Formation Process of the Passivating Products from Arsenopyrite Bioleaching by Acidithiobacillus ferrooxidans in 9K Culture Medium
Abstract
1. Introduction
2. Thermodynamic Calculations
3. Experimental
3.1. Minerals, Strain and Media
3.2. Bioleaching Experiment
3.3. Analytical Methods
4. Results and Discussion
4.1. Bioleaching Behaviour of Arsenopyrite
4.1.1. Bioleaching Kinetics of Arsenopyrite
4.1.2. Phase Transformation of Arsenopyrite During Bioleaching
4.1.3. Thermodynamics of Arsenopyrite Dissolution
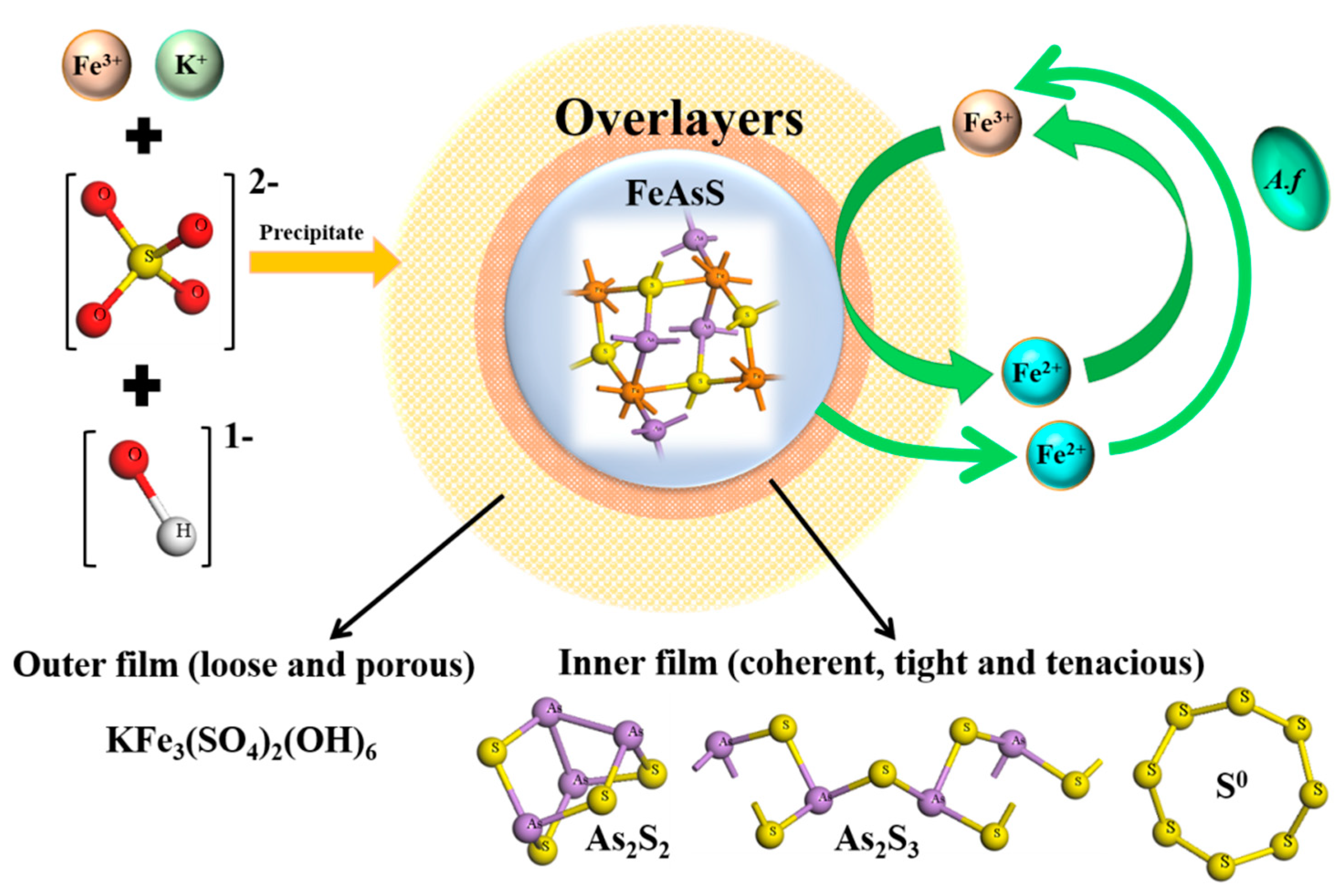
4.2. Formation of Surface Overlayers on Arsenopyrite
4.3. Possible Passivation Mechanisms for the Bioleaching of Arsenopyrite
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Species | ΔG°298 (kJ/mol) | Species | ΔG°298 (kJ/mol) | Species | ΔG°298 (kJ/mol) |
|---|---|---|---|---|---|
| FeAsS | −49.7616 | As2S2 | −68.5508 | S | 0 |
| FeAsO4 | −772.727 | As2S3 | −91.4907 | S2− | 86.00982 |
| Fe3(AsO4)2 | −1766.73 | As2O3 | −576.899 | S22− | 79.76167 |
| Fe(OH)2 | −492.158 | As2O4 | −701.161 | SO32− | −486.755 |
| Fe(OH)3 | −705.885 | As2O5 | −782.437 | S2O32− | −518.87 |
| FeO OH | −489.439 | As4O6 | −1152.42 | S2O42− | −600.825 |
| Fe3+ | −17.1907 | AsO2− | −349.991 | S2O52− | −791.217 |
| Fe2+ | −91.5644 | AsO43− | -648.477 | S2O62− | −969.453 |
| FeOH2+ | −242.064 | As(OH)4− | −824.457 | S2O72− | −795.432 |
| FeOH+ | −275.615 | HAsO3− | −606.638 | S2O82− | −1115.35 |
| Fe(OH)2+ | −452.391 | HAsO42− | −714.732 | HS2− | 11.51053 |
| Fe2(OH)24+ | −467.733 | H2AsO3− | −587.149 | HSO3− | −527.84 |
| H3OFe3(SO4)2(OH)6 b | −3230.36 | H2AsO4− | −753.399 | HS− | 12.44438 |
| H3AsO3 (a) | −638.142 | HS2O3− | −532.363 | ||
| H2O | −237.177 | H3AsO4 (a) | −764.001 | H2S (a) | −27.656 |
| HAsO2 (a) | −402.951 |
References
- Márquez, M.A.; Ospina, J.D.; Morales, A.L. New insights about the bacterial oxidation of arsenopyrite: A mineralogical scope. Miner. Eng. 2012, 39, 248–254. [Google Scholar] [CrossRef]
- Miller, P.; Brown, A.R.G. Bacterial Oxidation of Refractory Gold Concentrates. In Gold Ore Processing: Project Development and Operations; Adams, M.D., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 359–372. [Google Scholar]
- Rawlings, D.E. High level arsenic resistance in bacteria present in biooxidation tanks used to treat gold-bearing arsenopyrite concentrates: A review. Trans. Nonferrous Met. Soc. China 2008, 18, 1311–1318. [Google Scholar] [CrossRef]
- Climo, M.; Watling, H.R.; Bronswijk, W.V. Biooxidation as pre-treatment for a telluride-rich refractory gold concentrate. Miner. Eng. 2000, 13, 1219–1229. [Google Scholar] [CrossRef]
- Espitia, S.L.M.; Lapidus, G.T. Pretreatment of a refractory arsenopyritic gold ore using hydroxyl ion. Hydrometallurgy 2015, 153, 106–113. [Google Scholar] [CrossRef]
- Espitia, S.L.M.; Lapidus, G.T. Improved gold extraction from a refractory arsenopyritic ore by chemical pretreatments. Hydrometallurgy 2014, 1, 573–580. [Google Scholar]
- Kaksonen, A.H.; Mudunuru, B.M.; Hackl, R. The role of microorganisms in gold processing and recovery—A review. Hydrometallurgy 2014, 142, 70–83. [Google Scholar] [CrossRef]
- Nguyen, V.K.; Lee, M.H.; Park, H.J.; Lee, J.U. Bioleaching of arsenic and heavy metals from mine tailings by pure and mixed cultures of Acidithiobacillus spp. J. Ind. Eng. Chem. 2015, 21, 451–458. [Google Scholar] [CrossRef]
- Park, J.; Han, Y.; Lee, E.; Choi, U.; Yoo, K.; Song, Y.; Kim, H. Bioleaching of highly concentrated arsenic mine tailings by Acidithiobacillus ferrooxidans. Sep. Purif. Technol. 2014, 133, 291–296. [Google Scholar] [CrossRef]
- Corkhill, C.L.; Wincott, P.L.; Lloyd, J.R.; Vaughan, D.J. The oxidative dissolution of arsenopyrite (FeAsS) and enargite (Cu3AsS4) by Leptospirillum ferrooxidans. Geochim. Cosmochim. Acta 2008, 72, 5616–5633. [Google Scholar] [CrossRef]
- Fantauzzi, M.; Licheri, C.; Atzei, D.; Loi, G.; Elsener, B.; Rossi, G.; Rossi, A. Arsenopyrite and pyrite bioleaching: Evidence from XPS, XRD and ICP techniques. Anal. Bioanal. Chem. 2011, 401, 2237–2248. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.A.; Koval, S.F.; Nesbitt, H.W. Surface alteration of arsenopyrite (FeAsS) by Thiobacillus ferrooxidans. Geochim. Cosmochim. Acta 2003, 67, 955–965. [Google Scholar] [CrossRef]
- Lázaro, I.; Cruz, R.; González, I.; Monroy, M. Electrochemical oxidation of arsenopyrite in acidic media. Int. J. Miner. Process. 1997, 50, 63–75. [Google Scholar] [CrossRef]
- Min, X.; Cai, L.; Chen, W.; Zhang, C.; Zhong, H.; Kuang, Z. Bioleaching of refractory gold ore (II)—Mechanism on bioleaching of arsenopyrite by Thiobacillus ferrooxidans. Trans. Nonferrous Met. Soc. China 2002, 12, 142–146. [Google Scholar]
- Ramírez-Aldaba, H.; Valles, O.P.; Vazquez-Arenas, J.; Rojas-Contreras, J.A.; Valdez-Pérez, D.; Ruiz-Baca, E.; Meraz-Rodríguez, M.; Sosa-Rodríguez, F.S.; Rodríguez, Á.G.; Lara, R.H. Chemical and surface analysis during evolution of arsenopyrite oxidation by Acidithiobacillus thiooxidans in the presence and absence of supplementary arsenic. Sci. Total. Environ. 2016, 566, 1106–1119. [Google Scholar] [CrossRef] [PubMed]
- Corkhill, C.L.; Vaughan, D.J. Arsenopyrite oxidation—A review. Appl. Geochem. 2009, 24, 2342–2361. [Google Scholar] [CrossRef]
- Cruz, R.; Lázaro, I.; Rodríguez, J.M.; Monroy, M.; González, I. Surface characterization of arsenopyrite in acidic medium by triangular scan voltammetry on carbon paste electrodes. Hydrometallurgy 1997, 46, 303–319. [Google Scholar] [CrossRef]
- Roine, A. Outokumpu HSC Chemistry for Windows: Chemical reaction and equilibrium software with extensive thermochemical database. In User’s Guide, Version 6.0; Outokumpu Research Oy: Pori, Finland, 2006. [Google Scholar]
- Liu, J.Y.; Tao, X.X.; Cai, P. Study of formation of jarosite mediated by Thiobacillus ferrooxidans in 9K medium. Proc. Earth Planet. Sci. 2009, 1, 706–712. [Google Scholar] [CrossRef]
- Wang, H.; Bigham, J.M.; Jones, F.S.; Tuovinen, O.H. Synthesis and properties of ammoniojarosites prepared with iron-oxidizing acidophilic microorganisms at 22–65 °C. Geochim. Cosmochim. Acta 2007, 71, 155–164. [Google Scholar] [CrossRef]
- Barin, I. Thermochemical Data of Pure Substances, 3rd ed.; VCH: Weinheim, Germany, 1995. [Google Scholar]
- Puigdomenech, I. Make equilibrium diagrams using sophisticated algorithms (MEDUSA). In Inorganic Chemistry; Royal Institute of Technology: Stockholm, Sweden, 2004. [Google Scholar]

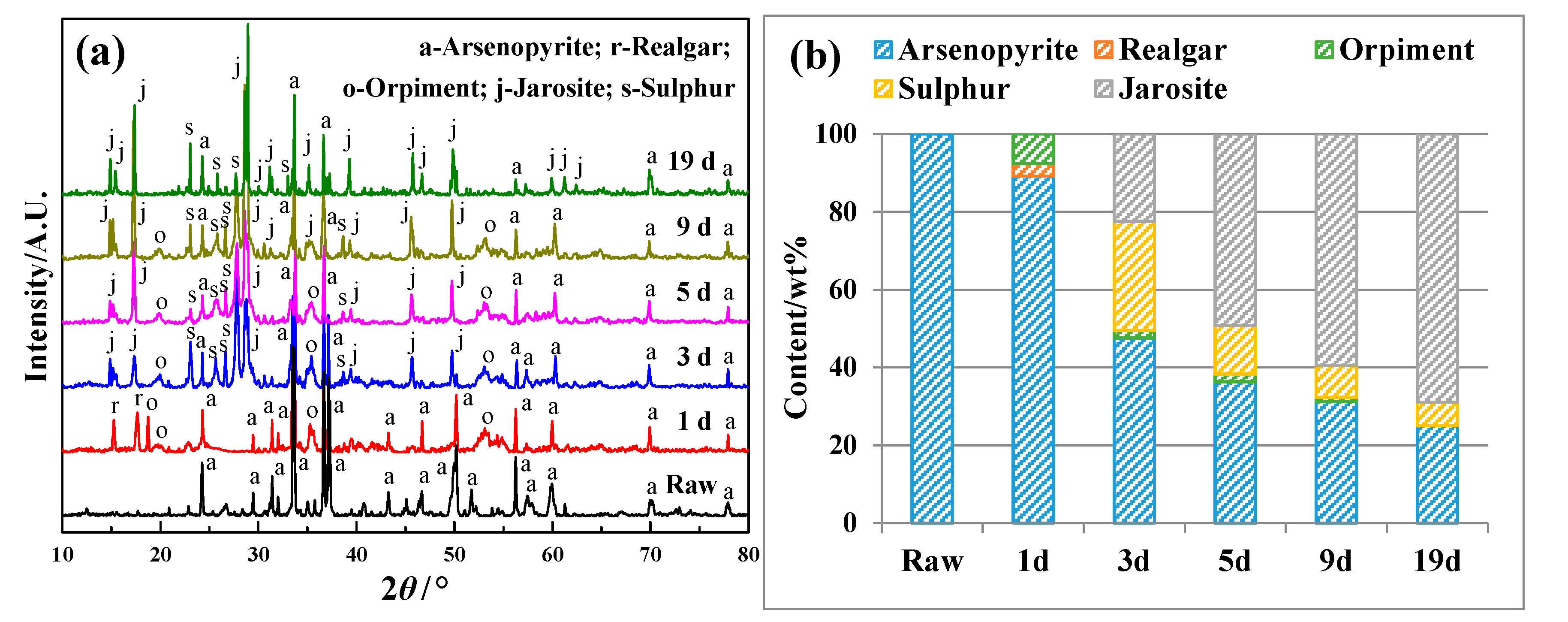
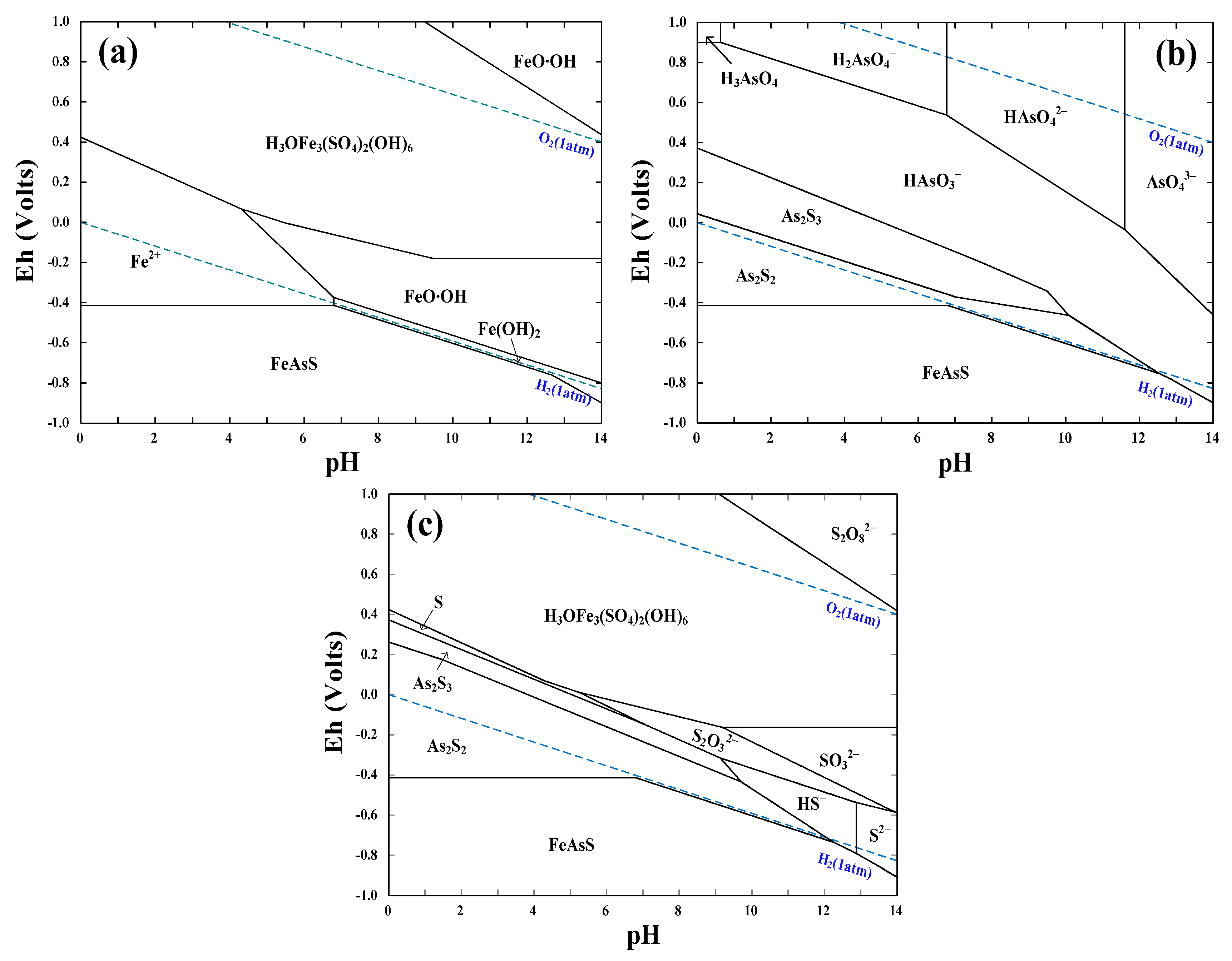
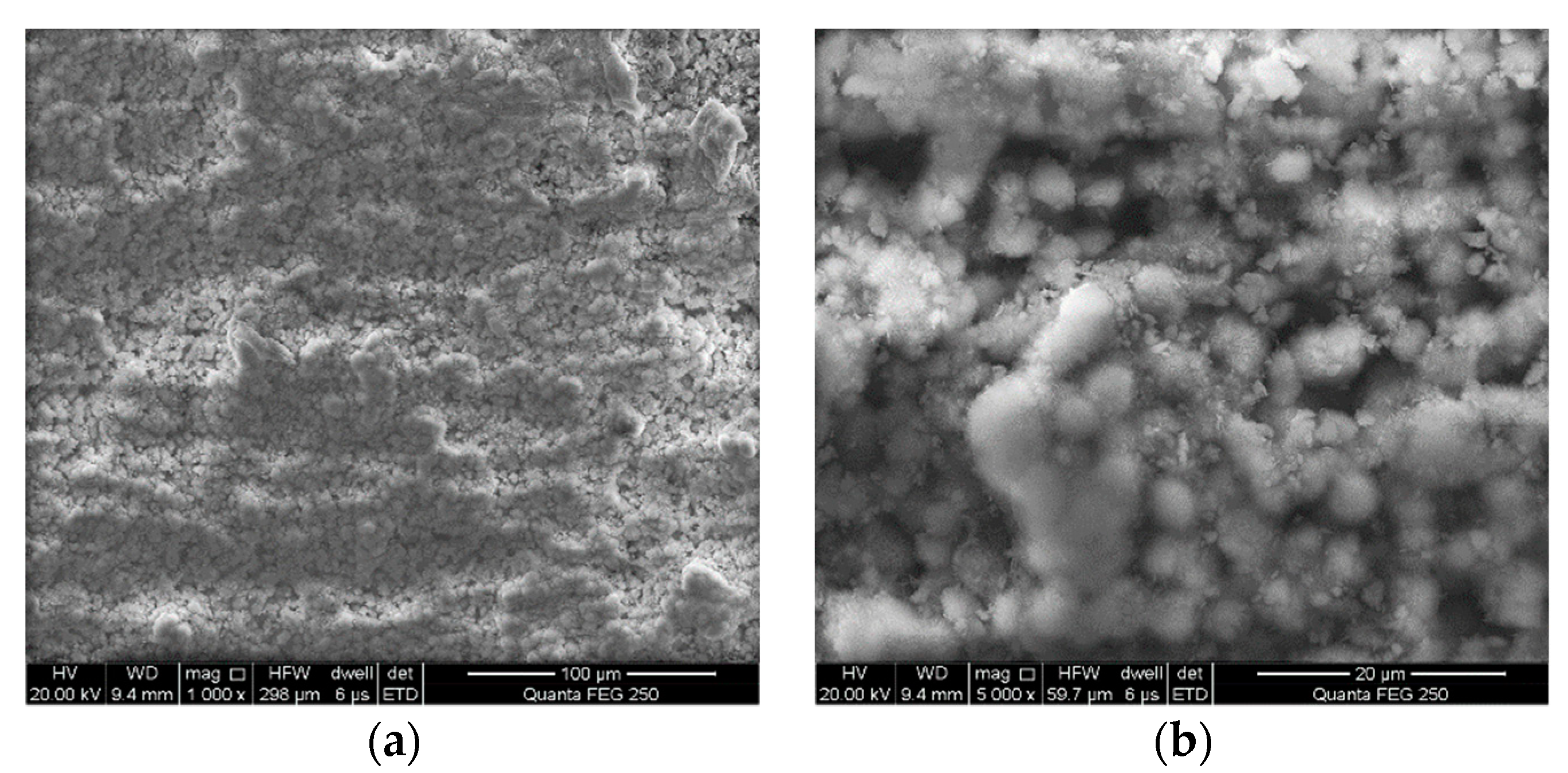
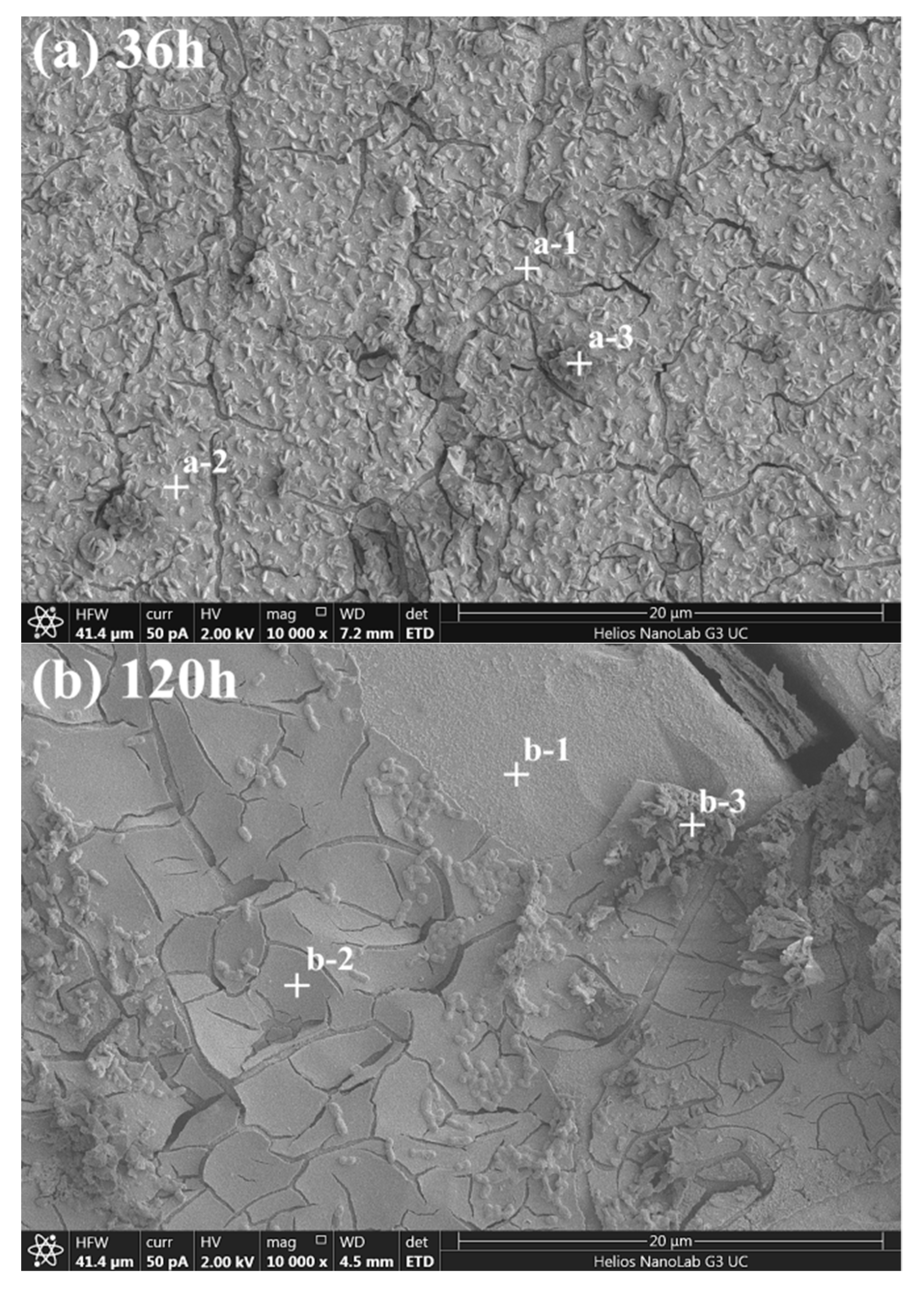
| Bioleaching Time/h | Spots a | Content/at% | |||
|---|---|---|---|---|---|
| Fe | As | S | O | ||
| 36 | a-1 | 33.12 | 34.96 | 27.14 | 4.78 |
| 36 | a-2 | 10.79 | 32.97 | 31.69 | 24.55 |
| 36 | a-3 | 34.16 | 10.70 | 17.11 | 38.03 |
| 120 | b-1 | 31.80 | 30.12 | 31.97 | 6.11 |
| 120 | b-2 | 12.21 | 21.43 | 22.78 | 43.58 |
| 120 | b-3 | 21.62 | 6.17 | 15.68 | 56.53 |
| Reaction Equations | ∆Gr0/(kJ/mol) a | No. |
|---|---|---|
| 6FeAsS + 7H2O + 22Fe3+ = 28Fe2+ + 2As2S2 + 2AsO2– + S2O32– + 14H+ | −1582.753 | 1 |
| 3FeAsS + 2H2O + 9Fe3+ = 12Fe2+ + As2S3 + AsO2– + 4H+ | −761.900 | 2 |
| FeAsS + 3H2O + 6Fe3+= 7Fe2+ + S0 + HAsO3– + 5H+ | −383.152 | 3 |
| 3As2S2 + 4H2O + 6Fe3+ = 6Fe2++ 2As2S3+ 2AsO2– + 8H+ | −174.846 | 4 |
| As2S2 + 6H2O + 8Fe3+ = 8Fe2++ 2S0 + 2HAsO3– + 10H+ | −316.652 | 5 |
| As2S3 + 6H2O + 8Fe3+ = 8Fe2++ 3S0 + 2HAsO3– + 10H+ | −293.712 | 6 |
| 3Fe3+ + K+ + 2SO42– + 6OH– = KFe3(SO4)2(OH)6 | <0 b | 7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Li, Q.; Zhang, Y.; Yang, Y.; Xu, B.; Jiang, T. Formation Process of the Passivating Products from Arsenopyrite Bioleaching by Acidithiobacillus ferrooxidans in 9K Culture Medium. Metals 2019, 9, 1320. https://doi.org/10.3390/met9121320
Liu X, Li Q, Zhang Y, Yang Y, Xu B, Jiang T. Formation Process of the Passivating Products from Arsenopyrite Bioleaching by Acidithiobacillus ferrooxidans in 9K Culture Medium. Metals. 2019; 9(12):1320. https://doi.org/10.3390/met9121320
Chicago/Turabian StyleLiu, Xiaoliang, Qian Li, Yan Zhang, Yongbin Yang, Bin Xu, and Tao Jiang. 2019. "Formation Process of the Passivating Products from Arsenopyrite Bioleaching by Acidithiobacillus ferrooxidans in 9K Culture Medium" Metals 9, no. 12: 1320. https://doi.org/10.3390/met9121320
APA StyleLiu, X., Li, Q., Zhang, Y., Yang, Y., Xu, B., & Jiang, T. (2019). Formation Process of the Passivating Products from Arsenopyrite Bioleaching by Acidithiobacillus ferrooxidans in 9K Culture Medium. Metals, 9(12), 1320. https://doi.org/10.3390/met9121320





