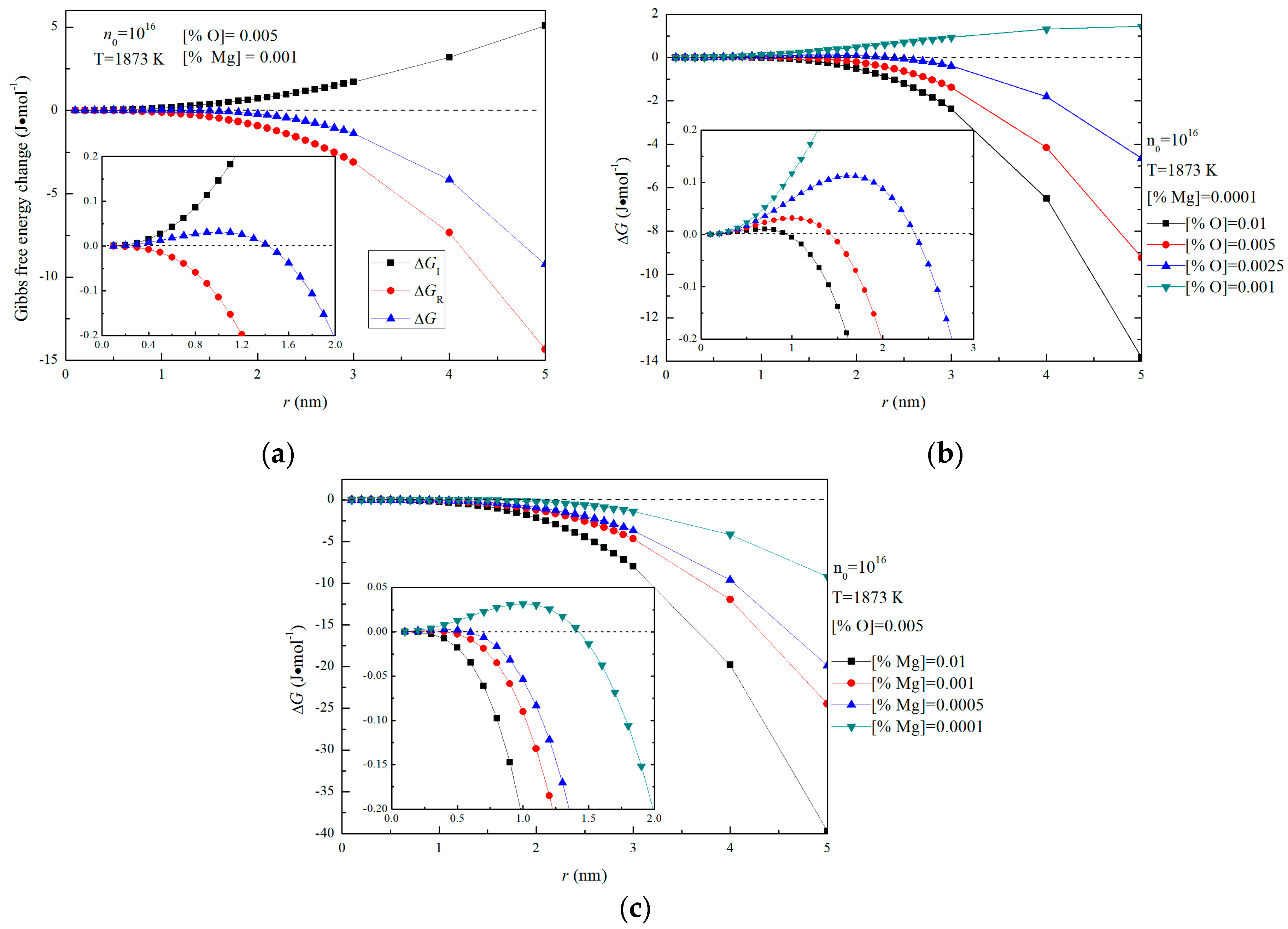
Figure 5a shows the Gibbs free energies changes for the formation of magnesia in Fe-O-Mg melt in the case of initial [%Mg] = 0.001 and [%O] = 0.005. The interfacial free energy ∆GI is positive and becomes larger with increasing size, due not only to the increase of 4πr2, but also to the increase of σ0. Such a result indicates that the energy barrier for the formation of magnesia in liquid iron increases with the increasing size. In contrast, the ∆GR is negative and is the thermodynamic driving force for the formation of magnesia in Fe-O-Mg melt. Since lnY is negative and constant for given initial magnesium and oxygen contents, ∆GR decrease as m increases. However, according to the Equation (12), ∆GR is larger in the case of a lower oxygen or magnesium contents because Y becomes smaller at lower oxygen or magnesium contents. This result means that the thermodynamic driving force for the formation of magnesia decreases as oxygen or magnesium contents decrease.
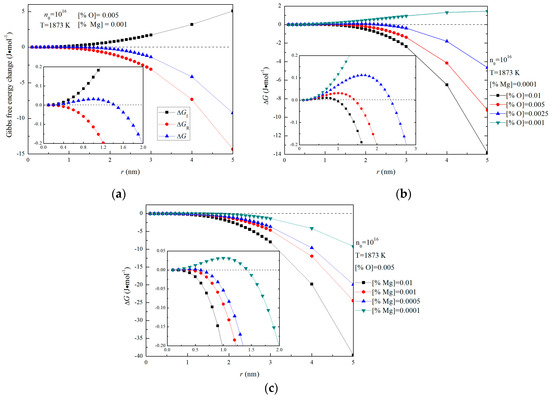
Figure 5.
Gibbs free energy changes for the formation of magnesia with n0 = 1016 in Fe-O-Mg melt, (a) ∆GI, ∆GR and ∆GM for [%Mg] = 0.001 and [%O] = 0.005, (b) Gibbs free energy changes for various initial magnesium contents ([%O] = 0.01, 0.005, 0.0025 and 0.001) and 0.0001 mass pct Mg, (c) Gibbs free energy changes for various initial magnesium contents ([%Mg] = 0.01, 0.001, 0.0005 and 0.0001 ) and 0.005 mass pct O.
Figure 5b,c show the Gibbs free energy changes for the formation of magnesia with various initial oxygen and magnesium contents in Fe-O-Mg melt. Figure 5b is the case of initial [%Mg] = 0.0001, and Figure 5c is the case of initial [%O] = 0.005. Figure 5b shows that ∆G at [%O] = 0.01, [%O] = 0.005 and [%O] = 0.0025 are negative with respect to r, except in the small-radius region, whereas ∆G at [%O] = 0.001 is positive. This result indicates that nucleation of magnesia does not occur at [%O] = 0.001. The critical nucleation radius for [%O] = 0.01, [O%] = 0.005 and [%O] = 0.0025 are 0.8 nm, 1 nm and 1.6 nm. This result means that the critical nucleation radius increase as initial oxygen decrease. In other words, the energy barrier for the formation of magnesia increases as initial oxygen decrease. Figure 5c shows that ∆G at [%Mg] = 0.01 and [%Mg] = 0.001 are negative with respect to r, whereas ∆G at [%Mg] = 0.0005 and [%Mg] = 0.0001 is positive in the small-radius region. This result indicates that nucleation of magnesia can occur easily at [%Mg] = 0.01 and [%Mg] = 0.001. In addition, the critical nucleation radius for [%Mg] = 0.01, [%Mg] = 0.001 and [%Mg] = 0.0005 are 0.3 nm, 0.4 nm, and 1 nm, which indicate that the critical nucleation radius also increases as initial magnesium decreases. Therefore, the supersaturation ratio of [Mg] and [O] in Fe-O-Mg melt is essential for the formation of solid magnesia in Mg-deoxidation process.
Suito and Ohta [45] reported that the experimental and calculated values for the critical degree of supersaturation (Y*) are 8.4 and 280, respectively. In the initial stage of Mg-deoxidation, magnesia can form spontaneously because of the high supersaturation ratio of magnesium and oxygen. As the Mg-deoxidation reaction proceeds, however, the thermodynamic driving force decreases gradually with the decreasing supersaturation ratio. In the final stage of Mg-deoxidation, magnesia is very difficult to nucleate and magnesia nucleus is also very difficult to grow up into bulk magnesia by attracting the surrounding Mg and O because of the low supersaturation ratio of magnesium and oxygen. On the other hand, it is difficult for small magnesia inclusions (or nano-magnesia inclusions) to float up because the probability of collision among small magnesia inclusions is so low that few small magnesia inclusions can grow up. Consequently, lots of small magnesia inclusions can suspend in liquid iron for a long time. The oxygen that exceeds the equilibrium value is referred to as excess oxygen. Wasai and Mukai [24] suggested the suspension of small inclusions is a possible cause of excess oxygen and this excess oxygen should be in the supersaturated state. Therefore, the existence of residual metastable magnesia may be the reason for the supersaturation ratio or the excess oxygen for MgO formation in liquid iron. There is existing metastable magnesia that refers to the excess oxygen, which cannot transform into bulk-magnesia in the Mg-deoxidized melt at the steelmaking temperature. The excess oxygen could consist of two sections: The oxygen in metastable magnesia (such as nano-magnesia), and the oxygen in equilibrium with the metastable magnesia. In order to understand the formation and growth mechanism of magnesia in liquid iron, the Gibbs free energy changes for the formation of nano-MgO in liquid iron are calculated in the next section.
3.3. Gibbs Free Energy Changes for the Formation of Nano-MgO in Liquid Iron
Nano-MgO is the intermediate product of the crystallization of bulk magnesia inclusion in the Mg-deoxidized steel. The formation of nano-MgO can be described as a deoxidation reaction
Then, nano-MgO continues to grow up into stable bulk MgO inclusions. The change from nanoparticle to bulk particle can also be expressed as
[Mg] + [O] = nano-MgO
nano-MgO → MgO (bulk)
Based on Equations (1), (16), and (17), the change of Gibbs free energy for forming nano-magnesia with different size can be calculated as
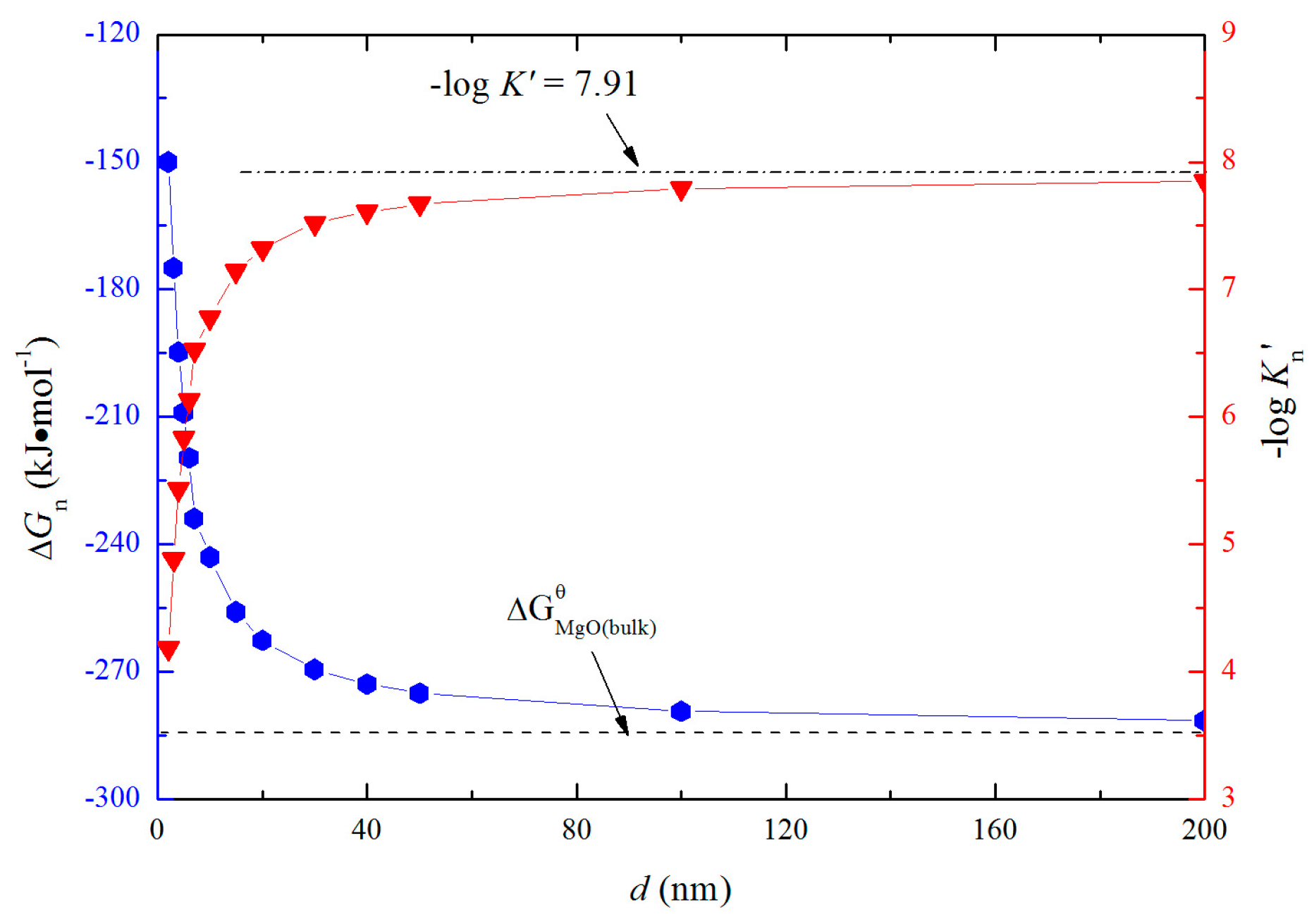
where ΔGn is Gibbs free energy change of the formation of nano-magnesia with size of n nm in Equation (16). is Gibbs free energy change for Equation (1). Gn is Gibbs free energy of nano-magnesia with size of n nm, is Gibbs free energy of MgO (bulk). It can be seen from Figure 6 that the concentrations of [O] and [Mg] in molten iron are at a very low range [%O] < 0.01, [%Mg] < 0.032. Thus, Fe-O-Mg melt is similar to the infinite dilute solution, and the value of log K is close to -log K′. The Gibbs free energy change of Equation (6) can be estimated as . According to the experimental data, the solubility product constants of Mg-deoxidaiton in liquid iron could be obtained at 1873 K. As shown in Figure 6, the value of −logK′ ranges from 4.46 to 7.91. Therefore, in this work, the equilibrium constant of bulk magnesia in liquid iron at 1873 K is taken as log K = 7.91.
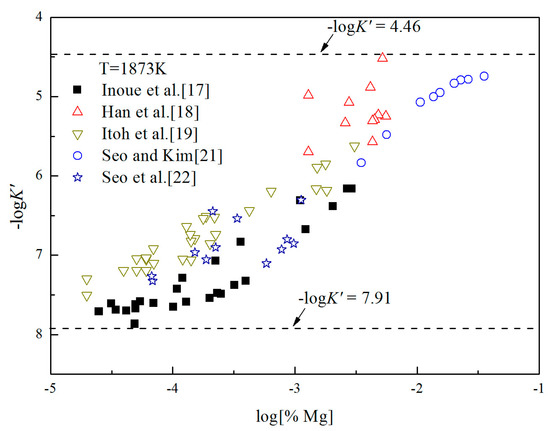
Figure 6.
Apparent equilibrium constants of Mg-deoxidaiton in liquid iron at 1873 K.
According to Equation (18), ΔGn can be obtained and shown in Figure 7. The solubility product of magnesium and oxygen for nano-MgO in liquid iron called as Kn′, and the value of −logKn′ ranges from 4.18 to 7.91 is similar to the value of −logK′. The value of −logKn′ increases with the increasing size of nano-MgO product. Such a result indicates that the thermodynamic relationship between the nano-MgO and liquid iron are gradually close to the final equilibrium between bulk magnesia and liquid iron with the increasing magnesia products size during the growth process. It may be the reason why the equilibrium constants obtained by various researchers are different to each other.
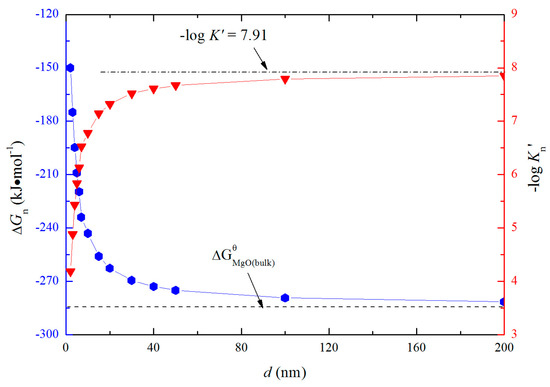
Figure 7.
Solubility product of magnesium and oxygen and Gibbs free energy change for nano-magnesia forming in liquid iron at 1873 K.
3.4. Multi-Equilibria Thermodynamics of MgO in Liquid Iron
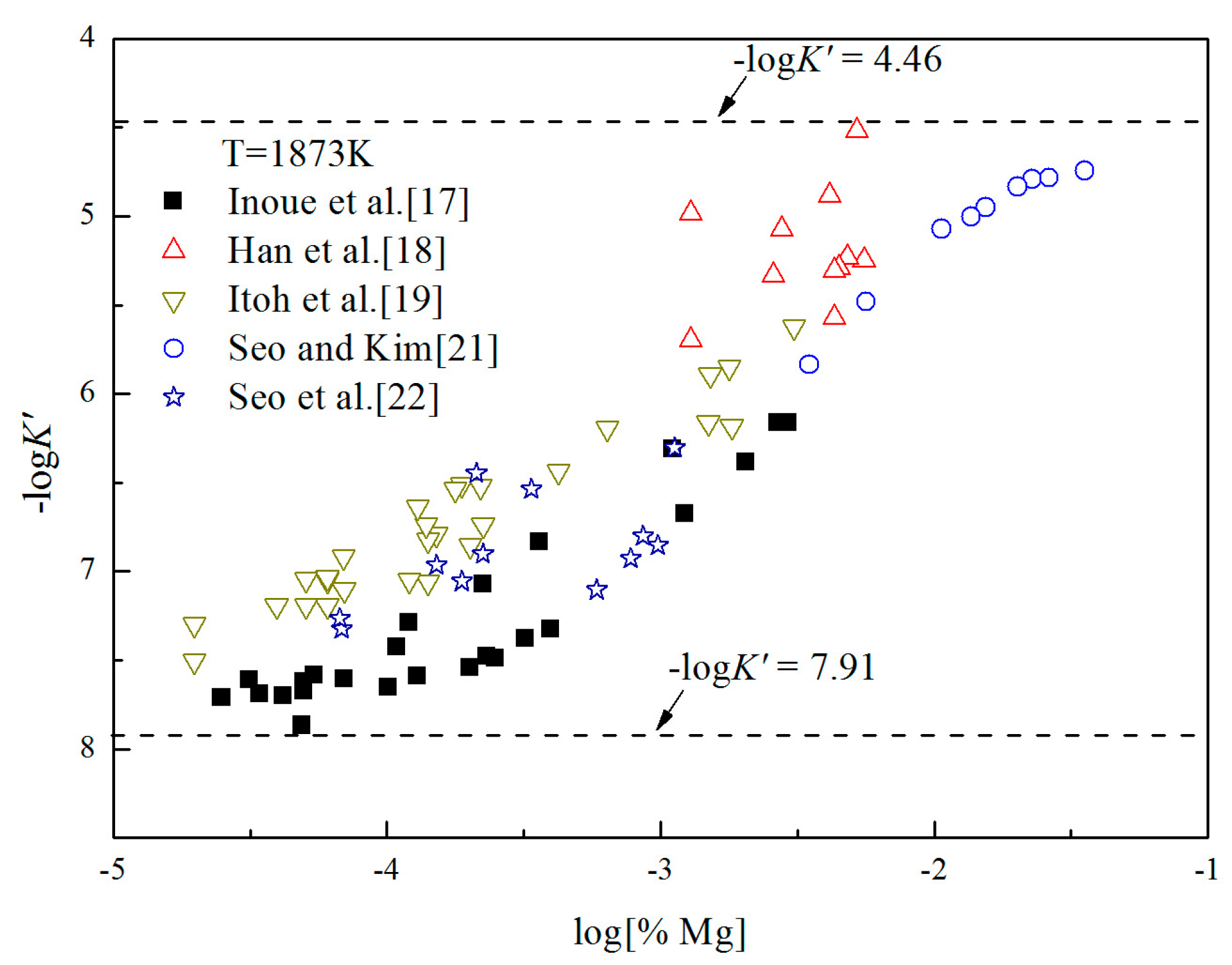
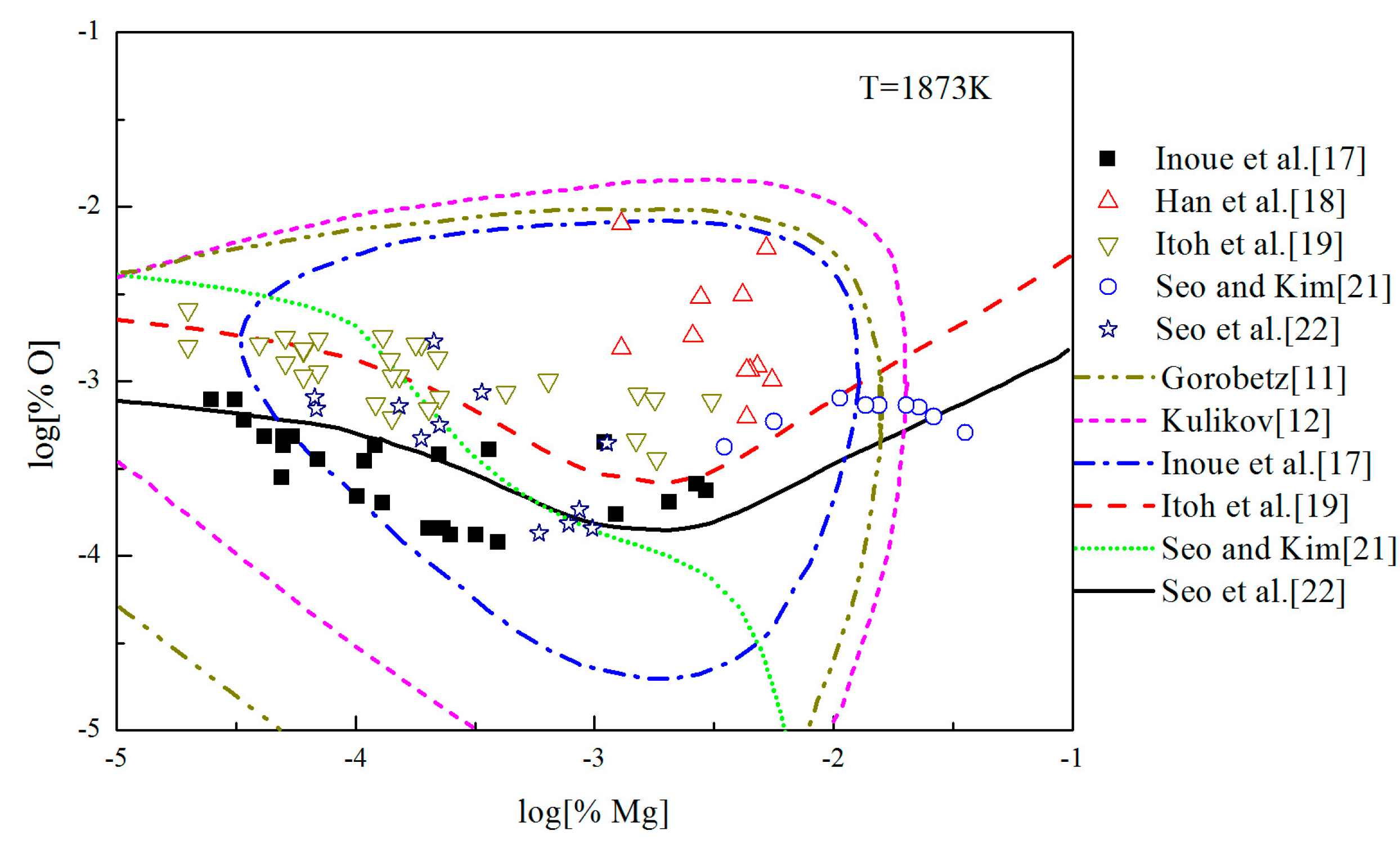
The Mg-deoxidation equilibrium in liquid iron has been investigated by Seo and Kim [21], Itoh et al. [22], and other researchers [17,18,19], and the Mg–O relationships are shown in Figure 8. Han et al. [18] and Seo and Kim [21] held their deoxidation experiments in a closed MgO crucible with initially containing several hundred parts per million oxygen by using Mg vapor. Similar experiments were performed by Itoh et al. [19] in open dolomite crucibles under a mixture atmosphere of argon and hydrogen in a high frequency induction furnace. Seo et al. [22] held their experiments in a specially designed high frequency induction furnace for a strong agitation of melt by adding Ni-Mg alloys. As shown in Figure 8, the equilibrium concentration of [Mg] are fluctuating within the range of 1 × 10−5 < [%Mg] < 0.039 in equilibrium with solid MgO. Moreover, the equilibrium concentration of [O] decreases with the increasing [%Mg] when the equilibrium concentration [%Mg] is <0.001, but the equilibrium concentration of [O] increases with the increasing [%Mg] when the equilibrium concentration [%Mg] is >0.001. In addition, the difference among the equilibrium concentrations of [O] at the same concentration of [Mg] is more than one order of magnitude.
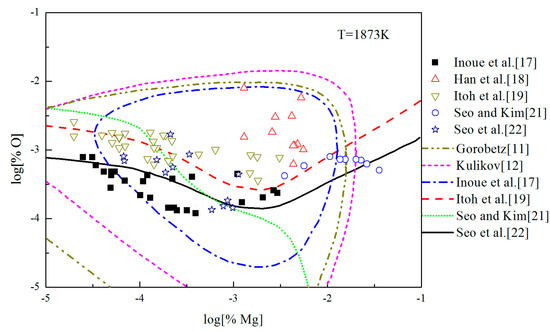
Figure 8.
Equilibrium experiments of Mg-deoxidation in liquid iron at 1873 K.
In order to describe the thermodynamic equilibrium rule of Mg-deoxidation in 1873 K, many researchers [11,12,17,19,21,22] gave the different thermodynamic equilibrium curves plotted as lines in Figure 8. It should be noted that the Mg-deoxidation equilibrium relation proposed by Gorobetz et al. [11], Kulikov et al. [12], and Inoue et al. [17] are oval-shaped. This means that one concentration of Mg corresponds to two concentrations of oxygen and vice versa. Moreover, the equilibrium relation of Mg-deoxidation proposed by Seo and Kim [21] is a monotone decreasing function even when the equilibrium concentration of [%Mg] > 0.001. Such a difference between predicted result and measured result in reference [21] comes from an incomplete expression of the activities of dissolved magnesium and oxygen. The equilibrium relations between [Mg] and [O] proposed by Itoh et al. [19] and Seo et al. [22] have a "V" shape. Their equilibrium curves descend with the increasing concentration of [%Mg] and agree well with their equilibrium experiment data when the equilibrium concentration of [%Mg] < 0.001. However, it is impossible to describe all the experiments data by one curve. Jung et al. [46] used the associated solution model to describe the Mg-deoxidation in liquid iron, and suggested that the [Mg] and [O] atoms could not be independently or randomly distributed, but had a strong tendency to form dissolved associated compound Mg–O, etc., as a kind of metastable phase. Thus, it can be suggested that the thermodynamics of Mg-deoxidaiton reaction in liquid iron has a close relationship with that of metastable phase, such as dissolved associated compound Mg-O, nano-MgO, etc.
Wang et al. [25,47,48] suggested that the formation of inclusions in molten steel follows a two-step nucleation mechanism. Firstly, the deoxidizers react with dissolved oxygen in molten steel to form various metastable structures, and then the metastable structures transform into stable crystal. This fact has been proved by several experiments [49,50]. Wasai et al. [49] found a series of nanoscale alumina and silica in Al-deoxidation experiments by ultra-rapid cooling methods. Zhao et al. [50] found some nanoscale alumina in Al-deoxidation experiments by the same method. Therefore, the metastable magnesia forms at first in the process of Mg-deoxidation. Then, the metastable magnesia grows up into bulk magnesia. However, it is suggested that the deoxidation reaction in liquid iron is very difficult to reach the final equilibrium between bulk inclusion and liquid iron because of low supersaturation, and the products of deoxidation reaction may be various metastable inclusions in many cases [25,47,48].
As discussed in Section 3.2, nanoscale magnesia is difficult to grow up into the final bulk magnesia at the final stage of Mg-deoxidation. Therefore, it is difficult for the Mg-deoxidation reaction to reach final thermodynamic equilibrium, so maybe the experiments in previous literatures do not all reach the equilibrium state between bulk magnesia and liquid iron, but in multi-equilibria between bulk-, nano-MgO, and liquid iron. In other words, the thermodynamic difference of Mg-deoxidation proposed by different researchers is caused by the different metastable magnesia, and these various experimental data may come from the different thermodynamic states.
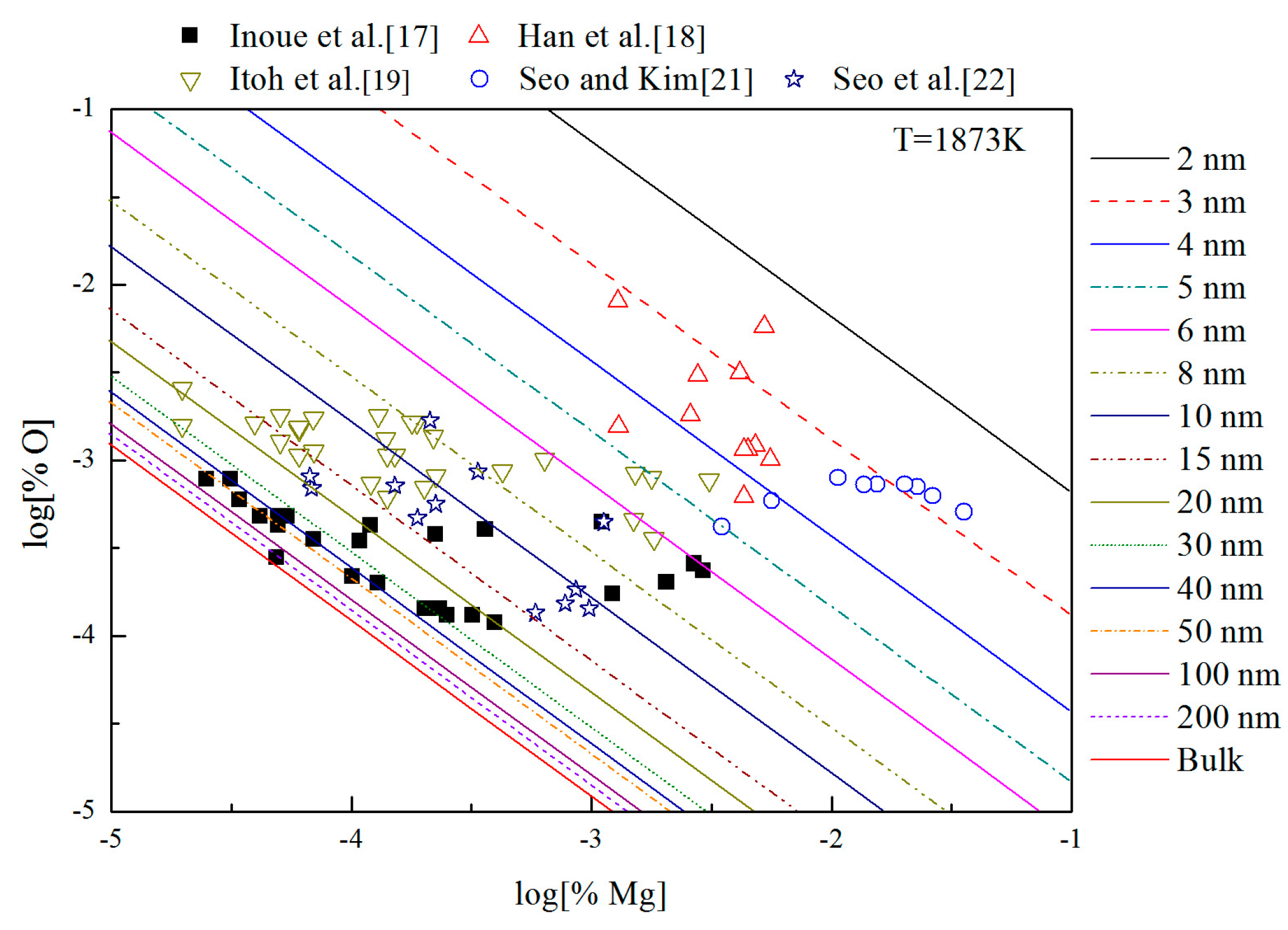
Figure 9 shows the thermodynamic curves of nano-MgO in liquid iron during the Mg-deoxidation process at 1873 K. All the experimental data are covered by the region between thermodynamic curves of bulk-magnesia and 2 nm magnesia. This fact suggested that the experiments by various researchers are in different thermodynamic state. Some of these experiments are in equilibrium with bulk-magnesia, while most of these experiments do not reach the equilibrium state between bulk magnesia and liquid iron, but in quasi-equilibria between nano-magnesia and liquid iron. It is suggested that the equilibrium reaction product should be nanoscale magnesia, but not bulk-magnesia in most of these equilibrium experiments. In addition, the nano-magnesia thermodynamic curves are close to the bulk-magnesia equilibrium curve gradually with the increase of magnesia inclusion size. It indicates that the Mg-deoxidation reaction is gradually closer to the final equilibrium. This is the reason why the various previous Mg-deoxidation experimental data are different to each other in cases of the same concentration of [Mg].
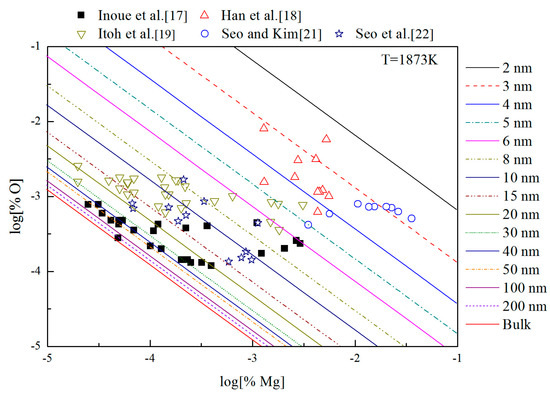
Figure 9.
Thermodynamic curves of nano-magnesia in liquid iron at 1873 K.
4. Conclusions
The present work developed a thermodynamic modeling for Mg-deoxidation reaction between nano-magnesia inclusion and liquid iron, and performed calculations of the thermodynamic properties for nano-magnesia. The conclusions are as follows:
- (1)
- It is difficult for nano-magnesia to grow up into final bulk magnesia in final stage of Mg-deoxidation in liquid iron. The existence of the residual nanoscale magnesia is one of the important reason for the supersaturation ratio or the excess oxygen for MgO formation in liquid iron.
- (2)
- Numerical calculation results suggest that the solubility product of magnesium and oxygen for nano-MgO in liquid iron increased with the increasing magnesia products size. The experimental data about Mg-deoxidation in liquid iron are covered by the region between the thermodynamic curves of 2 nm magnesia and bulk-magnesia.
- (3)
- The previous Mg-deoxidation experiments are in the different thermodynamic states, and many previous experiments are close to the final equilibrium between bulk magnesia and liquid iron, but do not reach the final equilibrium because their partial product is nano-magnesia.
Author Contributions
Investigation, Y.X.; Methodology, Y.X., H.L., G.W.; Project administration and funding acquisition, H.L., G.W. and Q.W.; Writing—original draft, Y.X.; Writing—review & editing, H.L., G.W.; Providing ideas, B.Y., Q.W. and W.J.
Funding
This paper was supported by the National Natural Science Foundation of China and Shanghai Baosteel (Grant No. U1460108), National Natural Science Foundation of China (Grant No. 51874170, 51604253 and 51634004) and the Fundamental Research Funds for the Central Universities (N170906004).
Acknowledgments
We acknowledge the funding support from National Natural Science Foundation of China and Shanghai Baosteel (Grant No. U1460108), National Natural Science Foundation of China (Grant No. 51874170, 51604253 and 51634004), Fundamental Research Funds for the Central Universities (N170906004).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jiang, Z.; Zhuang, Y.; Li, Y.; Li, S. Effect of modification treatment on inclusions in 430 stainless steel by Mg-Al alloys. J. Iron Steel Res. Int. 2013, 20, 6–10. [Google Scholar] [CrossRef]
- Wang, L.; Yang, S.; Li, J.; Zhang, S.; Ju, J. Effect of Mg addition on the refinement and homogenized distribution of Inclusions in steel with different Al contents. Metall. Mater. Trans. B 2017, 48, 805–818. [Google Scholar] [CrossRef]
- Liu, C.; Yagi, M.; Gao, X.; Kim, S.J.; Huang, F.; Ueda, S.; Kitamura, S. Kinetics of transformation of Al2O3 to MgO·Al2O3 spinel inclusions in Mg-Containing steel. Metall. Mater. Trans. B 2018, 49, 113–122. [Google Scholar] [CrossRef]
- Zhang, T.; Min, Y.; Liu, C.; Jiang, M. Effect of Mg addition on the evolution of inclusions in Al-Ca deoxidized melts. ISIJ Int. 2015, 55, 1541–1548. [Google Scholar] [CrossRef]
- Ma, W.; Bao, Y.; Wang, M.; Zhao, L. Effect of Mg and Ca treatment on behavior and particle size of inclusions in bearing steels. ISIJ Int. 2014, 54, 536–542. [Google Scholar] [CrossRef]
- Lowe, J.H.; Mitchell, A. Zero Inclusion Steels. In Proceedings of the Clean Steel: Superclean Steel-Conference Proceedings, London, UK, 6–7 March 1995; p. 223. [Google Scholar]
- Sarma, D.S.; Karasev, A.V.; Jonsson, P.G. On the role of non-metallic inclusions in the nucleation of acicular ferrite in steels. ISIJ Int. 2009, 49, 1063–1074. [Google Scholar] [CrossRef]
- Liu, Z.; Kobayashi, Y.; Yin, F.; Kuwabara, M.; Nagai, K. Nucleation of acicular ferrite on sulfide inclusion during rapid solidification of low carbon steel. ISIJ Int. 2007, 47, 1781–1788. [Google Scholar] [CrossRef]
- Lupis, C.H.P.; Elliott, J.F. Generalized interaction coefficients: Part I: Definitions. Acta Metall. 1966, 14, 529–538. [Google Scholar] [CrossRef]
- Sigworth, G.K.; Elliott, J.F. The thermodynamics of liquid dilute iron alloys. Metal Sci. 1974, 8, 298–310. [Google Scholar] [CrossRef]
- Gorobets, A.P. Investigation into the thermodynamics of the deoxidation of iron by a magnesium solution. Metall. Koksokhim 1980, 69, 34–37. [Google Scholar]
- Kulikov, I.S. Iron deoxidation by alkaline-earth metals. Izv. Akad. Nauk SSSR Met. 1985, 6, 9–15. [Google Scholar]
- Nadif, M.; Gatellier, C. Influence of calcium or manganese on the solubility of oxygen and sulphur in liquid steel. Rev. Metall. CIT 1986, 83, 377–394. [Google Scholar] [CrossRef]
- Turkdogan, E.T. Possible failure of emf oxygen sensor in liquid iron containing dissolved calcium or magnesium. Steel Res. 1991, 62, 379–382. [Google Scholar] [CrossRef]
- Satoh, N.; Taniguchi, T.; Mishima, S.; Oka, T.; Miki, T.; Hino, M. Prediction of nonmetallic inclusion formation in Fe-40mass% Ni-5mass% Cr alloy production process. Tetsu-to-Hagane 2009, 95, 827–836. [Google Scholar] [CrossRef]
- Teplitskii, E.B.; Vladimirov, L.P. Thermodynamics of the Interaction of Mg with O in an Fe Melt. Zh. Fiz. Khim 1977, 51, 831–833. [Google Scholar]
- Inoue, R.; Suito, H. Thermodynamics of O, N, and S in liquid Fe equilibrated with CaO-Al2O3-MgO slags. Metall. Mater. Trans. B 1994, 25, 235–244. [Google Scholar] [CrossRef]
- Han, Q.; Zhou, D. Determination of dissolved sulfur and Mg-S, Mg-O equilibria in molten iron. Steel Res. 1997, 68, 9–14. [Google Scholar] [CrossRef]
- Itoh, H.; Hino, M.; Ban-Ya, S. Thermodynamics on the formation of spinel nonmetallic inclusion in liquid steel. Metall. Mater. Trans. B 1997, 28, 953–956. [Google Scholar] [CrossRef]
- Ohta, H.; Suito, H. Deoxidation equilibria of calcium and magnesium in liquid iron. Metall. Mater. Trans. B 1997, 28, 1131–1139. [Google Scholar] [CrossRef]
- Seo, J.D.; Kim, S.H. Thermodynamic assessment of Mg deoxidation reaction of liquid iron and equilibria of [Mg]-[Al]-[O] and [Mg]-[S]-[O]. Steel Res. 2000, 71, 101–106. [Google Scholar] [CrossRef]
- Seo, W.G.; Han, W.H.; Kim, J.S. Deoxidation equilibria among Mg, Al and O in liquid iron in the presence of MgO· Al2O3 spinel. ISIJ Int. 2003, 43, 201–208. [Google Scholar] [CrossRef]
- Xiao, Y.; Lei, H.; Yang, B.; Wang, G.; Wang, Q.; Jin, W. Nucleation and growth for magnesia inclusion in Fe-O-Mg melt. RSC Adv. 2018, 8, 38336. [Google Scholar] [CrossRef]
- Wasai, K.; Mukai, K. Thermodynamics of nucleation and supersaturation for the aluminum-deoxidation reaction in liquid iron. Metall. Mater. Trans. B 1999, 49, 1065–1074. [Google Scholar] [CrossRef]
- Wang, G.; Wang, Q.; Li, S.; Ai, X.; Li, D. A Multi-step thermodynamic model for alumina formation during aluminum deoxidation in Fe-O-Al melt. Acta Metall. Sin. 2015, 28, 272–280. [Google Scholar] [CrossRef]
- Zong, N.; Yang, L.; He, P. Learning about the nucleation pathway of MgO·Al2O3 spinel from an Fe-O-Al-Mg melt using a two-step nucleation mechanism. RSC Adv. 2015, 5, 48382–48390. [Google Scholar] [CrossRef]
- Meng, Q.; Zhou, N.; Rong, Y.; Chen, S.; Hsu, T.Y. Size effect on the Fe nanocrystalline phase transformation. Acta Mater. 2002, 50, 4563–4570. [Google Scholar] [CrossRef]
- Zu, X.; Birringer, R.; Herr, U.; Gleiter, H. X-ray diffraction studies of the structure of nanometer-sized crystalline materials. Phys. Rev. B 1987, 35, 9085. [Google Scholar] [CrossRef]
- Sui, M.; Lu, K.; Deng, W.; Xiong, L.; Patu, S.; He, Y. Positron-lifetime study of polycrystalline Ni-P alloys with ultrafine grains. Phys. Rev. B 1991, 44, 6466. [Google Scholar] [CrossRef]
- Herr, U. Metastable phases in interface controlled materials. Contemp. Phys. 2000, 41, 93–104. [Google Scholar] [CrossRef]
- Dash, J.G. Surface melting. Contemp. Phys. 1989, 30, 89–100. [Google Scholar] [CrossRef]
- Thomas, G.J.; Siegel, R.W. Grain boundaries in nanophase palladium: High resolution electron microscopy and image simulation. Scr. Metall. 1990, 24, 201–206. [Google Scholar] [CrossRef]
- Waniewska, A.S.; Greneche, J.M. Magnetic interfaces in Fe-based nanocrystalline alloys determined by Mössbauer spectrometry. Phys. Rev. B 1997, 56, 8491. [Google Scholar] [CrossRef]
- Phillpot, S.R.; Wolf, D.; Gleiter, H. Molecular dynamics study of the synthesis and characterization of a fully dense, three-dimensional nanocrystalline material. J. Appl. Phys. 1995, 78, 847–861. [Google Scholar] [CrossRef]
- Chen, M.; Andrew, R.F.; Dixon, D.A. Structures and Stabilities of (MgO)n Nanoclusters. J. Phys. Chem. A 2014, 118, 3136–3146. [Google Scholar] [CrossRef] [PubMed]
- Haertelt, M.; Fielicke, A.; Meijer, G.; Kwapien, K.; Sierka, M.; Sauer, J. Structure determination of neutral MgO clusters-hexagonal nanotubes and cages. Phys. Chem. Chem. Phys. 2012, 14, 2849–2856. [Google Scholar] [CrossRef] [PubMed]
- Kwapien, K.; Sierka, M.; Dobler, J.; Sauer, J. Reactions of H2, CH4, C2H6, and C3H8 with [(MgO)n]+ clusters studied by density functional theory. ChemCatChem 2010, 2, 819–826. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, Y.H.; Song, Y.; Chen, H.S. Structures and bonding characters of (MgO)3n (n = 2-8) clusters. J. Phys. B At. Mol. Opt. Phys. 2014, 47, 355–359. [Google Scholar] [CrossRef]
- Gueddim, A.; Bouarissa, N.; Villesuzanne, A. First-principles determination of structural properties of MgO. Phys. Scr. 2009, 80, 055702. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]
- Wilson, E.B.; Decius, J.C.; Cross, P.C. Molecular Vibrations; Dover: New York, NY, USA, 1980. [Google Scholar]
- Hirano, T. MOPAC Manual, 7th ed.; Stewart, J.J.P., Ed.; US Air Force Academy: Colorado Springs, CO, USA, 1993. [Google Scholar]
- Nakajima, K. Estimation of interfacial tensions between phases in the molten iron-slag-inclusion (alumina) system. Tetsu-to-Hagané 1994, 80, 383–388. [Google Scholar] [CrossRef]
- Defay, R.; Prigogine, I. Surface Tension and Adsorption, Translated by D.H. Everet; John Wiley and Sons, Inc.: New York, NY, USA, 1966; p. 319. [Google Scholar]
- Suito, H.; Ohta, H. Characteristics of particle size distribution in early stage of deoxidation. ISIJ Int. 2006, 46, 33–41. [Google Scholar] [CrossRef]
- Jung, I.H.; Decterov, S.A.; Pelton, A.D. A thermodynamic model for deoxidation equilibria in steel. Metall. Mater. Trans. B 2004, 35, 493–507. [Google Scholar] [CrossRef]
- Wang, G.C.; Xiao, Y.Y.; Zhao, C.M.; Li, J.; Shang, D.L. Atomic cluster aggregates in nucleation of solid alumina inclusion in the aluminum deoxidation for liquid iron. Metall. Mater. Trans. B 2018, 49, 282–290. [Google Scholar] [CrossRef]
- Wang, G.C.; Wang, Q.; Li, S.L.; Ai, X.G.; Fan, C.G. Evidence of multi-step nucleation leading to various crystallization pathways from an Fe-O-Al melt. Sci. Rep. 2014, 4, 5082. [Google Scholar] [CrossRef] [PubMed]
- Wasai, K.; Mukai, K.; Miyanaga, A. Observation of inclusion in aluminum deoxidized iron. ISIJ Int. 2002, 42, 459–466. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, G.; Shang, D.; Lei, H.; Wang, Q.; Cao, L. Mechanisms on superfine alumina inclusions formation by Al-deoxidation reaction for liquid iron. Steel Res Int. 2018, 89, 18000255. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).