Synthesis of Poly-Alumino-Ferric Sulphate Coagulant from Acid Mine Drainage by Precipitation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Procedure
2.2.1. Iron and Aluminium Co-Precipitation
2.2.2. Metal Analysis
2.3. Water Treatment by Coagulation
3. Results and Discussion
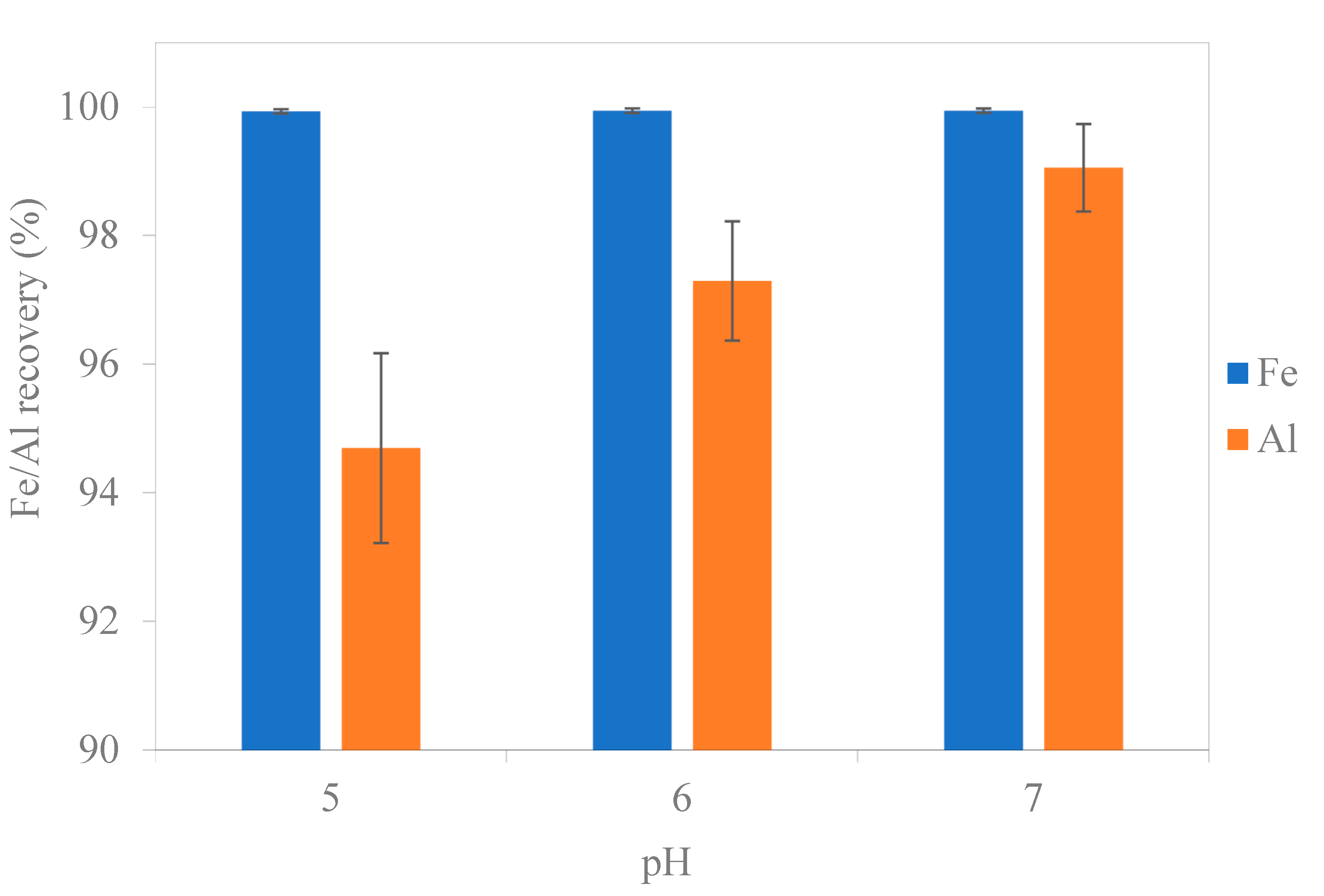
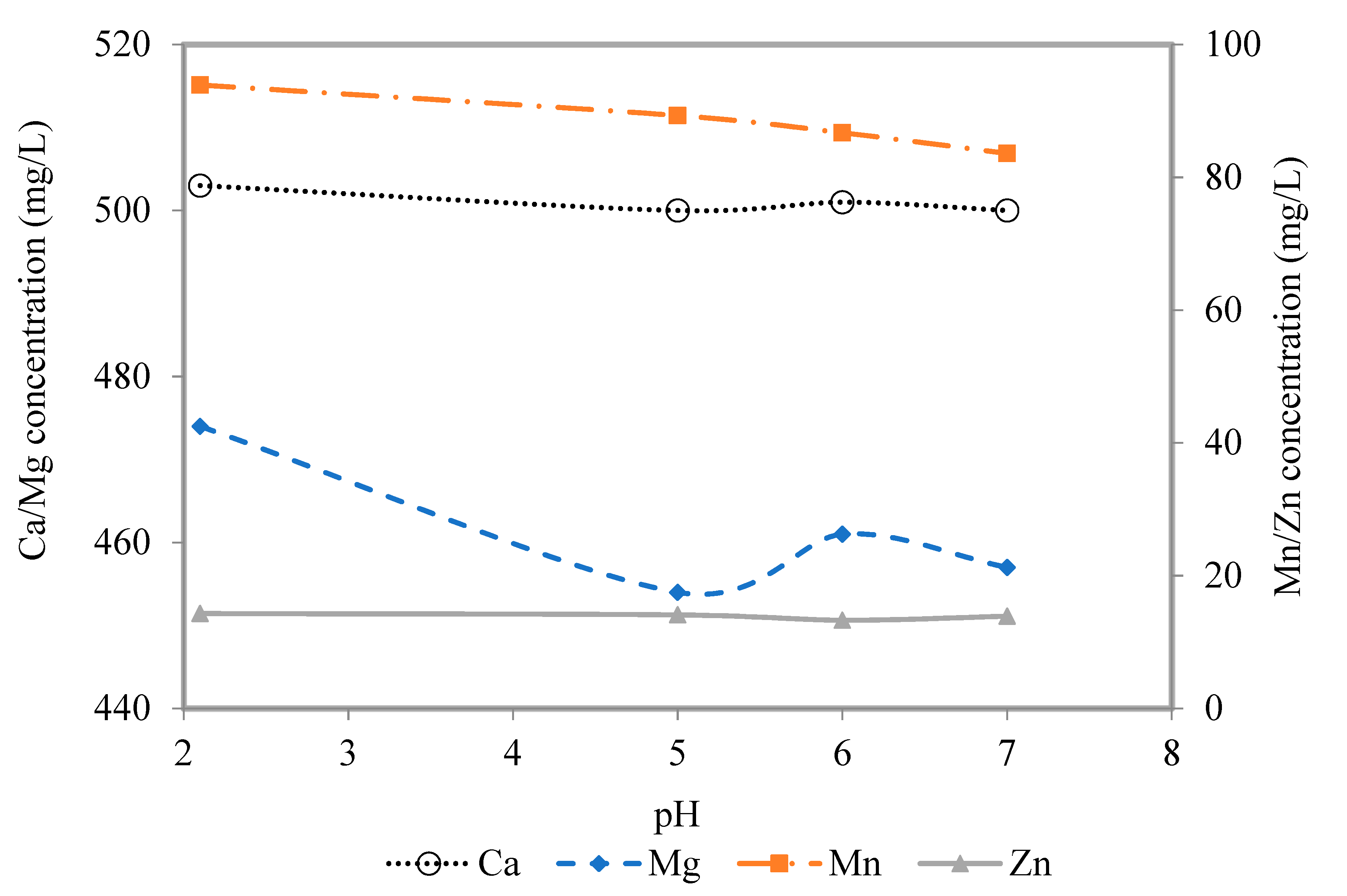
3.1. Precipitation
3.2. Coagulation
3.2.1. Coagulant Production and Testing
3.2.2. Effect of the Coagulant on Turbidity and Chemical Oxygen Demand Removal
3.2.3. Effect of the Coagulants on Electric Conductivity
3.2.4. Effect of the Coagulant Dose on Total Dissolved Solids
4. Conclusion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Manders, P.; Godfrey, L.; Hobbs, P. Briefing Note: Acid Mine Drainage in South Africa; CSIR: Pretoria, South Africa, 2009. [Google Scholar]
- Jacobs, J.A.; Lehr, J.H.; Testa, S.M. Acid Mine Drainage, Rock Drainage, and Acid Sulfate Soils: Causes, Assessment, Prediction, Prevention, and Remediation; John Wiley & Sons: New York, NY, USA, 2014. [Google Scholar]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters; John Wiley & Sons: New York, NY, USA, 2012. [Google Scholar]
- Fripp, J.; Ziemkiewicz, D.P.F.; Charkavorki, H. Acid Mine Drainage Treatment; ERDC TN-EMRRP-SR-14; Ecosystem Management and Restoration Research Program: Baltimore District, MD, USA, 2000; Volume 7. [Google Scholar]
- Garland, R. Acid mine drainage-can it affect human health? Quest 2011, 7, 46–47. [Google Scholar]
- Monachese, M.; Burton, J.P.; Reid, G. Bioremediation and Tolerance of Humans to Heavy Metals through Microbial Processes: A Potential Role for Probiotics? Appl. Environ. Microbiol. 2012, 78, 6397–6404. [Google Scholar] [CrossRef] [PubMed]
- Van Dyk, L.D.; Simate, G.S.; Seepe, L.; Sibanda, V.; Shemi, A.; Ndlovu, S. The removal of Co2+, V3+ and Cr3+ from waste effluents using cassava waste. S. Afr. J. Chem. Eng. 2013, 18, 51–69. [Google Scholar]
- Singh, R.; Gautam, N.; Mishra, A.; Gupta, R. Heavy metals and living systems: An overview. Indian J. Pharmacol. 2011, 43, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Coulton, R.; Bullen, C.; Hallett, C. The design and optimisation of active mine water treatment plants. Land Contam. Reclam. 2003, 11, 273–279. [Google Scholar] [CrossRef]
- Johnson, D.B.; Hallberg, K.B. Acid mine drainage remediation options: A review. Sci. Total Environ. 2005, 338, 3–14. [Google Scholar] [CrossRef]
- Menezes, J.; Silva, R.; Arce, I.; Schneider, I.; Silva, R.D.A. Production of a poly-alumino-iron sulphate coagulant by chemical precipitation of a coal mining acid drainage. Miner. Eng. 2010, 23, 249–251. [Google Scholar] [CrossRef]
- Simate, G.S.; Ndlovu, S. Acid mine drainage: Challenges and opportunities. J. Environ. Chem. Eng. 2014, 2, 1785–1803. [Google Scholar] [CrossRef]
- Ryan, M.; Kney, A.; Carley, T. A study of selective precipitation techniques used to recover refined iron oxide pigments for the production of paint from a synthetic acid mine drainage solution. Appl. Geochem. 2017, 79, 27–35. [Google Scholar] [CrossRef]
- Hedin, R.S. Recovery of marketable iron oxide from mine drainage in the USA. Land Contam. Reclam. 2003, 11, 93–97. [Google Scholar] [CrossRef]
- Macingova, E.; Luptakova, A. Recovery of iron from acid mine drainage in the form of oxides. Inżynieria Miner. 2014, 15, 193–198. [Google Scholar]
- Wei, X.; Viadero, R.C. Synthesis of magnetite nanoparticles with ferric iron recovered from acid mine drainage: Implications for environmental engineering. Colloids Surf. A Physicochem. Eng. Asp. 2007, 294, 280–286. [Google Scholar] [CrossRef]
- Marcello, R.; Galato, S.; Peterson, M.; Riella, H.; Bernardin, A.; Bernardin, A. Inorganic pigments made from the recycling of coal mine drainage treatment sludge. J. Environ. Manag. 2008, 88, 1280–1284. [Google Scholar] [CrossRef] [PubMed]
- Michalková, E.; Schwarz, M.; Pulišová, P.; Máša, B.; Sudovský, P. Metals Recovery from Acid Mine Drainage and Possibilities for their Utilization. Pol. J. Environ. Stud. 2013, 22, 1111–1118. [Google Scholar]
- Park, S.M.; Yoo, J.C.; Ji, S.W.; Yang, J.S.; Baek, K. Selective recovery of dissolved Fe, Al, Cu, and Zn in acid mine drainage based on modeling to predict precipitation pH. Environ. Sci. Pollut. Res. 2015, 22, 3013–3022. [Google Scholar] [CrossRef] [PubMed]
- Sahinkaya, E.; Gungor, M.; Bayrakdar, A.; Yucesoy, Z.; Uyanik, S. Separate recovery of copper and zinc from acid mine drainage using biogenic sulfide. J. Hazard. Mater. 2009, 171, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Viadero, R.C.; Buzby, K.M. Recovery of Iron and Aluminum from Acid Mine Drainage by Selective Precipitation. Environ. Eng. Sci. 2005, 22, 745–755. [Google Scholar] [CrossRef]
- Nleya, Y. Removal of Toxic Metals and Recovery of Acid from Acid Mine Drainage Using Acid Retardation and Adsorption Processes. Ph.D.; Thesis, University of Witwatersrand, Johannesburg, South Africa, 2016. Available online: http://wiredspace.wits.ac.za/handle/10539/21051 (accessed on 2 October 2019).
- Buzzi, D.C.; Viegas, L.S.; Rodrigues, M.A.S.; Bernardes, A.M.; Tenório, J.A.S. Water recovery from acid mine drainage by electrodialysis. Miner. Eng. 2013, 40, 82–89. [Google Scholar] [CrossRef]
- Martí-Calatayud, M.; Buzzi, D.; Gabaldón, M.G.; Ortega, E.; Bernardes, A.; Tenório, J.; Pérez-Herranz, V.; Bernardes, A. Sulfuric acid recovery from acid mine drainage by means of electrodialysis. Desalination 2014, 343, 120–127. [Google Scholar] [CrossRef]
- Masindi, V. Recovery of drinking water and valuable minerals from acid mine drainage using an integration of magnesite, lime, soda ash, CO2 and reverse osmosis treatment processes. J. Environ. Chem. Eng. 2017, 5, 3136–3142. [Google Scholar] [CrossRef]
- Silsbee, M. The Use of Sludge Generated by the Neutralization of Acid Mine Drainage in the Cement Industry; EPD06021; United States Environmental Protection Agency: Washington, DC, USA, 2006.
- Evenson, C.; Nairn, R. Enhancing phosphorus sorption capacity with treatment wetland iron oxyhydroxides. In Proceedings of the 17th National Meeting of the American Society for Surface Mining and Reclamation, Tampa, FL, USA, 11–15 June 2000. [Google Scholar]
- Dudeney, A.W.; Neville, K.J.; Tarasova, I.; Heath, A.O.; Smith, S.R. Utilisation of Ochreous Sludge as a Soil Amendment. In Proceedings of the Symposium: Mine Water, Newcastle, UK, 1 July 2004; pp. 19–23. [Google Scholar]
- Saunders, F.M.; Roeder, L.M. Coagulant Recovery: A Critical Assessment; The Water Research Foundation: Denver, CO, USA, 1991. [Google Scholar]
- Baes, C.; Mesmer, R. The Hydrolysis of Cations; Wiley: New York, NY, USA, 1976. [Google Scholar]
- Flynn, C.M. Hydrolysis of inorganic iron (III) salts. Chem. Rev. 1984, 84, 31–41. [Google Scholar] [CrossRef]
- Salama, E.-S.; Kim, J.R.; Ji, M.-K.; Cho, D.-W.; Abou-Shanab, R.A.; Kabra, A.N.; Jeon, B.-H. Application of acid mine drainage for coagulation/flocculation of microalgal biomass. Bioresour. Technol. 2015, 186, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Lopes, F.A.; Menezes, J.C.; Schneider, I.A. Acid Mine Drainage as Source of Iron for the Treatment of Sewage by Coagulation and Fenton’s Reaction; IMWA: Aachen, Germany, 2011. [Google Scholar]
- Rao, S.R.; Gehr, R.; Riendeau, M.; Lu, D.; Finch, J.A. Acid mine drainage as a coagulant. Min. Eng. 1992, 5, 1011–1020. [Google Scholar] [CrossRef]
- Rao, S.R.; Leroux, M.; Finch, J. Resource Recovery from Acid Mine Drainage. In Metals Removal from Acidic Drainage-Chemical Methods; MEND Project 3-21.2a; Noranda Technology Center: Pointe Claire, QC, Canada, 1996. [Google Scholar]
- Jiang, J.-Q.; Graham, N.J.D. Development of Optimal Poly-Alumino—Iron Sulphate Coagulant. J. Environ. Eng. 2003, 129, 699–708. [Google Scholar] [CrossRef]
- Snoeyink, V.L.; Jenkins, D. Water Chemistry; John Wiley: New York, NY, USA, 1980. [Google Scholar]
- Mendham, J. Vogels Textbook of Quantitative Chemical Analysis; Pearson Education: New Delhi, India, 2006. [Google Scholar]
- Tabak, H.H.; Scharp, R.; Burckle, J.; Kawahara, F.K.; Govind, R. Advances in biotreatment of acid mine drainage and biorecovery of metals: 1. Metal precipitation for recovery and recycle. Biodegradation 2003, 14, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Clescerl, L.; Greenberg, A.; Eaton, A. Standard Methods for Examining Water and Wastewater; APHA: Washington, DC, USA; AWWA: Washington, DC, USA; WEF: Washington, DC, USA, 1999. [Google Scholar]
- Gitari, W.M.; Petrik, L.F.; Akinyemi, S.A. Treatment of Acid Mine Drainage with Coal Fly Ash: Exploring the Solution Chemistry and Product Water Quality. In Coal Fly Ash Beneficiation—Treatment of Acid Mine Drainage with Coal Fly Ash; Akinyemi, S.A., Gitari, M.W., Eds.; InTech: Limpopo, South Africa, 2018. [Google Scholar] [CrossRef]
- Petrik, L.F.; Fatoba, O.O.; Missengue, R. Treatment of Mine Water Using a Combination of Coal Fly Ash and Flocculants in a Jet Loop Reactor System; WRC Report No. 2129/1/18; Water Research Commission: Pretoria, South Africa, 2017. [Google Scholar]
- National Gazette. National Gazette No. 36820, 6 September 2013, Vol. 579. Available online: https://www.greengazette.co.za/documents/national-gazette-36820-of-06-september-2013-vol-579_20130906-GGN-36820.pdf (accessed on 20 September 2019).
- Cheng, W.P.; Chi, F.H.; Yu, R.F.; Lee, Y.C. Using Chitosan as a Coagulant in Recovery of Organic Matters from the Mash and Lauter Wastewater of Brewery. J. Polym. Environ. 2005, 13, 383–388. [Google Scholar] [CrossRef]
- Tolkou, A.; Zouboulis, A. Synthesis and coagulation performance of composite poly-aluminum-ferric-silicate-chloride coagulants in water and wastewater. Desalin. Water Treat. 2015, 53, 3309–3318. [Google Scholar] [CrossRef]
- Wang, D.; Tang, H. Modified inorganic polymer flocculant-PFSi: Its preparation, characterization and coagulation behavior. Water Res. 2001, 35, 3418–3428. [Google Scholar] [CrossRef]
- Xing, Z.-P.; Sun, D.-Z. Treatment of antibiotic fermentation wastewater by combined polyferric sulfate coagulation, Fenton and sedimentation process. J. Hazard. Mater. 2009, 168, 1264–1268. [Google Scholar] [CrossRef]
- Oyem, H.; Oyem, I.; Ezeweali, D. Temperature, pH, Electrical Conductivity, Total Dissolved Solids and Chemical Oxygen Demand of Groundwater in Boji-BojiAgbor/Owa Area and Immediate Suburbs. Res. J. Environ. Sci. 2014, 8, 444–450. [Google Scholar] [CrossRef]
- Aniyikaiye, T.E.; Oluseyi, T.; Odiyo, J.O.; Edokpayi, J.N. Physico-Chemical Analysis of Wastewater Discharge from Selected Paint Industries in Lagos, Nigeria. Int. J. Environ. Res. Public Health 2019, 16, 1235. [Google Scholar] [CrossRef]
- Miranda, R.; Latour, I.; Hörsken, A.; Jarabo, R.; Blanco, A. Enhanced Silica Removal by Polyamine- and Polyacrylamide-Polyaluminum Hybrid Coagulants. Chem. Eng. Technol. 2015, 38, 2045–2053. [Google Scholar] [CrossRef]
- Kazi, T.; Virupakshi, A. Treatment of tannery wastewater using natural coagulants. Int. J. Environ. Res. Public Health 2013, 2, 4061–4068. [Google Scholar]
- Beyene, H.D.; Hailegebrial, T.D.; Dirersa, W.B. Investigation of Coagulation Activity of Cactus Powder in Water Treatment. J. Appl. Chem. 2016, 2016, 1–9. [Google Scholar]



| Factors | Values |
|---|---|
| Reaction temperature, °C | 25 |
| Fe oxidation time, hours | 24 |
| Type of oxidant | O2 |
| Agitation, rpm | 300 |
| Precipitation pH | 5.0, 6.0, 7.0 |
| Precipitation time, hours | 1 |
| NaOH concentration, molar | 4 |
| H2SO4 concentration, molar | 0.1 |
| Metal Concentration, mg/L | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | pH | Fe (Total) | Al | Ca | Mg | Mn | Zn | Ni | Co | Cu |
| Raw AMD | 2.1 | 4290.0 | 396.0 | 503.0 | 474.0 | 93.9 | 14.3 | 1.7 | 1.4 | 0.5 |
| SA effluent standard a | 5.5–9.5 | 0.3 | NA | NA | NA | 0.1 | 0.1 | NA | NA | 0.01 |
| Effluent after precipitation | 5.0 | 2.7 | 14.0 | 500.0 | 454.0 | 89.3 | 14.1 | 1.6 | 1.4 | 0.5 |
| SD | NA | 0.03 | 1.50 | 2.01 | 9.53 | 2.10 | 2.13 | NA | NA | NA |
| 6.0 | 2.1 | 9.0 | 501 | 461.0 | 86.7 | 13.3 | 1.6 | 1.4 | 0.5 | |
| SD | 0.04 | 0.92 | 1.15 | 3.60 | 3.10 | 1.67 | NA | NA | NA | |
| 7.0 | 2.1 | 3.7 | 500 | 457.0 | 83.6 | 13.9 | 1.6 | 1.4 | 0.5 | |
| SD | 0.03 | 0.67 | 2.02 | 4.58 | 1.87 | 3.87 | NA | NA | NA | |
| Parameter | AMD-PAFS | PFS b | PAS b |
|---|---|---|---|
| Fe (Total), mg/L | 88,768.84 | 115,000 | 112.5 |
| Al, mg/L | 9876.54 | 4419 | 47,668 |
| Mg, mg/L | 444.43 | 160.6 | 0.38 |
| Ca, mg/L | 6.1 | 56.8 | 8.4 |
| Mn, mg/L | 102.23 | 1585 | 1.3 |
| Zn, mg/L | ND | 22.4 | 3.80 |
| Ni, mg/L | ND | ND | ND |
| Co, mg/L | ND | ND | ND |
| Cu, mg/L | ND | 11.5 | <0.0004 |
| SO42−, mg/L | 122,000 | 130,800 | 53,000 |
| Parameter | Value |
|---|---|
| pH | 7.1 ± 0.2 |
| COD, mg/L | 3160 ± 90 |
| TDS, mg/L | 1810 ± 30 |
| Turbidity, FAU | 99 ± 7 |
| Electric conductivity, µS/cm | 3510 ± 133 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mwewa, B.; Stopić, S.; Ndlovu, S.; Simate, G.S.; Xakalashe, B.; Friedrich, B. Synthesis of Poly-Alumino-Ferric Sulphate Coagulant from Acid Mine Drainage by Precipitation. Metals 2019, 9, 1166. https://doi.org/10.3390/met9111166
Mwewa B, Stopić S, Ndlovu S, Simate GS, Xakalashe B, Friedrich B. Synthesis of Poly-Alumino-Ferric Sulphate Coagulant from Acid Mine Drainage by Precipitation. Metals. 2019; 9(11):1166. https://doi.org/10.3390/met9111166
Chicago/Turabian StyleMwewa, Brian, Srećko Stopić, Sehliselo Ndlovu, Geoffrey S. Simate, Buhle Xakalashe, and Bernd Friedrich. 2019. "Synthesis of Poly-Alumino-Ferric Sulphate Coagulant from Acid Mine Drainage by Precipitation" Metals 9, no. 11: 1166. https://doi.org/10.3390/met9111166
APA StyleMwewa, B., Stopić, S., Ndlovu, S., Simate, G. S., Xakalashe, B., & Friedrich, B. (2019). Synthesis of Poly-Alumino-Ferric Sulphate Coagulant from Acid Mine Drainage by Precipitation. Metals, 9(11), 1166. https://doi.org/10.3390/met9111166









