Investigation of Primary Carbides in a Commercial-Sized Electroslag Remelting Ingot of H13 Steel
Abstract
:1. Introduction
2. Experimental Procedure
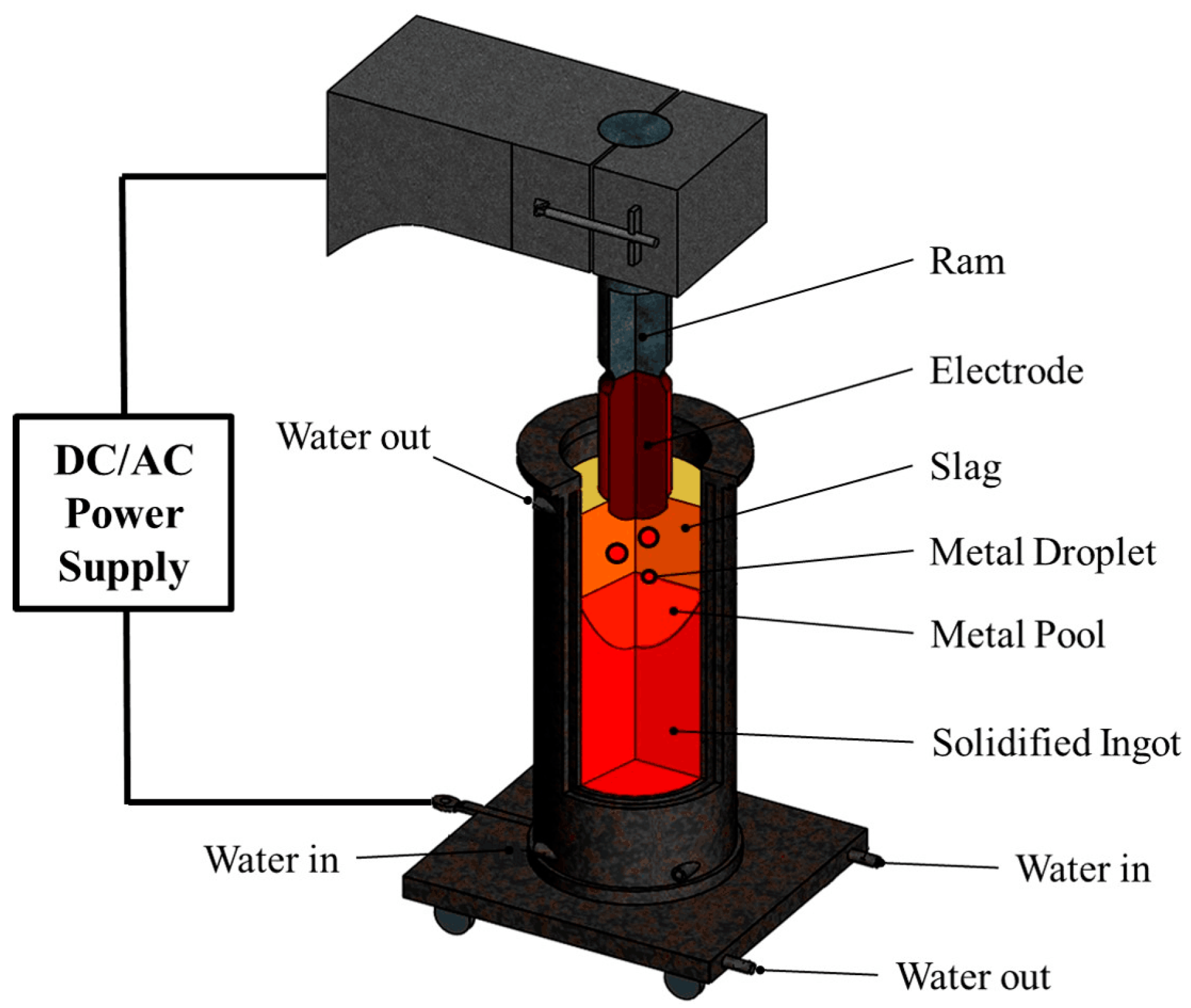
2.1. Preparation of the ESR Ingot
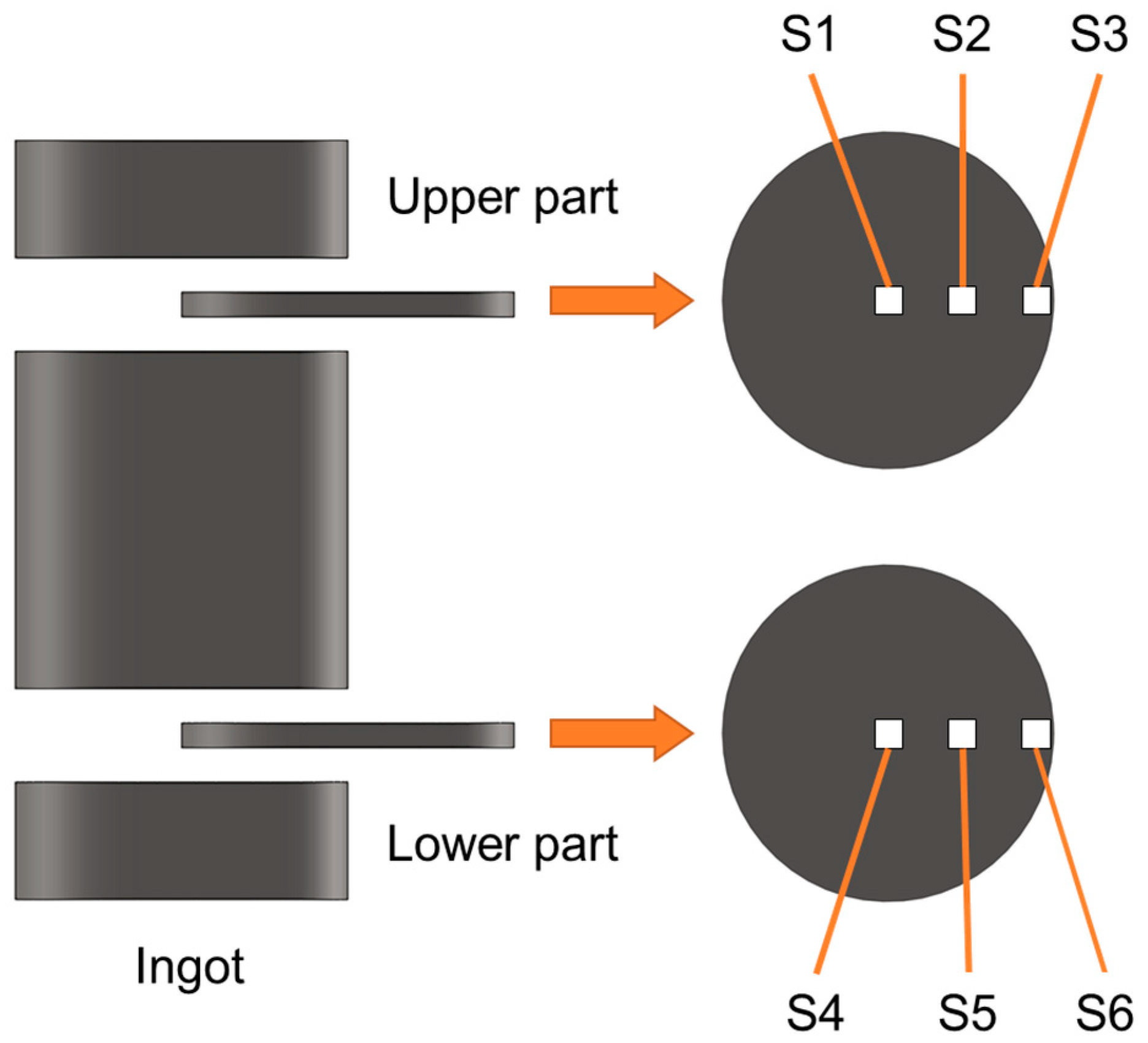
2.2. Specimen Preparation
2.3. Analyzing Approaches
3. Results and Discussion
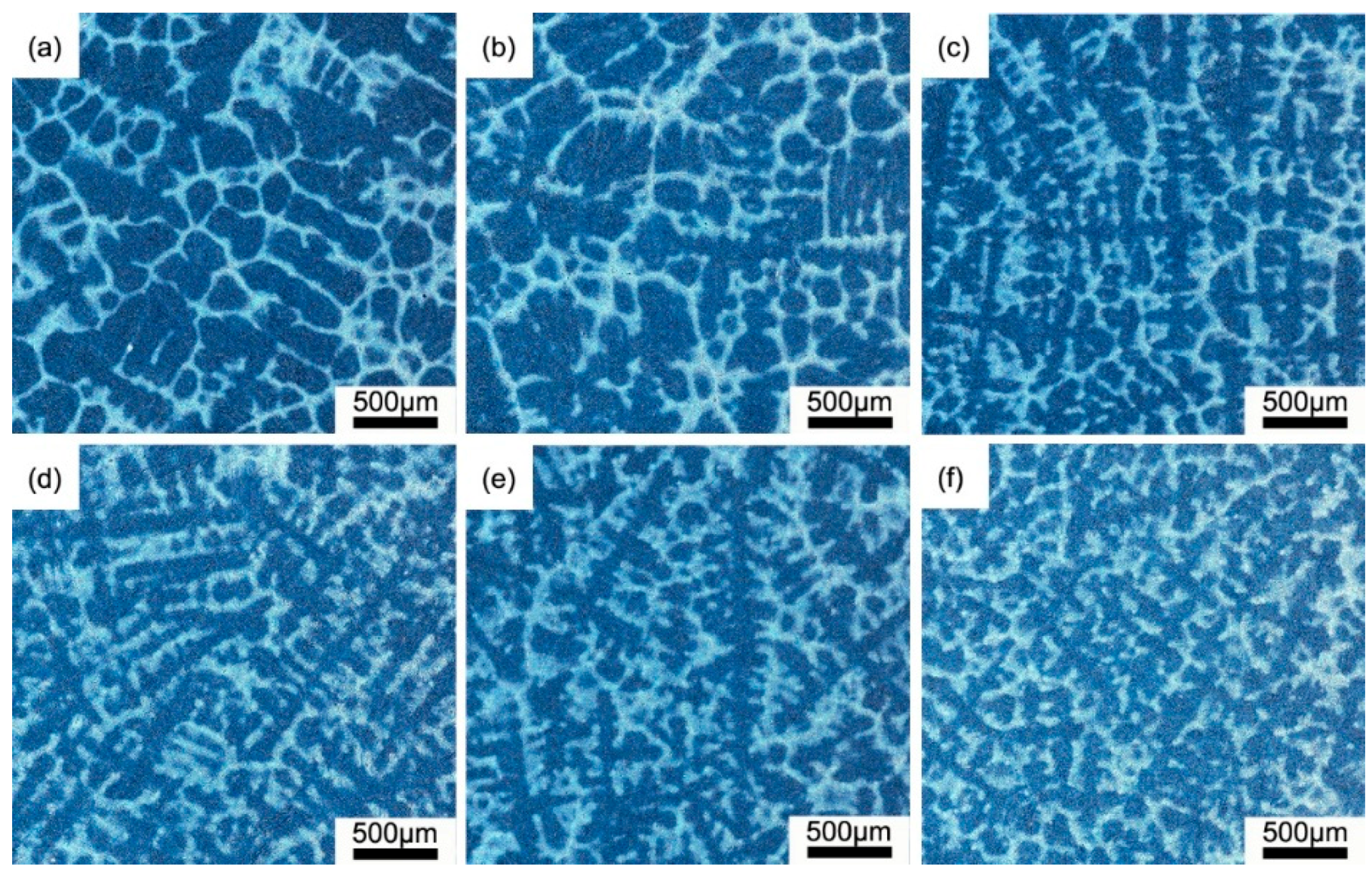
3.1. Characteristics of Primary Carbide
3.2. Distribution of Primary Carbide
3.3. Microstructure of H13 ESR Ingot
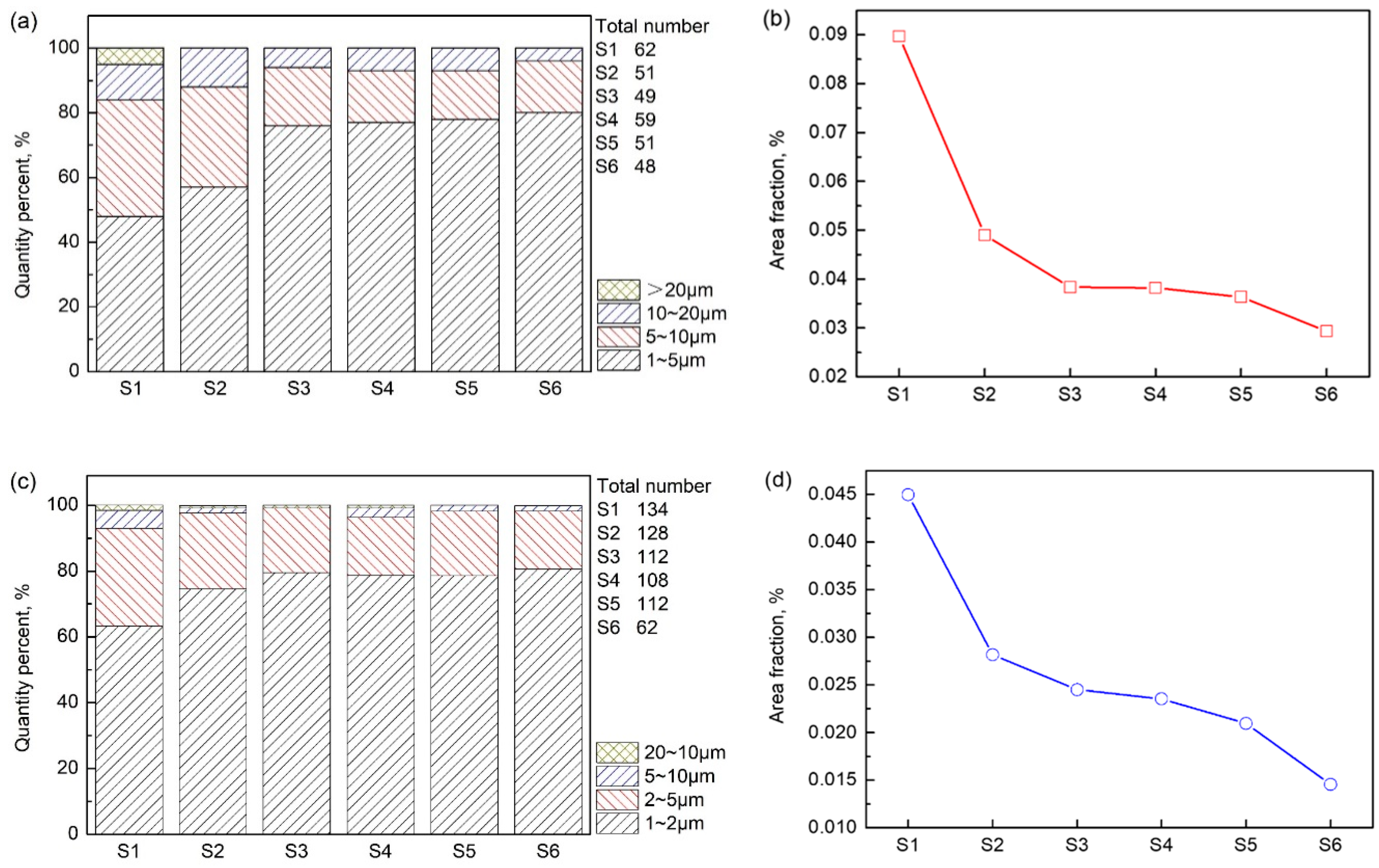
3.4. Size and Area of Primary Carbide
3.5. Heterogeneous Nucleation of Primary Carbide
4. Conclusions
- Two types of primary carbide, V-rich and Mo-rich primary carbides, are observed in the H13 ESR ingot, which mainly distribute in the interdendritic region.
- A net-like structure, caused by the enrichment of the solutes within the interdendritic region, is found in the matrix of the H13 ESR ingot. The primary carbide would be formed in the center of the net-like structure. Furthermore, the dendritic grain of the lower part of the H13 ESR ingot is finer than that in the upper part, and also dendritic grain at the outer surface of the ingot is finer than that at the center of the ingot.
- The quantity: the area fraction and the size of the two primary carbides tend to decrease from the center of the H13 ESR ingot to the outer surface. Additionally, the V-rich primary carbide is obviously larger than the Mo-rich primary carbide. A stronger cooling would reduce the size of the primary carbides.
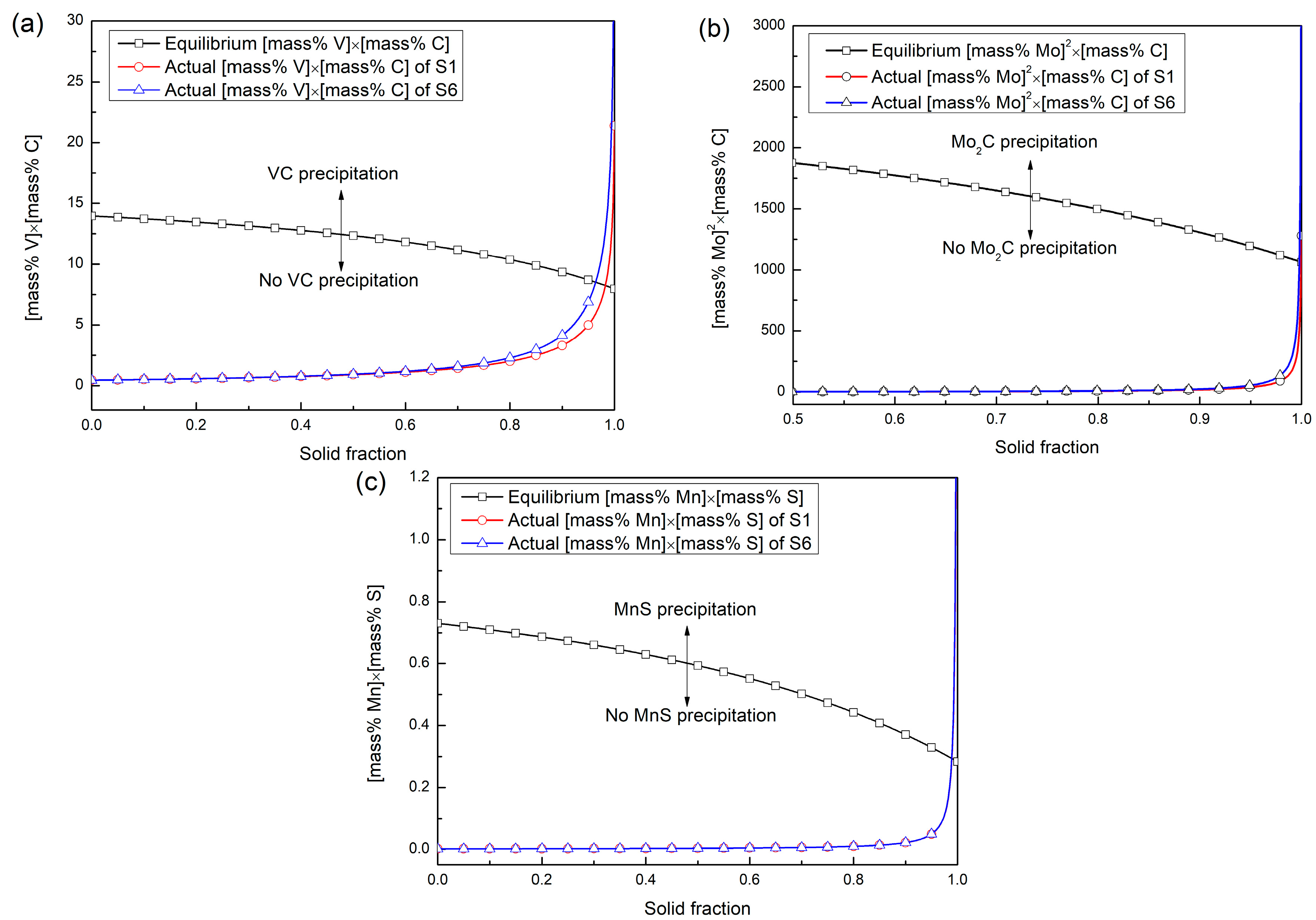
- The Al2O3 inclusion can promote the nucleation of the V-rich primary carbide, while the MnS inclusion can encourage the nucleation of the Mo-rich primary carbide. The solid fraction that the V-rich begins to precipitate in the investigated steel ranges from 0.965 to 0.983, and that for the Mo-rich primary carbide and the MnS inclusion change from 0.9990 to 0.9998 and from 0.989 to 0.990, respectively. The CaO∙Al2O3 inclusion cannot act as the nucleation site for the precipitation of the two primary carbides.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Delagnes, D.; Lamesle, P.; Mathon, M.H.; Mebarki, N.; Levaillant, C. Influence of silicon content on the precipitation of secondary carbides and fatigue properties of a 5%Cr tempered martensitic steel. Mater. Sci. Eng. A 2005, 394, 435–444. [Google Scholar] [CrossRef]
- Hu, X.B.; Li, L.; Wu, X.C.; Zhang, M. Coarsening behavior of M23C6 carbides after ageing or thermal fatigue in AISI H13 steel with niobium. Int. J. Fatigue 2006, 28, 175–182. [Google Scholar] [CrossRef]
- Fuchs, K.D. Hot-work tool steels with improved properties for die casting applications. In Proceedings of the 6th International Tooling Conference, Karlstad, Sweden, 10–13 September 2002. [Google Scholar]
- Medvedeva, A.; Bergström, J.; Gunnarsson, S.; Andersson, J. High-temperature properties and microstructural stability of hot-work tool steels. Mater. Sci. Eng. A 2009, 523, 39–46. [Google Scholar] [CrossRef]
- Ma, D.S.; Zhou, J.; Chen, Z.Z.; Zhang, Z.K.; Chen, Q.A.; Li, D.H. Influence of thermal homogenization treatment on structure and impact toughness of H13 ESR steel. J. Iron Steel Res. Int. 2009, 16, 56–60. [Google Scholar] [CrossRef]
- Tridello, A.; Paolino, D.S.; Chiandussi, G.; Rossetto, M. Effect of electroslag remelting on the VHCF response of an AISI H13 steel. Fatigue Fract. Eng. Mater. 2017, 40, 1783–1794. [Google Scholar] [CrossRef]
- Liu, J.H.; Wang, G.X.; Bao, Y.P.; Yang, Y.; Yao, W.; Cui, X.N. Inclusion variations of hot working die steel H13 in refining process. J. Iron Steel Res. Int. 2012, 19, 1–7. [Google Scholar] [CrossRef]
- Wang, Q.; He, Z.; Li, B.K.; Tsukihashi, F. A general coupled mathematical model of electromagnetic phenomena, two-phase flow, and heat transfer in electroslag remelting process including conducting in the mold. Metall. Mater. Trans. B 2014, 45, 2425–2441. [Google Scholar] [CrossRef]
- Kheirandish, S.; Noorian, A. Effect of niobium on microstructure of cast AISI H13 hot work tool steel. J. Iron Steel Res. Int. 2008, 15, 61–66. [Google Scholar] [CrossRef]
- Li, J.; Li, J.; Shi, C.B.; Wang, L.L.; Wu, Z.; Wang, H. Effect of trace magnesium on carbide improvement in H13 steel. Can. Metall. Q. 2016, 55, 321–327. [Google Scholar] [CrossRef]
- Atkinson, H.V.; Shi, G. Characterization of inclusions in clean steels: A review including the statistics of extremes methods. Prog. Mater. Sci. 2003, 48, 457–520. [Google Scholar] [CrossRef]
- Yao, D.; Li, J.; Li, J.H.; Zhu, Q.T. Effect of cold rolling on morphology of carbides and properties of 7Cr17MoV stainless steel. Mater. Manuf. Process. 2015, 30, 111–115. [Google Scholar] [CrossRef]
- Sun, X.L.; Guo, H.J.; Chen, X.C.; Ning, A.G.; Du, G.W.; Shi, C.B. Formation Mechanism of Primary Carbide in H13 Steel During Electroslag Remelting Process. Iron Steel 2014, 49, 68–73. [Google Scholar]
- Mao, M.T.; Guo, H.J.; Wang, F.; Sun, X.L. Effect of cooling rate on the solidification microstructure and characteristics of primary carbides in H13 steel. ISIJ Int. 2019, 59, 848–857. [Google Scholar] [CrossRef]
- Lan, J.; He, J.J.; Ding, W.J.; Wang, Q.D.; Zhu, Y.P. Effect of rare earth metals on the microstructure and impact toughness of a cast 0.4C–5Cr–1.2Mo–1.0V Steel. ISIJ Int. 2000, 40, 1275–1282. [Google Scholar] [CrossRef]
- Shi, C.B.; Zhu, Q.T.; Yu, W.T.; Song, H.D.; Li, J. Effect of oxide inclusions modification during electroslag remelting on primary carbides and toughness of a high-carbon 17 mass% Cr tool steel. J. Mater. Eng. Perform. 2016, 25, 4785–4795. [Google Scholar] [CrossRef]
- Won, Y.M.; Thomas, B.G. Simple model of microsegregation during solidification of steels. Metall. Mater. Trans. A 2001, 32, 1755–1767. [Google Scholar] [CrossRef]
- Bramfitt, B.L. The effect of carbide and nitride additions on the heterogeneous nucleation behavior of liquid iron. Metall. Trans. 1970, 1, 1987–1995. [Google Scholar] [CrossRef]
- Huang, Y.; Xie, Y.; Cheng, G.G.; Chen, L.; Zhang, Y.D.; Yan, Q.Z. Effect of rare earth on large size heterogeneous nucleation carbide in H13 steel. J. Chin. Soc. Rare Earths 2017, 35, 782–789. [Google Scholar]
- Deng, Z.Y.; Yong, M.Y. Evolution mechanism of non-metallic inclusions in Al-killed alloyed steel during secondary refining process. ISIJ Int. 2013, 53, 450–458. [Google Scholar] [CrossRef]
- Zhang, X.W.; Zhang, L.F.; Yang, W.; Dong, Y.C.; Li, Y.Z. Thermodynamics and dynamics of MnS inclusions precipitation during solidification process in heavy rail steel. Iron Steel 2016, 51, 30–39. [Google Scholar]
- Choudhary, S.K.; Ghosh, A. Mathematical model for prediction of composition of inclusions formed during solidification of liquid steel. ISIJ Int. 2009, 49, 1819–1827. [Google Scholar] [CrossRef]
- Suzuki, M.; Yamaguchi, R.; Murakami, K.; Nakada, M. Inclusion particle growth during solidification of stainless steel. ISIJ Int. 2001, 41, 247–256. [Google Scholar] [CrossRef]
- Binran, T. Handbook of Iron and Steel; Iron and Steel Institute of Japan: Tokyo, Japan; Maruzen: Tokyo, Japan, 1980; p. 205. [Google Scholar]
- Huang, X.H. Theory of Iron and Steel Metallurgy; Metallurgical Industry Press: Beijing, China, 2014; p. 174. [Google Scholar]
- Liu, Y.; Zhang, Z.; Li, G.Q.; Wang, Q.; Wang, L.; Li, B.K. Evolution of desulfurization and characterization of inclusions in dual alloy ingot processed by electroslag remelting. Steel Res. Int. 2017, 88. [Google Scholar] [CrossRef]





| Ingot | C | Si | Mn | V | Mo | Cr | Al | T. O | S |
|---|---|---|---|---|---|---|---|---|---|
| Electrode | 0.41 | 0.97 | 0.36 | 1.05 | 1.32 | 5.27 | 0.016 | 0.0046 | 0.0080 |
| ESR Ingot | 0.41 | 0.86 | 0.36 | 1.05 | 1.32 | 5.27 | 0.018 | 0.0038 | 0.0040 |
| Phase | VC | Mo2C | Al2O3 | MnS |
|---|---|---|---|---|
| Lattice type | NaCl structure | Hexagonal crystal system | Hexagonal crystal system | NaCl structure |
| Lattice constant, nm | a1 = 0.4182 | a2 = 0.3 | a3 = 0.4812 | a4 = 0.522 |
| Match Plane | Modified Lattice Constant | Disregistry, % | |
|---|---|---|---|
| Al2O3 (0001) // VC (110) | 9.4% | ||
| Al2O3 (0001) // Mo2C (0001) | 19.8% | ||
| MnS (110) // VC (100) | 14.6% | ||
| MnS (110) // Mo2C (0001) | 7.9% | ||
| VC (110) // Mo2C (0001) | 8.6% |
| Element | ki | DS, cm2/s |
|---|---|---|
| Mn | 0.785 | |
| S | 0.035 | |
| C | 0.34 | |
| V | 0.63 | |
| Mo | 0.585 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Li, G.; Liu, Y.; Cao, Y.; Wang, F.; Wang, Q. Investigation of Primary Carbides in a Commercial-Sized Electroslag Remelting Ingot of H13 Steel. Metals 2019, 9, 1247. https://doi.org/10.3390/met9121247
Wang X, Li G, Liu Y, Cao Y, Wang F, Wang Q. Investigation of Primary Carbides in a Commercial-Sized Electroslag Remelting Ingot of H13 Steel. Metals. 2019; 9(12):1247. https://doi.org/10.3390/met9121247
Chicago/Turabian StyleWang, Xijie, Guangqiang Li, Yu Liu, Yulong Cao, Fang Wang, and Qiang Wang. 2019. "Investigation of Primary Carbides in a Commercial-Sized Electroslag Remelting Ingot of H13 Steel" Metals 9, no. 12: 1247. https://doi.org/10.3390/met9121247
APA StyleWang, X., Li, G., Liu, Y., Cao, Y., Wang, F., & Wang, Q. (2019). Investigation of Primary Carbides in a Commercial-Sized Electroslag Remelting Ingot of H13 Steel. Metals, 9(12), 1247. https://doi.org/10.3390/met9121247






