Sulfation Roasting of Nickel Oxide–Sulfide Mixed Ore Concentrate in the Presence of Ammonium Sulfate: Experimental and DFT Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Procedure
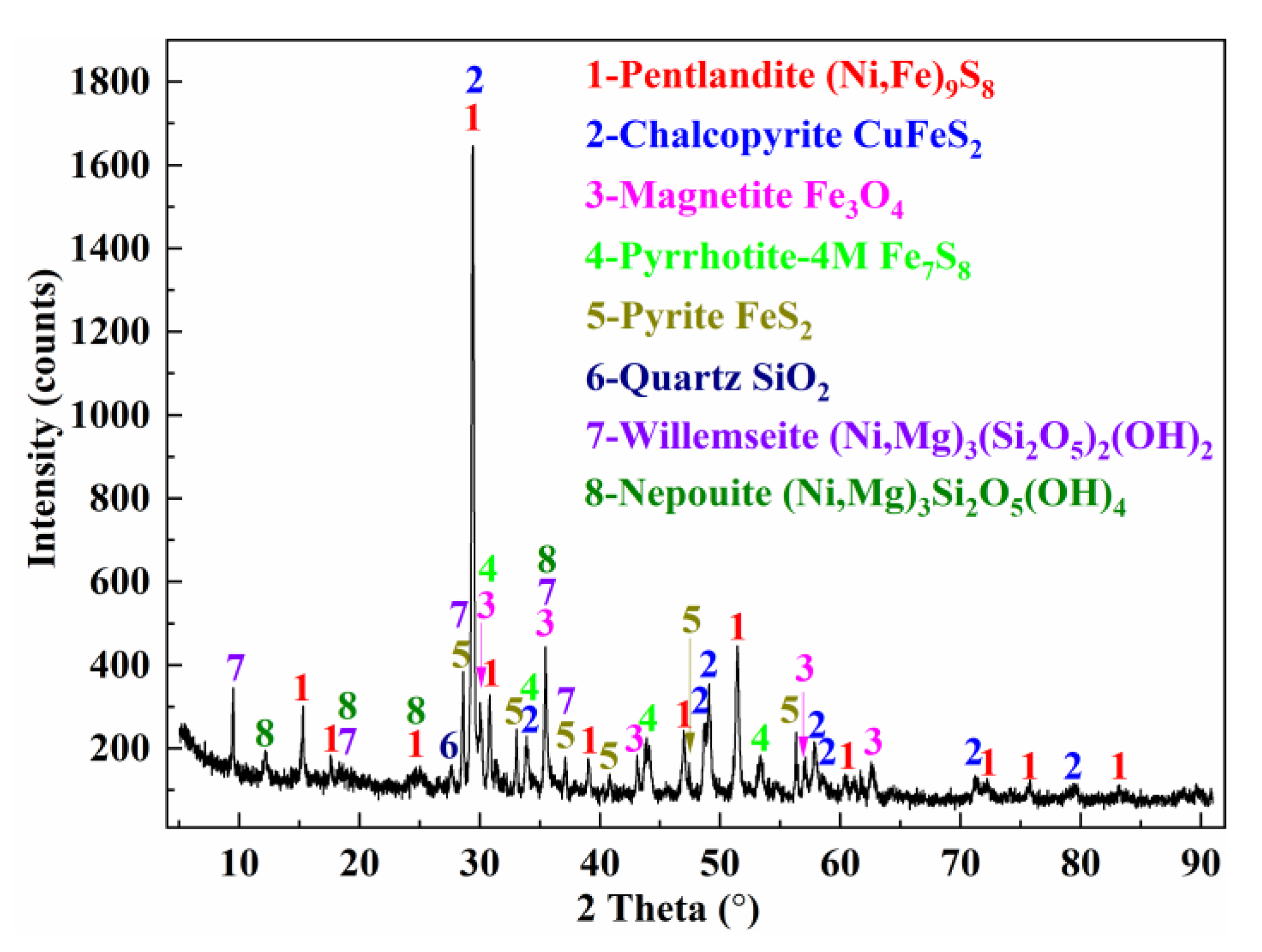
2.3. Characterization Methods
2.4. Computational Details
3. Results and Discussion
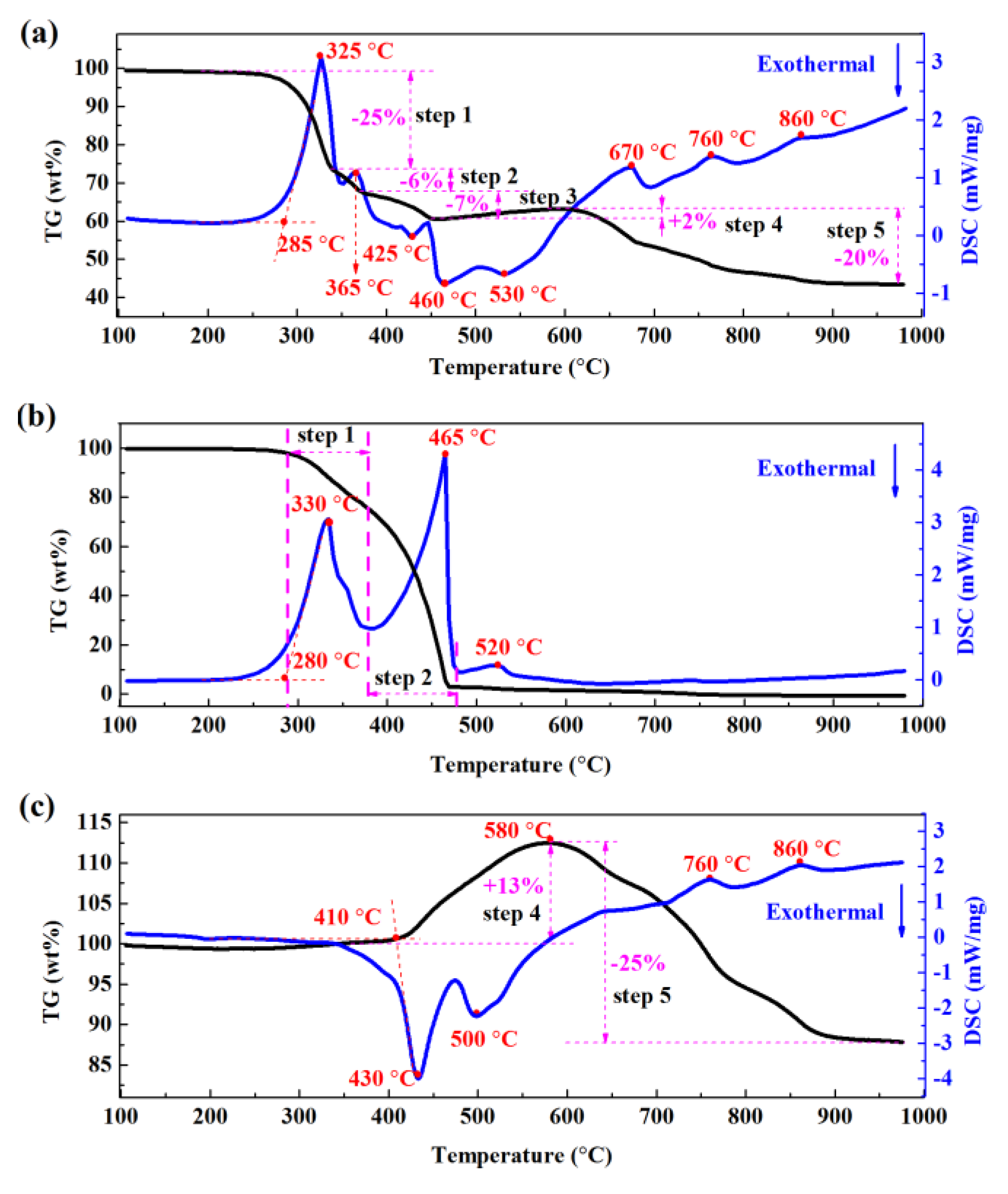
3.1. Thermal Analysis of the Roasting Process
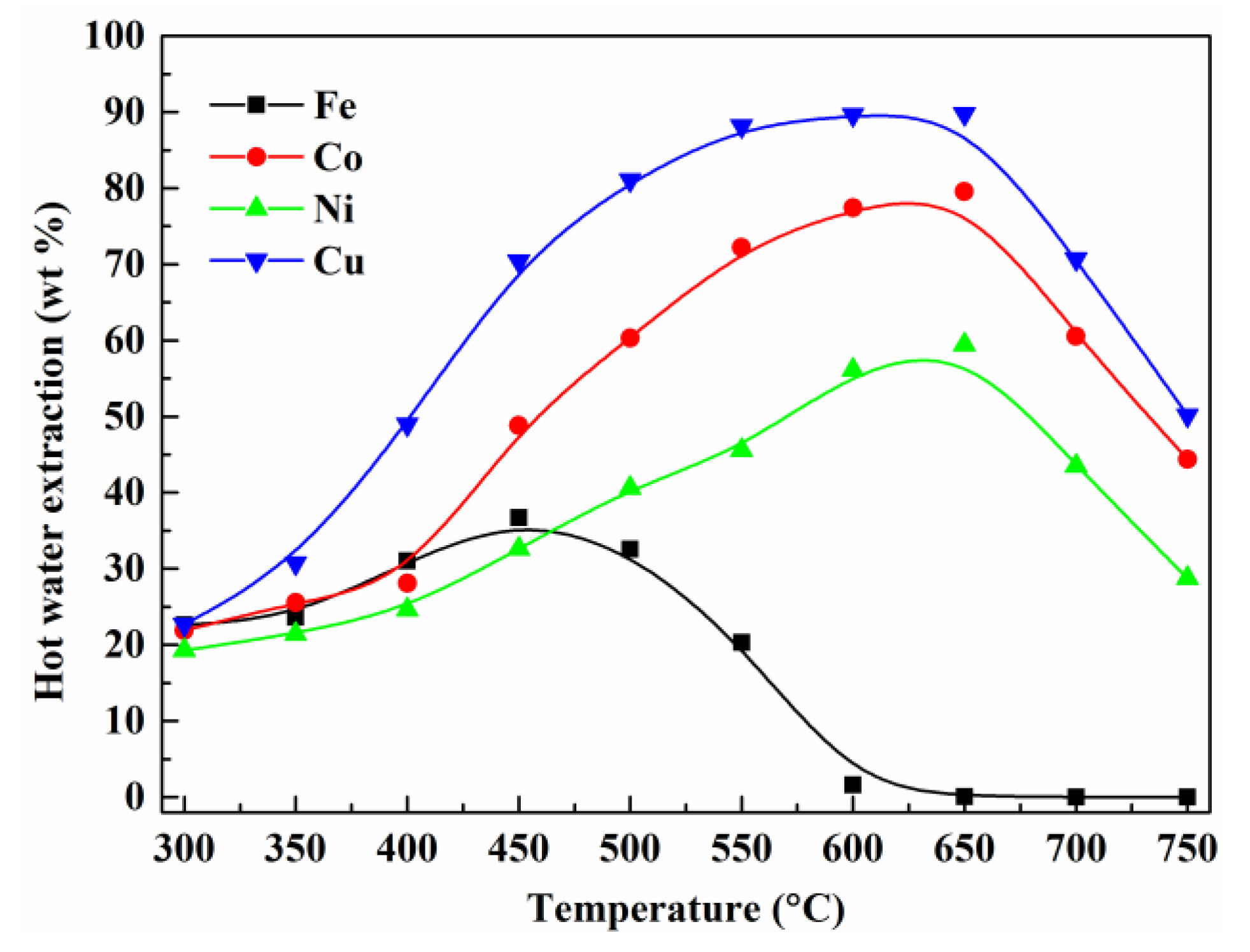
3.2. Effect of Roasting Temperature
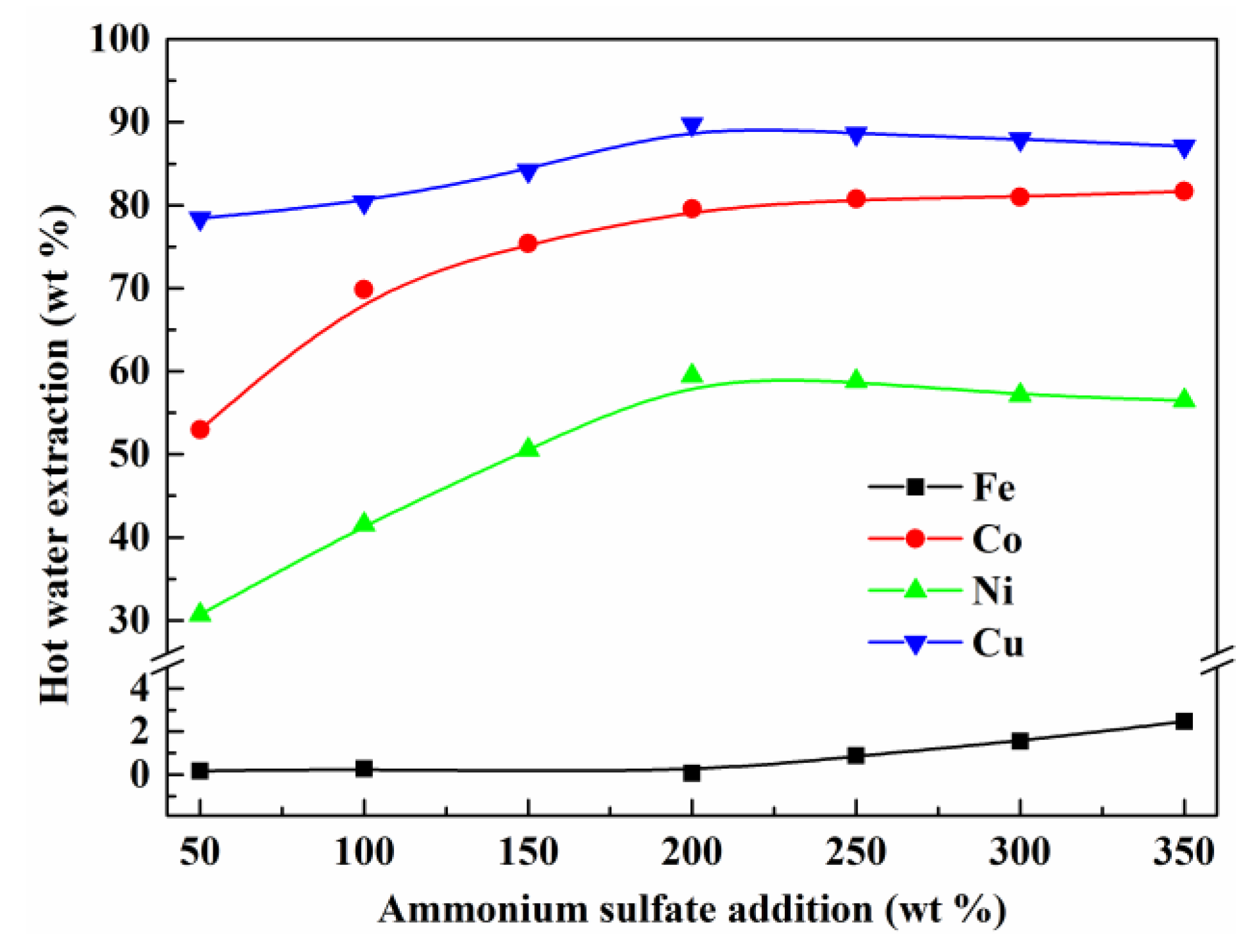
3.3. Effect of the Addition of Ammonium Sulfate
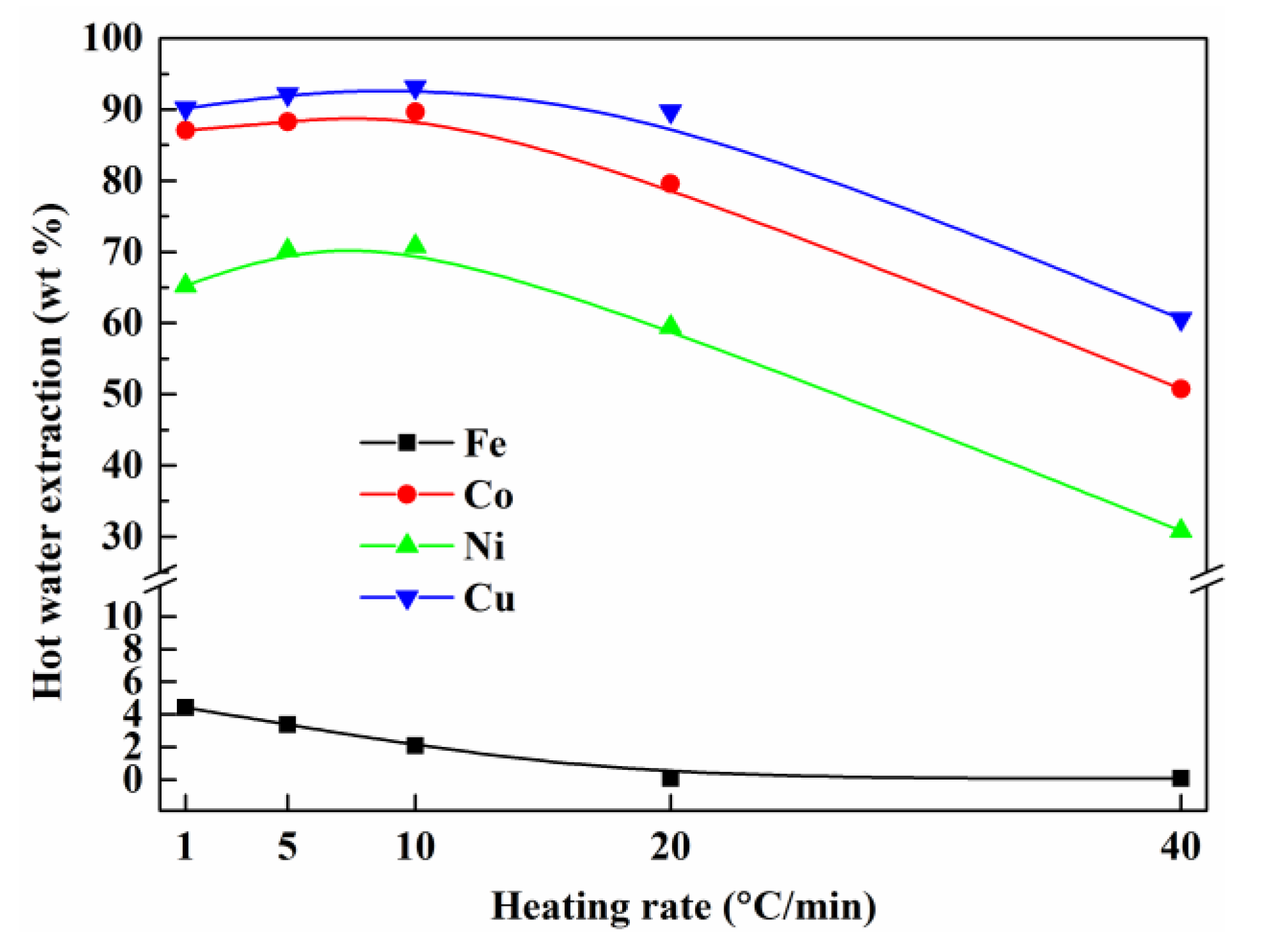
3.4. Effect of Heating Rate
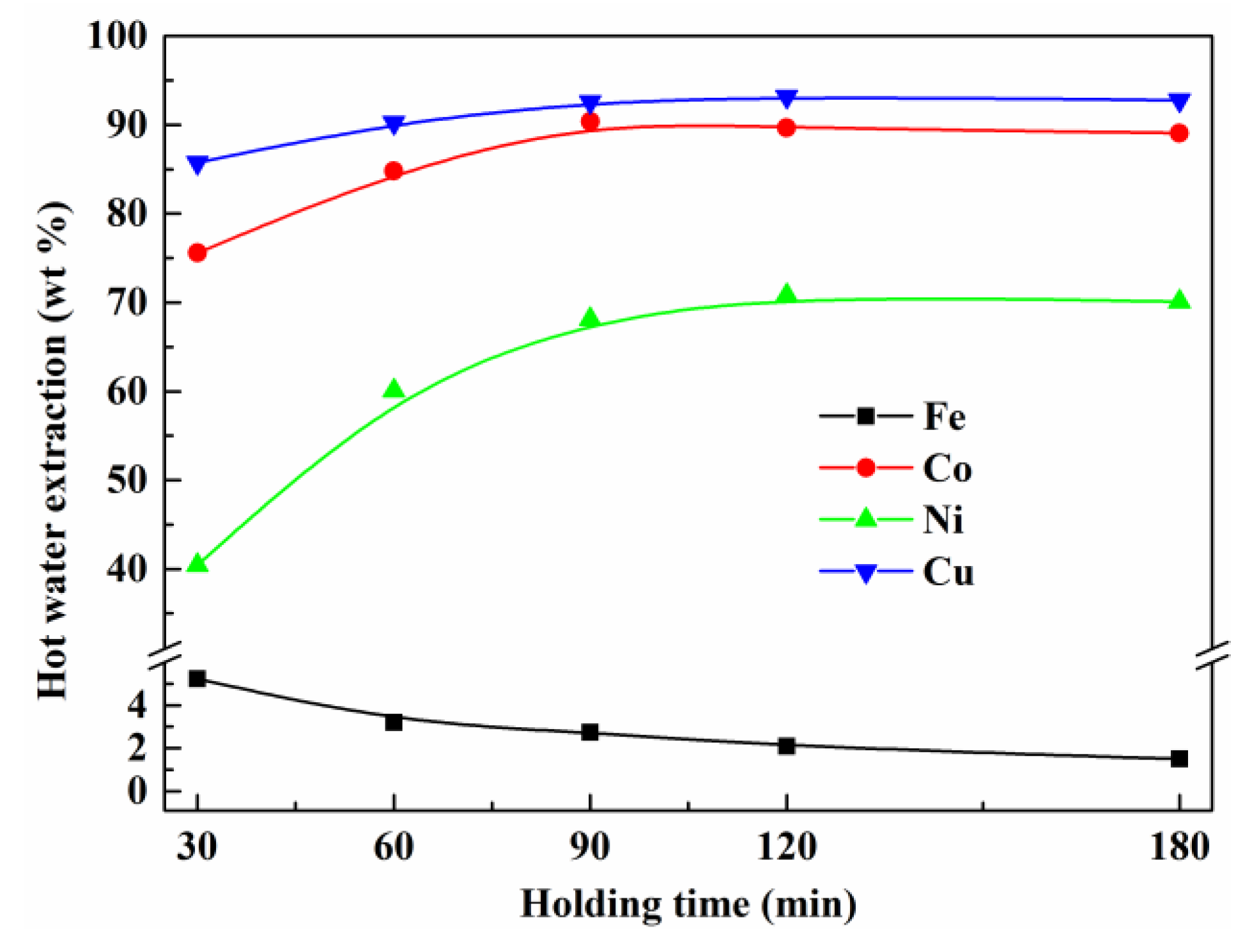
3.5. Effect of Holding Time
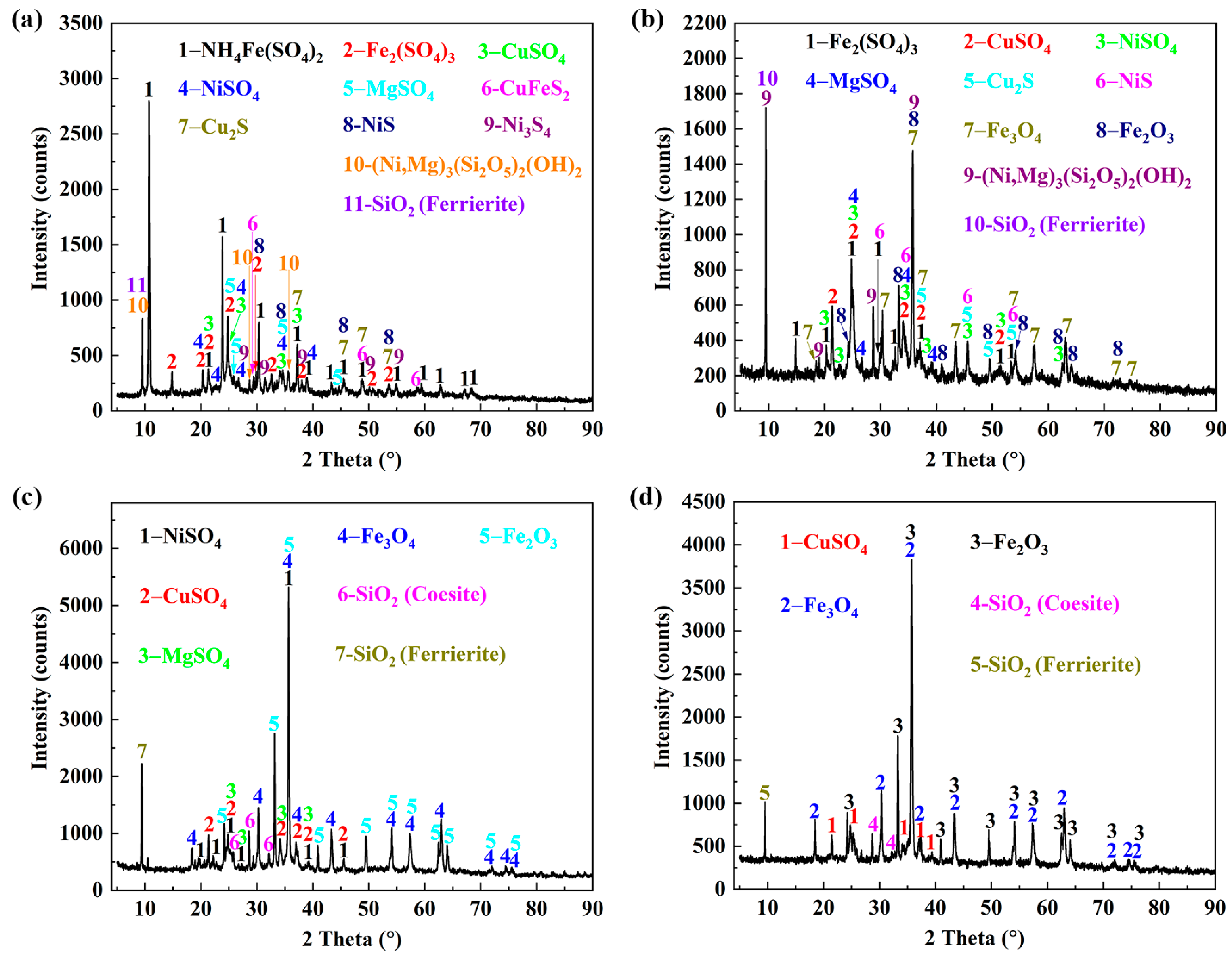
3.6. The Transformation Behaviors of the Crystal Phase and Microstructure of Roasting Products
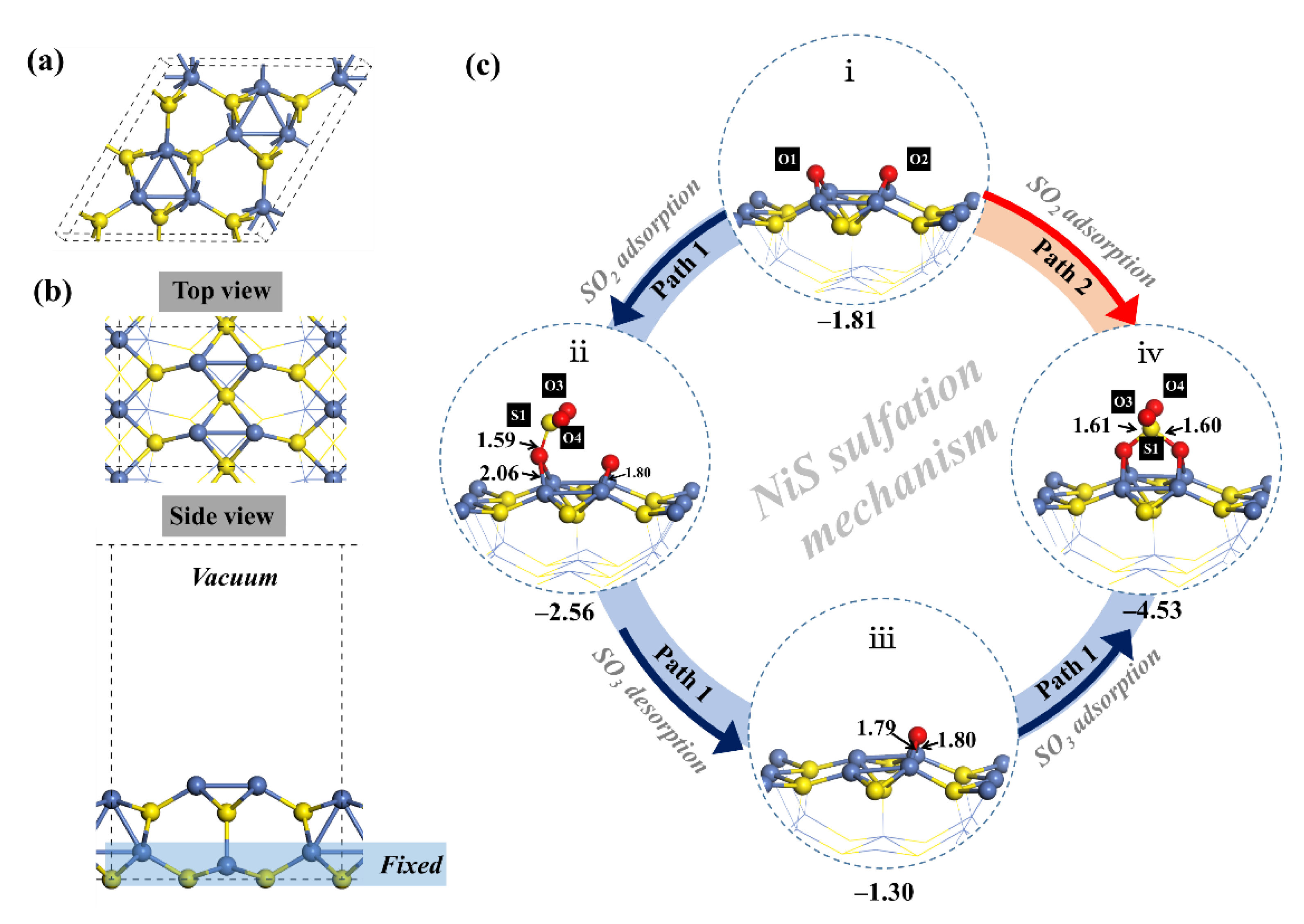
3.7. The Sulfation Mechanism for the NiS and Cu2S Surfaces by DFT Studies
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, G.; Zhou, Q.; Zhu, Z.; Luo, J.; Rao, M.; Peng, Z.; Jiang, T. Selective leaching of nickel and cobalt from limonitic laterite using phosphoric acid: An alternative for value-added processing of laterite. J. Clean. Prod. 2018, 189, 620–626. [Google Scholar] [CrossRef]
- Li, J.; Li, D.; Xu, Z.; Liao, C.; Liu, Y.; Zhong, B. Selective leaching of valuable metals from laterite nickel ore with ammonium chloride-hydrochloric acid solution. J. Clean. Prod. 2018, 179, 24–30. [Google Scholar] [CrossRef]
- Klyushnikov, A.M. Sulphuric-acid leaching of ural oxidized nickel ore with sodium sulfite and fluoride additives. J. Min. Sci. 2018, 54, 141–146. [Google Scholar] [CrossRef]
- Zeng, X.; Xu, M.; Li, J. Examining the sustainability of China’s nickel supply: 1950–2050. Resour. Conserv. Recycl. 2018, 139, 188–193. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, G.; Ostrovski, O.; Jahanshahi, S. Selective reduction of an Australian garnieritic laterite ore. Miner. Eng. 2019, 131, 79–89. [Google Scholar] [CrossRef]
- Rao, M.; Li, G.; Zhang, X.; Luo, J.; Peng, Z.; Jiang, T. Reductive roasting of nickel laterite ore with sodium sulfate for Fe-Ni production. Part I: Reduction/sulfidation characteristics. Sep. Sci. Technol. 2016, 51, 1408–1420. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, G.; Jahanshahi, S.; Ostrovski, O. Carbonylation of nickel and selectively reduced laterite ore. J. S. Afr. Inst. Min. Metall. 2018, 118. [Google Scholar] [CrossRef]
- Zhu, D.; Pan, L.; Guo, Z.; Pan, J.; Zhang, F. Utilization of limonitic nickel laterite to produce ferronickel concentrate by the selective reduction-magnetic separation process. Adv. Powder Technol. 2019, 30, 451–460. [Google Scholar] [CrossRef]
- Wang, X.-P.; Sun, T.-C.; Chen, C.; Kou, J. Effects of Na2SO4 on iron and nickel reduction in a high-iron and low-nickel laterite ore. Int. J. Miner. Metall. Mater. 2018, 25, 383–390. [Google Scholar] [CrossRef]
- Rodrigues, F.; Pickles, C.; Peacey, J.; Elliott, R.; Forster, J. Factors affecting the upgrading of a nickeliferous limonitic laterite ore by reduction roasting, thermal growth and magnetic separation. Minerals 2017, 7, 176. [Google Scholar] [CrossRef]
- Pickles, C.A.; Elliott, R. Thermodynamic analysis of selective reduction of nickeliferous limonitic laterite ore by carbon monoxide. Miner. Process. Extr. Metall. 2015, 124, 208–216. [Google Scholar] [CrossRef]
- Gao, L.; Liu, Z.; Pan, Y.; Ge, Y.; Feng, C.; Chu, M.; Tang, J. Separation and recovery of iron and nickel from low-grade laterite nickel ore using reduction roasting at Rotary Kiln followed by magnetic separation technique. Min. Metall. Explor. 2018, 36, 375–384. [Google Scholar] [CrossRef]
- Zhang, P.; Guo, Q.; Wei, G.; Meng, L.; Han, L.; Qu, J.; Qi, T. Extraction of metals from saprolitic laterite ore through pressure hydrochloric-acid selective leaching. Hydrometallurgy 2015, 157, 149–158. [Google Scholar] [CrossRef]
- Chang, Y.; Zhao, K.; Pesic, B. Selective leaching of nickel from prereduced limonitic laterite under moderate HPAL conditions—Part I: Dissolution. J. Min. Metall. Sect. B Metall. 2016, 52, 127–134. [Google Scholar] [CrossRef]
- Zhang, P.; Guo, Q.; Wei, G.; Meng, L.; Han, L.; Qu, J.; Qi, T. Leaching metals from saprolitic laterite ore using a ferric chloride solution. J. Clean. Prod. 2016, 112, 3531–3539. [Google Scholar] [CrossRef]
- Kobayashi, H.; Shoji, H.; Asano, S.; Imamura, M. Selective nickel leaching from nickel and cobalt mixed sulfide using sulfuric acid. Mater. Trans. 2018, 59, 1458–1464. [Google Scholar] [CrossRef]
- Luo, J.; Li, G.; Rao, M.; Peng, Z.; Zhang, Y.; Jiang, T. Atmospheric leaching characteristics of nickel and iron in limonitic laterite with sulfuric acid in the presence of sodium sulfite. Miner. Eng. 2015, 78, 38–44. [Google Scholar] [CrossRef]
- Oxley, A.; Smith, M.E.; Caceres, O. Why heap leach nickel laterites? Miner. Eng. 2016, 88, 53–60. [Google Scholar] [CrossRef]
- Mu, W.; Huang, Z.; Xin, H.; Luo, S.; Zhai, Y.; Xu, Q. Extraction of copper and nickel from low-grade nickel sulfide ore by low-temperature roasting, selective decomposition and water-leaching process. JOM 2019. [Google Scholar] [CrossRef]
- Mu, W.; Cui, F.; Huang, Z.; Zhai, Y.; Xu, Q.; Luo, S. Synchronous extraction of nickel and copper from a mixed oxide-sulfide nickel ore in a low-temperature roasting system. J. Clean. Prod. 2018, 177, 371–377. [Google Scholar] [CrossRef]
- Li, G.; Cheng, H.; Chen, S.; Lu, X.; Xu, Q.; Lu, C. Mechanism of Na2SO4 promoting nickel extraction from sulfide concentrates by sulfation roasting–water leaching. Metall. Mater. Trans. B 2018, 49, 1136–1148. [Google Scholar] [CrossRef]
- Mukherjee, T.K.; Menon, P.R.; Shukla, P.P.; Gupta, C.K. Chloridizing roasting process for a complex Sulfide concentrate. JOM 1985, 37, 29–33. [Google Scholar] [CrossRef]
- Swamy, Y.V.; Kar, B.B.; Mohanty, J.K. Physico-chemical characterization and sulphatization roasting of low-grade nickeliferous laterites. Hydrometallurgy 2003, 69, 89–98. [Google Scholar] [CrossRef]
- Yu, D.; Utigard, T.A.; Barati, M. Fluidized bed selective oxidation-sulfation roasting of nickel sulfide concentrate: Part II. sulfation roasting. Metall. Mater. Trans. B 2013, 45, 662–674. [Google Scholar] [CrossRef]
- Xu, C.; Cheng, H.; Li, G.; Lu, C.; Lu, X.; Zou, X.; Xu, Q. Extraction of metals from complex sulfide nickel concentrates by low-temperature chlorination roasting and water leaching. Int. J. Miner. Metall. Mater. 2017, 24, 377–385. [Google Scholar] [CrossRef]
- Cui, F.; Mu, W.; Wang, S.; Xin, H.; Xu, Q.; Zhai, Y. A sustainable and selective roasting and water-leaching process to simultaneously extract valuable metals from low-grade Ni-Cu matte. JOM 2018, 70, 1977–1984. [Google Scholar] [CrossRef]
- Guo, X.; Li, D.; Park, K.-H.; Tian, Q.; Wu, Z. Leaching behavior of metals from a limonitic nickel laterite using a sulfation–roasting–leaching process. Hydrometallurgy 2009, 99, 144–150. [Google Scholar] [CrossRef]
- Prasad, S.; Pandey, B.D. Alternative processes for treatment of chalcopyrite—A review. Miner. Eng. 1998, 11, 763–781. [Google Scholar] [CrossRef]
- Ingraham, T.R.; Kerby, R. Roasting in extractive metallurgy a thermodynamic and kinetic review. Can. Metall. Q. 1967, 6, 89–119. [Google Scholar] [CrossRef]
- Theys, L.F.; Lee, L.V. Sulfate roasting copper-cobalt sulfide concentrates. JOM 1958, 10, 134–136. [Google Scholar] [CrossRef]
- Tümen, F.; Bailey, N.T. Recovery of metal values from copper smelter slags by roasting with pyrite. Hydrometallurgy 1990, 25, 317–328. [Google Scholar] [CrossRef]
- Fletcher, A.; Shelef, M. The role of alkali sulfates in promoting the sulphation roasting of nickel sulfides. Unit Process Hydrometall. 1964, 24, 946–970. [Google Scholar]
- Altundoǧan, H.S.; Tümen, F. Metal recovery from copper converter slag by roasting with ferric sulphate. Hydrometallurgy 1997, 44, 261–267. [Google Scholar] [CrossRef]
- Sukla, L.B.; Panda, S.C.; Jena, P.K. Recovery of cobalt, nickel and copper from converter slag through roasting with ammonium sulphate and sulphuric acid. Hydrometallurgy 1986, 16, 153–165. [Google Scholar] [CrossRef]
- Yang, Z.; Li, Y.; Lou, Q.; Liu, D.; Ning, Y.; Yang, S.; Tang, Y.; Zhang, Y.; Tang, Z.; Wang, X. Release of uranium and other trace elements from coal ash by (NH4)2SO4 activation of amorphous phase. Fuel 2019, 239, 774–785. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, H.; Xue, X.-X.; Yuan, S. Separation and recovery of iron and rare earth from Bayan Obo tailings by magnetizing roasting and (NH4)2SO4 activation roasting. Metals 2017, 7, 195. [Google Scholar] [CrossRef]
- Liu, W.; Wang, X.; Lu, Z.; Yue, H.; Liang, B.; Lü, L.; Li, C. Preparation of synthetic rutile via selective sulfation of ilmenite with (NH4)2SO4 followed by targeted removal of impurities. Chin. J. Chem. Eng. 2017, 25, 821–828. [Google Scholar] [CrossRef]
- Shao, H.-M.; Shen, X.-Y.; Sun, Y.; Liu, Y.; Zhai, Y.-C. Reaction condition optimization and kinetic investigation of roasting zinc oxide ore using (NH4)2SO4. Int. J. Miner. Metall. Mater. 2016, 23, 1133–1140. [Google Scholar] [CrossRef]
- Cui, F.; Mu, W.; Wang, S.; Xin, H.; Xu, Q.; Zhai, Y.; Luo, S. Sodium sulfate activation mechanism on co-sulfating roasting to nickel-copper sulfide concentrate in metal extractions, microtopography and kinetics. Miner. Eng. 2018, 123, 104–116. [Google Scholar] [CrossRef]
- Li, Y.; Liu, H.; Peng, B.; Min, X.; Hu, M.; Peng, N.; Yuang, Y.; Lei, J. Study on separating of zinc and iron from zinc leaching residues by roasting with ammonium sulphate. Hydrometallurgy 2015, 158, 42–48. [Google Scholar] [CrossRef]
- Halstead, W.D. Thermal decomposition of ammonium sulphate. J. Appl. Chem. 2007, 20, 129–132. [Google Scholar] [CrossRef]
- Liu, X.; Feng, Y.; Li, H.; Yang, Z.; Cai, Z. Recovery of valuable metals from a low-grade nickel ore using an ammonium sulfate roasting-leaching process. Int. J. Miner. Metall. Mater. 2012, 19, 377–383. [Google Scholar] [CrossRef]
- Mkhonto, P.; Chauke, H.; Ngoepe, P. Ab initio studies of O2 adsorption on (110) nickel-rich pentlandite (Fe4Ni5S8) mineral surface. Minerals 2015, 5, 665–678. [Google Scholar] [CrossRef]
- Sit, P.H.; Cohen, M.H.; Selloni, A. Interaction of oxygen and water with the (100) surface of pyrite: mechanism of sulfur oxidation. J. Phys. Chem. Lett. 2012, 3, 2409–2414. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Hua, X.; Zheng, Y.; Lu, X.; Li, S.; Cheng, H.; Xu, Q. Oxidation mechanism of chalcopyrite revealed by X-ray photoelectron spectroscopy and first principles studies. Appl. Surf. Sci. 2018, 427, 233–241. [Google Scholar] [CrossRef]
- Xiong, X.; Lu, X.; Li, G.; Cheng, H.; Xu, Q.; Li, S. Energy dispersive spectrometry and first principles studies on the oxidation of pentlandite. Phys. Chem. Chem. Phys. 2018, 20, 12791–12798. [Google Scholar] [CrossRef]
- Li, G.; Cheng, H.; Xiong, X.; Lu, X.; Xu, C.; Lu, C.; Zou, X.; Xu, Q. In-situ XRD and EDS method study on the oxidation behaviour of Ni-Cu sulphide ore. Sci. Rep. 2017, 7, 3212. [Google Scholar] [CrossRef]
- Rajamani, V.T.; Prewitt, C. The crystal structure of millerite. Can. Mineral. 1974, 12, 253–257. [Google Scholar]
- Chen, J.; Long, X.; Zhao, C.; Kang, D.; Guo, J. DFT calculation on relaxation and electronic structure of sulfide minerals surfaces in presence of H2O molecule. J. Cent. S. Univ. 2014, 21, 3945–3954. [Google Scholar] [CrossRef]



| Fe | O | S | Ni | Mg | Cu | Si | Ca | Al | Co |
|---|---|---|---|---|---|---|---|---|---|
| 28.26 | 22.43 | 19.61 | 8.93 | 6.66 | 6.51 | 6.45 | 0.52 | 0.39 | 0.24 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Xiong, X.; Wang, L.; Che, L.; Wei, L.; Cheng, H.; Zou, X.; Xu, Q.; Zhou, Z.; Li, S.; et al. Sulfation Roasting of Nickel Oxide–Sulfide Mixed Ore Concentrate in the Presence of Ammonium Sulfate: Experimental and DFT Studies. Metals 2019, 9, 1256. https://doi.org/10.3390/met9121256
Li G, Xiong X, Wang L, Che L, Wei L, Cheng H, Zou X, Xu Q, Zhou Z, Li S, et al. Sulfation Roasting of Nickel Oxide–Sulfide Mixed Ore Concentrate in the Presence of Ammonium Sulfate: Experimental and DFT Studies. Metals. 2019; 9(12):1256. https://doi.org/10.3390/met9121256
Chicago/Turabian StyleLi, Guangshi, Xiaolu Xiong, Liping Wang, Lang Che, Lizhen Wei, Hongwei Cheng, Xingli Zou, Qian Xu, Zhongfu Zhou, Shenggang Li, and et al. 2019. "Sulfation Roasting of Nickel Oxide–Sulfide Mixed Ore Concentrate in the Presence of Ammonium Sulfate: Experimental and DFT Studies" Metals 9, no. 12: 1256. https://doi.org/10.3390/met9121256
APA StyleLi, G., Xiong, X., Wang, L., Che, L., Wei, L., Cheng, H., Zou, X., Xu, Q., Zhou, Z., Li, S., & Lu, X. (2019). Sulfation Roasting of Nickel Oxide–Sulfide Mixed Ore Concentrate in the Presence of Ammonium Sulfate: Experimental and DFT Studies. Metals, 9(12), 1256. https://doi.org/10.3390/met9121256





