Image Processing-Based Analysis of Temperature Dependent Interlayer in Brazed Fe-Cr-Al Alloy (Fecralloy®) with Ni-Based Filler Metal
Abstract
1. Introduction
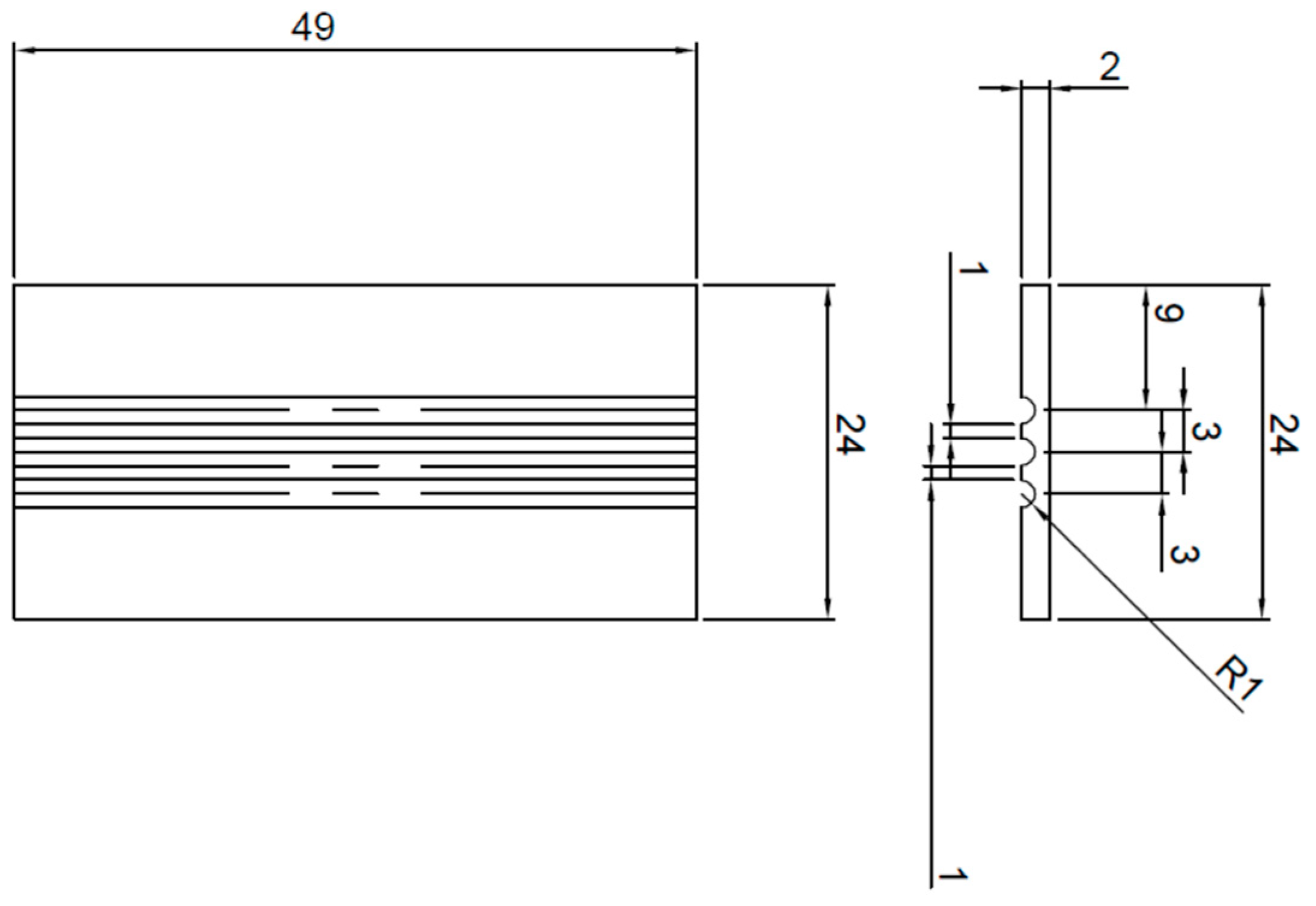
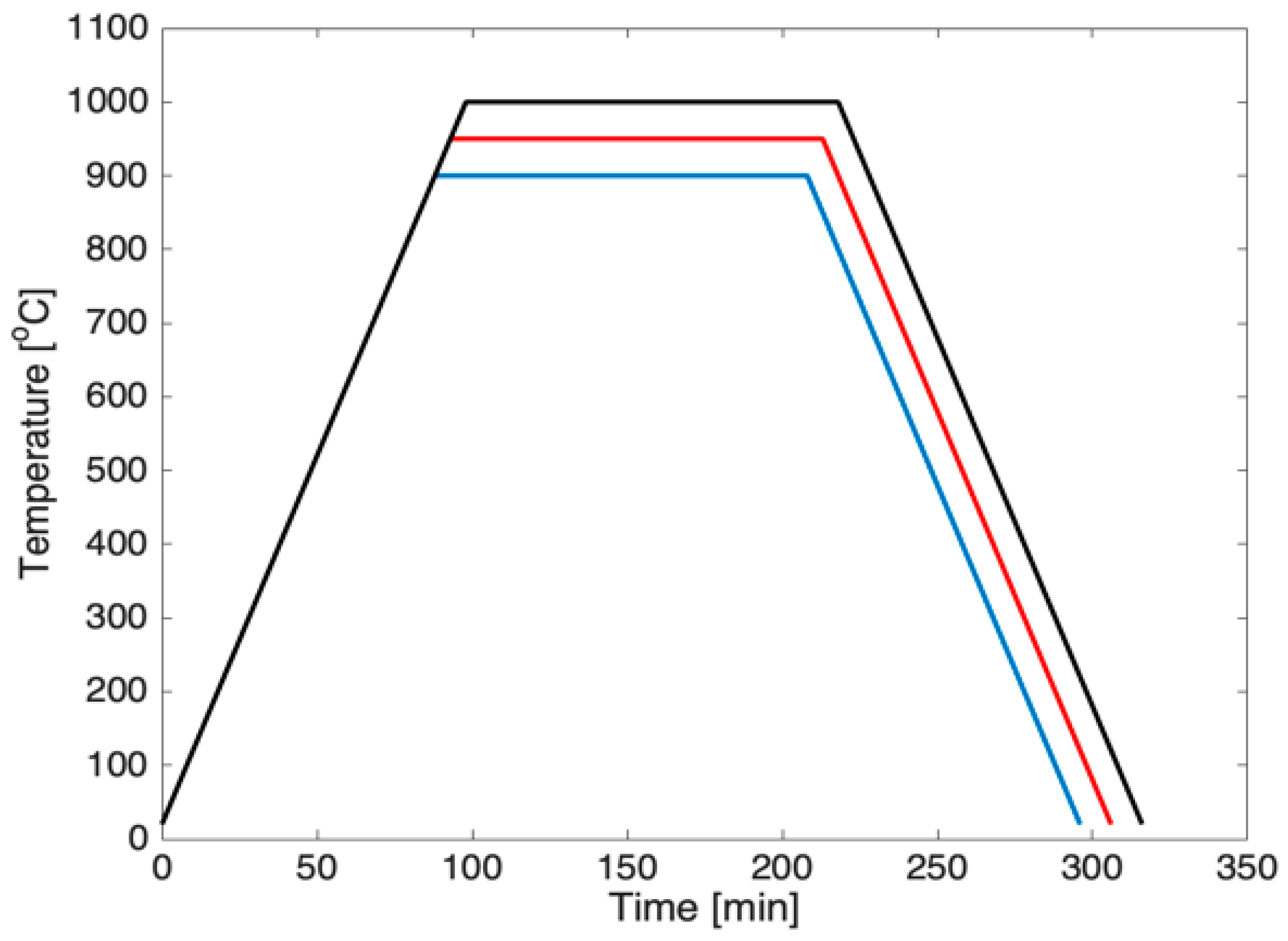
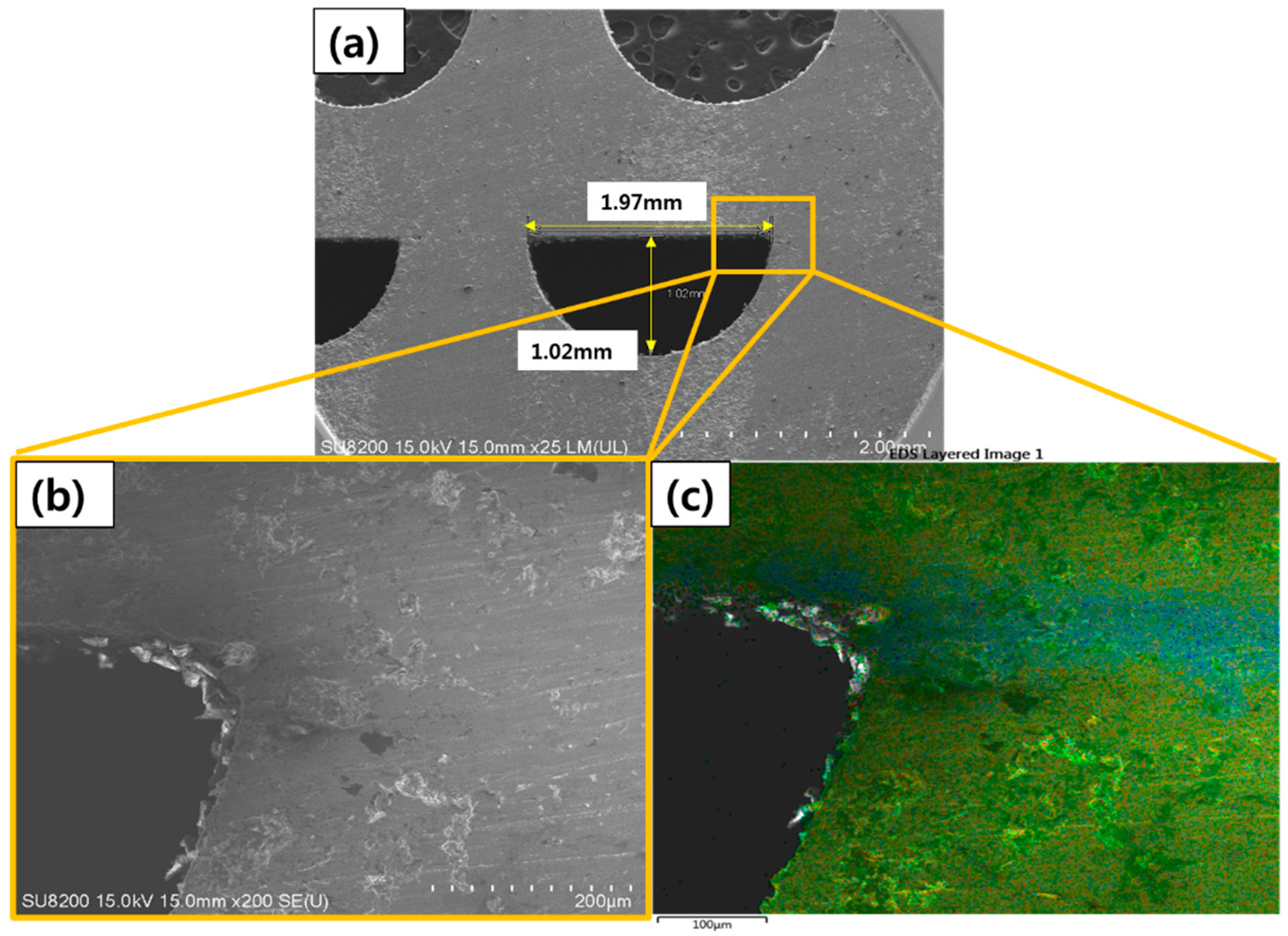
2. Experimental Procedure
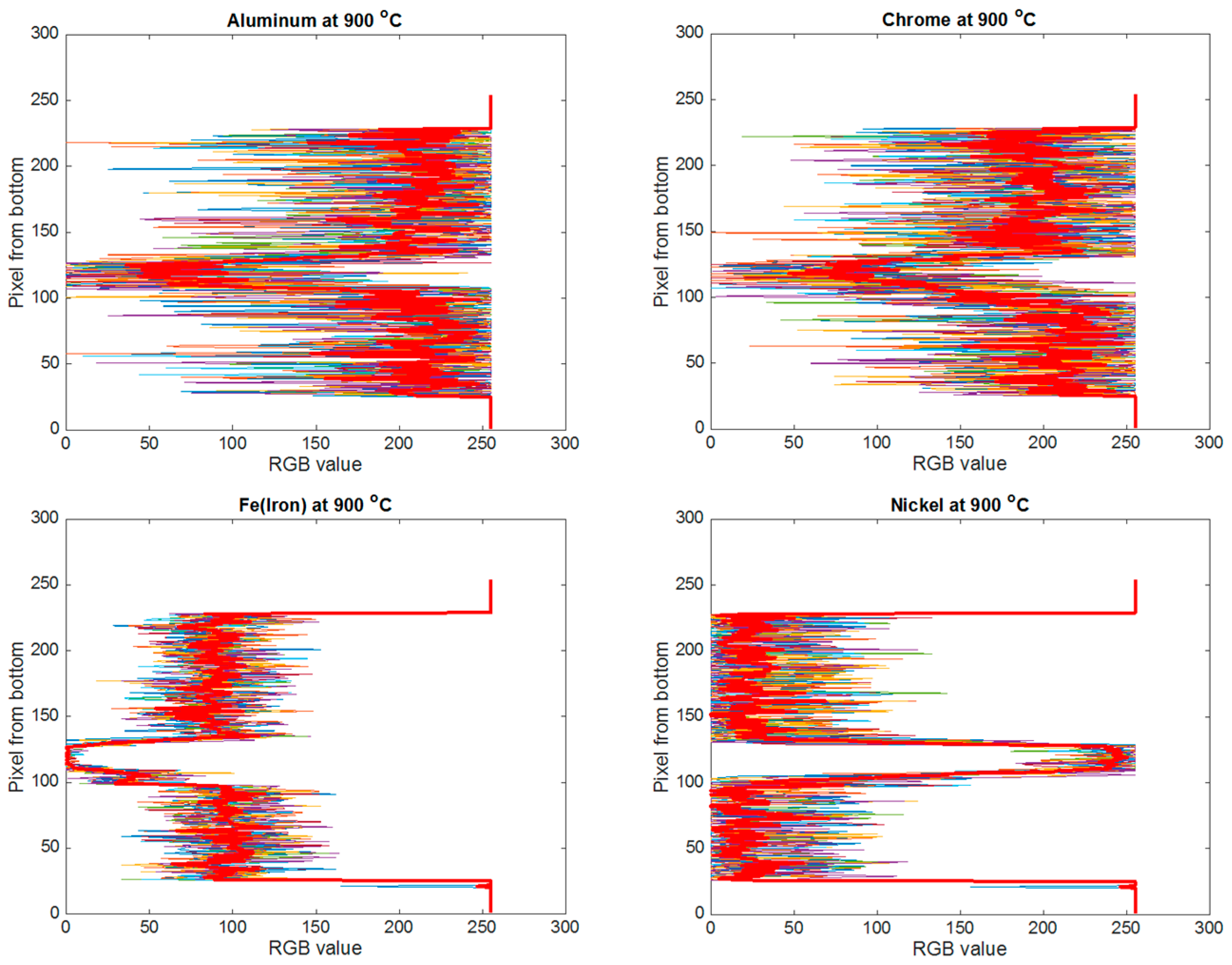
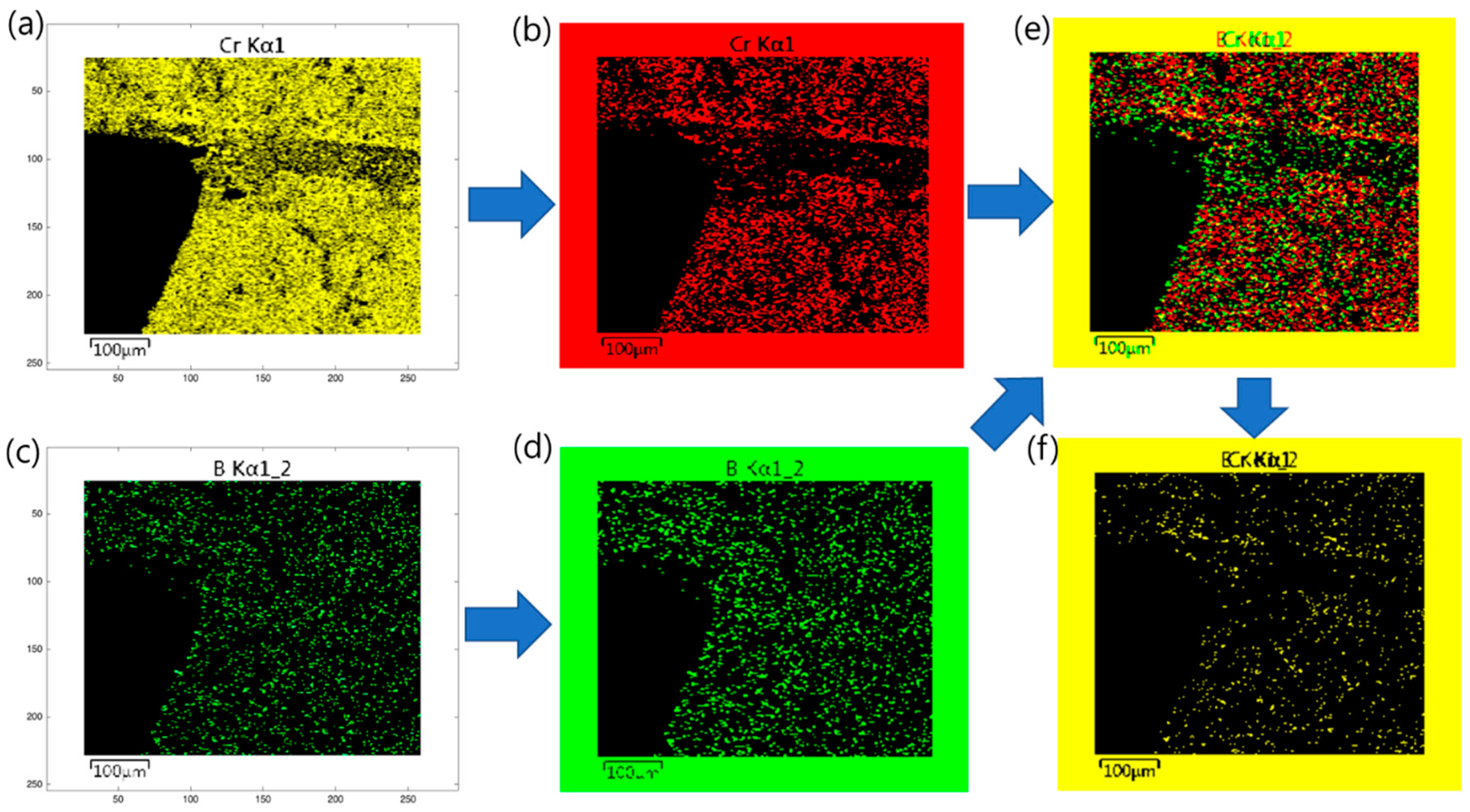
3. Image Processing Analysis
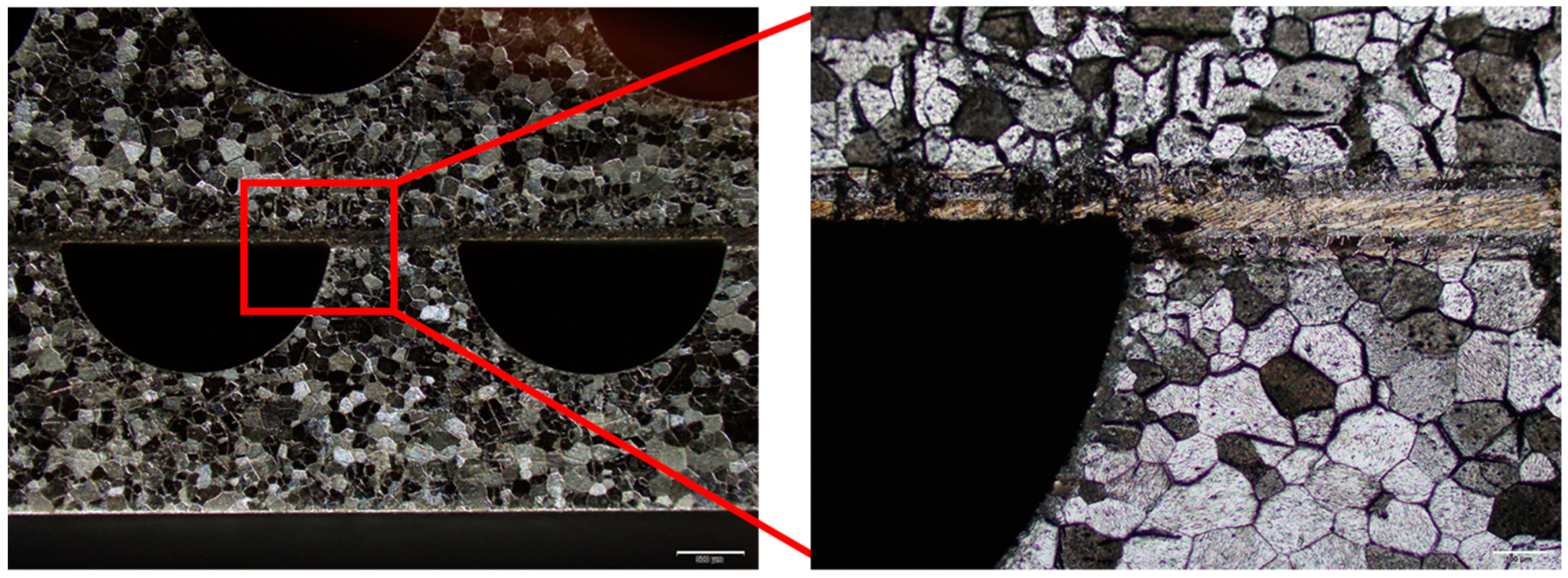
4. Results
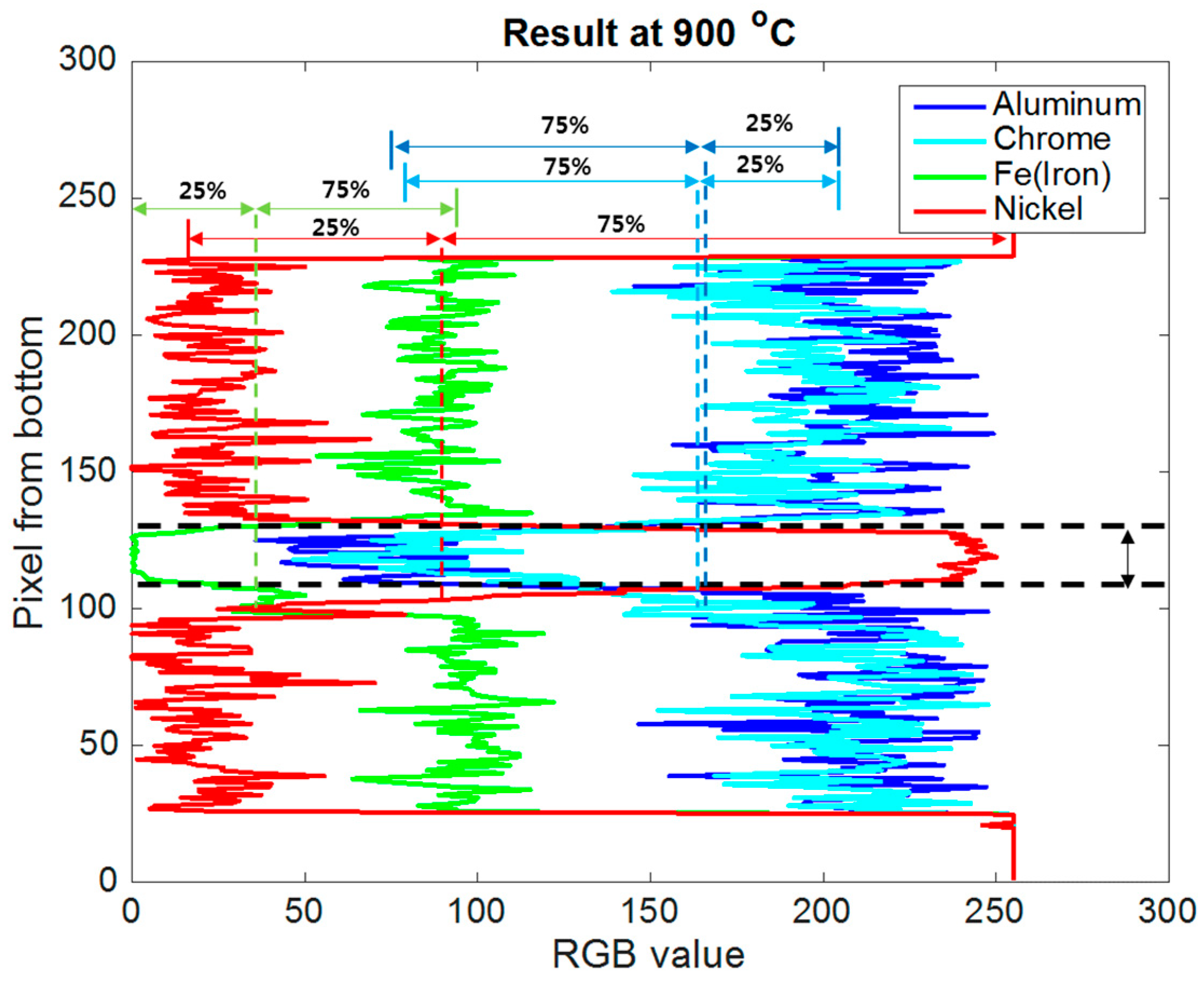
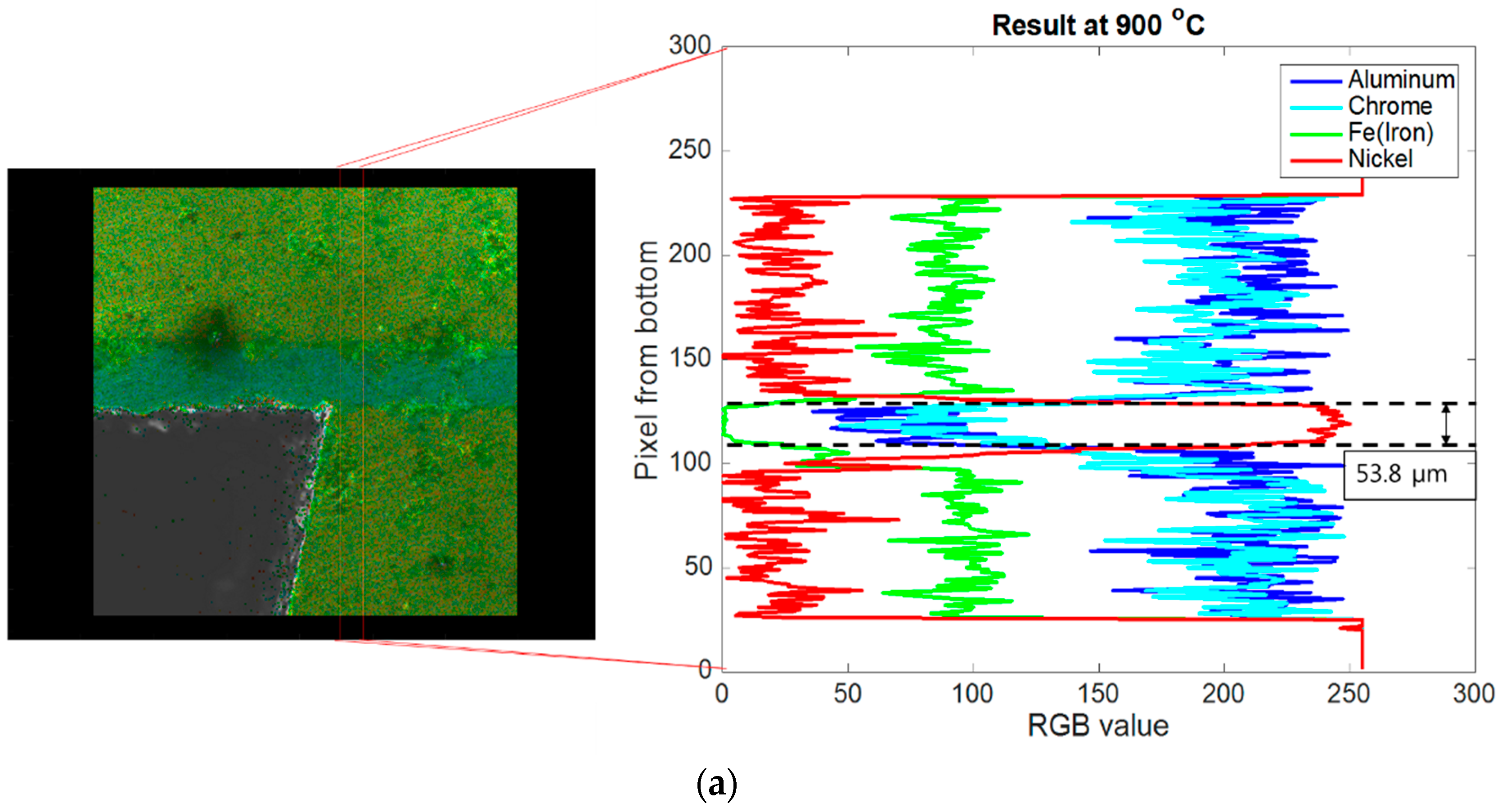
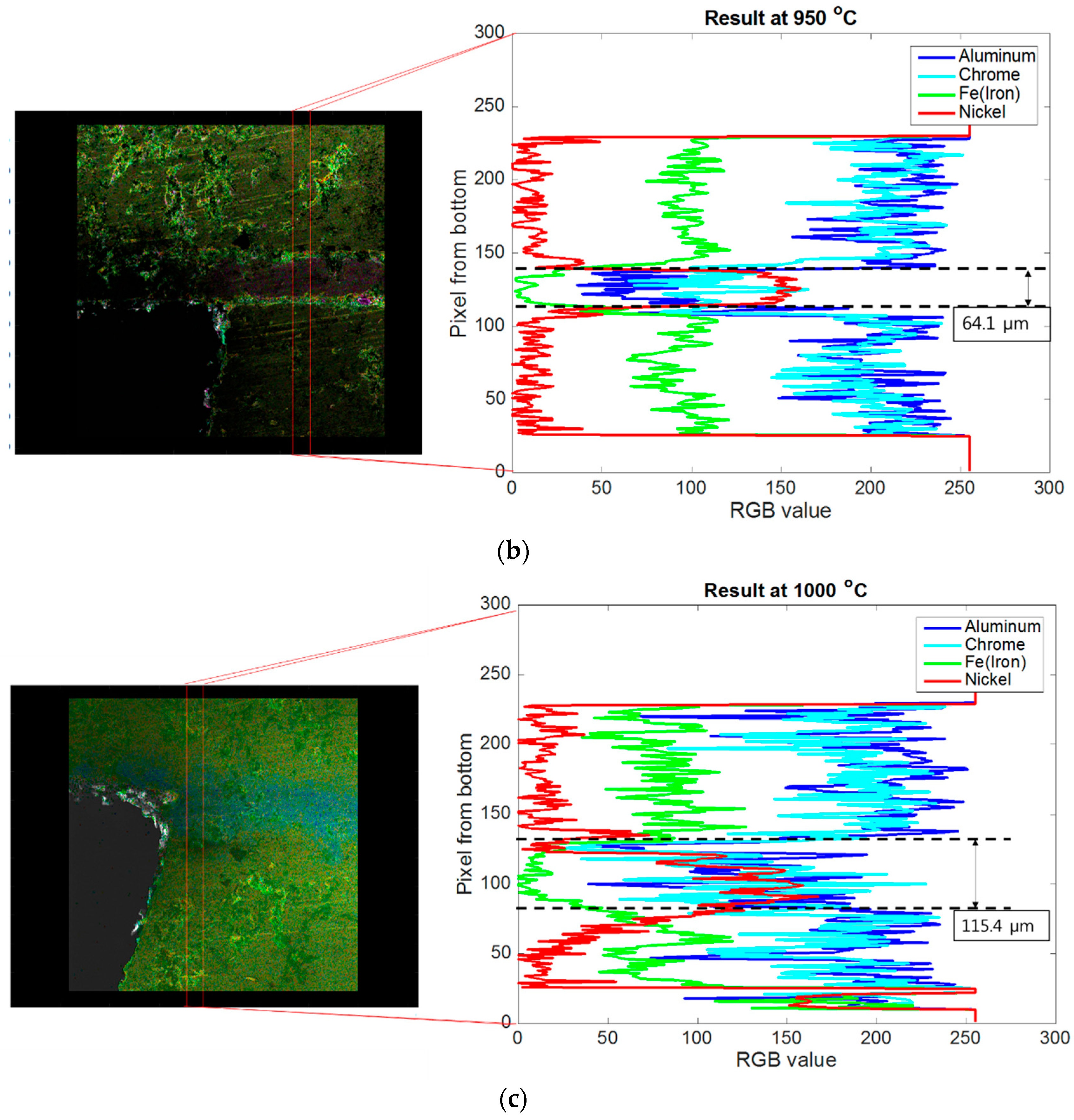
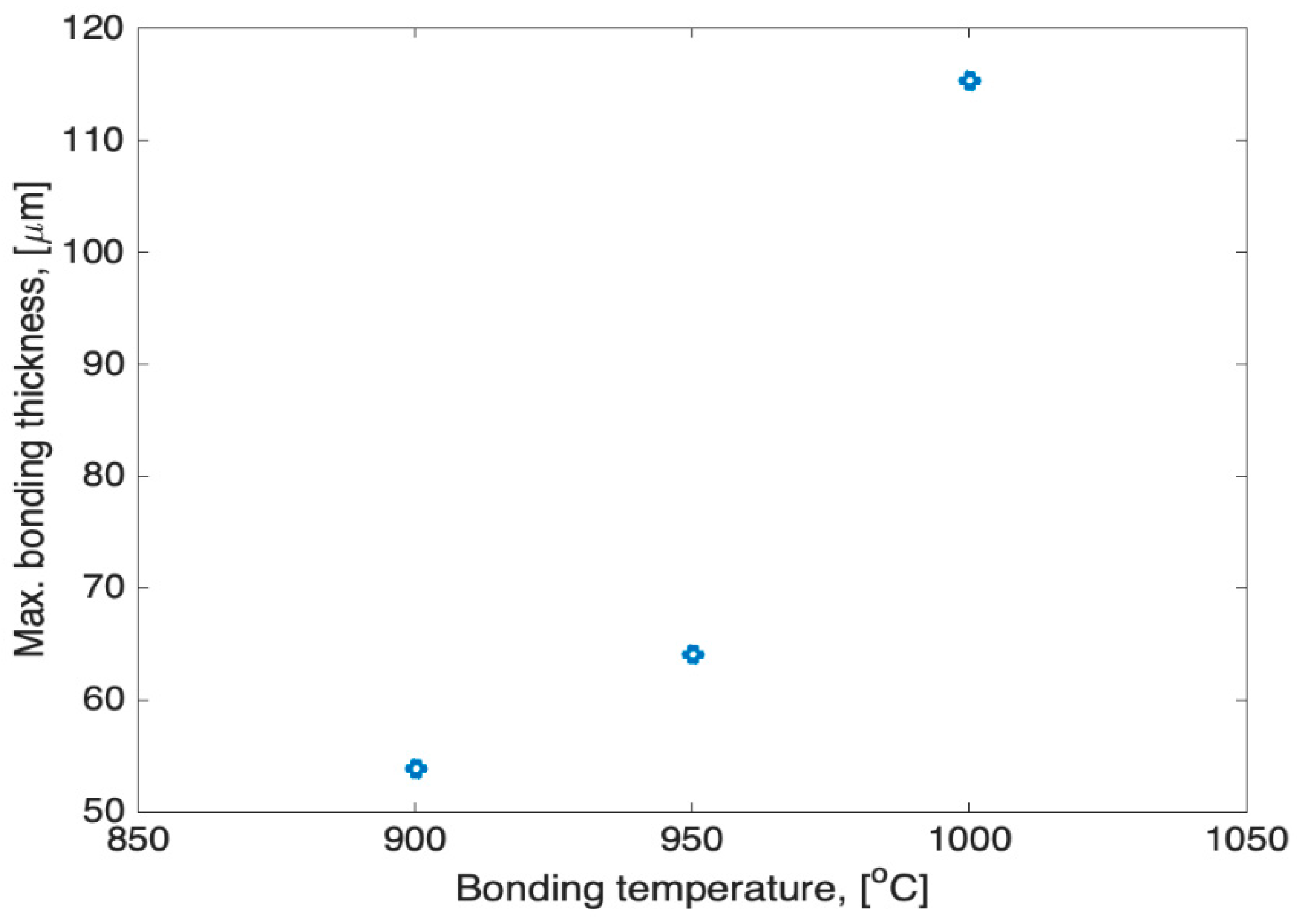
4.1. Brazing Bonding Thickness
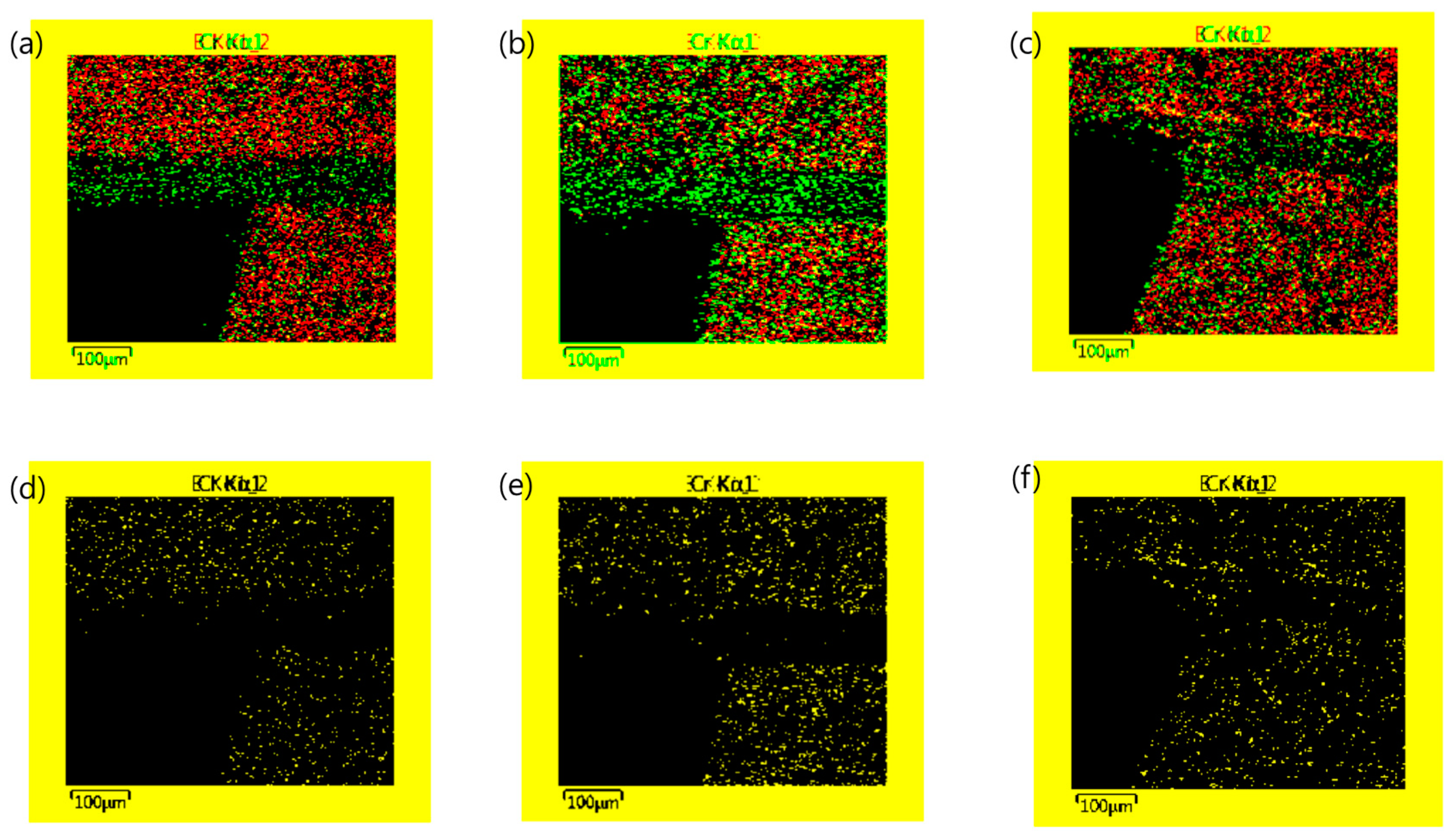
4.2. CrxBy and NixAly Phases
5. Conclusions
6. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Holladay, J.D. (Eds.) Microreactor Technology and Process. Intensification; American Chemical Society: Washington, DC, USA, 2005. [Google Scholar]
- Pfeifer, P.; Schubert, K.; Liauw, M.; Emig, G.; Liauw, M. Electrically Heated Microreactors for Methanol Steam Reforming. Chem. Eng. Res. Des. 2003, 81, 711–720. [Google Scholar] [CrossRef]
- Kolb, G.; Zapf, R.; Hessel, V.; Löwe, H. Propane steam reforming in micro-channels—Results from catalyst screening and optimisation. Appl. Catal. A Gen. 2004, 277, 155–166. [Google Scholar] [CrossRef]
- Men, Y.; Gnaser, H.; Zapf, R.; Hessel, V.; Ziegler, C.; Kolb, G. Steam reforming of methanol over Cu/CeO2/γ-Al2O3 catalysts in a microchannel reactor. Appl. Catal. A Gen. 2004, 277, 83–90. [Google Scholar] [CrossRef]
- Ming, Q. Steam reforming of hydrocarbon fuels. Catal. Today 2002, 77, 51–64. [Google Scholar] [CrossRef]
- Horny, C.; Kiwi-Minsker, L.; Renken, A. Micro-structured string-reactor for autothermal production of hydrogen. Chem. Eng. J. 2004, 101, 3–9. [Google Scholar] [CrossRef]
- Aartun, I.; Venvik, H.J.; Holmén, A.; Pfeifer, P.; Görke, O.; Schubert, K. Temperature profiles and residence time effects during catalytic partial oxidation and oxidative steam reforming of propane in metallic microchannel reactors. Catal. Today 2005, 110, 98–107. [Google Scholar] [CrossRef]
- Fichtner, M.; Mayer, J.; Wolf, D.; Schubert, K. Microstructured Rhodium Catalysts for the Partial Oxidation of Methane to Syngas under Pressure. Ind. Eng. Chem. Res. 2001, 40, 3475–3483. [Google Scholar] [CrossRef]
- Aartun, I.; Gjervan, T.; Venvik, H.; Görke, O.; Pfeifer, P.; Fathi, M.; Holmén, A.; Schubert, K. Catalytic conversion of propane to hydrogen in microstructured reactors. Chem. Eng. J. 2004, 101, 93–99. [Google Scholar] [CrossRef]
- Tonkovich, A.Y.; Zilka, J.L.; LaMont, M.J.; Wang, Y.; Wegeng, R.S. Microchannel Reactors for Fuel Processing Applications. I. Water Gas Shift Reactor. Chem. Eng. Sci. 1999, 54, 2947–2951. [Google Scholar] [CrossRef]
- De Miguel, N.; Manzanedo, J.; Thormann, J.; Pfeifer, P.; Arias, P.L. Ni Catalyst Coating on Fecralloy®Microchanneled Foils and Testing for Methane Steam Reforming. Chem. Eng. Technol. 2010, 33, 155–166. [Google Scholar] [CrossRef]
- Enger, B.C.; Walmsley, J.; Bjørgum, E.; Lødeng, R.; Pfeifer, P.; Schubert, K.; Holmén, A.; Venvik, H.J. Performance and SEM characterization of Rh impregnated microchannel reactors in the catalytic partial oxidation of methane and propane. Chem. Eng. J. 2008, 144, 489–501. [Google Scholar] [CrossRef]
- Koo, K.Y.; Joo, H.; Jung, U.H.; Choi, E.J.; You, S.M.; Yoon, W.L. Novel surface pretreatment for metal structured catalyst. Catal. Today 2011, 164, 52–57. [Google Scholar] [CrossRef]
- Schwartz, M.M. Brazing, 2nd ed.; ASM International: Novelty, OH, USA, 2003. [Google Scholar]
- Chakraborty, G.; Chaurasia, P.K.; Murugesan, S.; Albert, S.K.; Murugan, S.; Somasundaram, M. Effect of brazing temperature on the microstructure of martensitic–austenitic steel joints. Mater. Sci. Technol. 2017, 33, 1372–1378. [Google Scholar] [CrossRef]
- Chen, H.; Feng, K.; Wei, S.; Xiong, J.; Guo, Z.; Wang, H. Microstructure and properties of WC–Co/3Cr13 joints brazed using Ni electroplated interlayer. Int. J. Refract. Met. Hard Mater. 2012, 33, 70–74. [Google Scholar] [CrossRef]
- Leone, E.A.; Rabinkin, A.; Sarna, B. Microstructure of Thin-Gauge Austenitic and Ferritic Stainless Steel Joints Brazed using Metglas® Amorphous Foil. Weld. World 2006, 50, 3–15. [Google Scholar] [CrossRef]
- Park, D.Y.; Lee, S.K.; Kim, J.K.; Lee, S.N.; Park, S.J.; Oh, Y.J. Image processing-based analysis of interfacial phases in brazed stainless steel with Ni-based filler metal. Mater. Charact. 2017, 130, 278–284. [Google Scholar] [CrossRef]
- Park, D.Y.; Lee, S.K.; Oh, Y.J. Taguchi Analysis of Relation Between Tensile Strength and Interfacial Phases Quantified via Image Processing. Met. Mater. Trans. A 2018, 49, 4684–4699. [Google Scholar] [CrossRef]
- Kar, A.; Mandal, S.; Venkateswarlu, K.; Ray, A.K. Characterization of interface of Al2O3–304 stainless steel braze joint. Mater. Charact. 2007, 58, 555–562. [Google Scholar] [CrossRef]
- Kar, A.; Ray, A.K. Characterization of Al2O3–304 stainless steel braze joint interface. Mater. Lett. 2007, 61, 2982–2985. [Google Scholar] [CrossRef]
- Jadoon, A.; Ralph, B.; Hornsby, P. Metal to ceramic joining via a metallic interlayer bonding technique. J. Mater. Process. Technol. 2004, 152, 257–265. [Google Scholar] [CrossRef]
- Miab, R.J.; Hadian, A. Effect of brazing time on microstructure and mechanical properties of cubic boron nitride/steel joints. Ceram. Int. 2014, 40, 8519–8524. [Google Scholar] [CrossRef]
- Lemus-Ruiz, J.; Verduzco, J.; González-Sánchez, J.; López, V. Characterization, shear strength and corrosion resistance of self joining AISI 304 using a Ni Fe–Cr–Si metallic glass foil. J. Mater. Process. Technol. 2015, 223, 16–21. [Google Scholar] [CrossRef]
- Yuan, X.; Kim, M.B.; Cho, Y.H.; Kang, C.Y. Microstructures, Mechanical and Chemical Properties of Tlp-Bonded Joints in a Duplex Stainless Steel with Amorphous Ni-Based Insert Alloys. Metall. Mater. Trans. A 2012, 43, 1989–2001. [Google Scholar] [CrossRef]


| Chem. comp. [wt. %] | Fe | Cr | Al | C | Mn | Si | Y | Zr | B | Ni |
|---|---|---|---|---|---|---|---|---|---|---|
| Fecralloy | Bal. | 22.00 | 5.00 | 0.02 | 0.02 | 0.30 | 0.10 | 0.10 | - | - |
| MBF20 | 3.00 | 7.00 | - | 0.06 | - | 4.50 | - | - | 3.20 | Bal. |
| Bonding temp. (°C) | # of NixAly pixels | # of pixels in red sqr. | Intesity (# of NixAly pixels/# of pixels in red sqr., %) |
|---|---|---|---|
| 900 | 3790 | 6032 | 62.83% |
| 950 | 4989 | 7424 | 67.20% |
| 1000 | 7662 | 9060 | 84.57% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, J.-H.; Kim, Y.; Choi, H.W.; Choi, J.S.; Yoon, S.H. Image Processing-Based Analysis of Temperature Dependent Interlayer in Brazed Fe-Cr-Al Alloy (Fecralloy®) with Ni-Based Filler Metal. Metals 2019, 9, 1139. https://doi.org/10.3390/met9111139
Shin J-H, Kim Y, Choi HW, Choi JS, Yoon SH. Image Processing-Based Analysis of Temperature Dependent Interlayer in Brazed Fe-Cr-Al Alloy (Fecralloy®) with Ni-Based Filler Metal. Metals. 2019; 9(11):1139. https://doi.org/10.3390/met9111139
Chicago/Turabian StyleShin, Jeong-Heon, Young Kim, Hyun Woo Choi, Jun Seok Choi, and Seok Ho Yoon. 2019. "Image Processing-Based Analysis of Temperature Dependent Interlayer in Brazed Fe-Cr-Al Alloy (Fecralloy®) with Ni-Based Filler Metal" Metals 9, no. 11: 1139. https://doi.org/10.3390/met9111139
APA StyleShin, J.-H., Kim, Y., Choi, H. W., Choi, J. S., & Yoon, S. H. (2019). Image Processing-Based Analysis of Temperature Dependent Interlayer in Brazed Fe-Cr-Al Alloy (Fecralloy®) with Ni-Based Filler Metal. Metals, 9(11), 1139. https://doi.org/10.3390/met9111139





