Pure Aluminum Structure and Mechanical Properties Modified by Al2O3 Nanoparticles and Ultrasonic Treatment
Abstract
:1. Introduction
2. Materials
2.1. Preparation of Nanoparticles
2.2. Alloy Casting
3. Methods
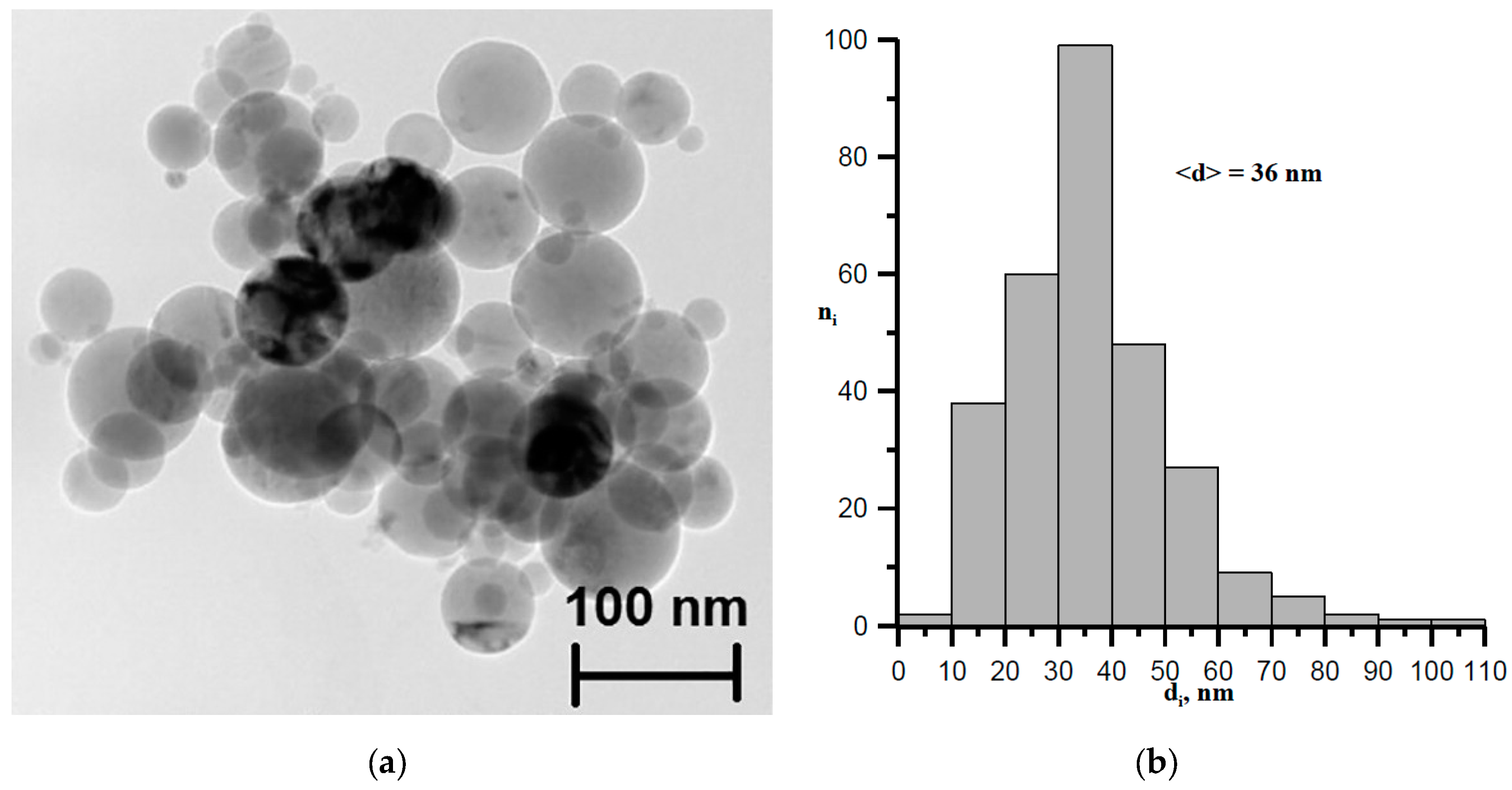
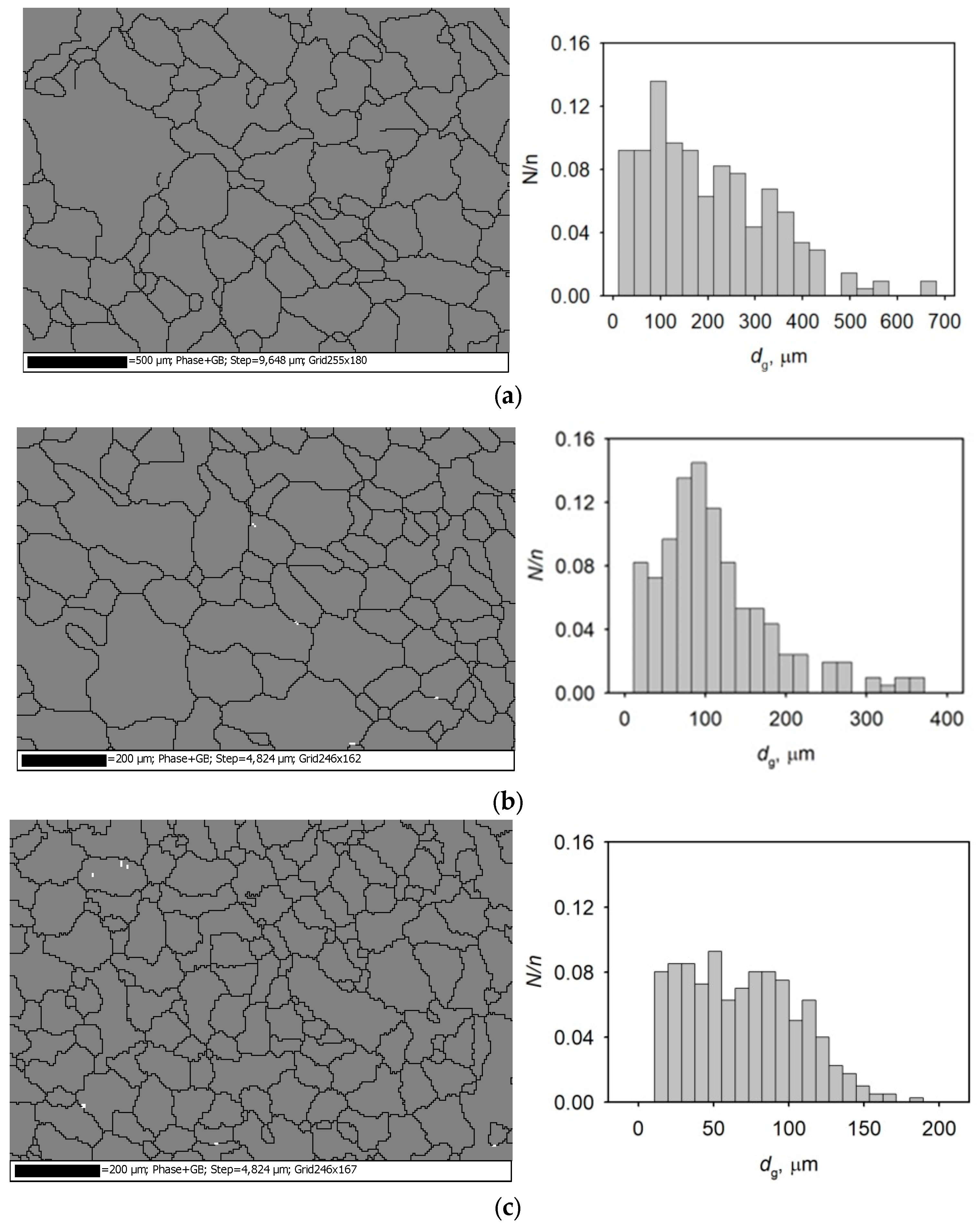
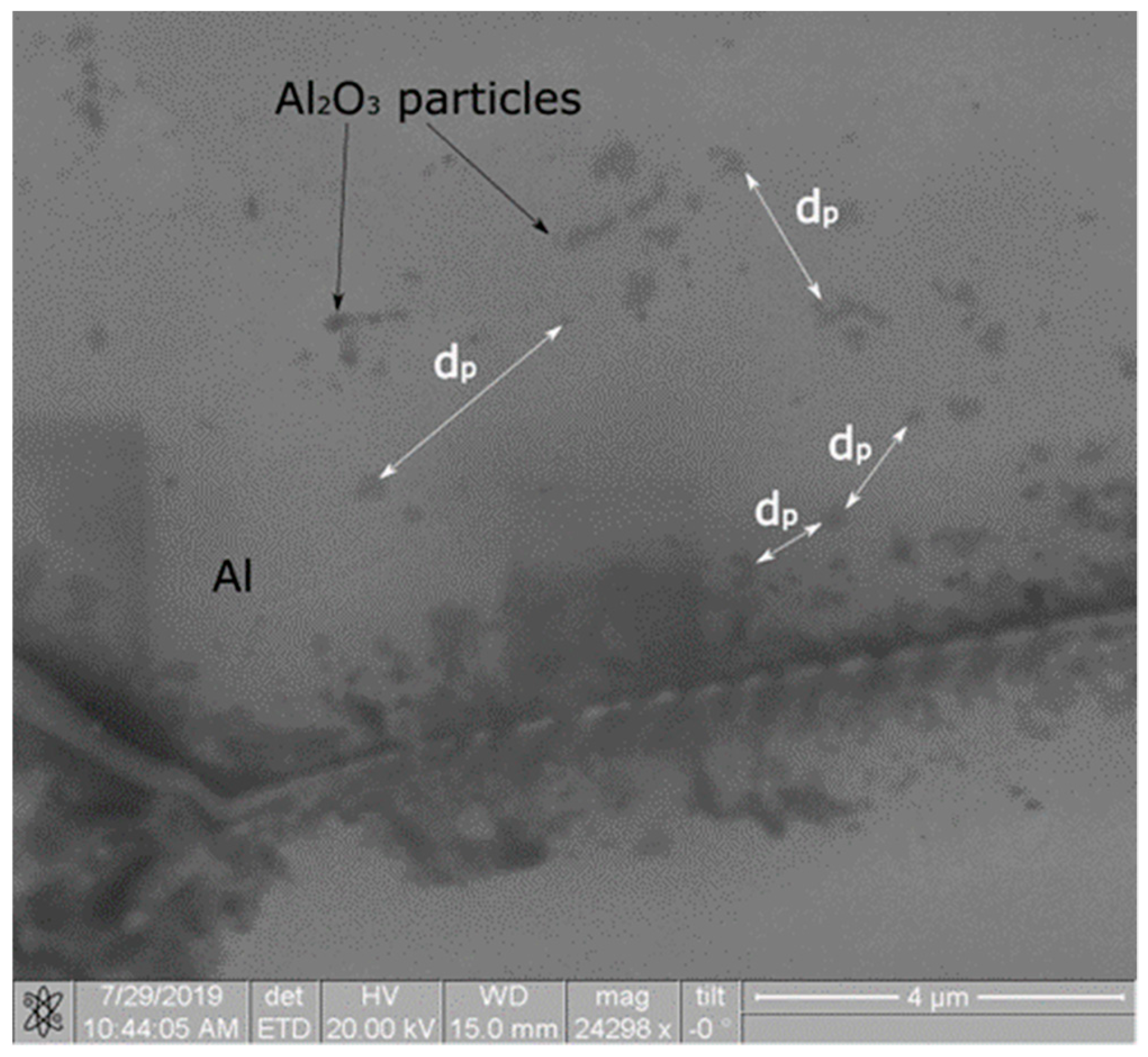
4. Experimental Results
5. Discussion
5.1. Hall–Petch Relationship
5.2. Orowan Strengthening
5.3. Particle-to-Matrix Load Transfer
5.4. Difference between the Thermal-Expansion Coefficients of the Particles and Aluminum Matrix
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Malaki, M.; Xu, W.; Kasar, A.K.; Menezes, P.L.; Dieringa, H.; Varma, R.S.; Gupta, M. Advanced Metal Matrix Nanocomposites. Metals 2019, 9, 330. [Google Scholar] [CrossRef]
- Promakhov, V.V.; Khmeleva, M.G.; Zhukov, I.A.; Platov, V.V.; Khrustalyov, A.P.; Vorozhtsov, A.B. Influence of Vibration Treatment and Modification of A356 Aluminum Alloy on Its Structure and Mechanical Properties. Metals 2019, 9, 87. [Google Scholar] [CrossRef]
- De Luca, A.; Dunand, D.C.; Seidman, D.N. Mechanical properties and optimization of the aging of a dilute Al-Sc-Er-Zr-Si alloy with a high Zr/Sc ratio. Acta Mater. 2016, 119, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Spierings, A.B.; Dawson, K.; Heeling, T.; Uggowitzer, P.J.; Schäublin, R.; Palm, F.; Wegener, K. Microstructural features of Sc-and Zr-modified Al-Mg alloys processed by selective laser melting. Mater. Des. 2017, 115, 52–63. [Google Scholar] [CrossRef]
- Gao, T.; Ceguerra, A.; Breen, A.; Liu, X.; Wu, Y.; Ringer, S. Precipitation behaviors of cubic and tetragonal Zr–rich phase in Al–(Si–) Zr alloys. J. Alloys Compd. 2016, 674, 125–130. [Google Scholar] [CrossRef]
- Shirvanimoghaddam, K.; Hamim, S.U.; Akbari, M.K.; Fakhrhoseini, S.M.; Khayyam, H.; Pakseresht, A.H.; Ghasali, E.; Zabet, M.; Munir, K.S.; Jia, S.; et al. Carbon fiber reinforced metal matrix composites: Fabrication processes and properties. Compos. Part A Appl. Sci. Manuf. 2017, 92, 70–96. [Google Scholar] [CrossRef]
- Chen, B.; Shen, J.; Ye, X.; Jia, L.; Li, S.; Umeda, J.; Takahashi, M.; Kondoh, K. Length effect of carbon nanotubes on the strengthening mechanisms in metal matrix composites. Acta Mater. 2017, 140, 317–325. [Google Scholar] [CrossRef]
- Maruyama, B.; Hunt, W.P. Discontinuously reinforced aluminum: Current status and future direction. JOM 1999, 11, 59–61. [Google Scholar] [CrossRef]
- Umasankar, V.; Xavior, M.A.; Karthikeyan, S. Experimental evaluation of the influence of processing parameters on the mechanical properties of SiC particle reinforced AA6061 aluminium alloy matrix composite by powder processing. J. Alloys Compd. 2014, 582, 380–386. [Google Scholar] [CrossRef]
- Khrustalyov, A.P.; Garkushin, G.V.; Zhukov, I.A.; Razorenov, S.V.; Vorozhtsov, A.B. Quasi-Static and Plate Impact Loading of Cast Magnesium Alloy ML5 Reinforced with Aluminum Nitride Nanoparticles. Metals 2019, 9, 715. [Google Scholar] [CrossRef]
- Mirza, F.A.; Chen, D.L. A Unified Model for the Prediction of Yield Strength in Particulate-Reinforced Metal Matrix Nanocomposites. Materials 2015, 8, 5138–5153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dieringa, H.; Katsarou, L.; Buzolin, R.; Szakács, G.; Horstmann, M.; Wolff, M.; Mendis, C.; Vorozhtsov, S.; StJohn, D. Ultrasound Assisted Casting of an AM60 Based Metal Matrix Nanocomposite, Its Properties, and Recyclability. Metals 2017, 7, 388. [Google Scholar] [CrossRef]
- Casati, R.; Vedani, M. Metal Matrix Composites Reinforced by Nano-Particles—A Review. Metals 2014, 4, 65–83. [Google Scholar] [CrossRef]
- Akbari, M.K.; Baharvandi, H.R.; Shirvanimoghaddam, K. Tensile and fracture behavior of nano/micro TiB2 particle reinforced casting A356 aluminum alloy composites. Mater. Des. 2015, 66, 150–161. [Google Scholar] [CrossRef]
- Katsarou, L.; Mounib, M.; Lefebvre, W.; Vorozhtsov, S.; Pavese, M.; Badini, C.; Molina-Aldareguia, J.M.; Jimenez, C.C.; Pérez Prado, M.T.; Dieringa, H. Microstructure, mechanical properties and creep of magnesium alloy Elektron21 reinforced with AlN nanoparticles by ultrasound-assisted stirring. Mater. Sci. Eng. A 2016, 659, 84–92. [Google Scholar] [CrossRef]
- Sreekumar, V.M.; Babu, N.H.; Eskin, D.G. Prospects of In-Situ α-Al2O3 as an Inoculant in Aluminum: A Feasibility Study. J. Mater. Eng. Perform. 2017, 26, 4166–4176. [Google Scholar] [CrossRef]
- Dieringa, H. Properties of magnesium alloys reinforced with nanoparticles and carbon nanotubes: A review. J. Mater. Sci. 2011, 46, 289–306. [Google Scholar] [CrossRef]
- Puga, H.; Costa, S.; Barbosa, J.; Ribeiro, S.; Prokic, M. Influence of ultrasonic melttreatment on microstructure and mechanical properties of AlSi9Cu3 alloy. J. Mater. Process. Technol. 2011, 211, 1729–1735. [Google Scholar] [CrossRef]
- Kudryashova, O.B.; Eskin, D.G.; Khrustalev, A.P.; Vorozhtsov, S.A. Ultrasonic effect on thepenetration of the metallic melt into submicron particles and their agglomerates. Russ. J. Non-Ferr. Met. 2017, 58, 427–433. [Google Scholar] [CrossRef]
- Zhang, F.; Jacobi, A.M. Aluminum surface wettability changes by pool boiling of nanofluids. Colloids Surf. A Physicochem. Eng. Asp. 2016, 506, 438–444. [Google Scholar] [CrossRef]
- Vorozhtsov, S.; Zhukov, I.; Promakhov, V.; Naydenkin, E.; Khrustalyov, A.; Vorozhtsov, A. The Influence of ScF3 Nanoparticles on the Physical and Mechanical Properties of New Metal Matrix Composites Based on A356 Aluminum Alloy. JOM 2016, 68, 3101–3106. [Google Scholar] [CrossRef]
- Zhukov, I.A.; Kozulin, A.A.; Khrustalyov, A.P.; Matveev, A.E.; Platov, V.V.; Vorozhtsov, A.B.; Zhukova, T.V.; Promakhov, V.V. The Impact of Particle Reinforcement with Al2O3, TiB2, and TiC and Severe Plastic Deformation Treatment on the Combination of Strength and Electrical Conductivity of Pure Aluminum. Metals 2019, 9, 65. [Google Scholar] [CrossRef]
- Ramakrishnan, N. An analytical study on strengthening of particulate reinforced metal matrix composites. Acta Mater. 1996, 44, 69–77. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, D.L. Consideration of Orowan strengthening effect in particulate-reinforced metal matrix nanocomposites: A model for predicting their yield strength. Scr. Mater. 2006, 54, 1321–1326. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, D.L. Contribution of Orowan strengthening effect in particulate-reinforced metal matrix nanocomposites. Mat. Sci. Eng. A 2008, 483–484, 148–152. [Google Scholar] [CrossRef]
- Shin, S.; Lee, D.; Lee, Y.-H.; Ko, S.; Park, H.; Lee, S.-B.; Cho, S.; Kim, Y.; Lee, S.-K.; Jo, I. High Temperature Mechanical Properties and Wear Performance of B4C/Al7075 Metal Matrix Composites. Metals 2019, 9, 1108. [Google Scholar] [CrossRef]
- Khrustalyov, A.P.; Kozulin, A.A.; Zhukov, I.A.; Khmeleva, M.G.; Vorozhtsov, A.B.; Eskin, D.; Chankitmunkong, S.; Platov, V.V.; Vasilyev, S.V. Influence of Titanium Diboride Particle Size on Structure and Mechanical Properties of an Al-Mg Alloy. Metals 2019, 9, 1030. [Google Scholar] [CrossRef]
- Belov, N.A. Effect of eutectic phases on the fracture behavior of high-strength castable aluminum alloys. Met. Sci. Heat Treat. 1995, 37, 237–242. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, T.; Zheng, Y.; Zhao, Y.; Kang, H.; Gao, L. Development of TiB2 reinforced aluminum foundry alloy based in situ composites—Part I: An improved halide salt route to fabricate Al–5 wt%TiB2 master composite. Mat. Sci. Eng. A 2014, 605, 301–309. [Google Scholar] [CrossRef]
- Vorozhtsov, S.A.; Eskin, D.G.; Tamayo, J.; Vorozhtsov, A.B.; Promakhov, V.V.; Averin, A.A.; Khrustalyov, A.P. The application of external fields to the manufacturing of novel dense composite master alloys and aluminum-based nanocomposites. Metal. Mater. Trans. A 2015, 46, 2870–2875. [Google Scholar] [CrossRef]

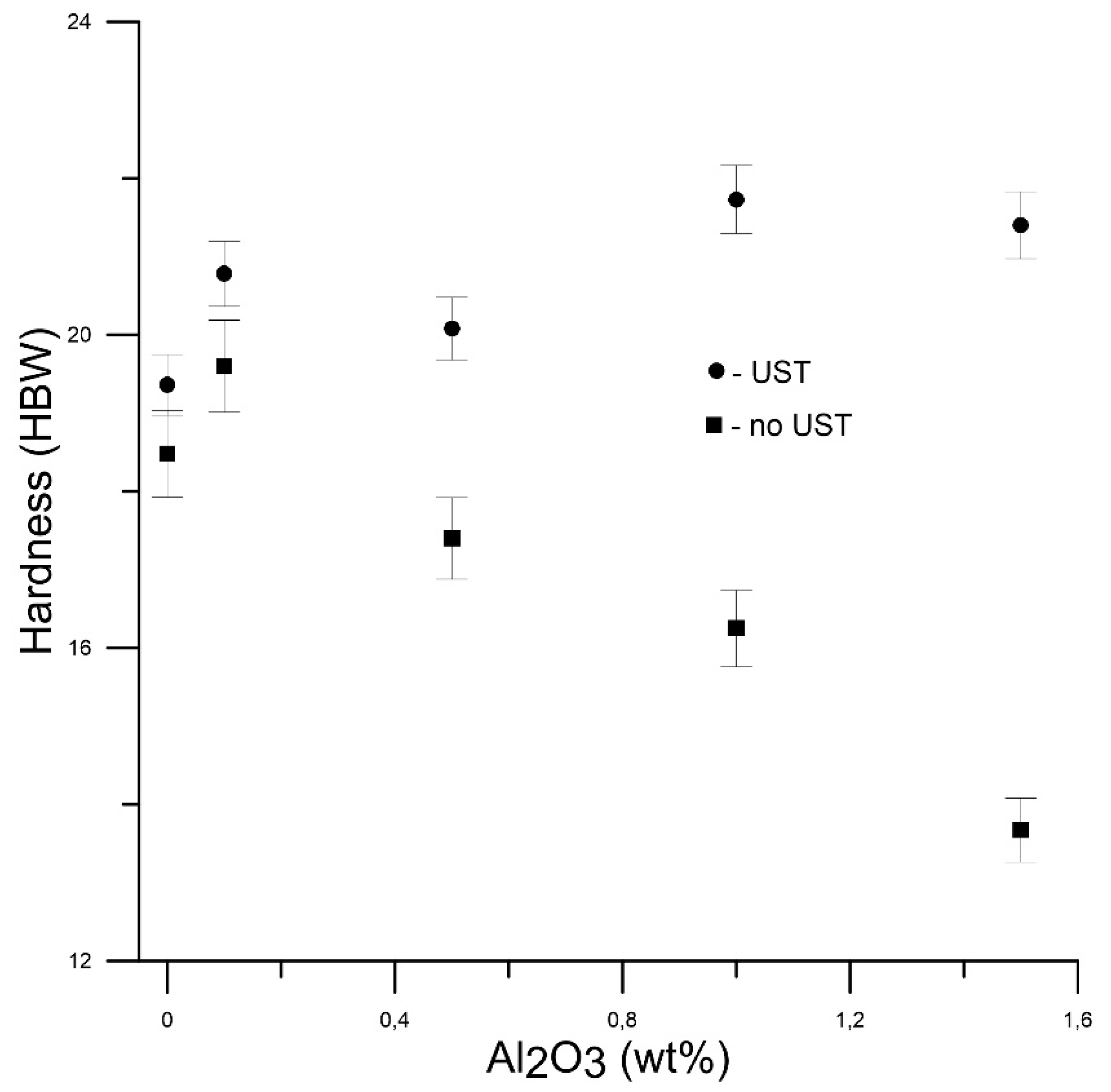
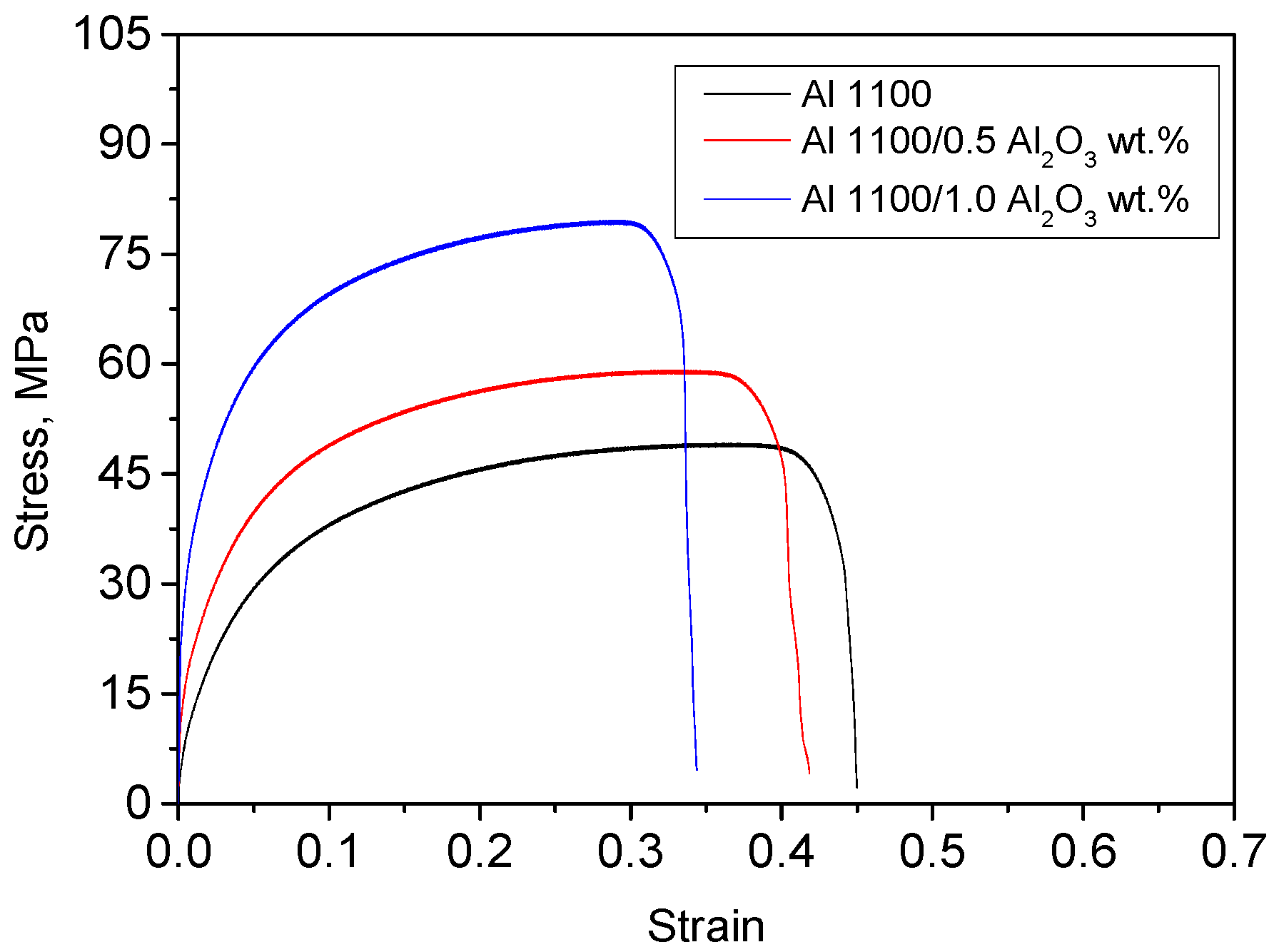
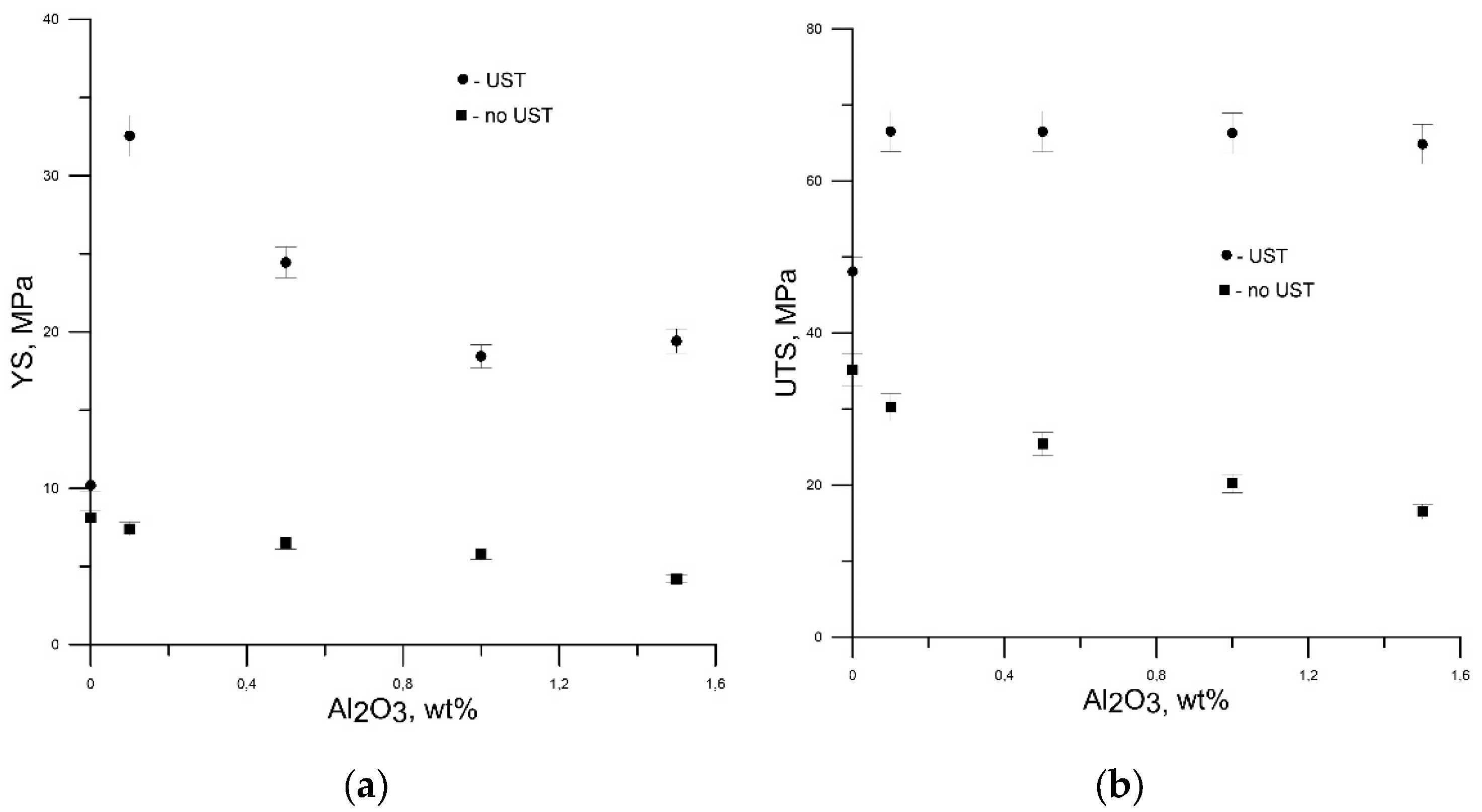
| Alloy Composition | Ρ × 103, g/mm3 | YS, MPa | UTS, MPa | εmax, % | Brinell Hardness |
|---|---|---|---|---|---|
| Initial state | 2.68 | 12.08 | 48.75 | 45 | 19.36 |
| 0.5 wt.% Al2O3 | 2.66 | 16.36 | 58.85 | 41.8 | 20.08 |
| 1 wt.% Al2O3 | 2.69 | 27.40 | 79.10 | 34.4 | 21.73 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhukov, I.A.; Kozulin, A.A.; Khrustalyov, A.P.; Kahidze, N.I.; Khmeleva, M.G.; Moskvichev, E.N.; Lychagin, D.V.; Vorozhtsov, A.B. Pure Aluminum Structure and Mechanical Properties Modified by Al2O3 Nanoparticles and Ultrasonic Treatment. Metals 2019, 9, 1199. https://doi.org/10.3390/met9111199
Zhukov IA, Kozulin AA, Khrustalyov AP, Kahidze NI, Khmeleva MG, Moskvichev EN, Lychagin DV, Vorozhtsov AB. Pure Aluminum Structure and Mechanical Properties Modified by Al2O3 Nanoparticles and Ultrasonic Treatment. Metals. 2019; 9(11):1199. https://doi.org/10.3390/met9111199
Chicago/Turabian StyleZhukov, Ilya A., Alexander A. Kozulin, Anton P. Khrustalyov, Nikolay I. Kahidze, Marina G. Khmeleva, Evgeny N. Moskvichev, Dmitry V. Lychagin, and Alexander B. Vorozhtsov. 2019. "Pure Aluminum Structure and Mechanical Properties Modified by Al2O3 Nanoparticles and Ultrasonic Treatment" Metals 9, no. 11: 1199. https://doi.org/10.3390/met9111199
APA StyleZhukov, I. A., Kozulin, A. A., Khrustalyov, A. P., Kahidze, N. I., Khmeleva, M. G., Moskvichev, E. N., Lychagin, D. V., & Vorozhtsov, A. B. (2019). Pure Aluminum Structure and Mechanical Properties Modified by Al2O3 Nanoparticles and Ultrasonic Treatment. Metals, 9(11), 1199. https://doi.org/10.3390/met9111199





