Effects of Relative Humidity on Crevice Corrosion Behavior of 304L Stainless-Steel Nuclear Material in a Chloride Environment
Abstract
1. Introduction
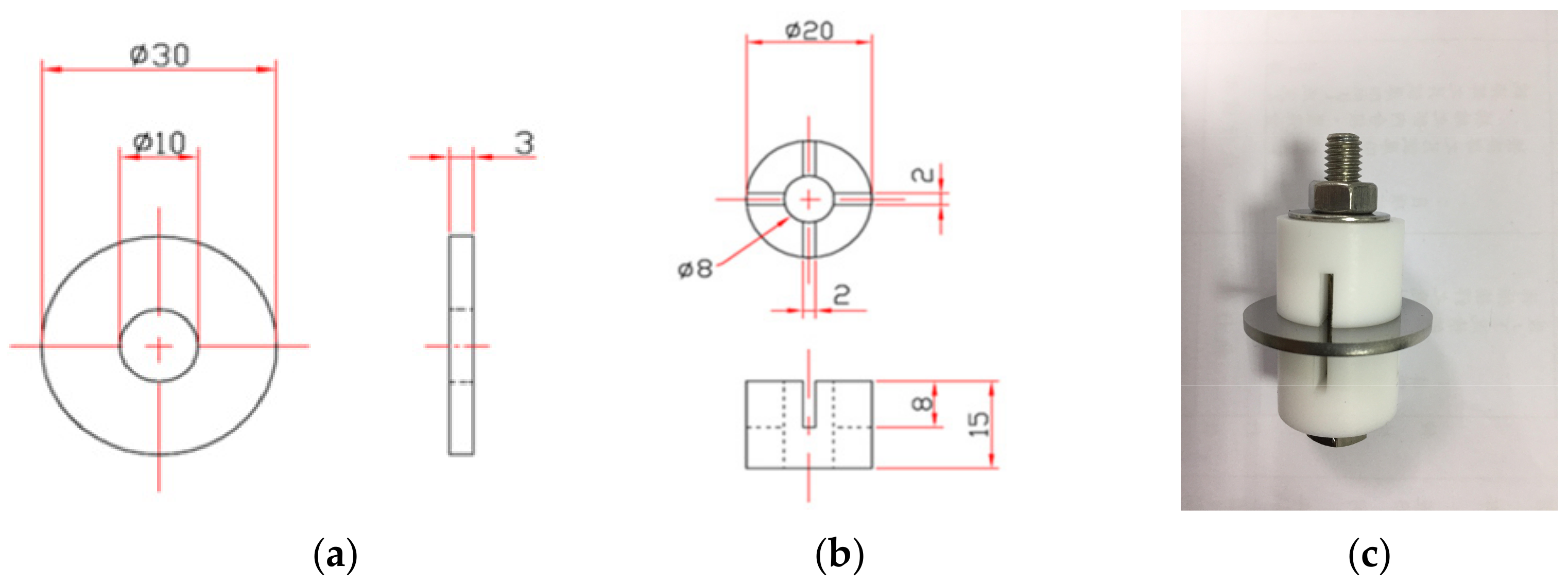
2. Experimental Setup
3. Results and Discussion
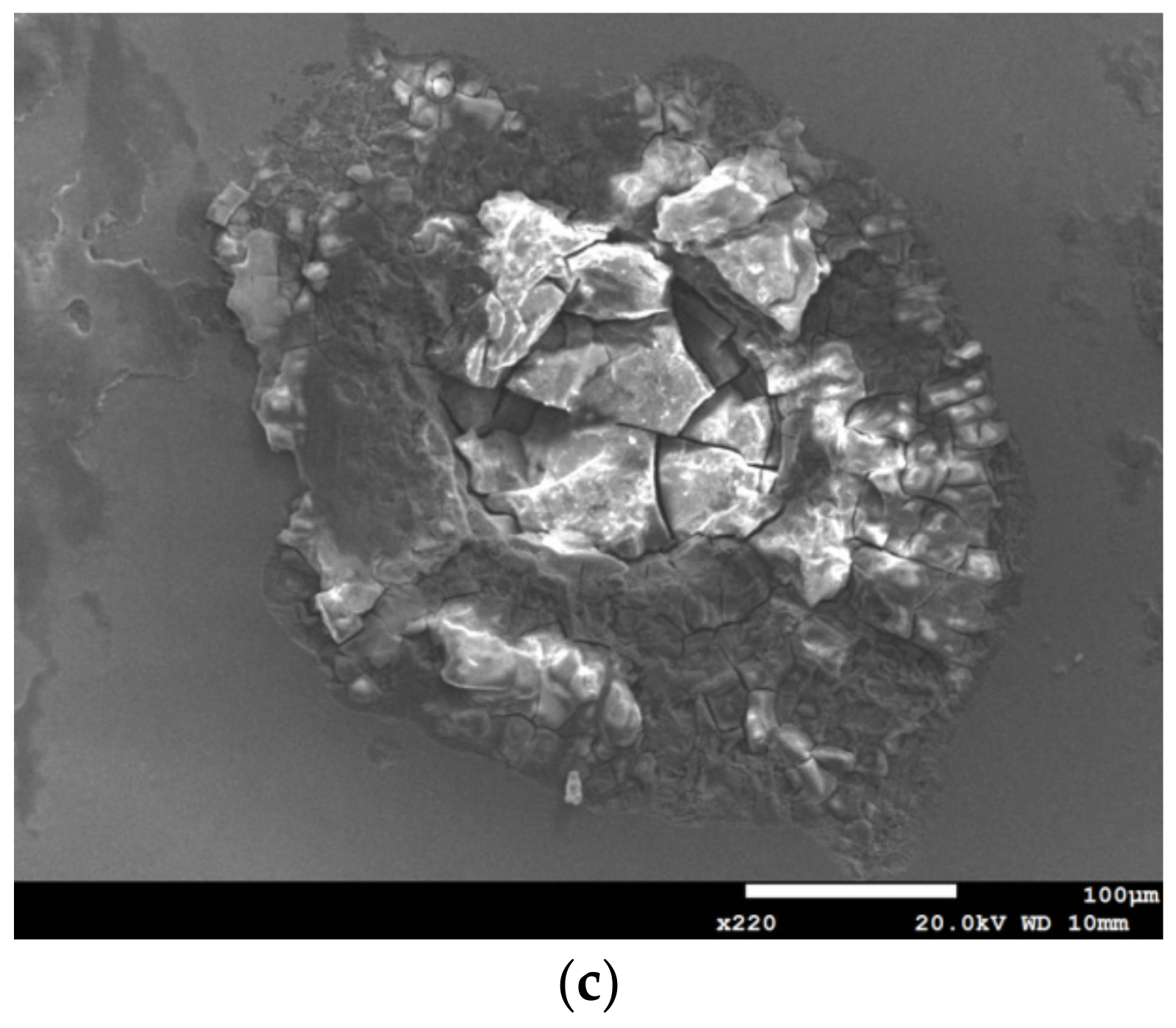
3.1. Surface Morphology Analysis
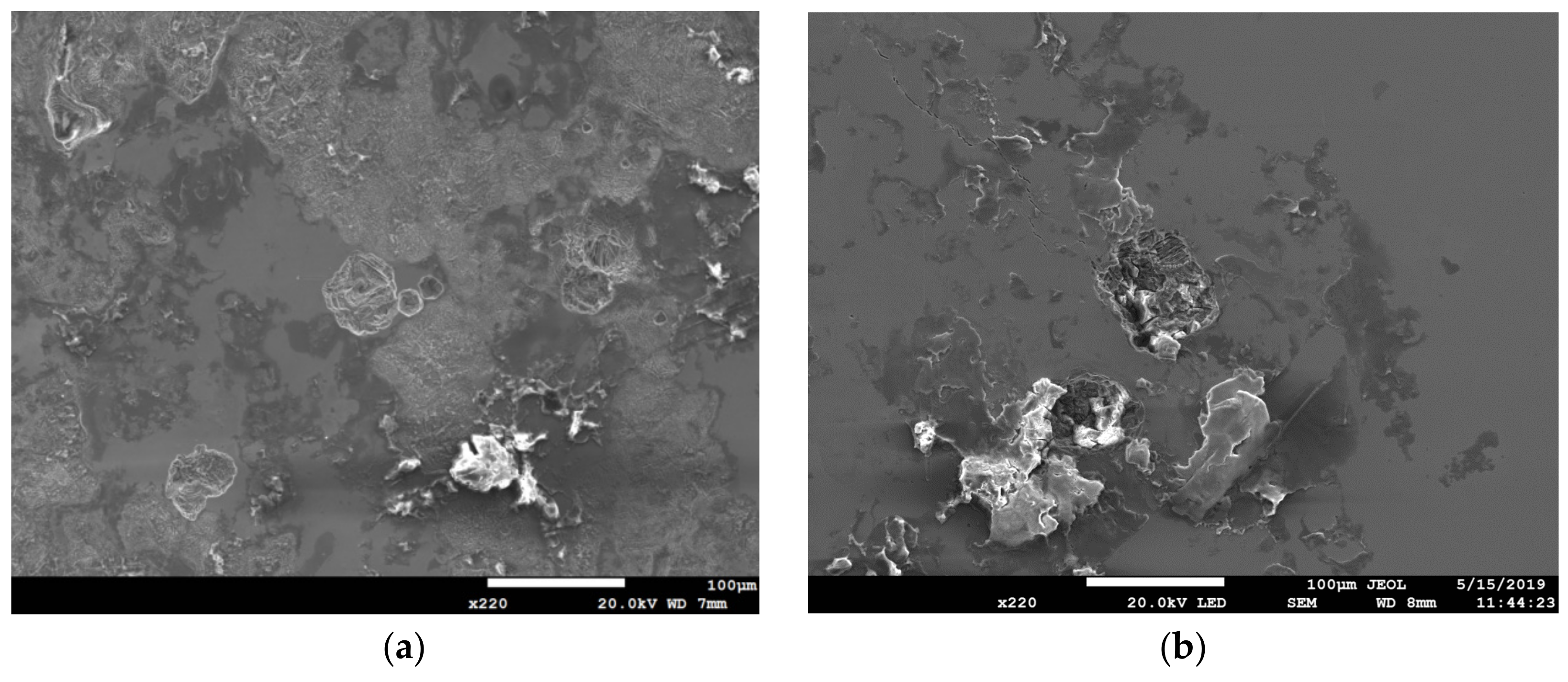
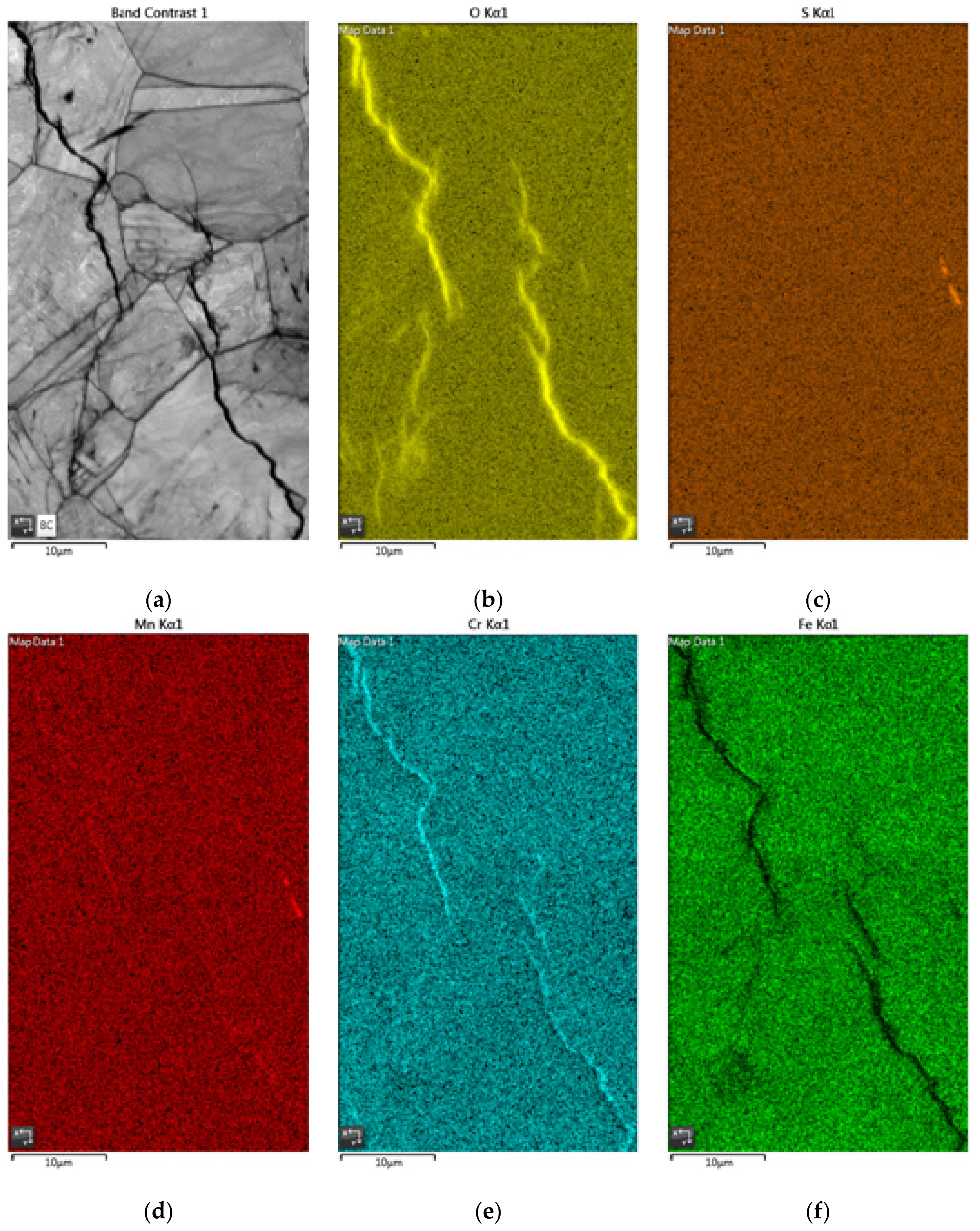
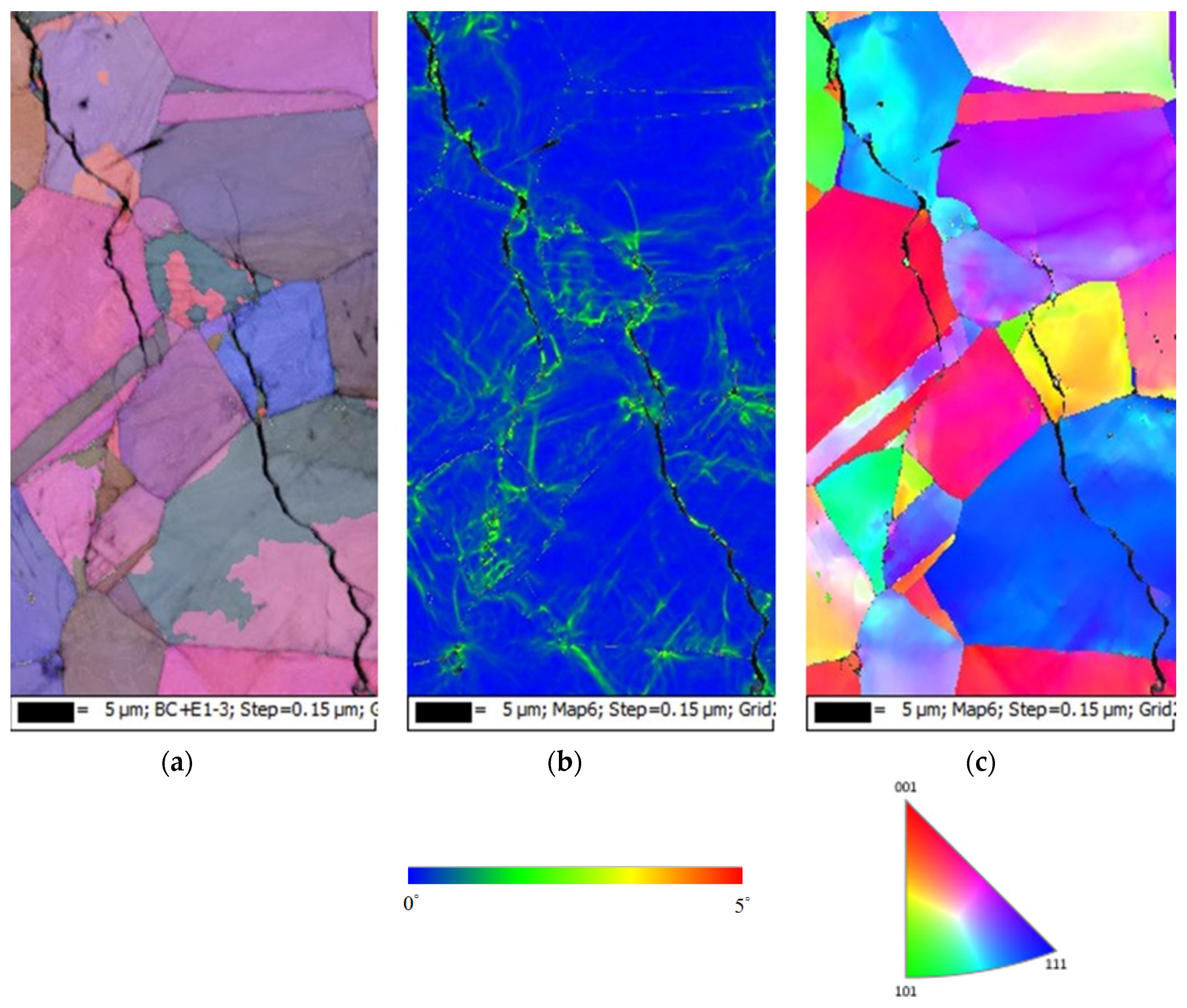
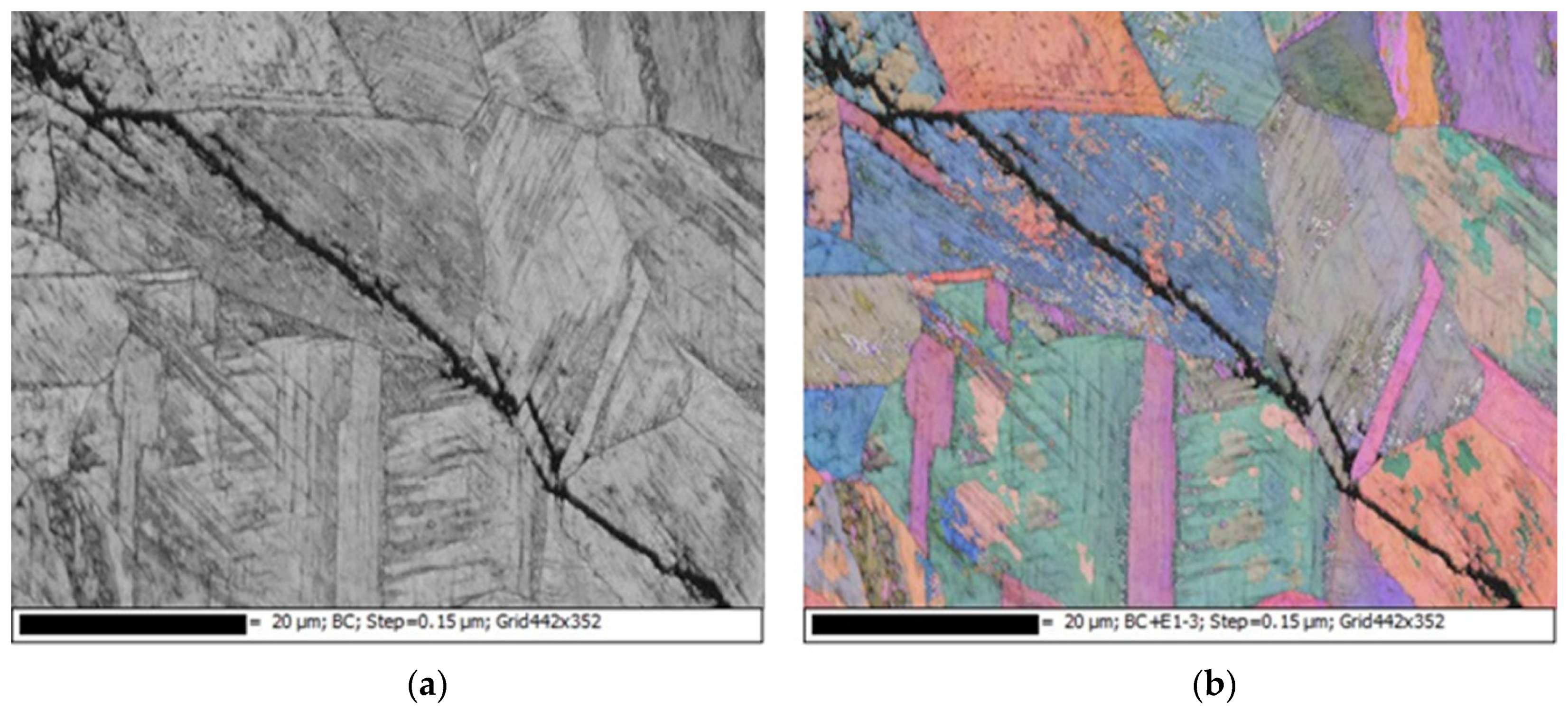
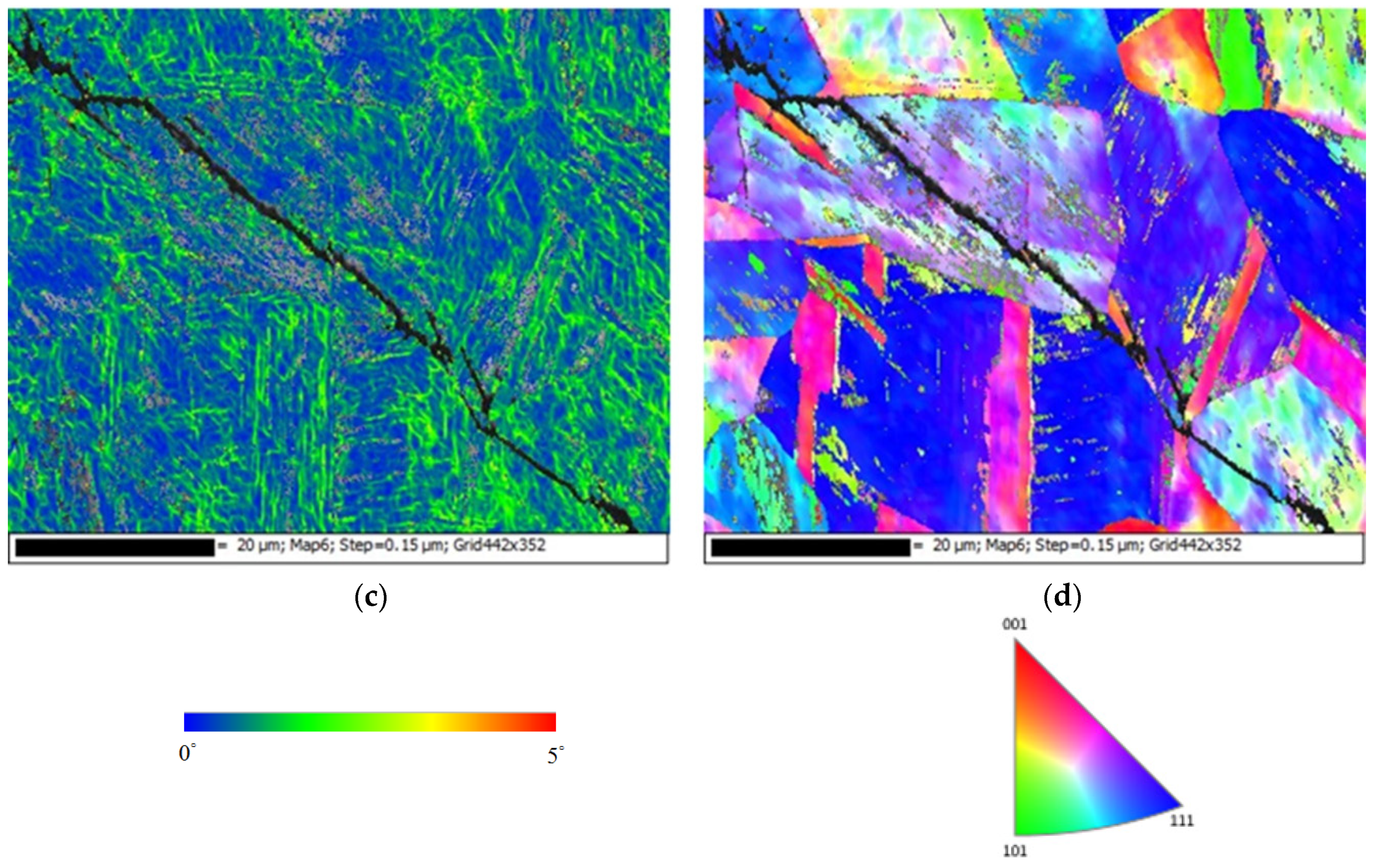
3.2. EDS and EBSD Analysis
4. Conclusions
- (1)
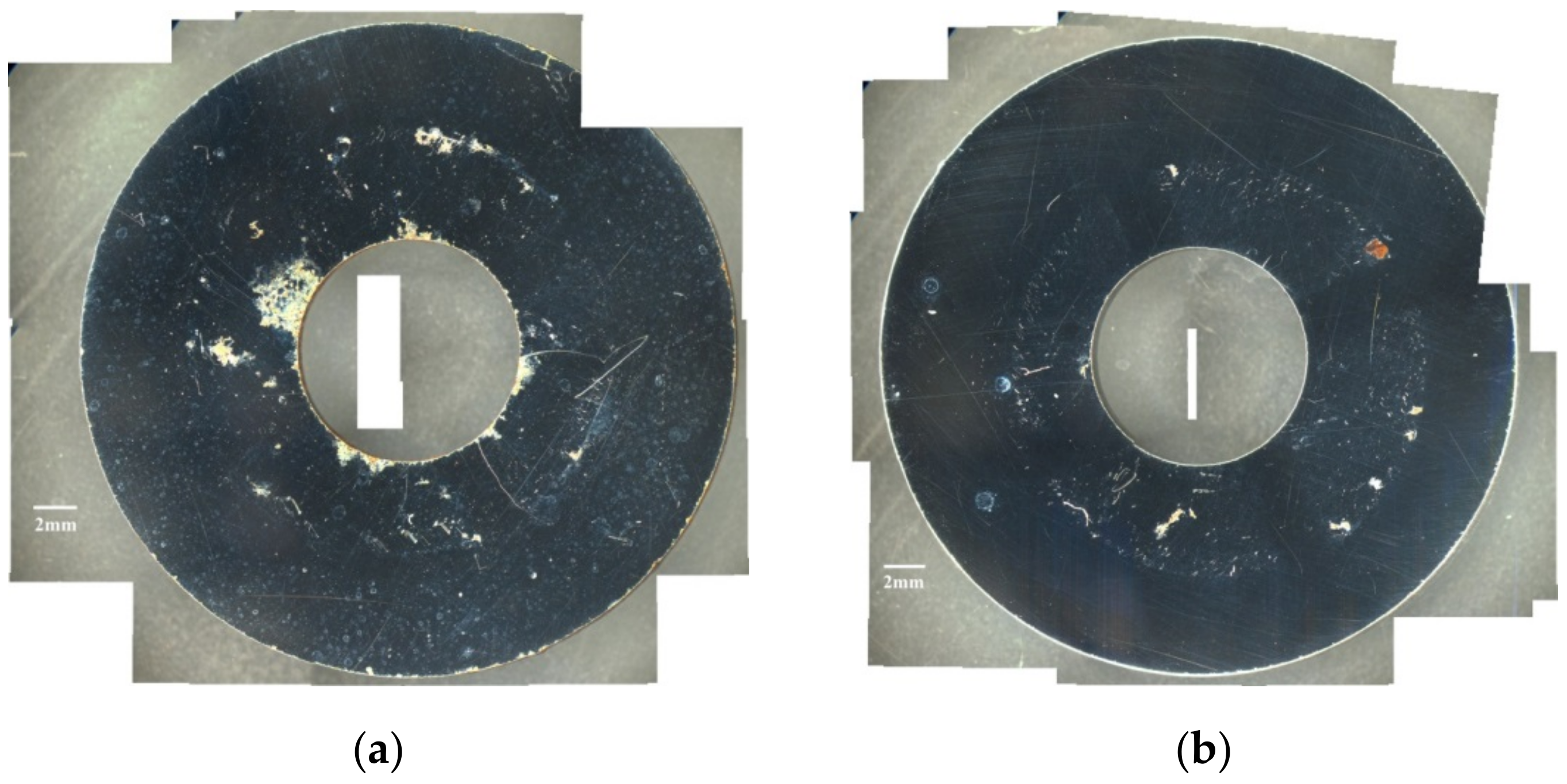
- When the chloride concentration was 0.1 g/m2, the specimen surfaces had minimum rusted areas at RH = 55% and no cracks were found in the corroded region at all three different levels of relative humidity. It may be that the 0.1 g/m2 chloride concentration was too low to initiate SCC at any tested RH.
- (2)
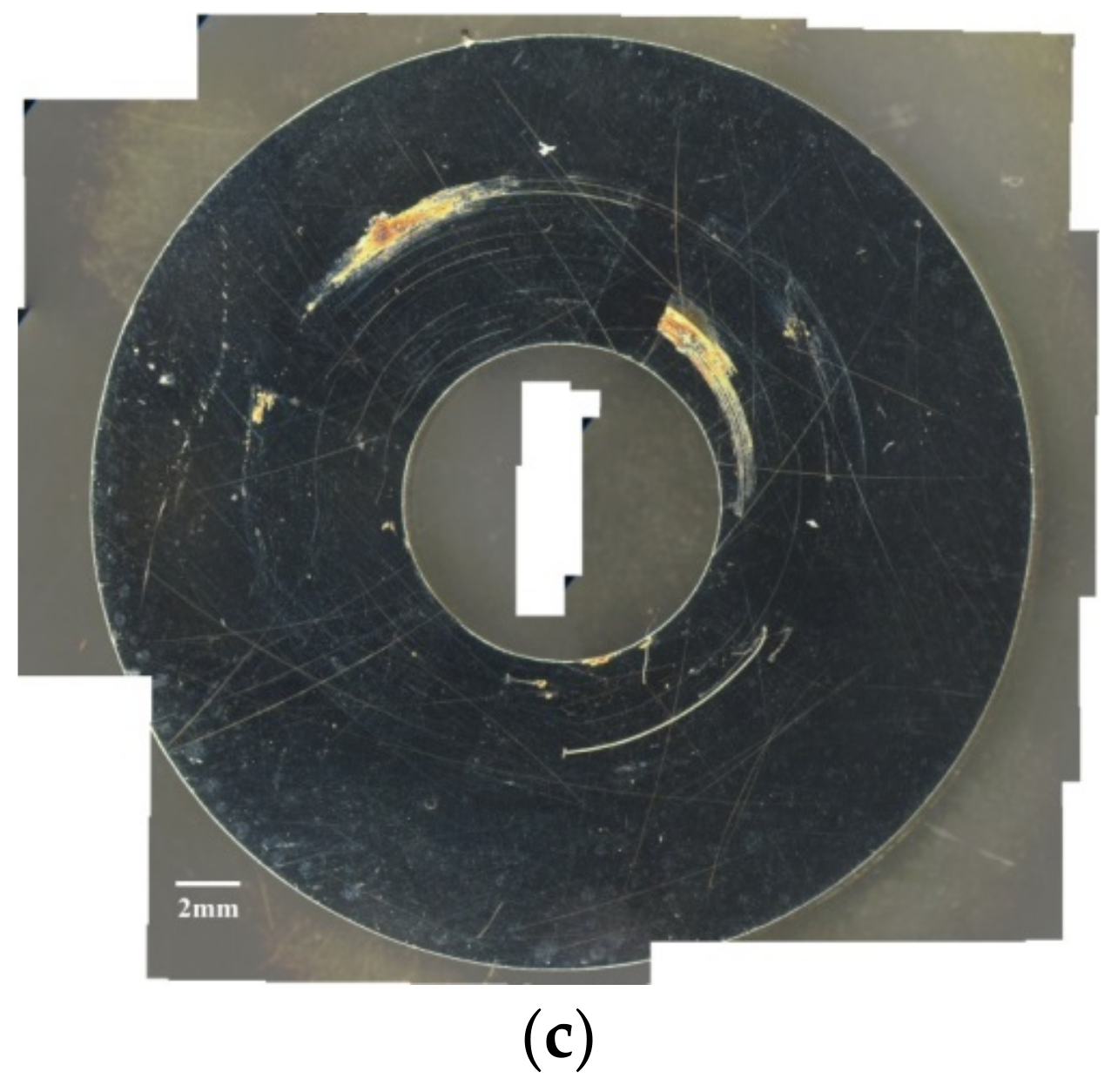
- When the chloride concentration was 1 g/m2, there was little or no difference for the rusted area under conditions with relative humidity of 45% and 55%, but the rusted area obviously increased when the relative humidity increased to 70%. Additionally, several rust spots and shallow corrosion were observed, but no crack was found in the corroded region with relative humidity of 45%, whereas the distinct SCC cracks were observed with relative humidity of 55% and 70%. This may be explained by a limited chloride transport into the crevice sites at the lowest RH (RH = 45%) with 1 g/m2 chloride concentration. The chloride may need a higher relative humidity environment to transport into the crevice sites due to the low chloride concentration. Thus, this suggests that the threshold relative humidity for SCC initiation of 304L stainless steel with 1 g/m2 chloride concentration at 45 °C is between 45% and 55%.
- (3)
- The type of SCC on the 304L stainless steel at 45 °C was transgranular SCC, which was verified by EBSD results.
Author Contributions
Funding
Conflicts of Interest
References
- Guo, L.; Street, S.R.; Mohammed-Ali, H.B.; Ghahari, M.; Mi, N.; Glanvill, S.; Plessis, A.D.; Reinhard, C.; Rayment, T.; Davenport, A.J. The effect of relative humidity change on atmospheric pitting corrosion of stainless steel 304L. Corros. Sci. 2019, 150, 110–120. [Google Scholar] [CrossRef]
- Meyer, R.M.; Pardini, A.; Cuta, J.; Adkins, H.; Casella, A.; Qiao, A.; Larche, M.R.; Diaz, A.; Doctor, S.R. NDE to Manage Atmospheric SCC in Canister for Dry Storage of Spent Fuel: An Assessment; PNNL-22495 401001060; Pacific Northwest National Laboratory: Richland, WA, USA, 1 September 2013.
- Tani, J.I.; Mayuzumi, M.; Hara, N. Stress corrosion cracking of stainless-steel canister for concrete cask storage of spent fuel. J. Nucl. Mater. 2008, 379, 42–47. [Google Scholar] [CrossRef]
- Cai, B.P.; Liu, Y.H.; Tian, X.J.; Wang, F.; Li, H.; Ji, R.J. An experimental study of crevice corrosion behavior of 316L stainless steel in artificial seawater. Corros. Sci. 2010, 52, 3235–3242. [Google Scholar] [CrossRef]
- Abdulsalam, M.I. Behavior of crevice corrosion in iron. Corros. Sci. 2005, 47, 1336–1351. [Google Scholar] [CrossRef]
- Oldfield, J.W.; Sutton, W.H. Crevice corrosion of stainless steel-2. Experimental studies. J. Br. Corros. 1978, 13, 104–111. [Google Scholar] [CrossRef]
- Sharland, S.N. A review of the theoretical modeling of crevice and pitting corrosion. Corros. Sci. 1987, 27, 289–323. [Google Scholar] [CrossRef]
- Chen, D.; Han, E.H.; Wu, X. Effects of crevice geometry on corrosion behavior of 304 stainless steel during crevice corrosion in high temperature pure water. Corros. Sci. 2016, 111, 518–530. [Google Scholar] [CrossRef]
- Yang, Y.Z.; Jiang, Y.M.; Li, J. In situ investigation of crevice corrosion on UNS S32101 duplex stainless steel in sodium chloride solution. Corros. Sci. 2013, 76, 163–169. [Google Scholar] [CrossRef]
- Shoji, S.; Ohnaka, N.; Furutani, Y.; Saitoh, T. Effects of relative humidity on atmospheric stress corrosion cracking of stainless steels. Corros. Eng. 1986, 35, 559–565. [Google Scholar] [CrossRef]
- Cook, A.B.; Lyon, S.B.; Stevens, N.P.C.; Gunther, M.; McFiggans, G.; Newman, R.C.; Engelberg, D.L. Assessing the risk of under-deposit chloride-induced stress corrosion cracking in austenitic stainless steel nuclear waste containers. Corros. Eng. Sci. Technol. 2014, 49, 529–534. [Google Scholar] [CrossRef]
- Albores-Silca, O.E.; Charles, E.A.; Padovani, C. Effect of chloride deposition on stress corrosion cracking of 316L stainless steel used for intermediate level radioactive waste containers. Corros. Eng. Sci. Technol. 2011, 46, 124–128. [Google Scholar] [CrossRef]
- Prosek, T.; Iverson, A.; Taxsen, C.; Thierry, D. Low temperature on stress corrosion cracking of stainless steels in the atmosphere in the presence of chloride deposits. Corrosion 2009, 65, 105–117. [Google Scholar] [CrossRef]
- Nash, B.K.; Kelly, R.G. Characterization of the crevice solution chemistry of 304stainless-steel. Corros. Sci. 1993, 35, 817–825. [Google Scholar] [CrossRef]
- White, S.P.; Weir, G.J.; Laycock, N.J. Calculating chemical concentrations during the initiation of crevice corrosion. Corros. Sci. 2000, 42, 605–629. [Google Scholar] [CrossRef]
- Zhong, C.; He, M.F.; Liu, L.; Chen, Y.J.; Shen, B.; Wu, Y.T.; Deng, Y.D.; Hu, W.B. Formation of an aluminum-alloyed coating on AZ91D magnesium alloy in molten salts at lower temperature. Surf. Coat. Tech. 2010, 205, 2412–2418. [Google Scholar] [CrossRef]




| Element | C | Si | S | Cr | Mn | Ni | Fe |
|---|---|---|---|---|---|---|---|
| wt% | 0.017 | 0.450 | 0.029 | 18.000 | 1.540 | 9.000 | Bal. |
| Composition | NaCl | MgCl2 | Na2SO4 | CaCl2 | KCl | NaHCO3 | KBr | H3BO3 | SrCl2 | NaF |
|---|---|---|---|---|---|---|---|---|---|---|
| wt% | 58.490 | 26.460 | 9.750 | 2.765 | 1.645 | 0.477 | 0.238 | 0.071 | 0.095 | 0.007 |
| wt% | O | S | Cl | Mn | Cr | Ni | Fe |
|---|---|---|---|---|---|---|---|
| A | 0.50 | 0.00 | 0.00 | 1.80 | 19.0 | 7.90 | 70.7 |
| B | 44.8 | 0.90 | 0.80 | 0.70 | 16.0 | 5.20 | 31.6 |
| C | 25.7 | 1.00 | 0.40 | 1.40 | 30.7 | 4.00 | 36.8 |
| D | 9.70 | 0.10 | 0.40 | 1.30 | 17.8 | 6.80 | 63.9 |
| E | 36.6 | 1.20 | 0.50 | 0.80 | 31.4 | 5.10 | 24.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeh, C.-P.; Tsai, K.-C.; Huang, J.-Y. Effects of Relative Humidity on Crevice Corrosion Behavior of 304L Stainless-Steel Nuclear Material in a Chloride Environment. Metals 2019, 9, 1185. https://doi.org/10.3390/met9111185
Yeh C-P, Tsai K-C, Huang J-Y. Effects of Relative Humidity on Crevice Corrosion Behavior of 304L Stainless-Steel Nuclear Material in a Chloride Environment. Metals. 2019; 9(11):1185. https://doi.org/10.3390/met9111185
Chicago/Turabian StyleYeh, Chun-Ping, Kun-Chao Tsai, and Jiunn-Yuan Huang. 2019. "Effects of Relative Humidity on Crevice Corrosion Behavior of 304L Stainless-Steel Nuclear Material in a Chloride Environment" Metals 9, no. 11: 1185. https://doi.org/10.3390/met9111185
APA StyleYeh, C.-P., Tsai, K.-C., & Huang, J.-Y. (2019). Effects of Relative Humidity on Crevice Corrosion Behavior of 304L Stainless-Steel Nuclear Material in a Chloride Environment. Metals, 9(11), 1185. https://doi.org/10.3390/met9111185




