The Effect of Laser Shock Peening on the Corrosion Behavior of Biocompatible Magnesium Alloy ZK60
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Material and Preparation of Samples
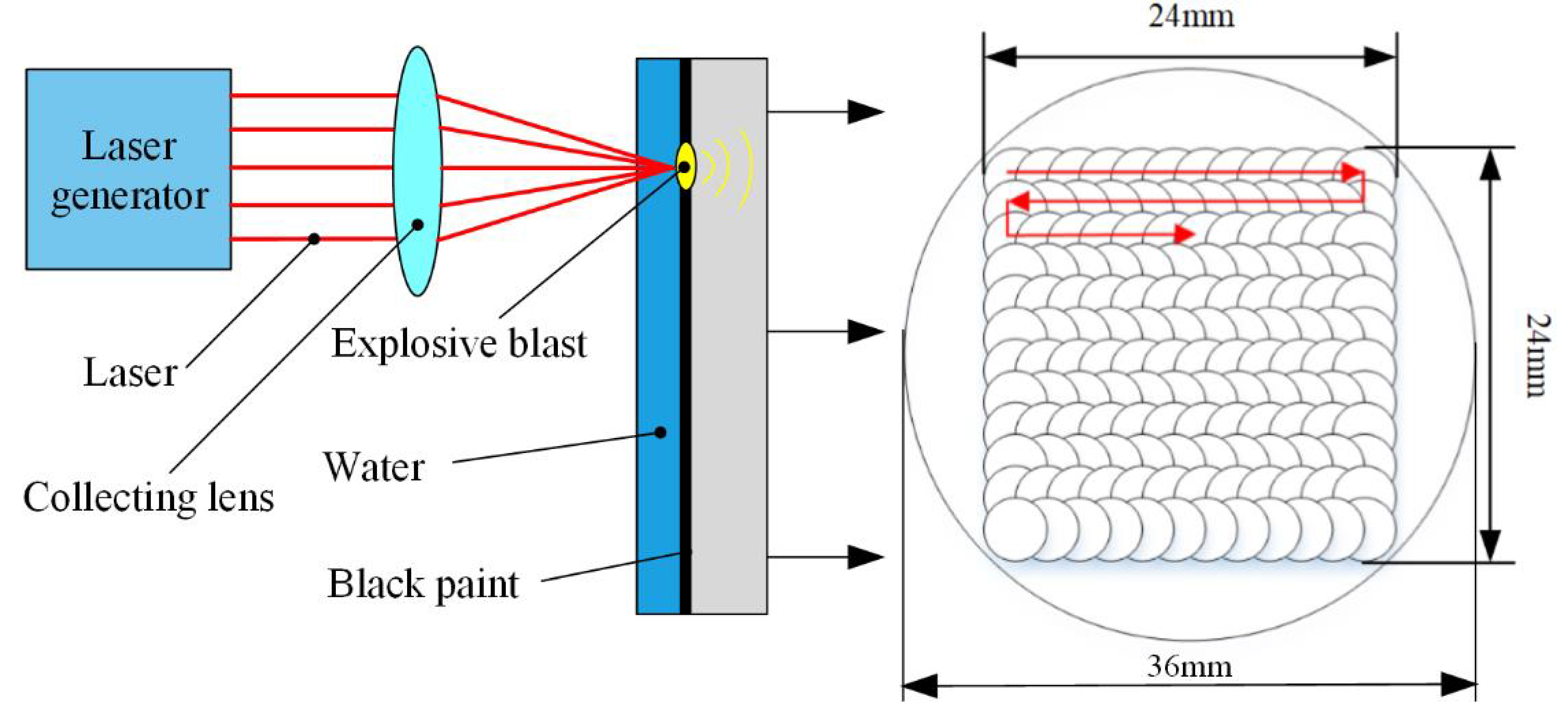
2.2. Laser Shock Peening Process
2.3. Surface Characterization
2.4. Simulated Body Fluid Immersion Testing
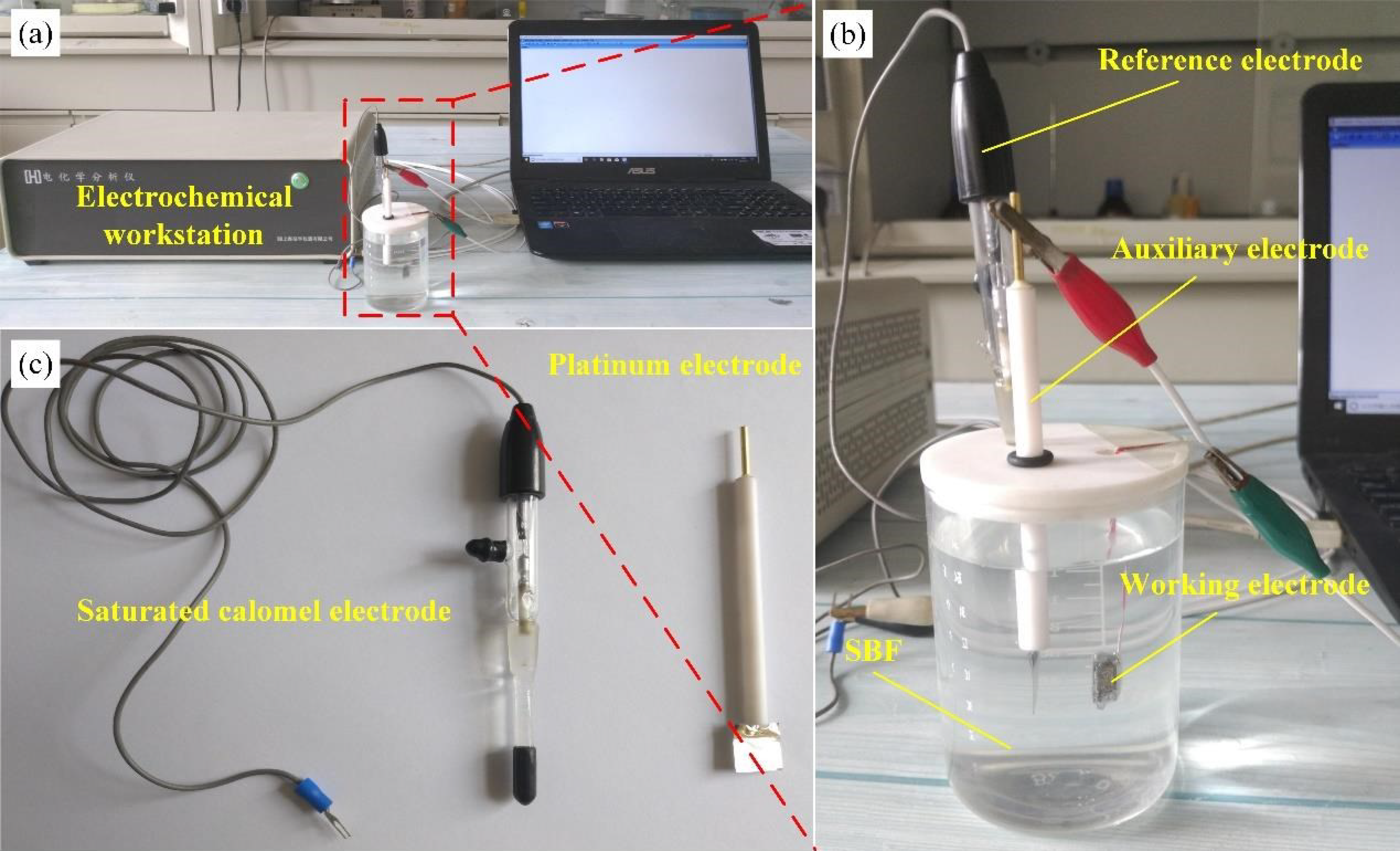
2.5. Electrochemical Corrosion Testing
3. Results and Discussion
3.1. Surface Topography and Roughness
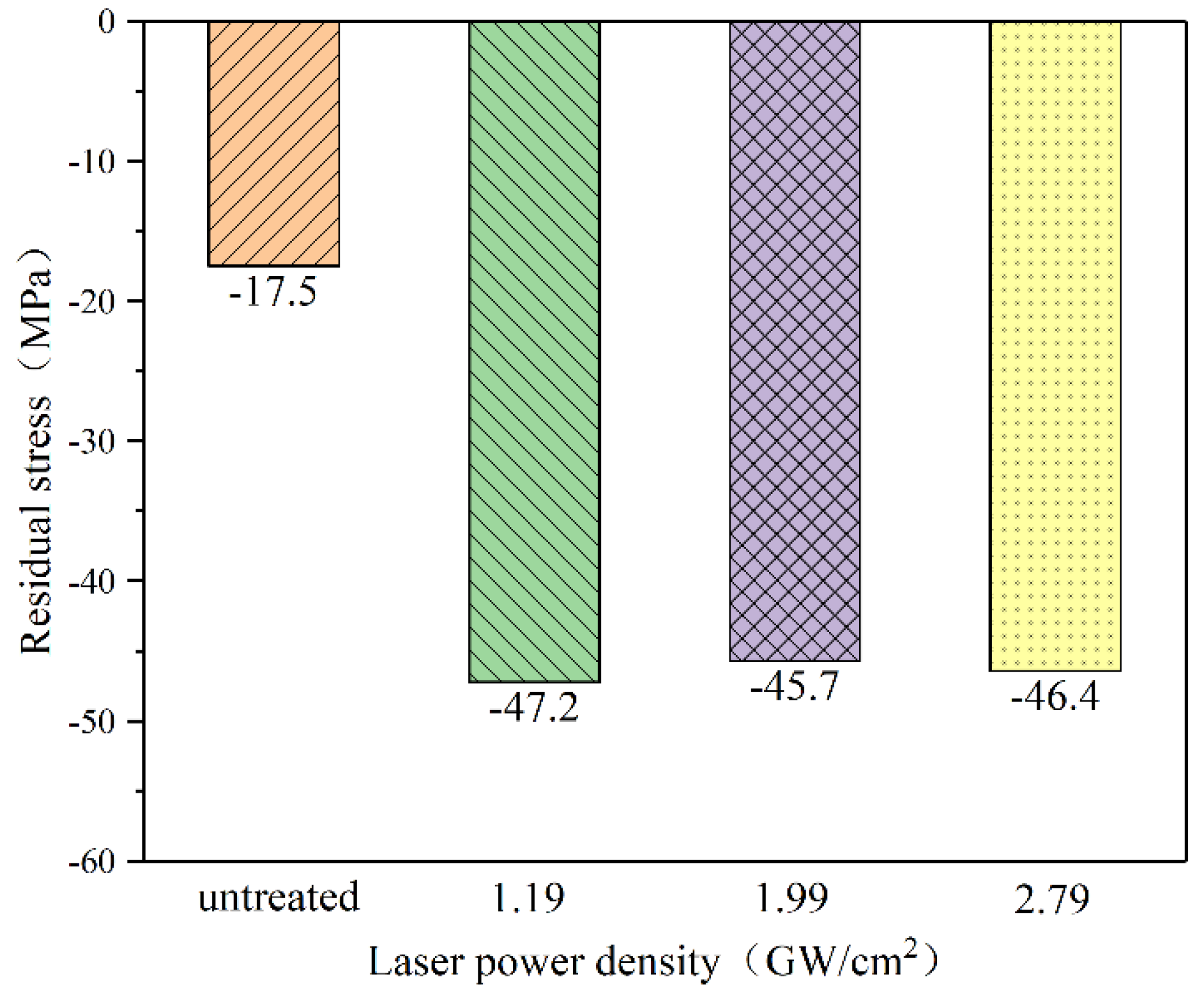
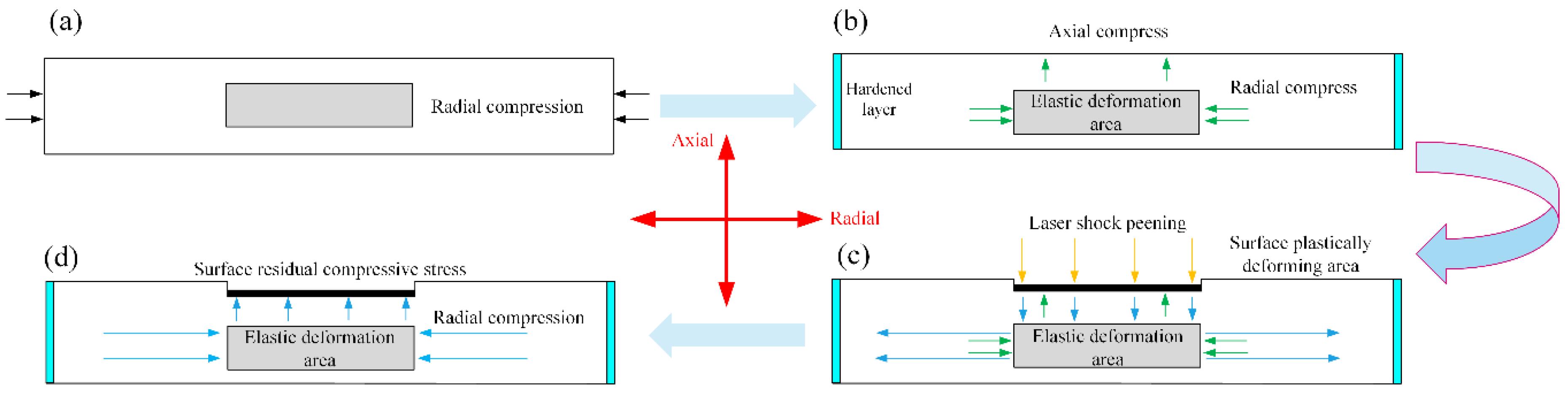
3.2. Residual Stress
3.3. Microstructure
3.4. Corrosion Behaviour
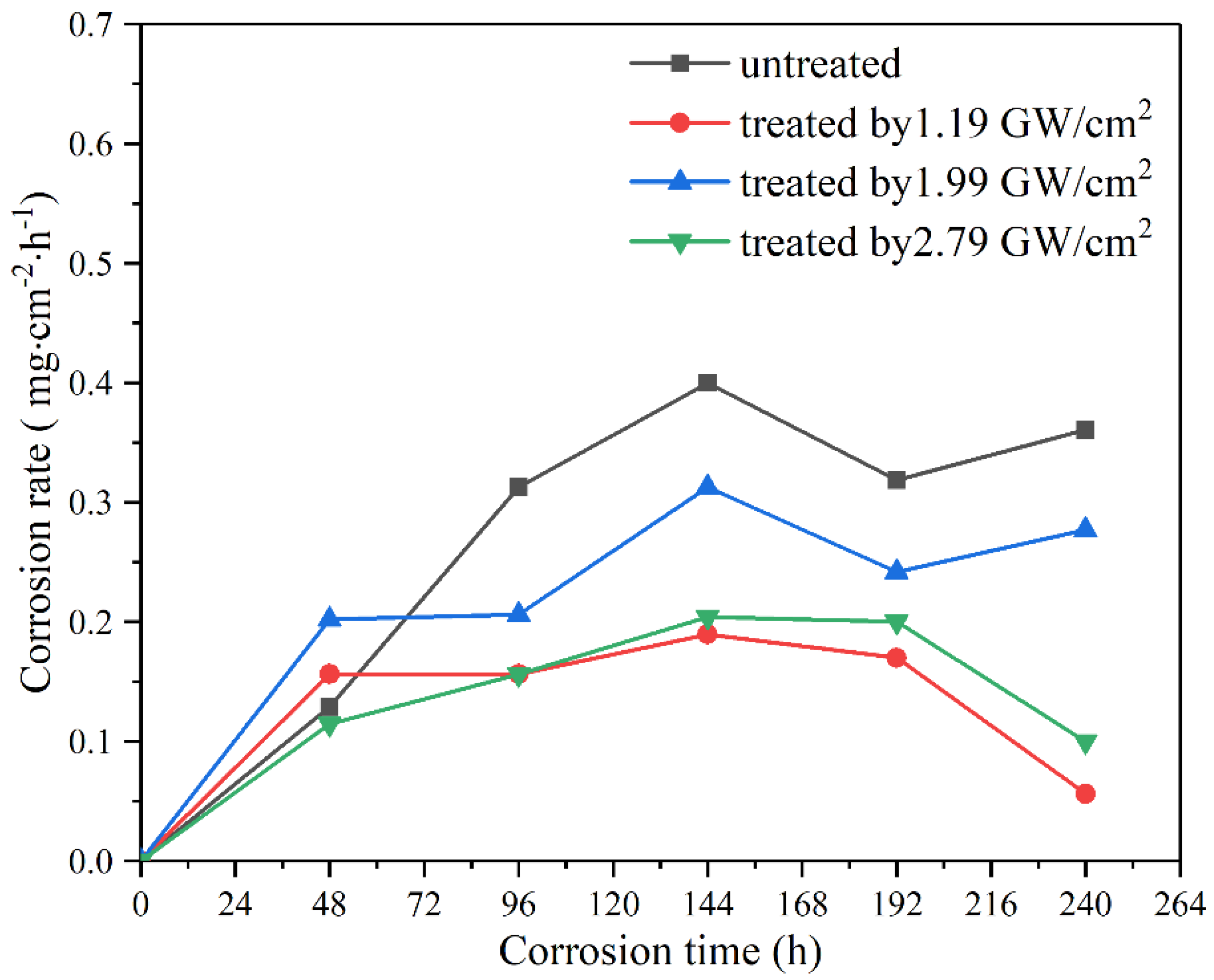
3.4.1. Degradation Rate and Weight Loss
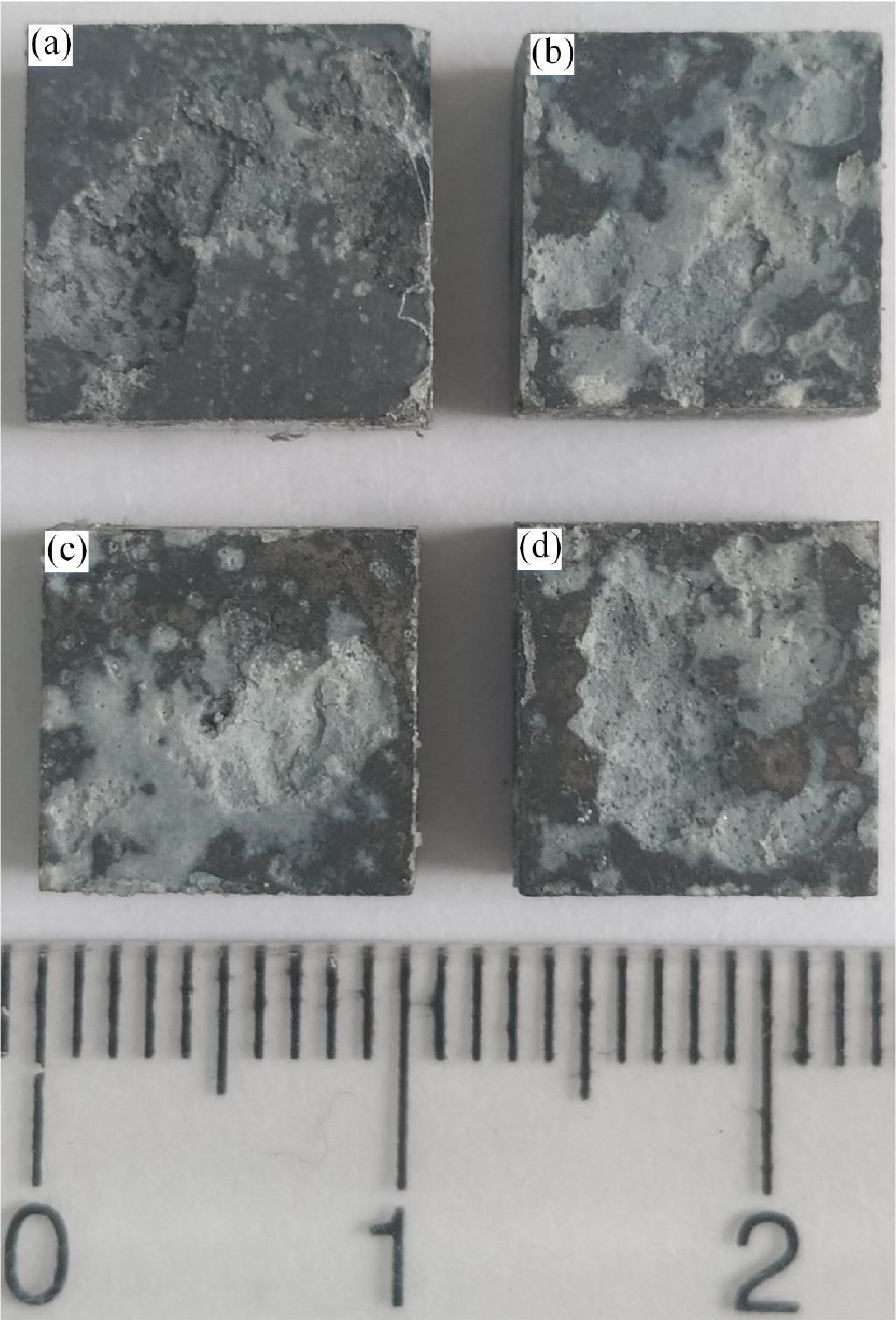
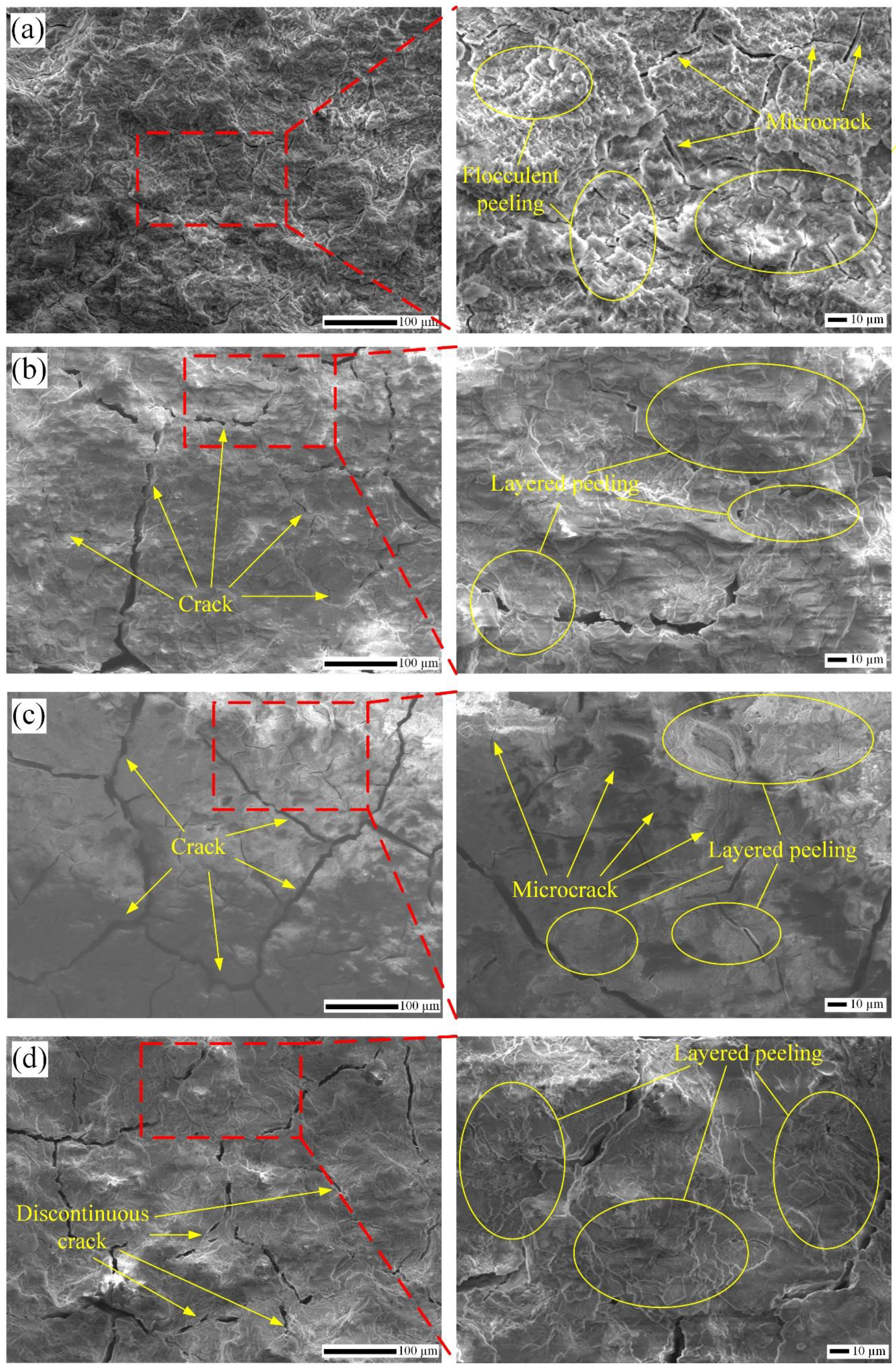
3.4.2. Corrosion Morphology Analysis
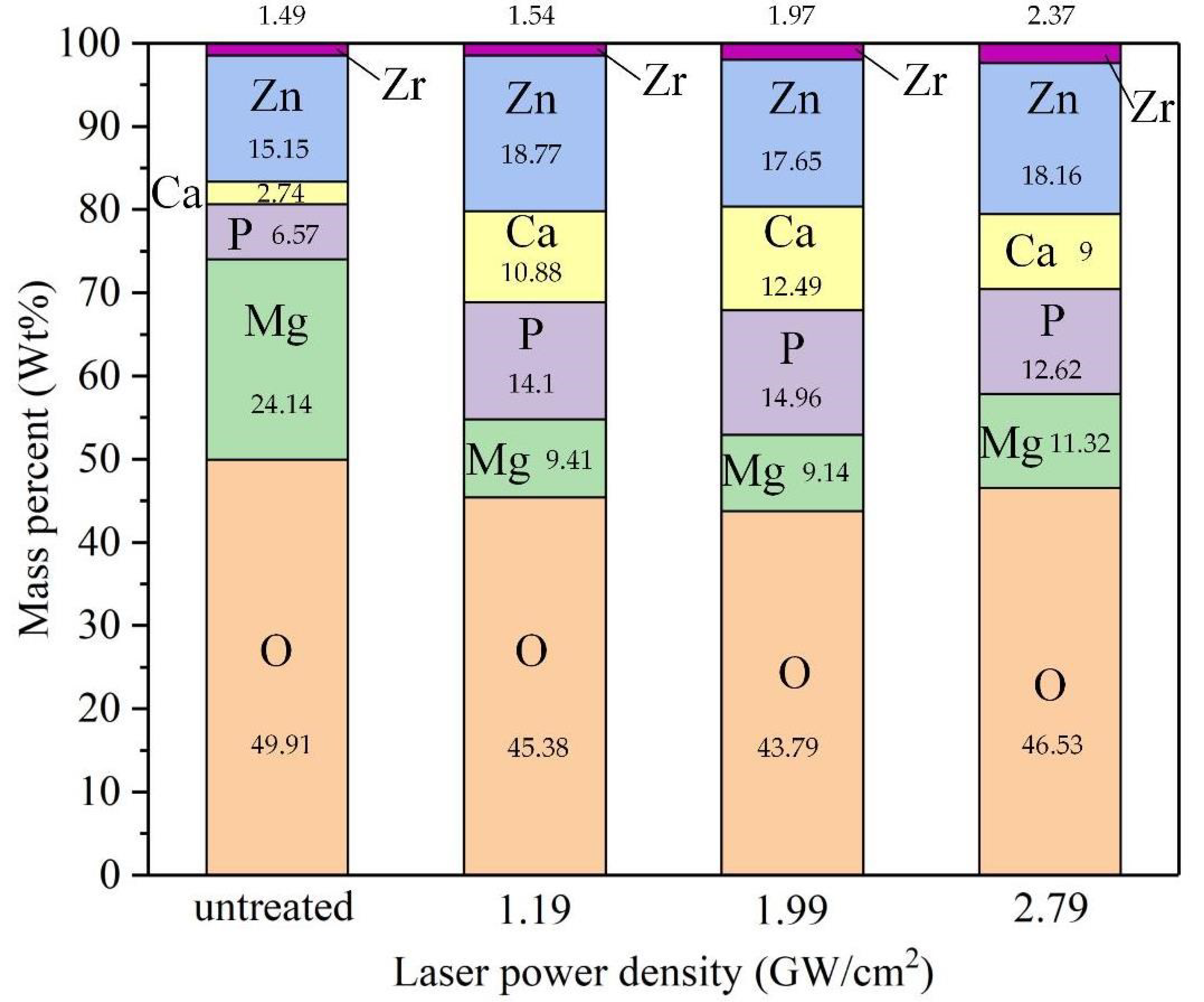
3.4.3. Corrosion Mechanism Analysis
3.5. Electrochemical Analysis
4. Conclusions
- (1)
- Plastic deformation increases with the increase of laser power density, which increases the value of surface roughness from the initial 0.2 µm to 6.11 µm.
- (2)
- LSP changes the surface residual stress field of magnesium alloy ZK60. The surface residual stress does not increase with the laser power density increases. When the laser power density is 1.19 GW/cm2, 1.99 GW/cm2, and 2.79 GW/cm2, the surface residual stress can be increased up to 47.2 MPa, 45.7 MPa, 46.4 MPa, respectively. Residual compressive stress is enhanced by a maximum of 1.7 times compared with the original sample. LSP can refine the size of the grains, and the average area of the surface grains decreases approximately as the power density increases. The average grains area falls from 45 μm2 to 17 μm2.
- (3)
- The degradation rate of magnesium alloy ZK60 in the SBF solution is decreased after LSP owing to a denser passivation film induced by higher residual compressive stress and grain refinement. In terms of the total weight loss, corrosion resistance increased by 52.1%, 45.1%, and 49%, respectively.
- (4)
- Corrosion cracks originate from corrosion pitting pits due to the influence of hydrogen embrittlement and stress concentration. Modified samples can improve corrosion pitting resistance and restrain crack initiation and propagation. The increase of calcium and phosphorus deposition is beneficial to improving the biocompatibility further.
- (5)
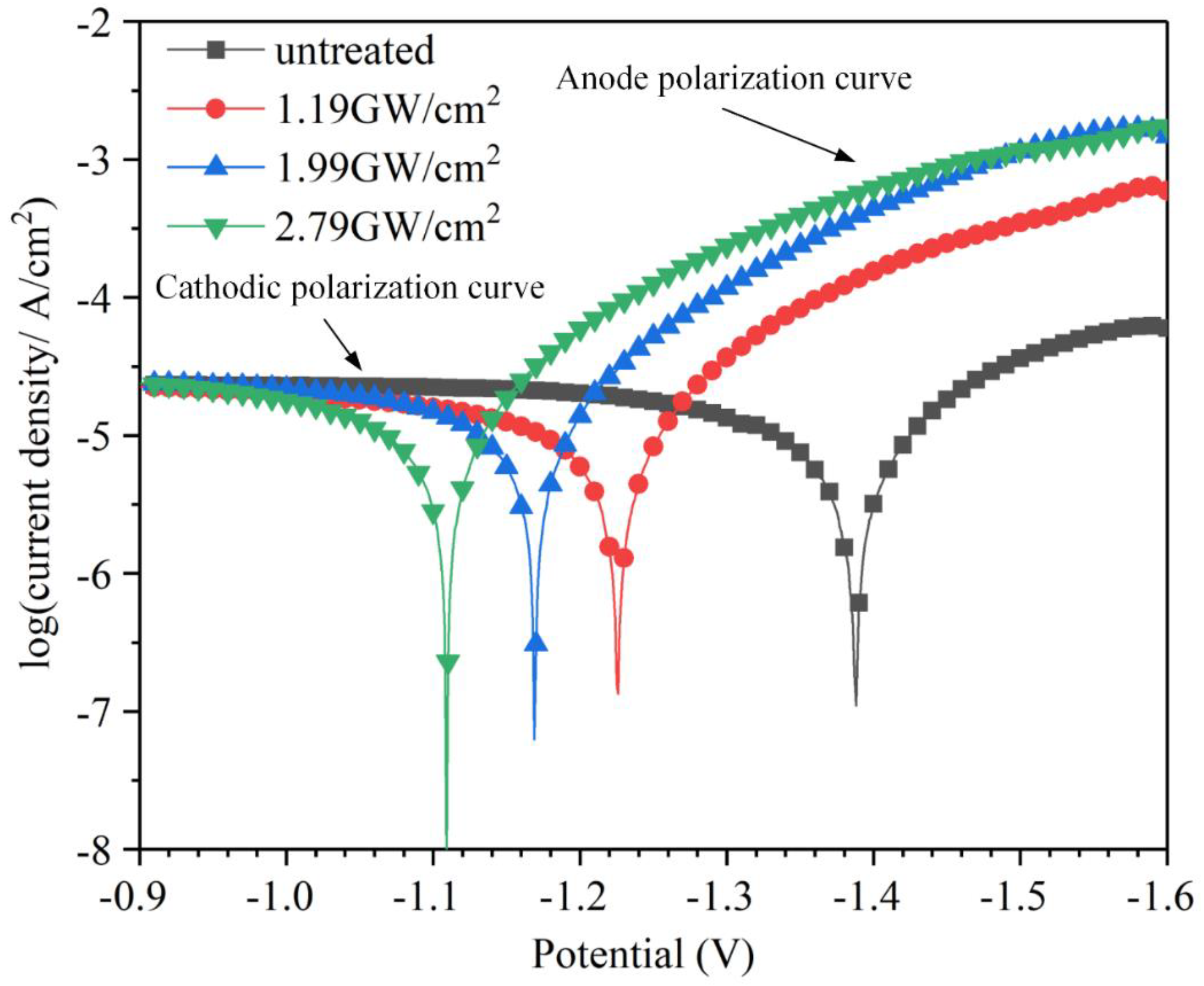
- Electrochemical experiments show that the corrosion potential increased from −1.3884 V to −1.1094 V, and the current density decreased from 1.378 × 10−5A/cm2 to 1.196 × 10−5A/cm2. The corrosion tendency decreased by 20.1% in maximally.
- (6)
- To summarize, when the power density is 1.19 GW/cm2, the magnesium alloy ZK60 can obtain superior corrosion resistance in the SBF solution.
Author Contributions
Funding
Conflicts of Interest
References
- Zeng, R.; Dietzel, W.; Witte, F.; Hort, N.; Blawert, C. Progress and challenge for magnesium alloys as biomaterials. Adv. Eng. Mater. 2008, 10, B3–B14. [Google Scholar] [CrossRef]
- Puleo, D.A.; Huh, W.W. Acute toxicity of metal ions in cultures of osteogenic cells derived from bone marrow stromal cells. J. Appl. Biomater. 1995, 6, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Poinern, G.E.J.; Brundavanam, S.; Fawcett, D. Biomedical magnesium alloys: A review of material properties, surface modifications and potential as a biodegradable orthopaedic implant. Am. J. Biomed. Eng. 2012, 2, 218–240. [Google Scholar] [CrossRef]
- Denkena, B.; Lucas, A. Biocompatible magnesium alloys as absorbable implant materials-adjusted surface and subsurface properties by machining processes. Cirp Ann. 2007, 56, 113–116. [Google Scholar] [CrossRef]
- Radha, R.; Sreekanth, D. Insight of magnesium alloys and composites for orthopedic implant applications—A review. J. Magnes. Alloys 2017, 5, 286–312. [Google Scholar] [CrossRef]
- Filli, L.; Luechinger, R.; Frauenfelder, T.; Beck, S.; Guggenberger, R.; Farshad-Amarcker, N.; Andreisek, G. Metal-induced artifacts in computed tomography and magnetic resonance imaging: Comparison of a biodegradable magnesium alloy versus titanium and stainless steel controls. Skelet. Radiol. 2015, 44, 849–856. [Google Scholar] [CrossRef]
- Vormann, J. Magnesium: Nutrition and metabolism. Mol. Asp. Med. 2003, 24, 27–37. [Google Scholar] [CrossRef]
- Persaud-Sharma, D.; Mcgoron, A. Biodegradable magnesium alloys: A review of material development and applications. J. Biomim. Biomater. Tissue Eng. 2012, 12, 25–39. [Google Scholar] [CrossRef]
- Shimizu, Y.; Yamamoto, A.; Mukai, T.; Shirai, Y.; Kano, M.; Kudo, T.; Kanetaka, H.; Kikuchi, M. Medical application of magnesium and its alloys as degradable biomaterials. Interface Oral Health Sci. 2009 2010, 318–320. [Google Scholar] [CrossRef]
- Gray, J.E.; Luan, B. Protective coatings on magnesium and its alloys—A critical review. J. Alloys Compd. 2002, 336, 88–113. [Google Scholar] [CrossRef]
- Lim, H.; Kim, P.; Jeong, H.; Jeong, S. Enhancement of abrasion and corrosion resistance of duplex stainless steel by laser shock peening. J. Mater. Process Technol. 2012, 212, 1347–1354. [Google Scholar] [CrossRef]
- Peyre, P.; Scherpereel, X.; Berthe, L.; Carboni, C.; Fabbro, R.; Béranger, G.; Lemaitre, C. Surface modifications induced in 316L steel by laser peening and shot-peening. Influence on pitting corrosion resistance. Mater. Sci. Eng. A 2000, 280, 294–302. [Google Scholar] [CrossRef]
- Caralapatti, V.K.; Narayanswamy, S. Analyzing the effect of high repetition laser shock peening on dynamic corrosion rate of magnesium. Opt. Laser Technol. 2017, 93, 165–174. [Google Scholar] [CrossRef]
- Ge, M.Z.; Xiang, J.Y. Effect of laser shock peening on microstructure and fatigue crack growth rate of AZ31B magnesium alloy. J. Alloys Compd. 2016, 680, 544–552. [Google Scholar] [CrossRef]
- Guo, Y.B.; Sealy, M.P.; Guo, C.S. Significant improvement of corrosion resistance of biodegradable metallic implants processed by laser shock peening. Cirp Ann. 2012, 61, 583–586. [Google Scholar] [CrossRef]
- Zhang, Y.K.; You, J.; Lu, J.Z.; Cui, C.Y.; Jiang, Y.F.; Ren, X.D. Effects of laser shock processing on stress corrosion cracking susceptibility of AZ31B magnesium alloy. Surf. Coat. Technol. 2010, 204, 3947–3953. [Google Scholar] [CrossRef]
- Witte, F.; Hort, N.; Vogt, C.; Cohen, S.; Kainer, K.U.; Wilumeit, R.; Feyerabend, F. Degradable biomaterials based on magnesium corrosion. Curr. Opin. Solid State Mater. Sci. 2008, 12, 63–72. [Google Scholar] [CrossRef]
- Qi, Z.R.; Zhang, Q.; Tan, L.L.; Lin, X.; Yin, Y.; Wang, X.L.; Yang, K.; Wang, Y. Comparison of degradation behavior and the associated bone response of ZK60 and PLLA in vivo. J. Biomed. Mater. Res. Part A 2014, 102, 1255–1263. [Google Scholar] [CrossRef]
- Hong, D.; Saha, P.; Chou, D.T.; Lee, B.; Collins, B.E.; Tan, Z.Q.; Dong, Z.Y.; Kumat, P.N. In vitro degradation and cytotoxicity response of Mg–4% Zn–0.5% Zr (ZK40) alloy as a potential biodegradable material. Acta Biomater. 2013, 9, 8534–8547. [Google Scholar] [CrossRef]
- Gu, X.N.; Li, N.; Zheng, Y.F.; Ruan, L. In vitro degradation performance and biological response of a Mg-Zn-Zr alloy. Mater. Sci. Eng., B 2011, 176, 1778–1784. [Google Scholar] [CrossRef]
- Kieswetter, K.; Schwartz, Z.; Dean, D.D.; Boyan, B.D. The Role of Implant Surface Characteristics in the Healing of Bone. Crit. Rev. Oral Biol. Med. 1996, 7, 329–345. [Google Scholar] [CrossRef] [PubMed]
- Peyre, P.; Fabbro, R.; Merrien, P.; Lieurade, H.P. Laser shock processing of aluminium alloys. Application to high cycle fatigue behavior. Mater. Sci. Eng., A 1996, 210, 102–113. [Google Scholar] [CrossRef]
- Chen, J.Y.; Zhang, Z.G.; Ouyang, J.L.; Chen, X.S.; Xu, Z.W.; Sun, X.T. Bioactivity and osteogenic cell response of TiO2 nanotubes coupled with nanoscale calcium phosphate via ultrasonification-assisted electrochemical deposition. Appl. Surf. Sci. 2014, 305, 24–32. [Google Scholar] [CrossRef]
- Song, G.L.; Atrens, A. Corrosion Mechanisms of Magnesium Alloys. Adv. Eng. Mater. 1999, 1, 11–33. [Google Scholar] [CrossRef]
- Kannan, M.B.; Dietzel, W. Pitting-induced hydrogen embrittlement of magnesium-aluminium alloy. Mater. Des. 2012, 42, 321–326. [Google Scholar] [CrossRef]
- Aung, N.N.; Zhou, W. Effect of grain size and twins on corrosion behaviour of AZ31B magnesium alloy. Corros. Sci. 2010, 52, 589–594. [Google Scholar] [CrossRef]
- Wu, S.X.; Wang, S.R.; Wang, G.Q.; Yu, X.C.; Liu, W.T.; Chang, Z.Q.; Dao, S.W. Microstructure, mechanical and corrosion properties of magnesium alloy bone plate treated by high-energy shot peening. Trans. Nonferrous Met. Soc. China 2019, 29, 1641–1652. [Google Scholar] [CrossRef]





| Mg | Zn | Zr | Mn | Fe | Cu | Ni |
|---|---|---|---|---|---|---|
| Bal. | 6.027 | 0.6758 | 0.006 | 0.005 | 0.006 | 0.003 |
| Number | Energy | Spot Diameter | Laser Density | Pulse Width | Overlap |
|---|---|---|---|---|---|
| 1 | 3 J | 4 mm | 1.19 GW/cm2 | 20 ns | 50% |
| 2 | 5 J | 4 mm | 1.99 GW/cm2 | 20 ns | 50% |
| 3 | 7 J | 4 mm | 2.79 GW/cm2 | 20 ns | 50% |
| Composition | Na+ | K+ | Mg2+ | Ca2+ | Cl− | HCO3− | HPO42− | SO42− |
|---|---|---|---|---|---|---|---|---|
| Content (mM/L) | 142 | 5.0 | 1.5 | 2.5 | 103.0 | 10.0 | 1.0 | 0.5 |
| Samples | Ecorr (V) | Icorr (A/cm2) |
|---|---|---|
| untreated | −1.3884 ± 0.04 | 1.378 × 10−5 |
| 1.19 GW/cm2 | −1.2256 ± 0.03 | 1.267 × 10−5 |
| 1.99 GW/cm2 | −1.1707 ± 0.05 | 1.230 × 10−5 |
| 2.79 GW/cm2 | −1.1094 ± 0.03 | 1.196 × 10−5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Wang, S.; Liu, W.; Xiao, T.; Zhu, G.; Sun, Z. The Effect of Laser Shock Peening on the Corrosion Behavior of Biocompatible Magnesium Alloy ZK60. Metals 2019, 9, 1237. https://doi.org/10.3390/met9111237
Guo Y, Wang S, Liu W, Xiao T, Zhu G, Sun Z. The Effect of Laser Shock Peening on the Corrosion Behavior of Biocompatible Magnesium Alloy ZK60. Metals. 2019; 9(11):1237. https://doi.org/10.3390/met9111237
Chicago/Turabian StyleGuo, Yu, Shouren Wang, Wentao Liu, Teng Xiao, Guodong Zhu, and Zhaolei Sun. 2019. "The Effect of Laser Shock Peening on the Corrosion Behavior of Biocompatible Magnesium Alloy ZK60" Metals 9, no. 11: 1237. https://doi.org/10.3390/met9111237
APA StyleGuo, Y., Wang, S., Liu, W., Xiao, T., Zhu, G., & Sun, Z. (2019). The Effect of Laser Shock Peening on the Corrosion Behavior of Biocompatible Magnesium Alloy ZK60. Metals, 9(11), 1237. https://doi.org/10.3390/met9111237




