Copper Extraction from Black Copper Ores through Modification of the Solution Potential in the Irrigation Solution
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
Sample Characterization
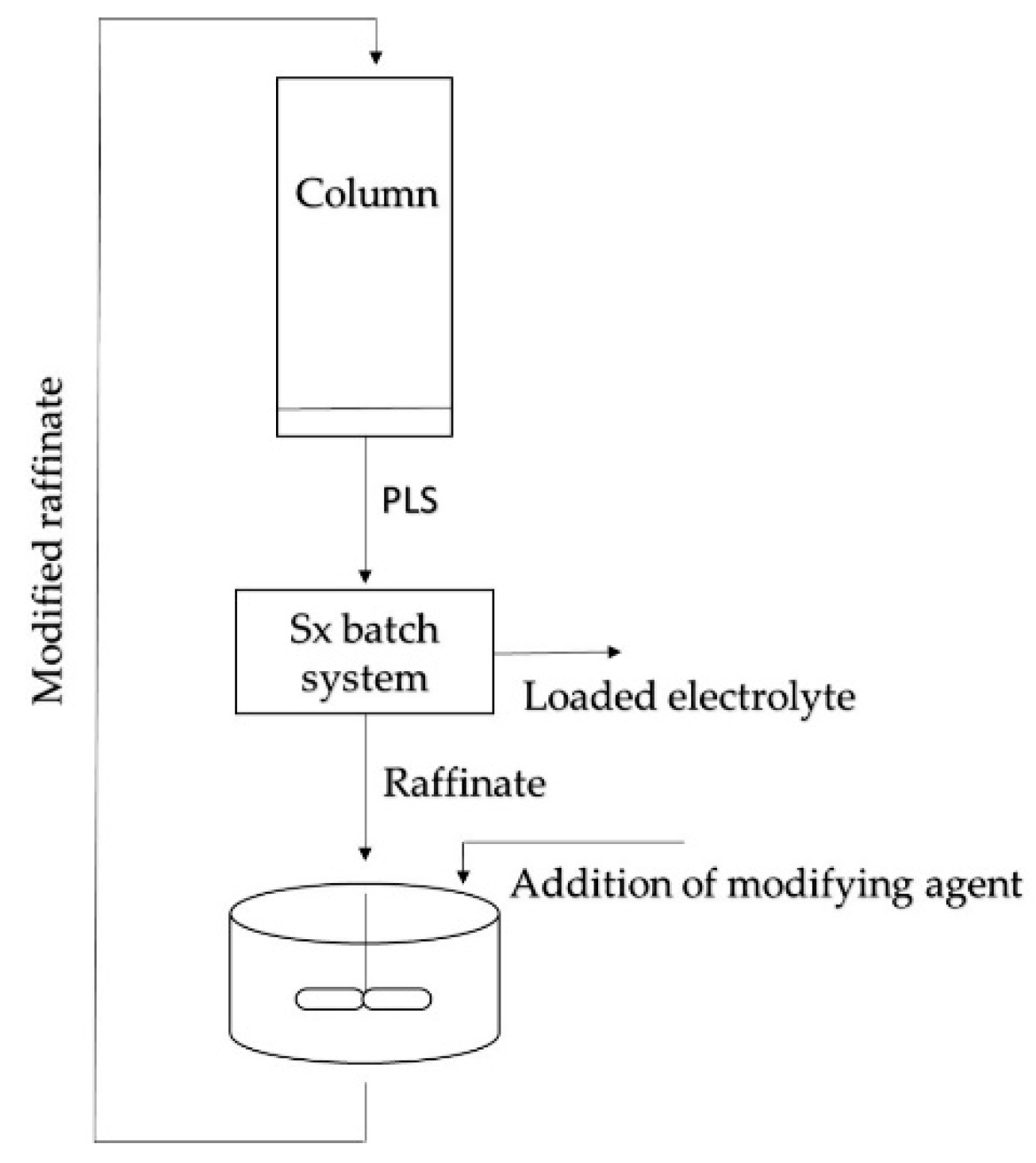
2.2. Experimental Procedure
2.2.1. ISO-pH Experiments
2.2.2. Column Leaching Test
3. Results and Discussion
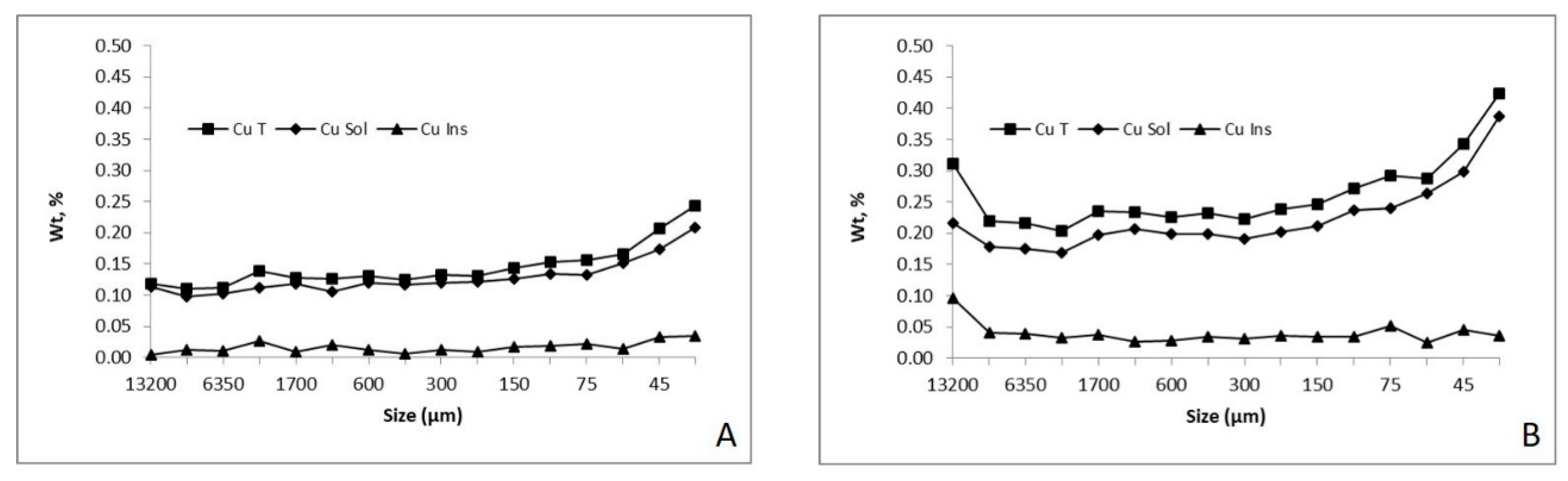
3.1. Mineral Characterization
3.2. Leaching Test Pretreatment
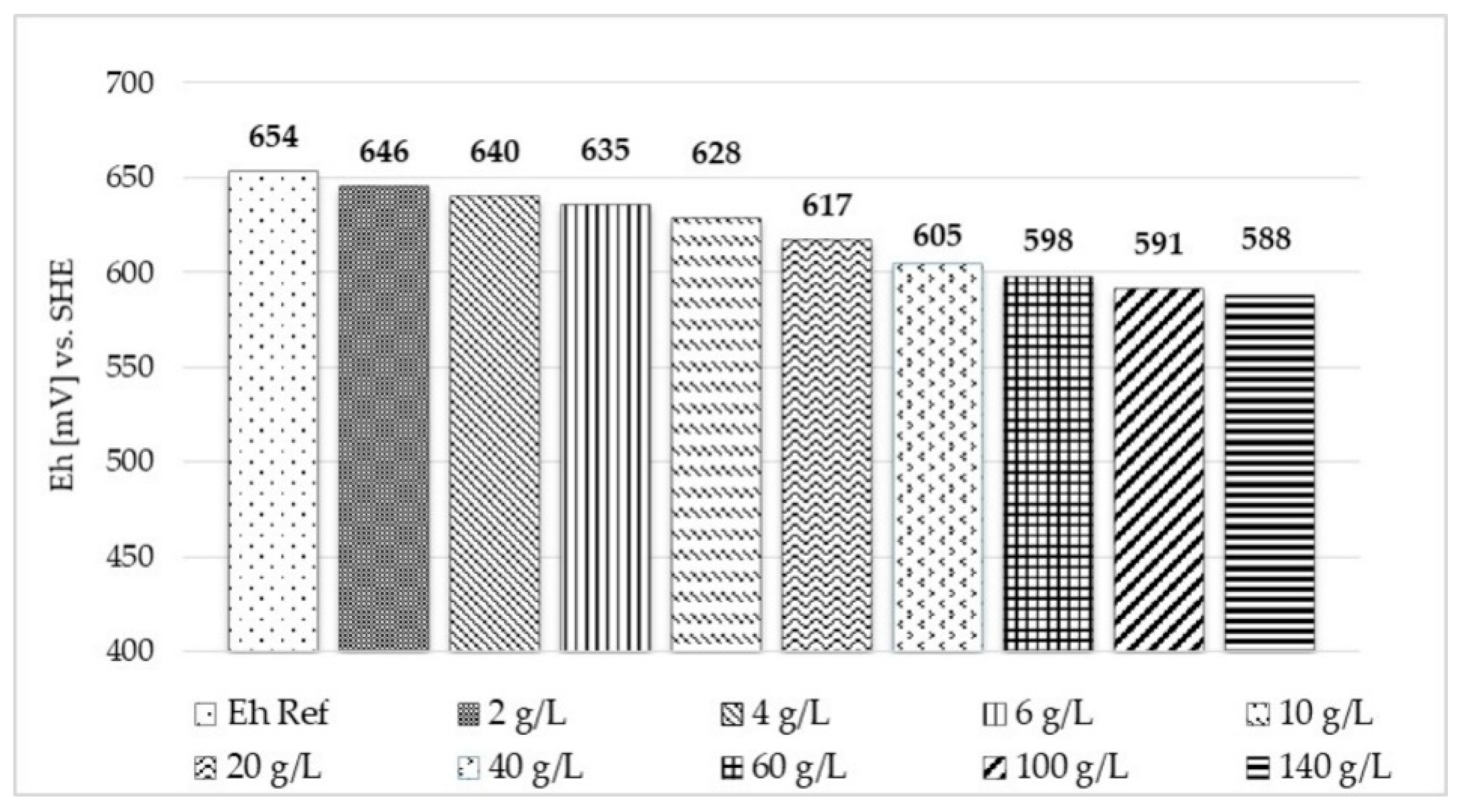
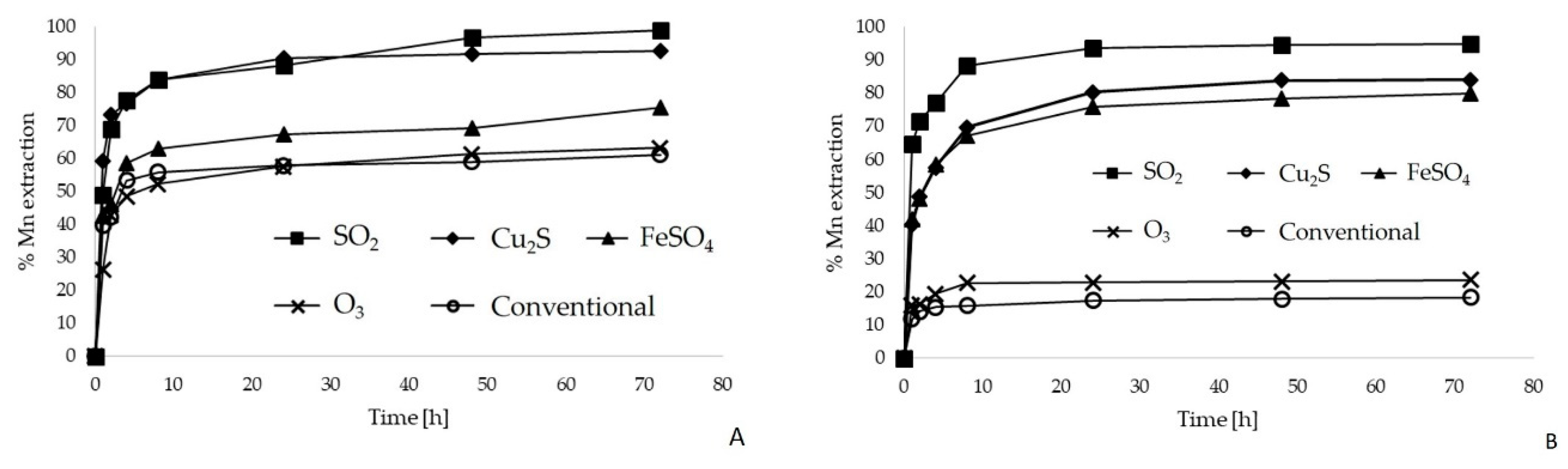
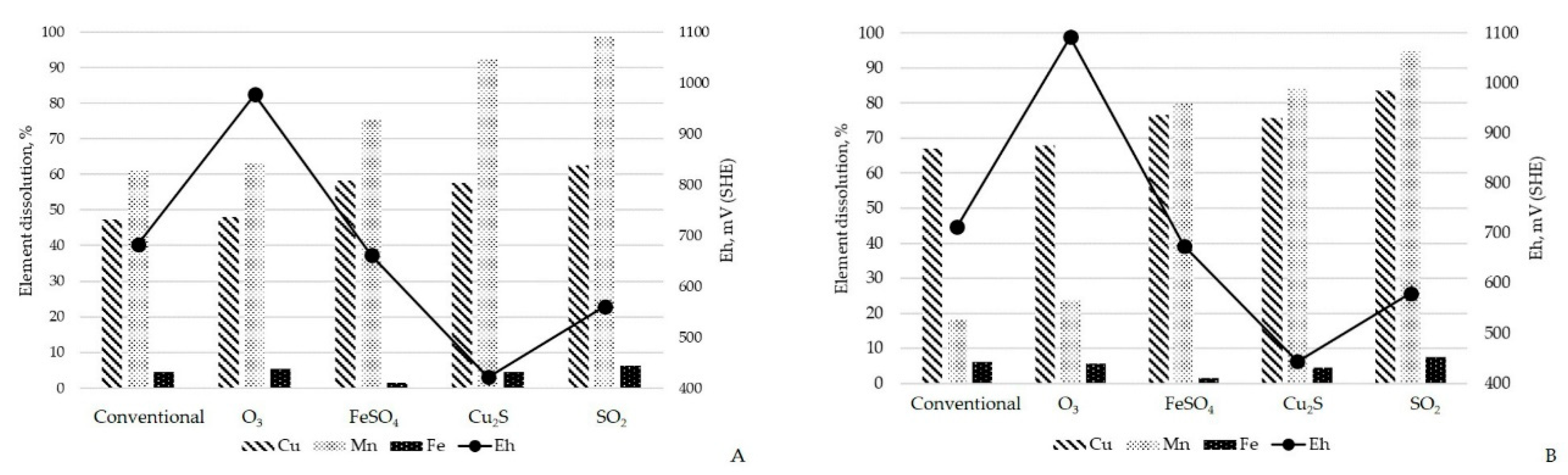
3.2.1. Eh Modifying Agents
3.2.2. Curing Test
3.3. Leaching Test
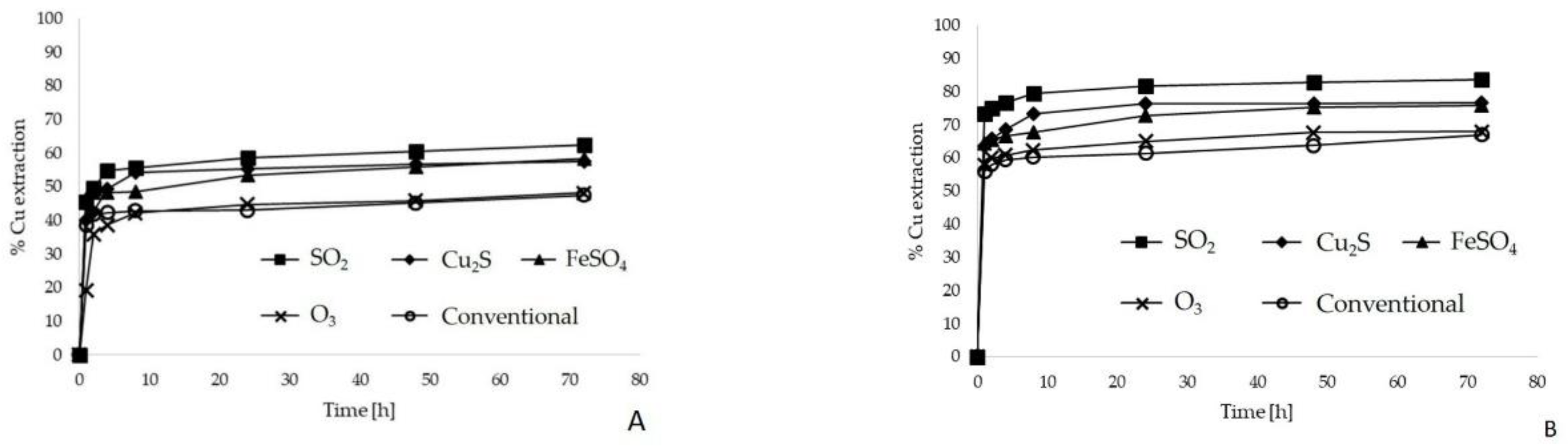
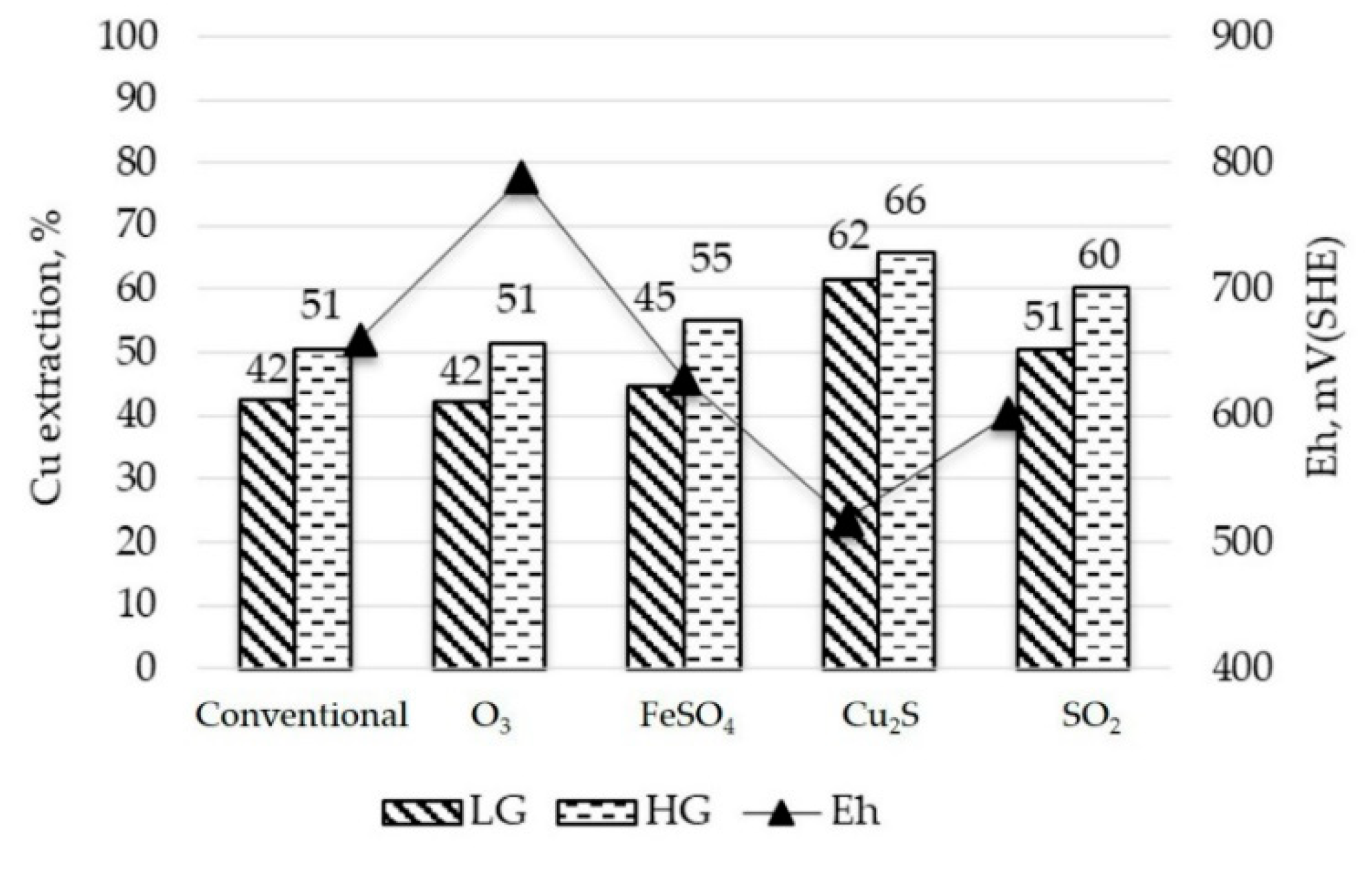
3.3.1. Leaching Bottle Test
3.3.2. Leaching Column Test
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fernández-Mort, A.; Riquelme, R.; Alonso-Zarza, A.M.; Campos, E.; Bissig, T.; Mpodozis, C.; Carretier, S.; Herrera, C.; Tapia, M.; Pizarro, H.; et al. A genetic model based on evapoconcentration for sediment-hosted exotic-Cu mineralization in arid environments: The case of the El Tesoro Central copper deposit, Atacama Desert, Chile. Miner. Depos. 2018, 53, 775–795. [Google Scholar] [CrossRef]
- Riquelme, R.; Tapia, M.; Campos, E.; Mpodozis, C. Supergene and exotic Cu mineralization occur during periods of landscape stability in the Centinela Mining District, Atacama Desert. Basin Res. 2017, 30, 395–425. [Google Scholar] [CrossRef]
- Munchmeyer, C. Los depósitos exóticos: Productos de migración lateral asociada a sistemas porfiricos. In Proceedings of the VII Congreso Geológico Chileno, Concepción, Chile, 17–21 October 1994; Universidad de Concepción: Concepción, Chile, 1994; pp. 1602–1606. [Google Scholar]
- Lillo, J.; Oyarzun, R.; Rivera, S. Giant versus small porphyry copper deposits of Cenozoic age in northern Chile: Adakitic versus normal calc-alkaline magmatism. Miner. Depos. 2001, 36, 794–798. [Google Scholar]
- Menzies, A.; Campos, E.; Hernández, V.; Sola, S.; Riquelme, R. Understanding Exotic-Cu Mineralisation Part II: Characterization of “ Black Copper ” ore (“ Cobre Negro ”). In Proceedings of the 13th SGA Biennial Meeting, Nancy, France, 24–27 August 2015. [Google Scholar]
- Benavente, O.; Hernández, M.; Melo, E.; Núñez, D.; Quezada, V.; Zepeda, Y. Copper Dissolution from Black Copper Ore under Oxidizing and Reducing Conditions. Metals 2019, 9, 799. [Google Scholar] [CrossRef]
- Pérez, K.; Toro, N.; Campos, E.; González, J.; Jeldres, R.I.; Nazer, A.; Rodriguez, M.H. Extraction of Mn from black copper using iron oxides from tailings and Fe2+ as reducing agents in acid medium. Metals 2019, 9, 1112. [Google Scholar] [CrossRef]
- Chávez, W. Supergene Oxidation of Copper Deposits: Zoning and Distribution of Copper Oxide Minerals. Soc. Econ. Geol. 2000, 41, 10–21. [Google Scholar]
- Pincheira, M.; Dagnini, A.; Kelm, U.; Helle, S. Copper Pitch Y Copper Wad: Contraste Entre Las Fases Presentes En Las Cabezas Y En Los Ripios En Pruebas De Mina sur, Chuquicamata. In Proceedings of the X Congreso Geológico Chileno, Concepción, Chile, 6–10 October 2003. [Google Scholar]
- Madhuchhanda, M.; Devi, N.B.; Rath, P.C.; Rao, K.S.; Paramguru, R.K.; Devi, N.B.; Rath, P.C.; Rao, K.S.; Paramguru, R.K. Leaching of Manganese Nodule in Hydrochloric Acid in Presence of Sphalerite. Can. Metall. Q. 2013, 4433, 49–59. [Google Scholar] [CrossRef]
- Saldaña, M.; Toro, N.; Castillo, J.; Hernández, P.; Trigueros, E.; Alessandro, N. Development of an Analytical Model for the Extraction of Manganese from Marine Nodules. Metals 2019, 9, 903. [Google Scholar] [CrossRef]
- Sparenberg, O. The Extractive Industries and Society Original article A historical perspective on deep-sea mining for manganese nodules, 1965–2019. Extr. Ind. Soc. 2019, 6, 842–854. [Google Scholar]
- Toro, N.; Herrera, N.; Castillo, J.; Torres, C.M. Initial Investigation into the Leaching of Manganese from Nodules at Room Temperature with the Use of Sulfuric Acid and the Addition of Foundry Slag—Part I. Minerals 2018, 8, 565. [Google Scholar] [CrossRef]
- Senanayake, G.A. mixed surface reaction kinetic model for the reductive leaching of manganese dioxide with acidic sulfur dioxide. Hydrometallurgy 2004, 73, 215–224. [Google Scholar] [CrossRef]
- Naik, P.K.; Sukla, L.B.; Das, S.C. Aqueous SO2 leaching studies on Nishikhal manganese ore through factorial experiment. Hydrometallurgy 2000, 54, 217–228. [Google Scholar] [CrossRef]
- Acharya, R.; Ghosh, M.K.; Anand, S.; Das, R.P. Leaching of metals from Indian ocean nodules in SO2-H2O-H2SO4-(NH4)2SO4 medium. Hydrometallurgy 1999, 53, 169–175. [Google Scholar] [CrossRef]
- Sun, D.; Xin, G.; Yao, L.; Yang, L.; Jiang, X.; Xin, G.; Yao, L. Manganese leaching in high concentration flue gas desulfurization process with semi-oxidized manganese ore. Chin. J. Chem. Eng. 2019, in press. [Google Scholar] [CrossRef]
- Sinha, M.K.; Purcell, W. Reducing agents in the leaching of manganese ores: A comprehensive review. Hydrometallurgy 2019, 187, 168–186. [Google Scholar] [CrossRef]
- Jana, R.K.; Singh, D.D.N.; Roy, S.K. Alcohol-modified hydrochloric acid leaching of sea nodules. Hydrometallurgy 1995, 38, 289–298. [Google Scholar] [CrossRef]
- Kanungo, S.B. Rate process of the reduction leaching of manganese nodules in dilute HCl in presence of pyrite. Part II: Leaching behavior of manganese. Hydrometallurgy 1999, 52, 331–347. [Google Scholar] [CrossRef]
- Bafghi, M.S.; Zakeri, A.; Ghasemi, Z.; Adeli, M. Reductive dissolution of manganese ore in sulfuric acid in the presence of iron metal. Hydrometallurgy 2008, 90, 207–212. [Google Scholar] [CrossRef]
- Stone, A. Microbial metabolites and the reductive dissolution of manganese oxides: Oxalate and pyruvate. Geochim. Cosmochim. Acta 1987, 51, 919–925. [Google Scholar] [CrossRef]
- Sahoo, R.N.; Naik, P.K.; Das, S.C. Leaching of manganese from low-grade manganese ore using oxalic acid as reductant in sulphuric acid solution. Hydrometallurgy 2001, 62, 157–163. [Google Scholar] [CrossRef]
- Hazek, M.N.E.; Lasheen, T.A.; Helal, A.S. Reductive leaching of manganese from low grade Sinai ore in HCl using H2O2 as reductant. Hydrometallurgy 2006, 84, 187–191. [Google Scholar] [CrossRef]
- Jiang, T.; Yang, Y.; Huang, Z.; Zhang, B.; Qiu, G. Leaching kinetics of pyrolusite from manganese-silver ores in the presence of hydrogen peroxide. Hydrometallurgy 2004, 72, 129–138. [Google Scholar] [CrossRef]
- Kojima, S.; OHMOTO, H. Hydrothermal synthesis of Wurtzite and Sphalerite at T = 350–250°C. Min. Geol. 1991, 41, 313–327. [Google Scholar]
- Dhawan, N.; Safarzadeh, M.S.; Miller, J.D.; Moats, M.S.; Rajamani, R.K. Crushed ore agglomeration and its control for heap leach operations. Miner. Eng. 2013, 41, 53–70. [Google Scholar] [CrossRef]
- Quezada, V.; Velásquez, L.; Roca, A.; Benavente, O.; Melo, E.; Keith, B. Effect of curing time on the dissolution of a secondary copper sulphide ore using alternative water resources. IOP Conf. Ser. Mater. Sci. Eng. 2018, 427, 012030. [Google Scholar] [CrossRef]
- Velásquez-Yévenes, L.; Quezada-Reyes, V. Influence of seawater and discard brine on the dissolution of copper ore and copper concentrate. Hydrometallurgy 2018, 180, 88–95. [Google Scholar] [CrossRef]
- Zakeri, A.; Bafghi, M.S.; Shahriari, S.; Das, S.C.; Sahoo, P.K.; Rao, P.K. Dissolution kinetics of manganese dioxide ore in sulfuric acid in the presence of ferrous ion. Iran. J. Mater. Sci. Eng. 2007, 8, 22–27. [Google Scholar]



| Sample | Cu(T) % | Cu(Sol) % | Cu(Ins) % | Fe % | Mn % |
|---|---|---|---|---|---|
| LG | 0.13 | 0.12 | 0.010 | 4.0 | 0.15 |
| HG | 0.25 | 0.20 | 0.050 | 2.4 | 0.38 |
| LG | HG | ||||
|---|---|---|---|---|---|
| Mineral | Proportion | Size (µm) | Mineral | Proportion | Size (µm) |
| Hematite | abundant | <760 | Limonite | abundant | <140 |
| Magnetite | abundant | <300 | Black copper | abundant | <60 |
| Limonite | abundant | - | Atacamite | abundant | - |
| Black copper | Minor | ~10 | Hematite | Minor | ~10 |
| Rutile | Minor | <50 | Rutile | Minor | <50 |
| Pyrite | Trace | ~30 | Chalcopyrite | Minor | <20 |
| - | - | - | Pyrite | Trace | ~50 |
| Wt % | ||||||
|---|---|---|---|---|---|---|
| Samples | O | Mn | Cu | Fe | K | Si |
| LG | 64 | 18 | 1.1 | 5.7 | 1.7 | 3.9 |
| HG | 42 | 38 | 4.2 | 7.5 | 2.5 | 5.9 |
| Mineral | Formula | LG (%) | HG (%) |
|---|---|---|---|
| Quartz | SiO2 | 24.1 | 25.4 |
| Orthoclase | KAlSi3O8 | 14.0 | 20.4 |
| Pyrite | FeS2 | 0.15 | 0.13 |
| Muscovite | KAl2(Si3AlO10)(OH)2 | 4.93 | 5.21 |
| Djurleite | Cu31S16 | 0.13 | - |
| Kaolinite | Al2Si2O5(OH)4 | 20.4 | 16.8 |
| Albite | NaAlSi3O8 | 19.9 | 13.5 |
| Chalcocite | Cu2S | 0.18 | 0.13 |
| Chlorite | (Mg,Fe)6(Si,Al)4O10(OH)8 | 10.9 | 15.3 |
| Jarosite | KFe3(SO4)2(OH)6 | 0.11 | 0.43 |
| Digenite | Cu9S5 | - | 0.12 |
| Gypsum | CaSO4*2H2O | - | - |
| Anhydrite | CaSO4 | 4.95 | 2.48 |
| Chalcopyrite | CuFeS2 | 0.13 | - |
| Tenorite | CuO | 0.10 | 0.15 |
| Samples | Conventional | O3 | FeSO4 | Cu2S | SO2 |
|---|---|---|---|---|---|
| LG | 13.3 | 16.8 | 13.5 | 11.6 | 9.20 |
| HG | 8.80 | 14.7 | 26.5 | 21.8 | 12.0 |
| Samples | Conventional | O3 | FeSO4 | Cu2S | SO2 |
|---|---|---|---|---|---|
| LG | 14.78 | 13.29 | 14.37 | 13.97 | 16.66 |
| HG | 12.60 | 10.80 | 13.93 | 10.31 | 11.12 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benavente, O.; Hernández, M.C.; Melo, E.; Ardiles, L.; Quezada, V.; Zepeda, Y. Copper Extraction from Black Copper Ores through Modification of the Solution Potential in the Irrigation Solution. Metals 2019, 9, 1339. https://doi.org/10.3390/met9121339
Benavente O, Hernández MC, Melo E, Ardiles L, Quezada V, Zepeda Y. Copper Extraction from Black Copper Ores through Modification of the Solution Potential in the Irrigation Solution. Metals. 2019; 9(12):1339. https://doi.org/10.3390/met9121339
Chicago/Turabian StyleBenavente, Oscar, Ma.Cecilia Hernández, Evelyn Melo, Luis Ardiles, Víctor Quezada, and Yuri Zepeda. 2019. "Copper Extraction from Black Copper Ores through Modification of the Solution Potential in the Irrigation Solution" Metals 9, no. 12: 1339. https://doi.org/10.3390/met9121339
APA StyleBenavente, O., Hernández, M. C., Melo, E., Ardiles, L., Quezada, V., & Zepeda, Y. (2019). Copper Extraction from Black Copper Ores through Modification of the Solution Potential in the Irrigation Solution. Metals, 9(12), 1339. https://doi.org/10.3390/met9121339







