Suppressing the Use of Critical Raw Materials in Joining of AISI 304 Stainless Steel Using Activated Tungsten Inert Gas Welding
Abstract
1. Introduction
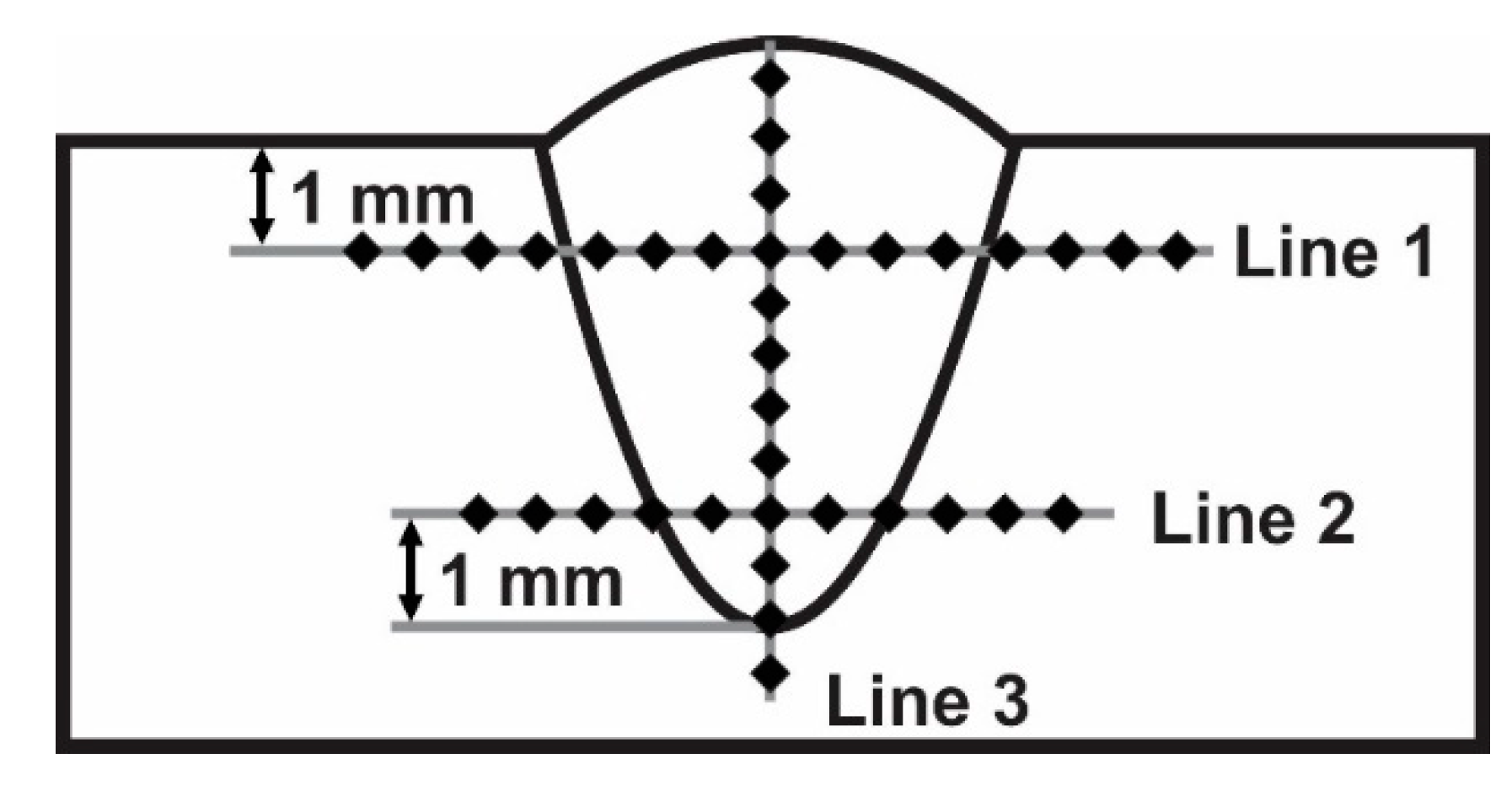
2. Materials and Methods
- (1)
- All-submicron particles (5M);
- (2)
- 20% nano and 80% submicron particles (4M1N);
- (3)
- 40% nano and 60% submicron particles (3M2N);
- (4)
- 60% nano and 40% submicron particles (2M3N);
- (5)
- 80% nano and 20% submicron particles (1M4N);
- (6)
- All-nanoparticles (designated as 5N).
3. Results
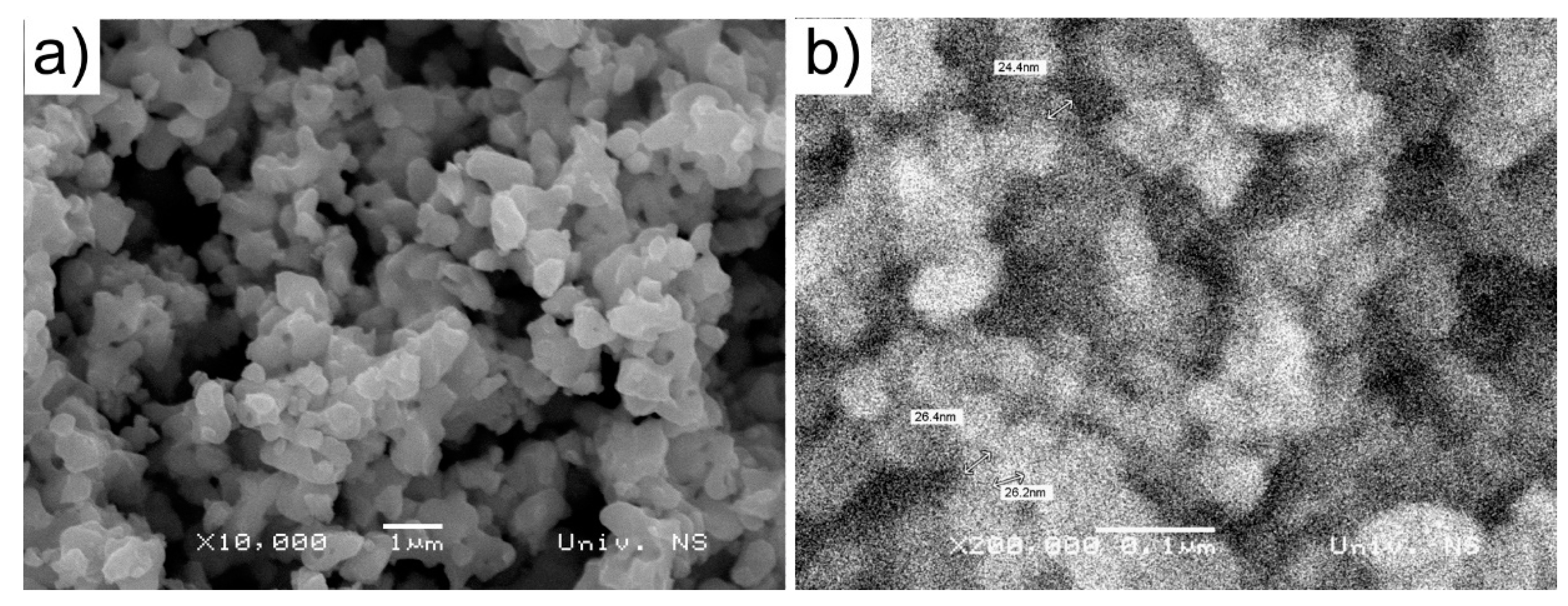
3.1. Particle Size Distribution in the Coating
3.2. Macro and Weld Bead Dimensions
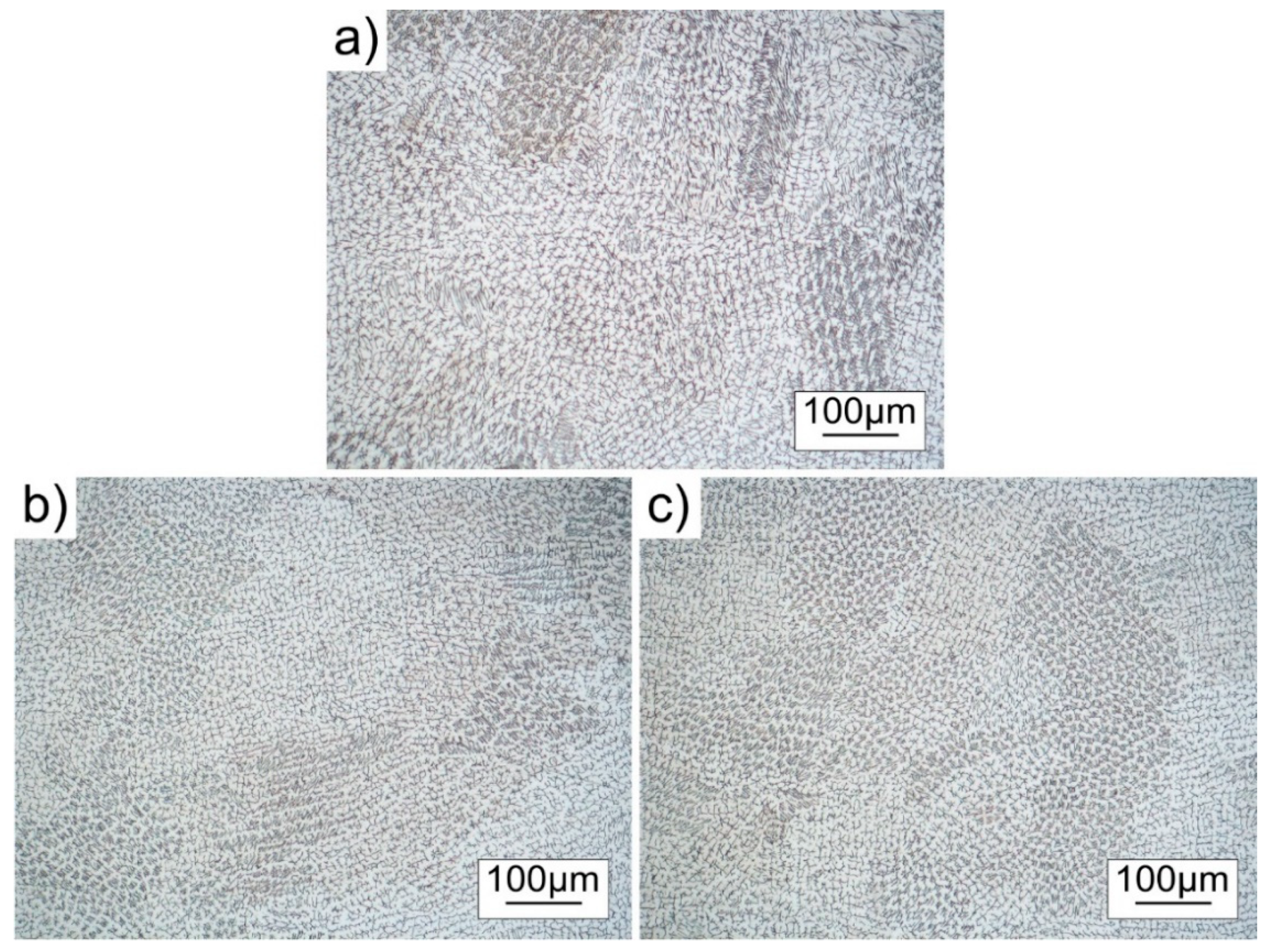
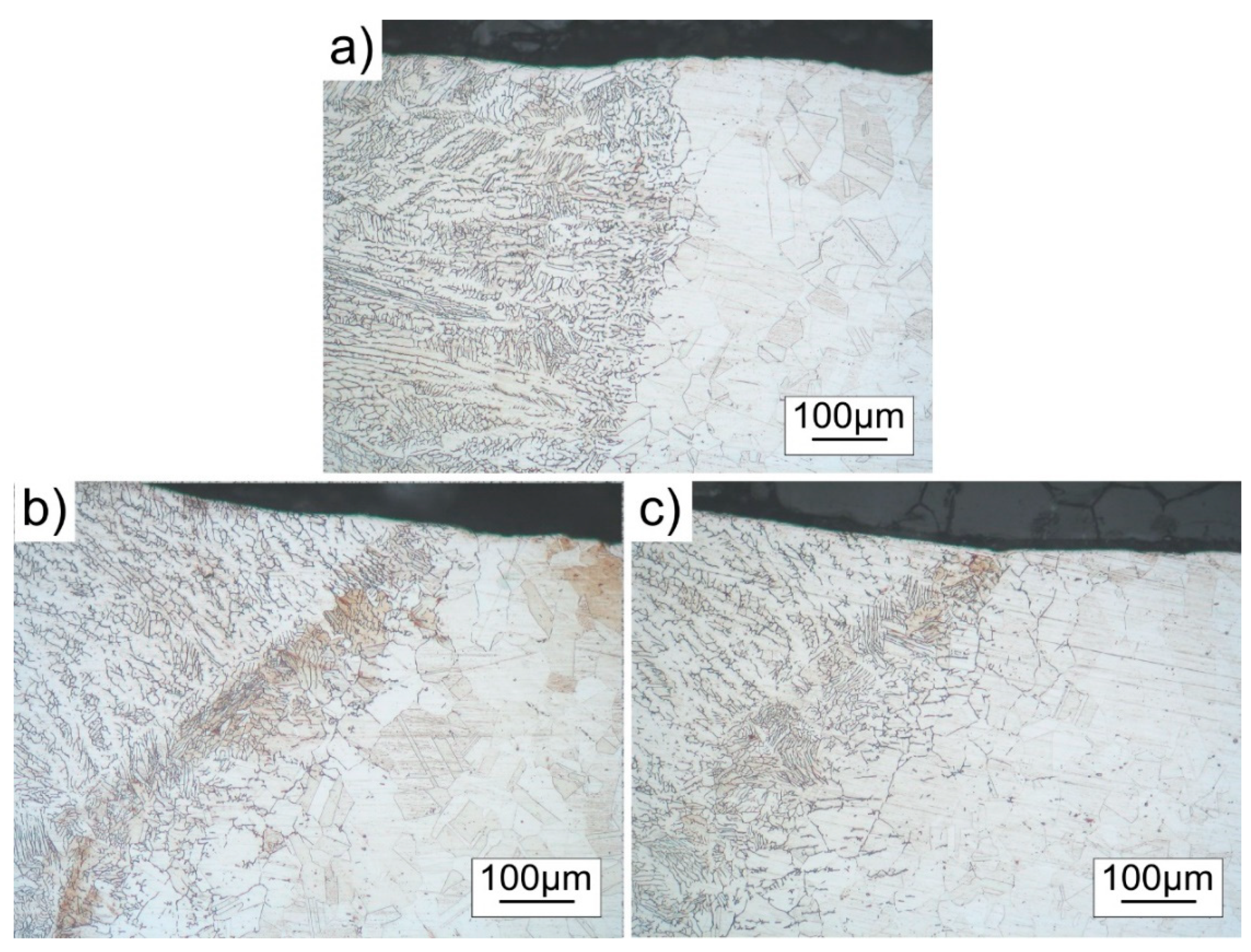
3.3. Microstructure
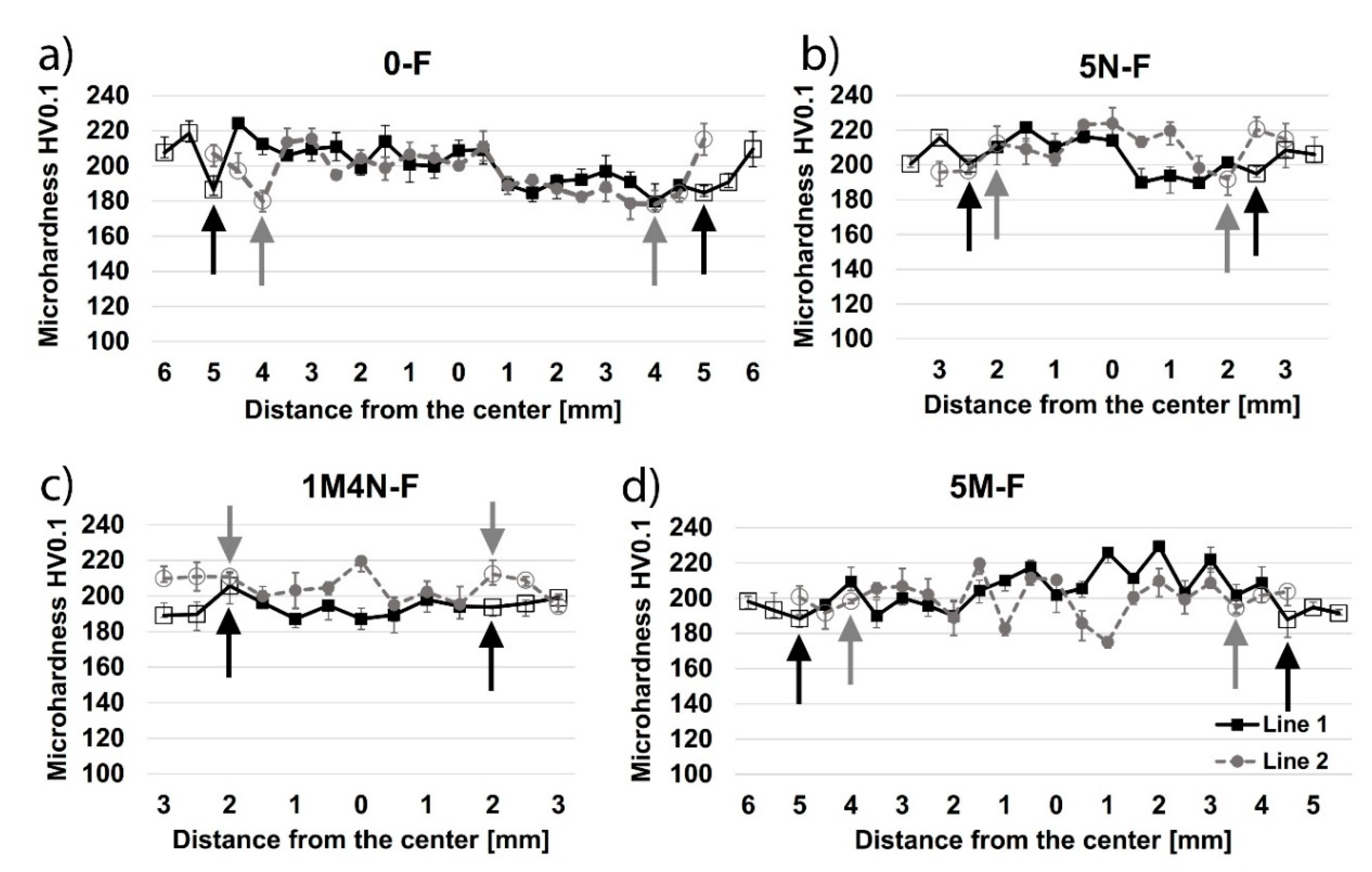
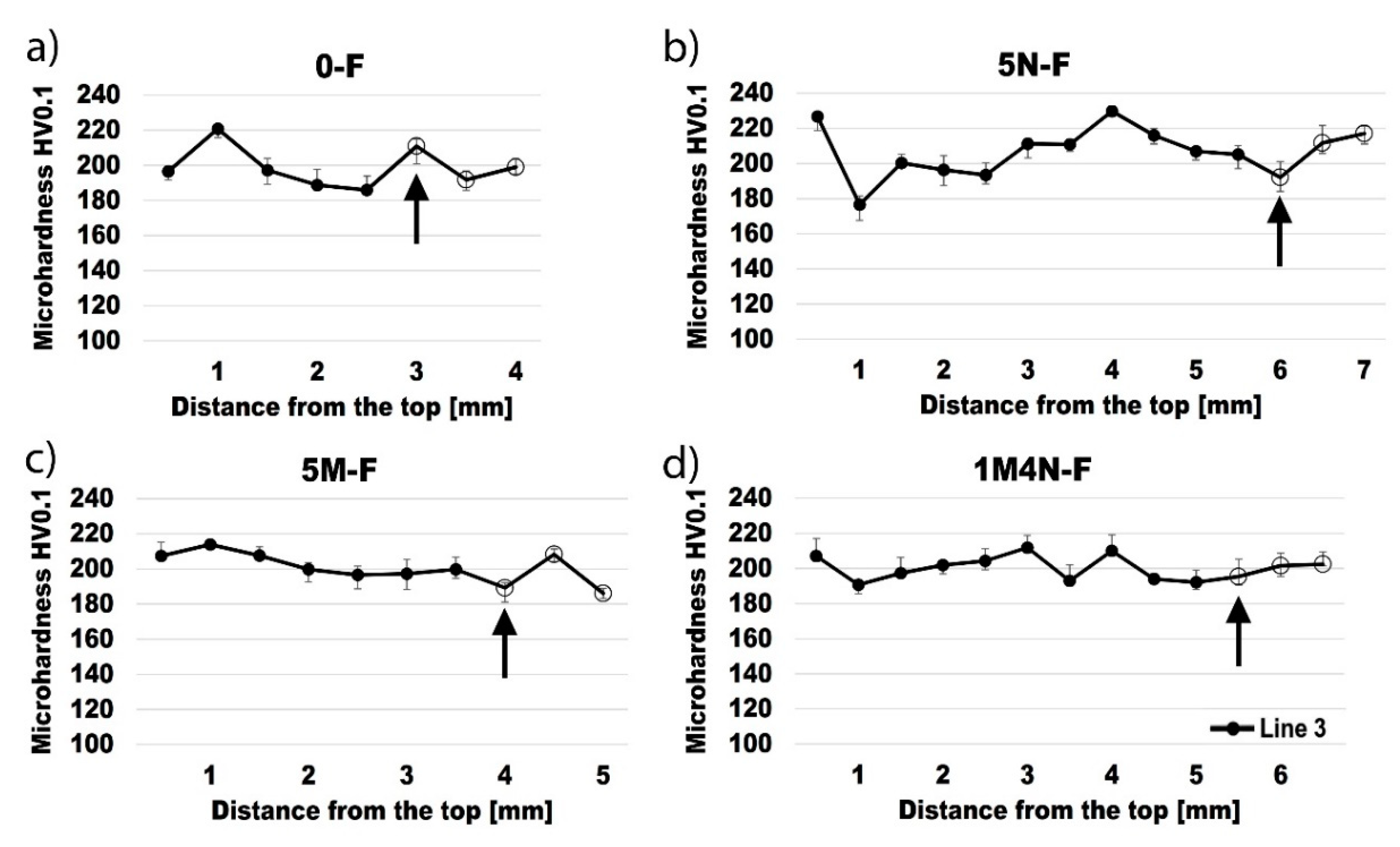
3.4. Vickers Microhardness
4. Discussion
5. Conclusions
- The correlation between the coating composition and weld metal surface areas could not be determined, although weld metal areas were larger with frustum and conical tips compared to blunt tip.
- Nanoparticles were more effective than submicron particles in increasing the penetration, but a mixture of nano- and micron-sized particles helped achieve the best weld. The main reason of this appears to be the collisions that occurred between agglomerates and submicron particles, resulting in a lower size of particles in the lowest range of sizes that were most effective in increasing the weld penetration.
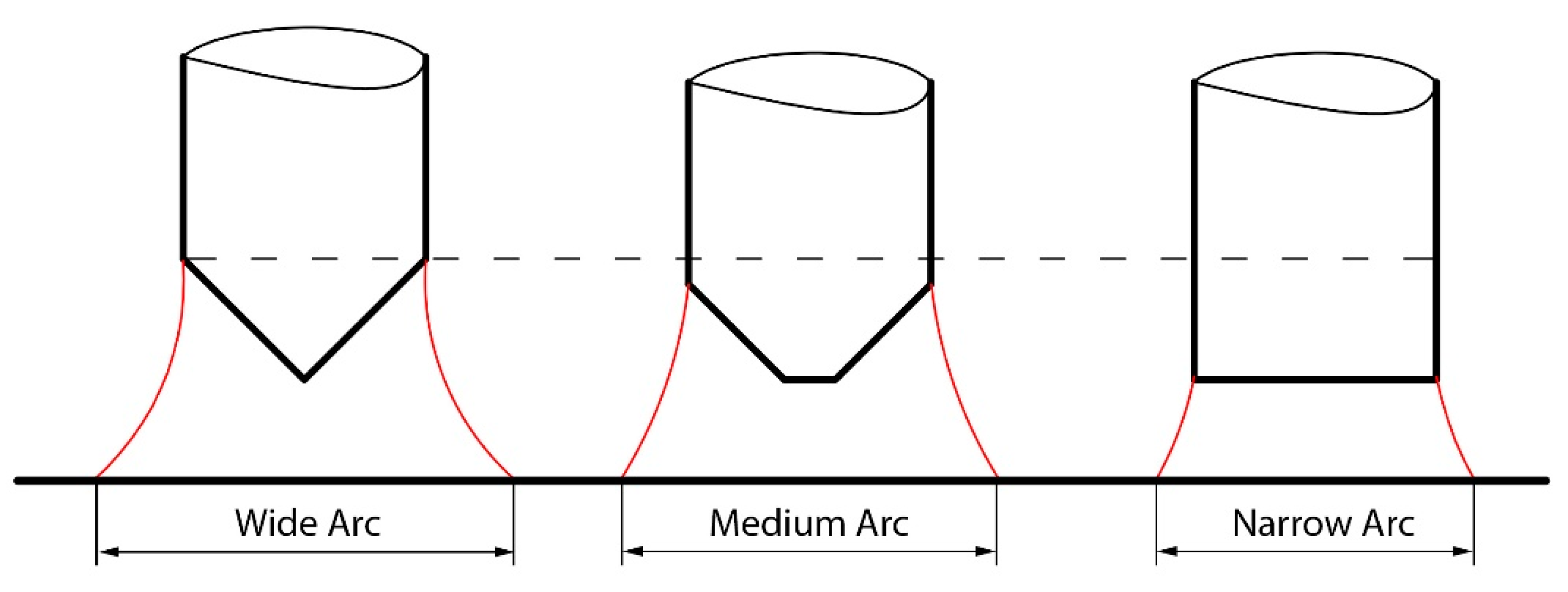
- Frustum and sharp electrodes proved to be more consistent in producing high penetration compared to blunt electrodes, due to a narrower arc that affected a narrower width of the coating applied to the base metal surface.
- Specimens welded without the coating showed an increased grain size near the fusion line in the base metal under the surface, resulting in a decreased hardness in this zone. Contrarily, specimens welded with the coating showed an increased grain size near the fusion line in the base metal under the weld metal, resulting in a decreased hardness in this zone.
- The main cause of a reduced hardness and increased grain size under the surface and under the weld metal may be attributed to high-temperature material flowing near these zones and heat transfer towards base metal.
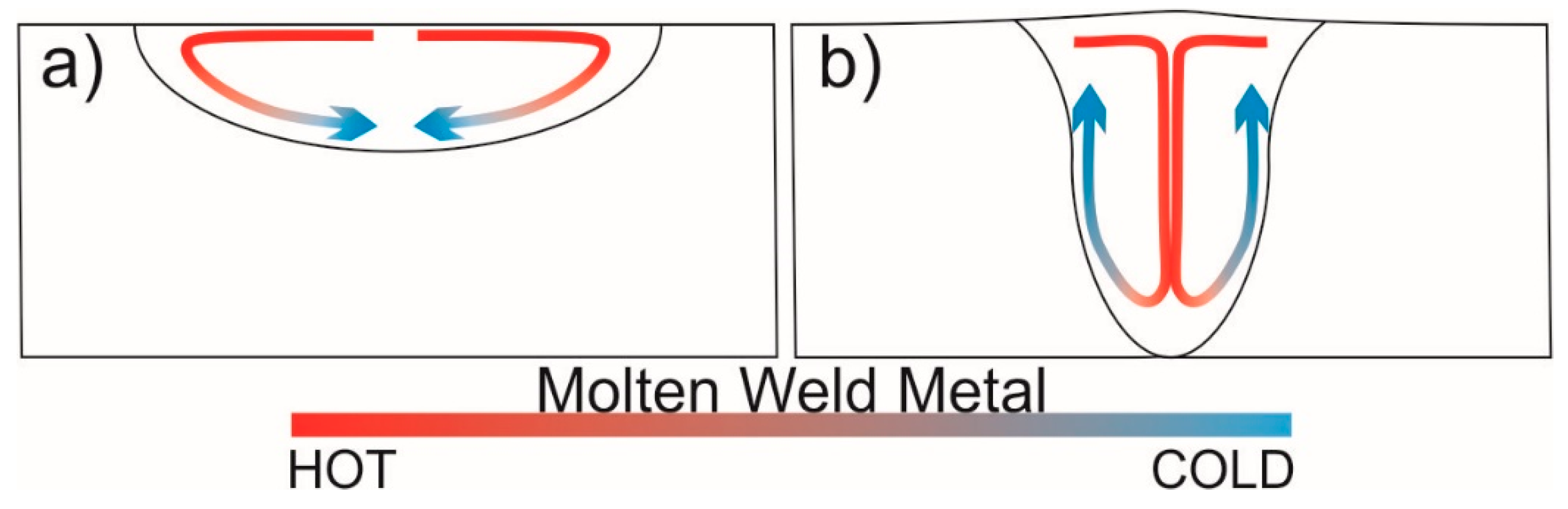
- Material flow direction in the weld pool was the result of Marangoni convection in TIG and reversed Marangoni convection in A-TIG. In TIG, the flow was towards the fusion boundary and along the fusion line, while in A-TIG, the flow towards the center of the weld and to the bottom of the weld was more pronounced resulting in an increase in the weld penetration depth.
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Tseng, K.-H.; Hsu, C.-Y. Performance of activated TIG process in austenitic stainless steel welds. J. Mater. Process. Technol. 2011, 211, 503–512. [Google Scholar] [CrossRef]
- Tseng, K.-H.; Lin, P.-Y. UNS S31603 Stainless Steel Tungsten Inert Gas Welds Made with Microparticle and Nanoparticle Oxides. Materials 2014, 7, 4755–4772. [Google Scholar] [CrossRef] [PubMed]
- Gurevich, S.M.; Zamkov, V.N.; Kushnirenko, N.A. Improving the penetration of titanium alloys when they are welded by argon tungsten arc process. Avtom. Svarka. 1965, 18, 1–5. [Google Scholar]
- Huang, H.Y. Argon–hydrogen shielding gas mixtures for activating coating-assisted gas tungsten arc welding. Metall. Mater. Trans. A 2010, 41, 2829–2835. [Google Scholar] [CrossRef]
- Huang, H.Y. Effects of shielding gas composition and activating coating on GTAW weldments. Mater. Des. 2009, 30, 2404–2409. [Google Scholar] [CrossRef]
- Paskell, T.D. Gas Tungsten Arc Welding Coating. U.S. Patent 5,804,792A, 9 August 1998. [Google Scholar]
- Tseng, K.H.; Wang, N.S. Welding Activated Coating for Structural Alloy Steels. U.S. Patent 20,160,167,178A1, 16 June 2016. [Google Scholar]
- Muthukumaran, V.; Bhaduri, A.K.; Raj, B. Penetration Enhancing Coating Formulation for Tungsten Inert Gas (TIG) Welding of Austenitic Stainless Steel and Its Application. U.S. Patent 8,097,826, 17 January 2012. [Google Scholar]
- Dhandha, K.H.; Badheka, V.J. Effect of activating coatings on weld bead morphologyof P91 steel bead-on-plate welds by coating assisted tungsten inert gas welding process. Mater. Manuf. Process. 2015, 17, 48–57. [Google Scholar] [CrossRef]
- Nayee, S.G.; Badheka, V.J. Effect of oxide-based coatings on mechanical and metallur-gical properties of Dissimilar activting coating assisted-tungsten inert gas welds. Mater. Manuf. Process. 2014, 16, 137–143. [Google Scholar] [CrossRef]
- Modenesi, P.J.; Apolinário, E.R.; Pereira, I.M. TIG welding with single-component coatings. J. Mater. Process. Technol. 2000, 99, 260–265. [Google Scholar] [CrossRef]
- Lin, H.L.; Wu, T.M. Effects of activating coating on weld bead geometry of Inconel718 alloy TIG welds. Mater. Manuf. Process. 2012, 27, 1457–1461. [Google Scholar] [CrossRef]
- Kou, S. Welding Metallurgy, 2nd ed.; Wiley—Interscience Publishers: Hoboken, NJ, USA, 2003; pp. 116–117. [Google Scholar]
- Vasudevan, M.; Bhaduri, A.K.; Raj, B.; Prasad, R.K. Genetic algorithm based com-putational model for optimizing the process parameters in A-TIG welding of 304LN and 316LN stainless steels. Mater. Manuf. Process. 2007, 22, 641–649. [Google Scholar] [CrossRef]
- Chandrasekhar, N.; Vasudevan, M. Intelligent modeling for optimization of A-TIG welding process. Mater. Manuf. Process. 2010, 25, 1341–1550. [Google Scholar] [CrossRef]
- Maduraimuthu, V.; Vasudevan, M.; Muthupandi, V.; Bhaduri, A.K.; Jayakumar, T. Study of the effect of activated coating on the microstructure and mechanical properties of mod. 9Cr-1Mo steel. Metall. Mater. Trans. 2012, 43, 123–132. [Google Scholar] [CrossRef]
- Sakthivel, T.; Vasudevan, M.; Laha, K.; Parameswaran, P.; Chandravathi, K.S.; Paneer-Selvi, S.; Maduraimuthu, V.; Mathew, M.D. Creep-rupture behavior of 9Cr-1.8W-0.5Mo-VNb P92 ferritic steel weld joint. Mater. Sci. Eng. 2014, 591, 111–120. [Google Scholar] [CrossRef]
- Vidyarthy, R.S.; Dwivedi, D.K. Activating coating tungsten inert gas welding for enhanced weld penetration. J. Manuf. Process. 2016, 22, 211–228. [Google Scholar] [CrossRef]
- Kuo, C.H.; Tseng, K.H.; Chou, C.P. Effect of activated TIG coating on performance of dissimilar welds between mild steel and stainless steel. Key Eng. Mater. 2011, 479, 74–80. [Google Scholar] [CrossRef]
- Vora, J.J.; Badheka, V.J. Improved Penetration with the Use of Oxide Coatings in Activated TIG Welding of Low Activation Ferritic/Martensitic Steel. Trans. Indian Inst. Met. 2016, 69, 1755–1764. [Google Scholar] [CrossRef]
- Vora, J.J.; Badheka, V.J. Experimental investigation on mechanism and weld morphology of activated TIG welded bead-on-plate weldments of reduced activation ferritic/martensitic steel using oxide coatings. J. Manuf. Process. 2015, 20, 224–233. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Takagi, R.; Shinoda, T. Effect of bismuth on weld joint penetration in austenitic stainless steel. Weld. Res. Suppl. 1992, 71, 283–290. [Google Scholar]
- Mills, K.C.; Keene, B.J.; Brooks, R.F.; Shirali, A. Marangoni effects in welding. Philos. Trans. R. Soc. Lond. Ser. A 1998, 356, 911–925. [Google Scholar] [CrossRef]
- Zou, Y.; Ueji, R.; Fujii, H. Effect of oxygen on weld shape and crystallographic orientation of duplex stainless steel weld using advanced A-TIG (AA-TIG) welding method. Mater. Charact. 2014, 91, 42–49. [Google Scholar] [CrossRef]
- Skvortsov, E.A. Role of electronegative elements in contraction of thearc discharge. Weld. Int. 1998, 12, 471–475. [Google Scholar] [CrossRef]
- Tanaka, M.; Shimizu, T.; Terasaki, T.; Ushio, M.; Koshiishi, F.; Yang, C.-L. Effects of activating coating on arc phenomena in gas tungstenarc welding. Sci. Technol. Weld Join. 2000, 5, 397–402. [Google Scholar] [CrossRef]
- Venkatesan, G.; Goeuge, J.; Sowmyasri, M.; Muthapandi, V. Effet of ternarz coatings on depth of penetration in A-TIG welding of AISI 409 ferritic stainless steel. Proc. Mater. Sci. 2014, 5, 2402–2410. [Google Scholar] [CrossRef]
- Report on Critical Raw Materials for EU, Report of the Ad-Hoc Working Group on Defining Critical Raw Materials for EU. May 2014. Available online: http://mima.geus.dk/report-on-critical-raw-materials en.pdf (accessed on 28 August 2019).
- Luisa Grilli, M.; Bellezze, T.; Gamsjäger, E.; Rinaldi, A.; Novak, P.; Balos, S.; Piticescu, R.; Letizia Ruello, M. Solutions for Critical Raw Materials under Extreme Conditions: A Review. Materials 2017, 10, 285. [Google Scholar] [CrossRef] [PubMed]
- Balos, S.; Dramicanin, M.; Janjatovic, P.; Zabunov, I.; Klobcar, D.; Busic, M.; Luisa Grilli, M. Metal oxide nanoparticle-based coating as a catalyzer for A-TIG welding: Critical raw material perspective. Metals 2019, 9, 567. [Google Scholar] [CrossRef]
- Key, J.F. Anode/Cathode Geometry and Shielding Gas Interrelationships in GTAW Electrode tip geometry and groove geometry must be compatible to ensure arc stability. In Proceedings of the 61st AWS Annual Meeting, Los Angeles, CA, USA, 13–18 April 1980; pp. 364–370. [Google Scholar]
- Mannion, B.; Heizman, J., III. Setting up and Determining Parameters for Orbital Tube Welding. Available online: http://www.pro-fusiononline.com/feedback/fab-may99.htm (accessed on 15 June 2019).
- Balos, S.; Pilic, B.; Markovic, D.; Pavlicevic, J.; Luzanin, O. Poly(Methyl-Methacrylate) Nanocomposites with Low Silica Addition. J. Prosthet. Dent. 2014, 111, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Balos, S.; Pilic, B.; Petrovic, D.; Petronijevic, B.; Sarcev, I. Flexural strength and modulus of autopolimerized poly(methyl methacrylate) with nanosilica. Vojnosaniteski Pregled. 2018, 75, 564–569. [Google Scholar] [CrossRef]
- Elshereksi, N.W.; Mohamed, S.H.; Arifin, A.; Mohd Ishak, Z.A. Effect of filler incorporation on the fracture toughness properties of denture base poly(methyl methacrylate). J. Phys. Sci. 2009, 20, 1–12. [Google Scholar]
- Dongguang, W.; Rajesh, D.; Robert, P. Mixing and characterization of nanosized powders: An assessment of different techniques. J. Nanopart. Res. 2002, 4, 21–41. [Google Scholar]
- Dramicanin, M.; Balos, S.; Janjatovic, P.; Zabunov, I.; Grabulov, V. Activated Flux TIG Welding of Stainless Steel Pipes. Chem. Ind. Chem. Eng. Q. 2019. [Google Scholar] [CrossRef]
- Roper, J.R.; Olson, D.L. Capillarity effects in the GTA weld penetration of 21-6-9 stainless steel. Weld J. 1978, 57, 103s–107s. [Google Scholar]
- Dong, C.; Zhu, Y.; Chai, G. Preliminary study on the mechanism of arc welding with the activating coating. Aeronaut. Manuf. Technol. Suppl. 2004, 6, 271–278. [Google Scholar]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balos, S.; Dramicanin, M.; Janjatovic, P.; Zabunov, I.; Pilic, B.; Goel, S.; Szutkowska, M. Suppressing the Use of Critical Raw Materials in Joining of AISI 304 Stainless Steel Using Activated Tungsten Inert Gas Welding. Metals 2019, 9, 1187. https://doi.org/10.3390/met9111187
Balos S, Dramicanin M, Janjatovic P, Zabunov I, Pilic B, Goel S, Szutkowska M. Suppressing the Use of Critical Raw Materials in Joining of AISI 304 Stainless Steel Using Activated Tungsten Inert Gas Welding. Metals. 2019; 9(11):1187. https://doi.org/10.3390/met9111187
Chicago/Turabian StyleBalos, Sebastian, Miroslav Dramicanin, Petar Janjatovic, Ivan Zabunov, Branka Pilic, Saurav Goel, and Magdalena Szutkowska. 2019. "Suppressing the Use of Critical Raw Materials in Joining of AISI 304 Stainless Steel Using Activated Tungsten Inert Gas Welding" Metals 9, no. 11: 1187. https://doi.org/10.3390/met9111187
APA StyleBalos, S., Dramicanin, M., Janjatovic, P., Zabunov, I., Pilic, B., Goel, S., & Szutkowska, M. (2019). Suppressing the Use of Critical Raw Materials in Joining of AISI 304 Stainless Steel Using Activated Tungsten Inert Gas Welding. Metals, 9(11), 1187. https://doi.org/10.3390/met9111187






