Electrochemical Impedance Analysis on Cryogenically Treated Dissimilar Metal Welding of 316L Stainless Steel and Monel 400 Alloy Using GTAW
Abstract
1. Introduction
- Gas tungsten arc welding of SS 316L and Monel 400 alloy using ENiCrFe-5 filler material;
- Post-weld heat treatment (annealing) and cryogenic treatment on weld specimens;
- Potentiodynamic and electrochemical analyses for corrosion resistance of the treated specimens;
- SEM and EDS analysis of the corroded specimens to understand the results obtained in the corrosion tests.
2. Materials and Methods
2.1. Materials
2.2. Welding and Post-Weld Treatments
2.3. Optical Microscopy, EDS, and SEM
2.4. Polarization Analysis and Electrochemical Impedance Spectroscopy Analysis
3. Results
3.1. Optical Microscopy, EDS, and SEM
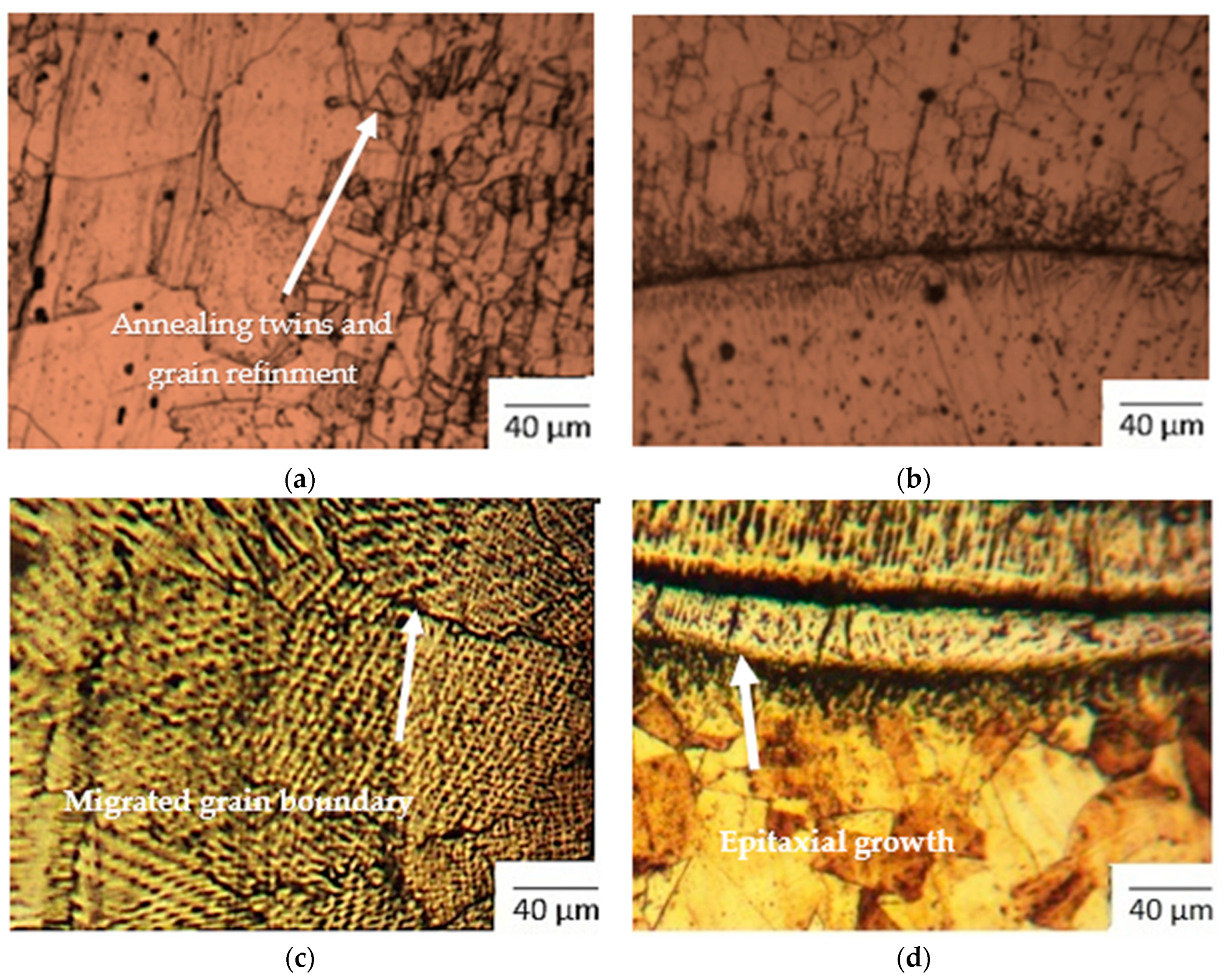
- SS 316L HAZ of the CT specimen has revealed annealing twins and grain refinement (Figure 3a). The grain size number was in the range of 11 to 13 (smaller is coarser), as per ASTM E112. The area fraction of carbide precipitates was found to be 2.52%, with an average carbide precipitate size of 1.335 µm.
- Figure 3b shows the optical image of SS 316L weld fusion zone, which has the grain number 13. Carbide precipitates for 2.68% of its area, with an average precipitate size of 2.78 µm, was observed.
- Migrated grain boundaries were observed at the weld zone in Figure 3c for the CT specimen. Similar results were presented by and Devendranath Ramkumar et al. in 2015 for SS/Inconel dissimilar metal welding [22]. Carbide precipitation was observed at 6.27%, with an average precipitate size of 3.811 µm. The grain size number was noted to be close to 8.
- Epitaxial growth in weld fusion zone was noted in Figure 3d for the CT specimen. Epitaxial growth was detected prominently at the fusion boundary of ERNiCrFe-5 weld with Monel 400. This phenomenon was also reported by Farahani et al. during welding of Inconel 617 alloy [23]. The epitaxial growth normally occurs in the weld and base materials which have a similar crystal microstructure. The grain size number of 14 was observed for the microstructure.
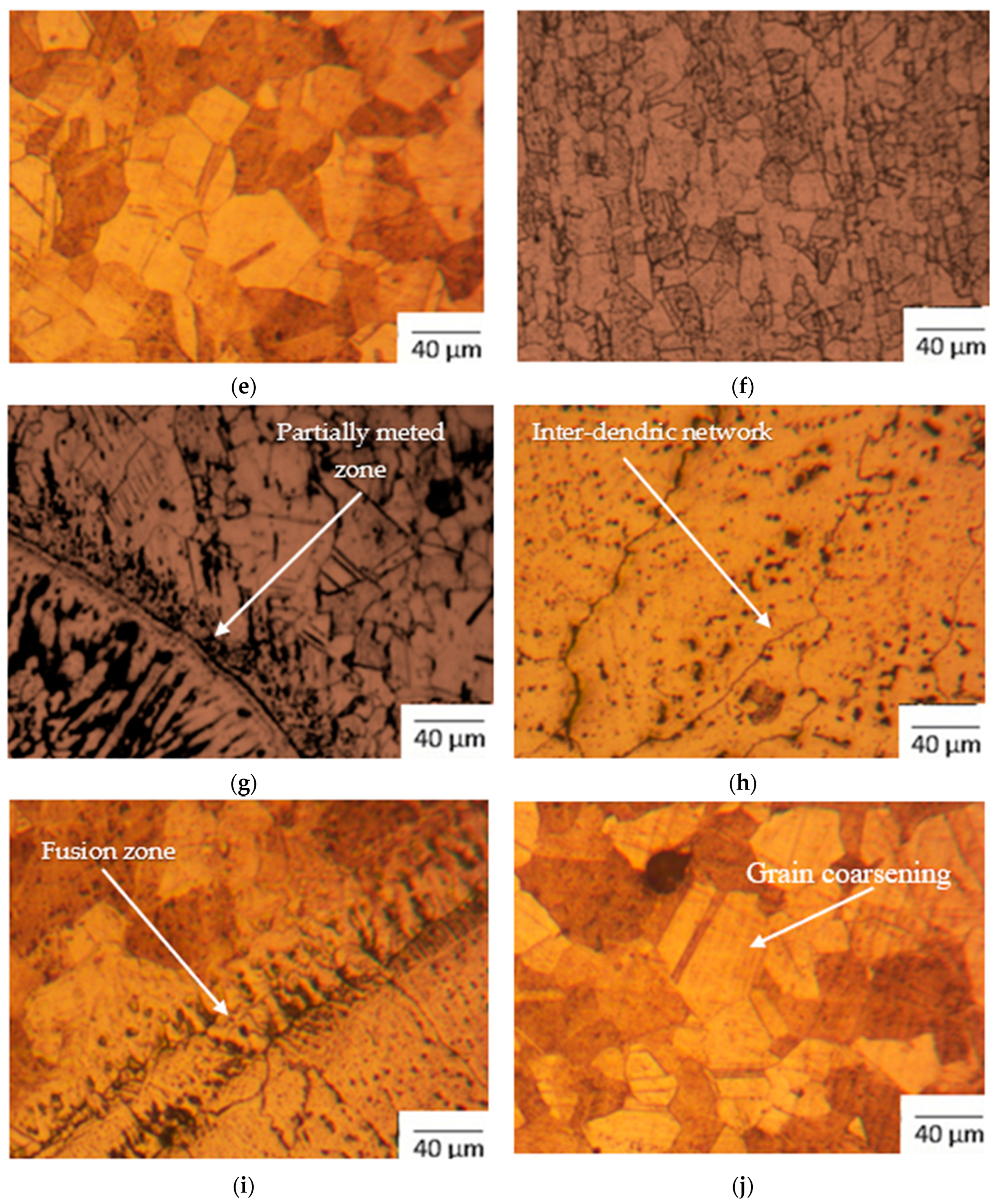
- Figure 3e of the CT specimen shows Monel 400 HAZ, which has a grain size number from 11 to 13.
- Figure 3f shows the optical image of SS 316L HAZ for the HT specimen, which has grain size number ranging from 13 to 14. The average carbide precipitate size was observed to be 1.73 µm, with 2.46% of precipitates with respect to area.
- Partially melted zones on the weld fusion are shown in Figure 3g for the SS 316L weld fusion zone of the HT specimen, which has grain size number ranging from 12 to 14. The average carbide precipitate size was observed to be 3.045 µm, with 3.97% area fraction of carbide precipitates.
- Weld region of the HT specimen has inter-dendritic network of the elements consisting of Ni, Cr, Fe, Cu, and Nb in Figure 3h. The grain size number is 7 and average carbide precipitate size was observed to be 4.66 µm, with 3.86% of carbide precipitates.
- Weld fusion of Monel was seen in Figure 3i for the HT specimen, which has a size grain number of 13.
- Grain coarsening is noticed in Monel 400 HAZ for the HT specimen, as seen in Figure 3j, with grain size number in the range of 11 to 14.
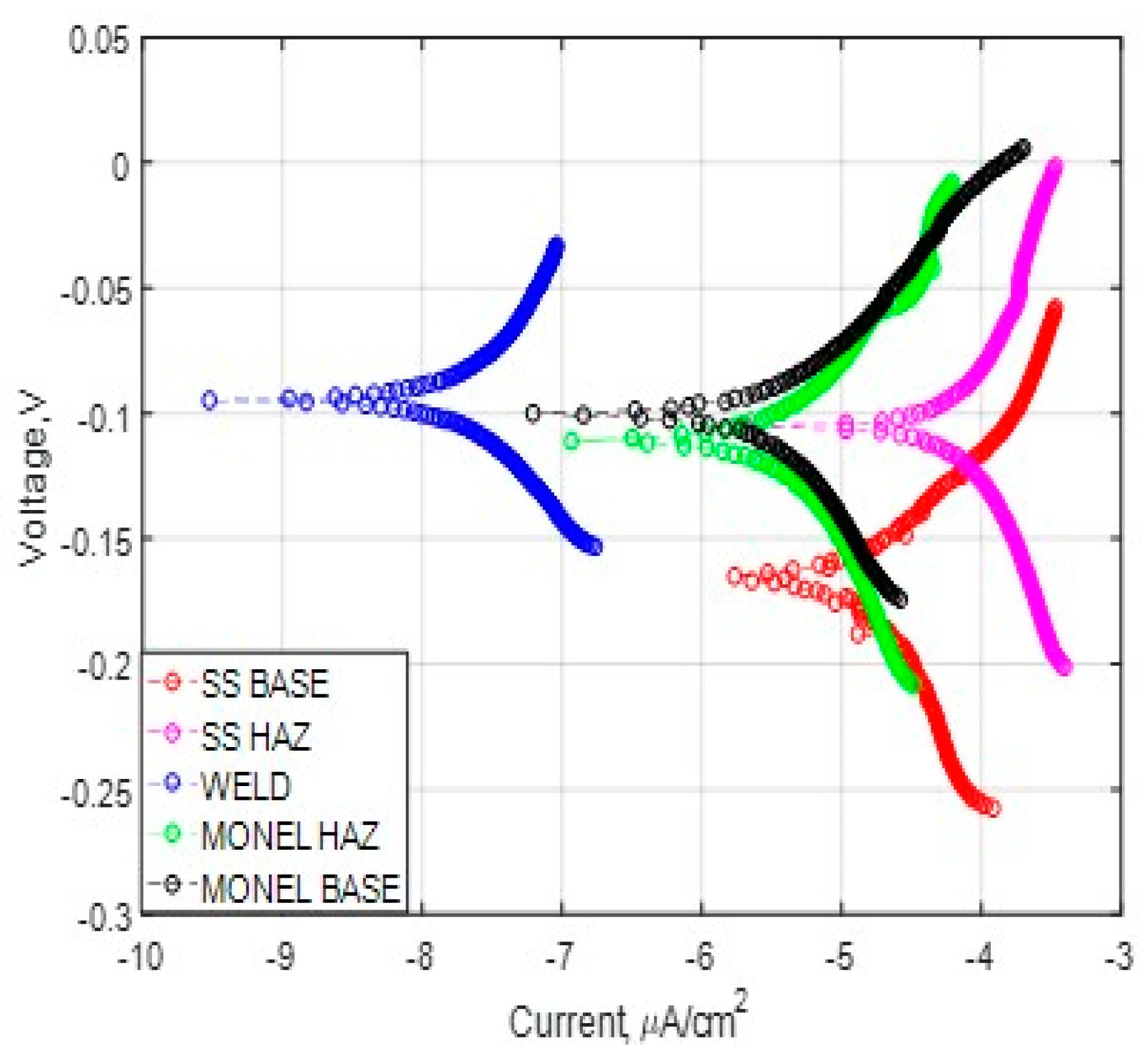
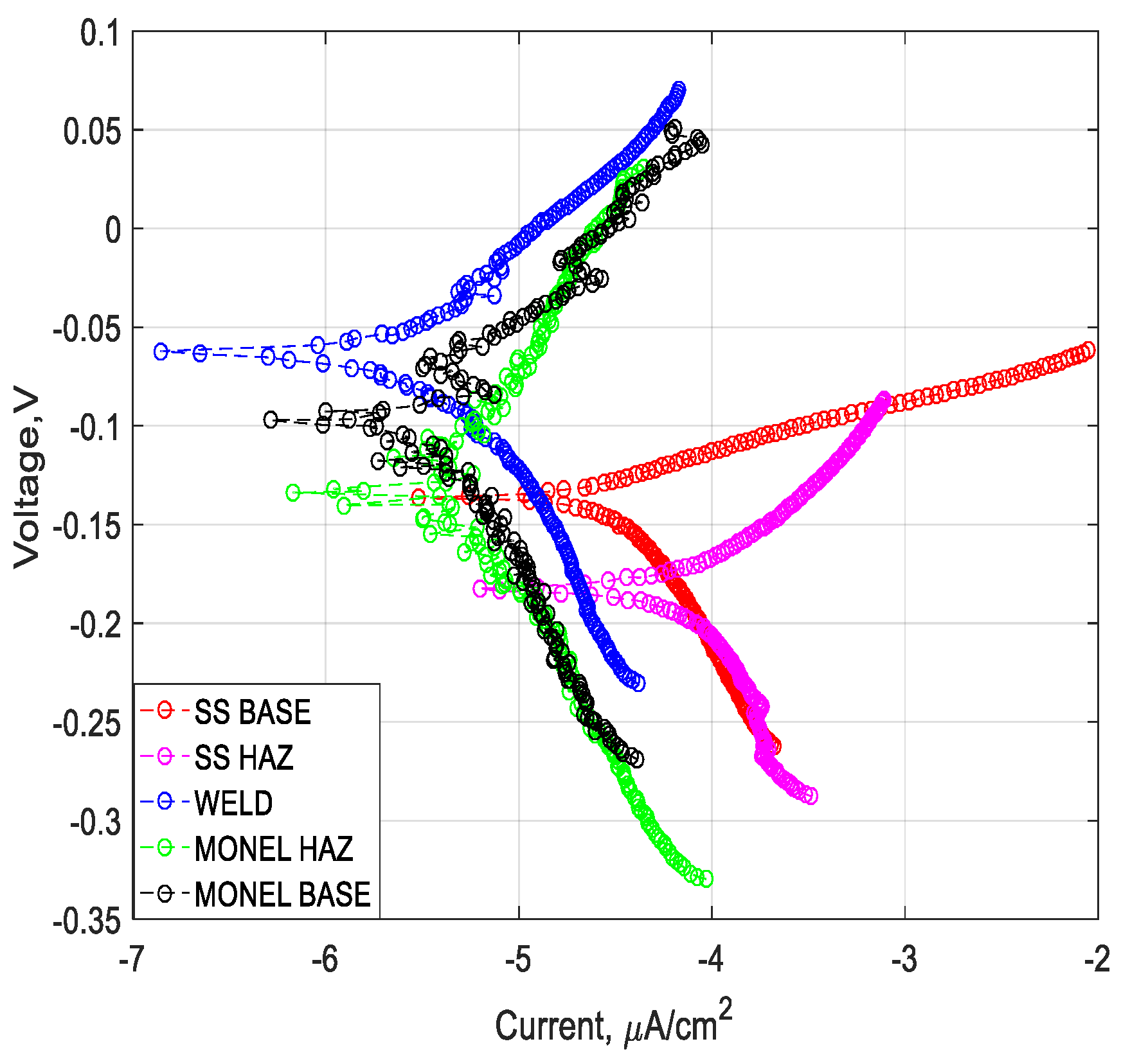
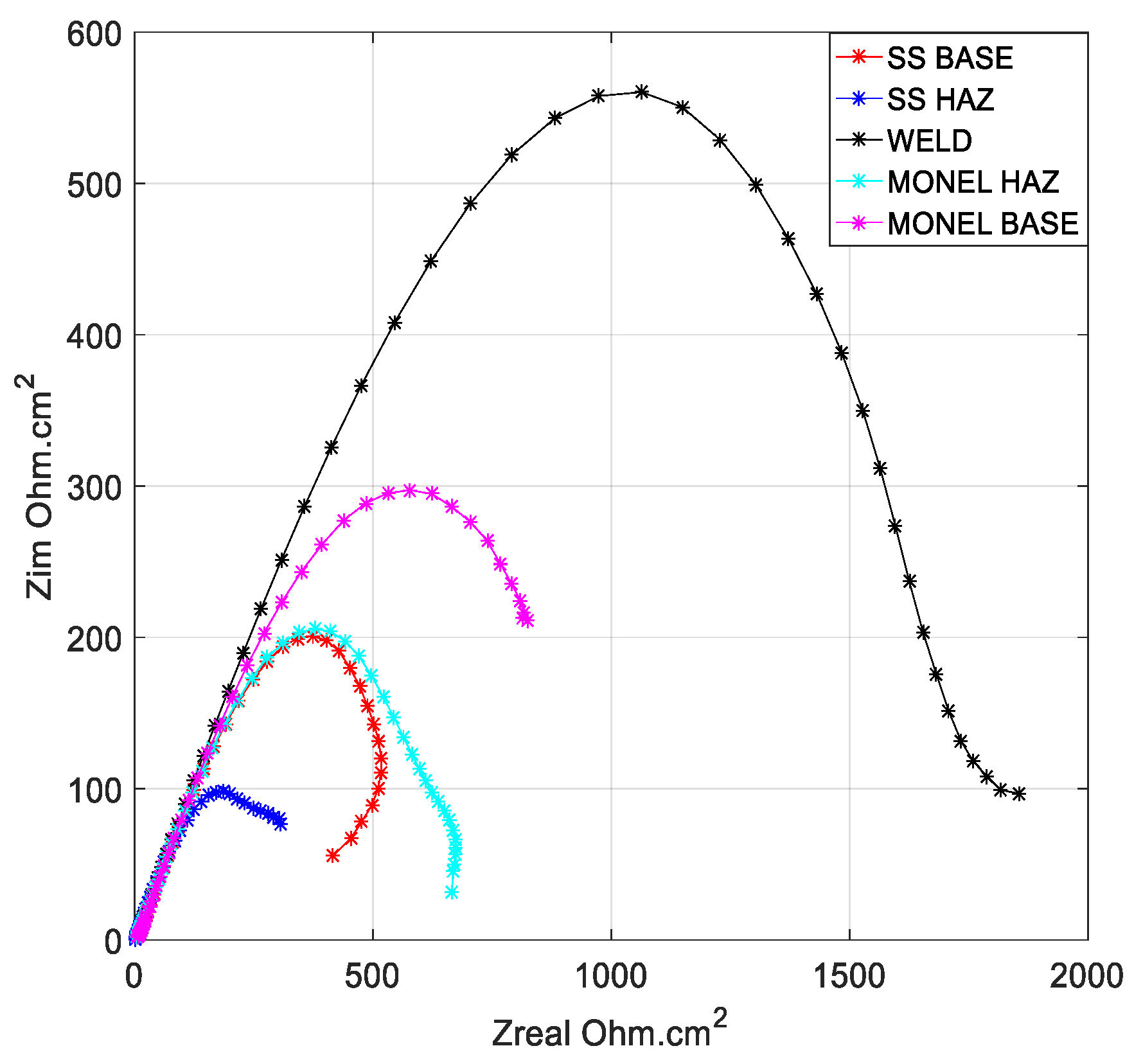
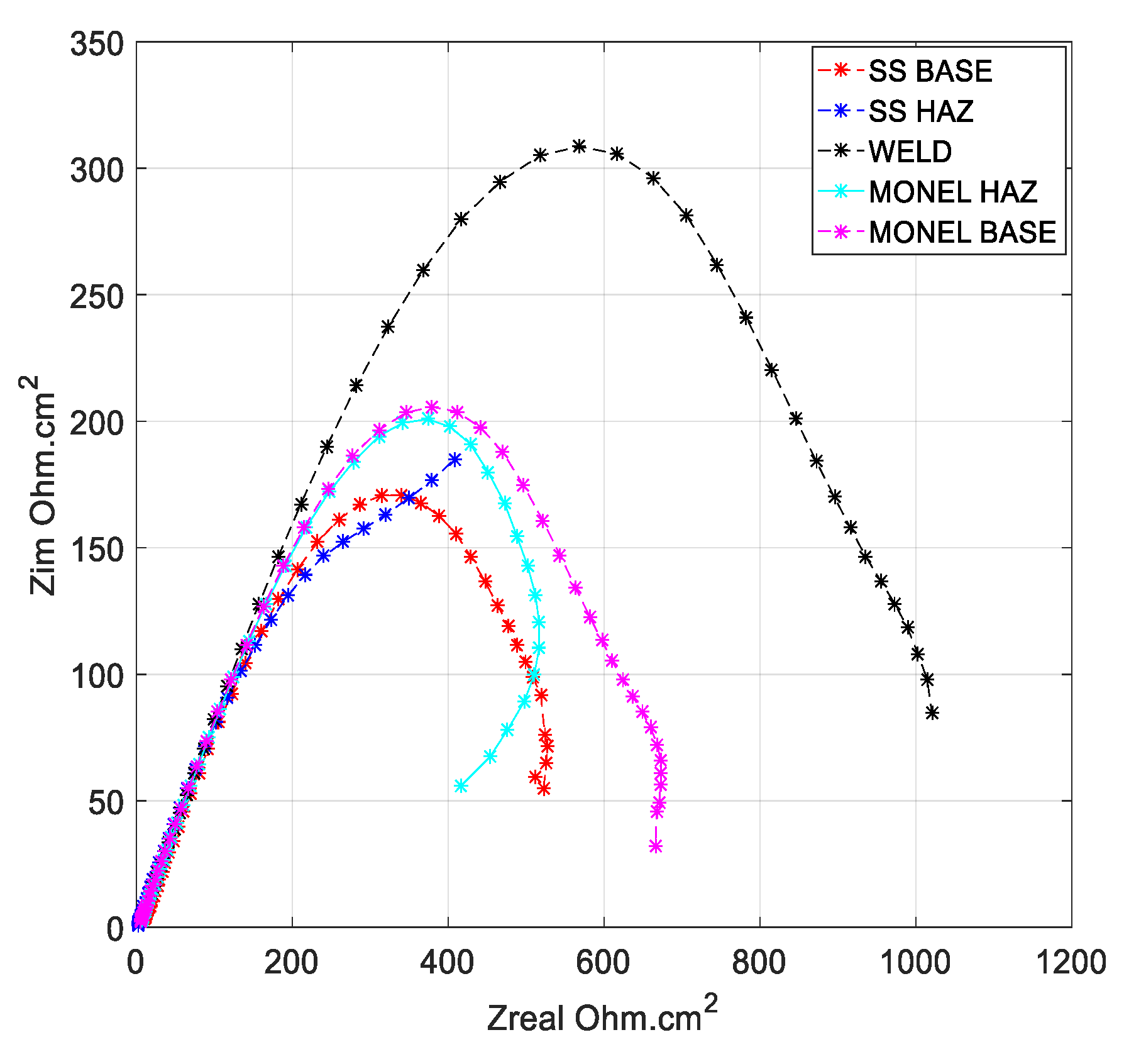
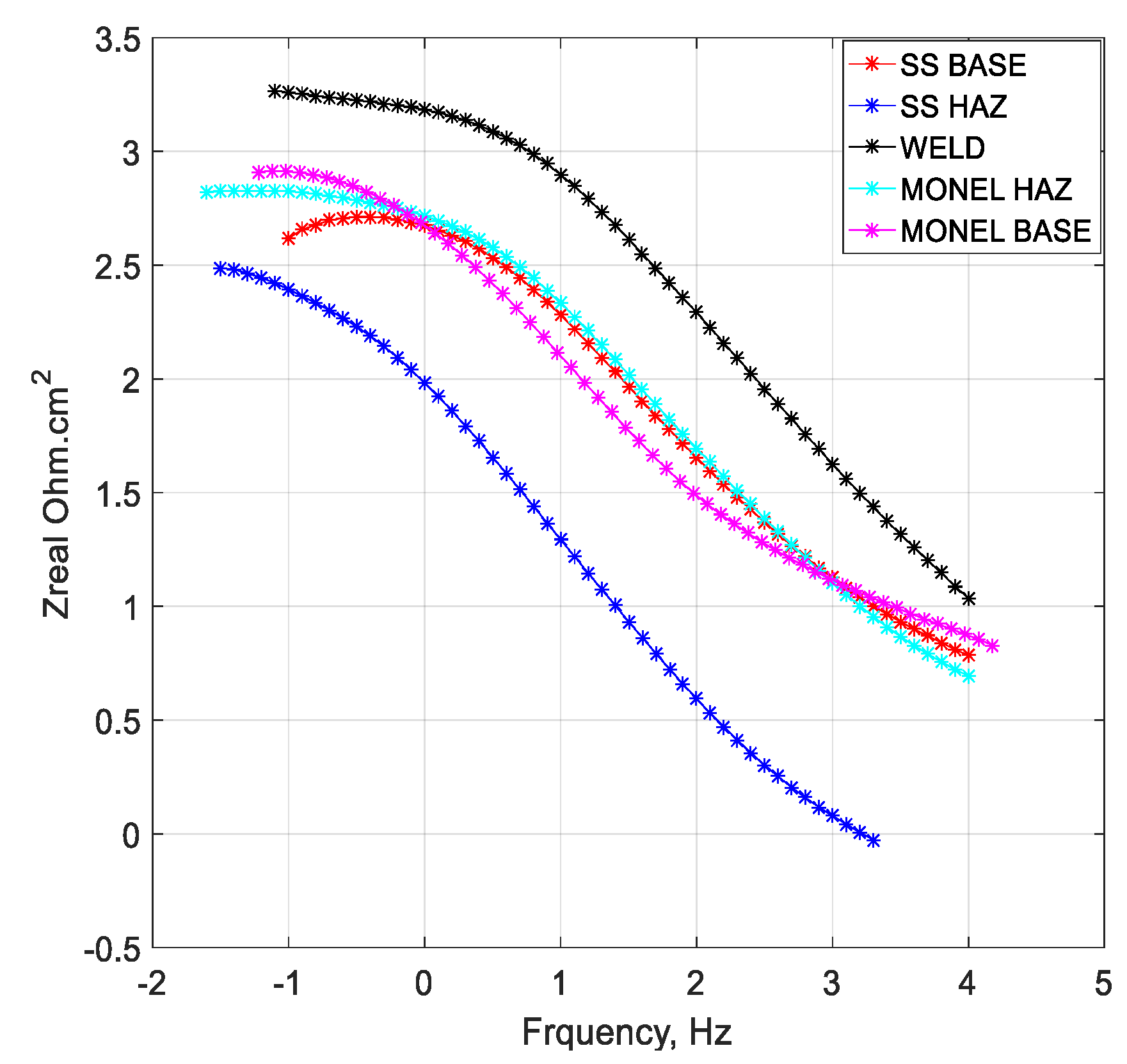
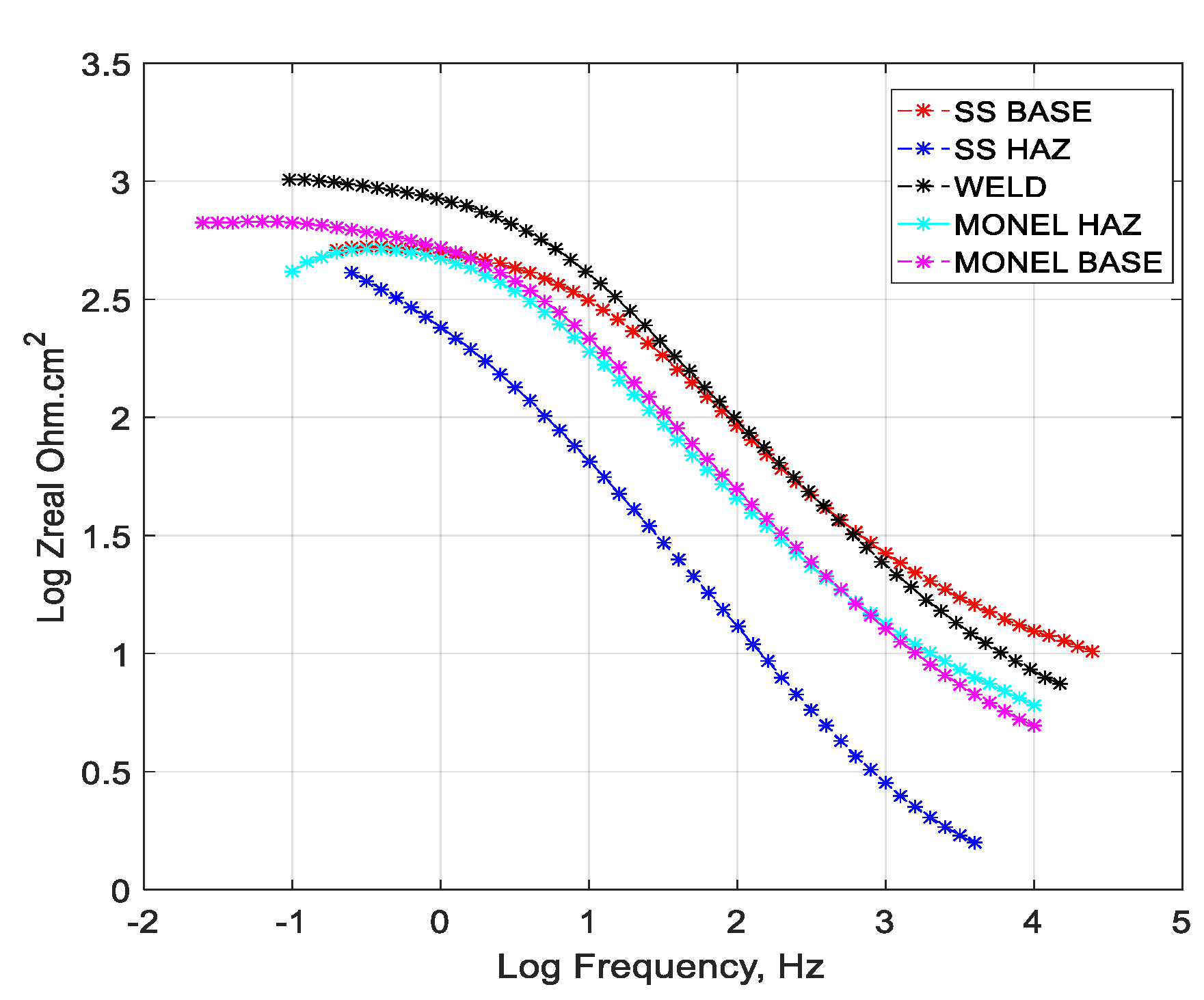
3.2. Polarization Analysis and Electrochemical Impedance Spectroscopy Analysis
3.2.1. Weld Zone
3.2.2. SS 316L HAZ
3.2.3. Monel 400 HAZ
3.2.4. SS 316L Base
3.2.5. Monel 400 Base
4. Discussion and Conclusions
- Polarization analysis and Electrochemical Impedance Spectroscopy Analysis are powerful techniques to investigate the corrosion resistance of dissimilar metal welds. In the present study, the results have similar trends.
- Chloride attack at localized location as corrosion is a serious problem of stainless steels when they are exposed to chloride solutions in 316L/Monel 400 dissimilar metal welds.
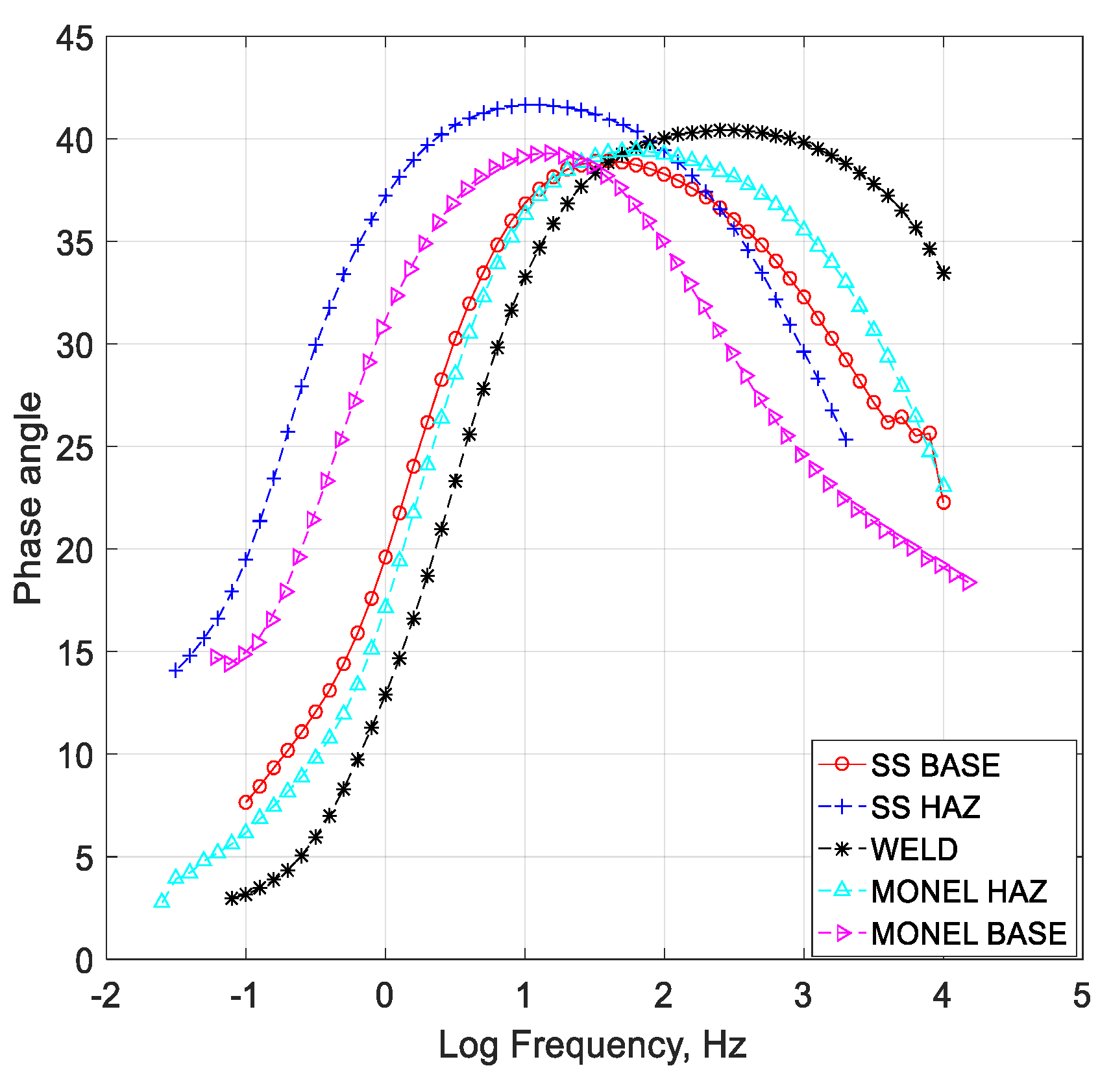
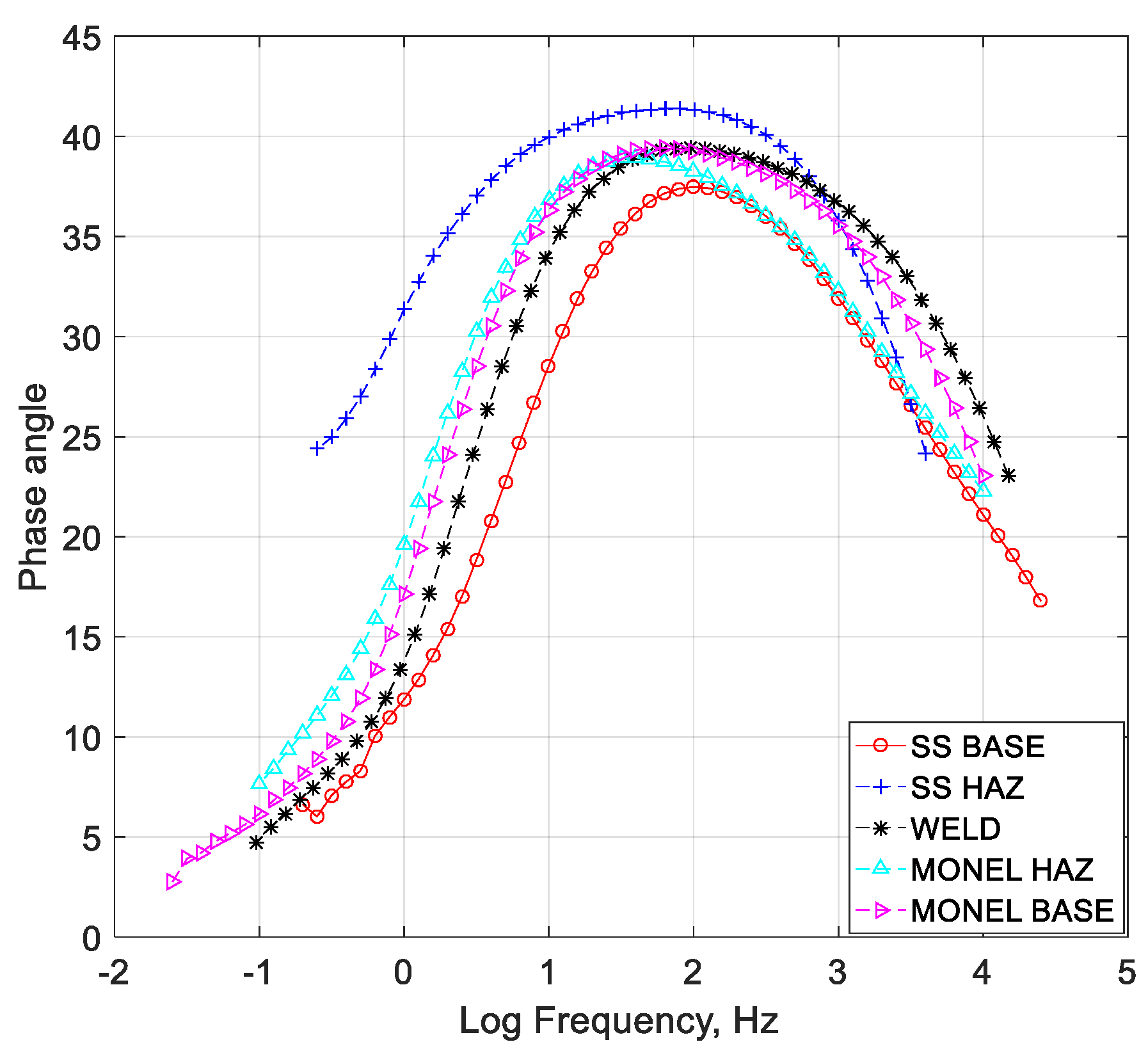
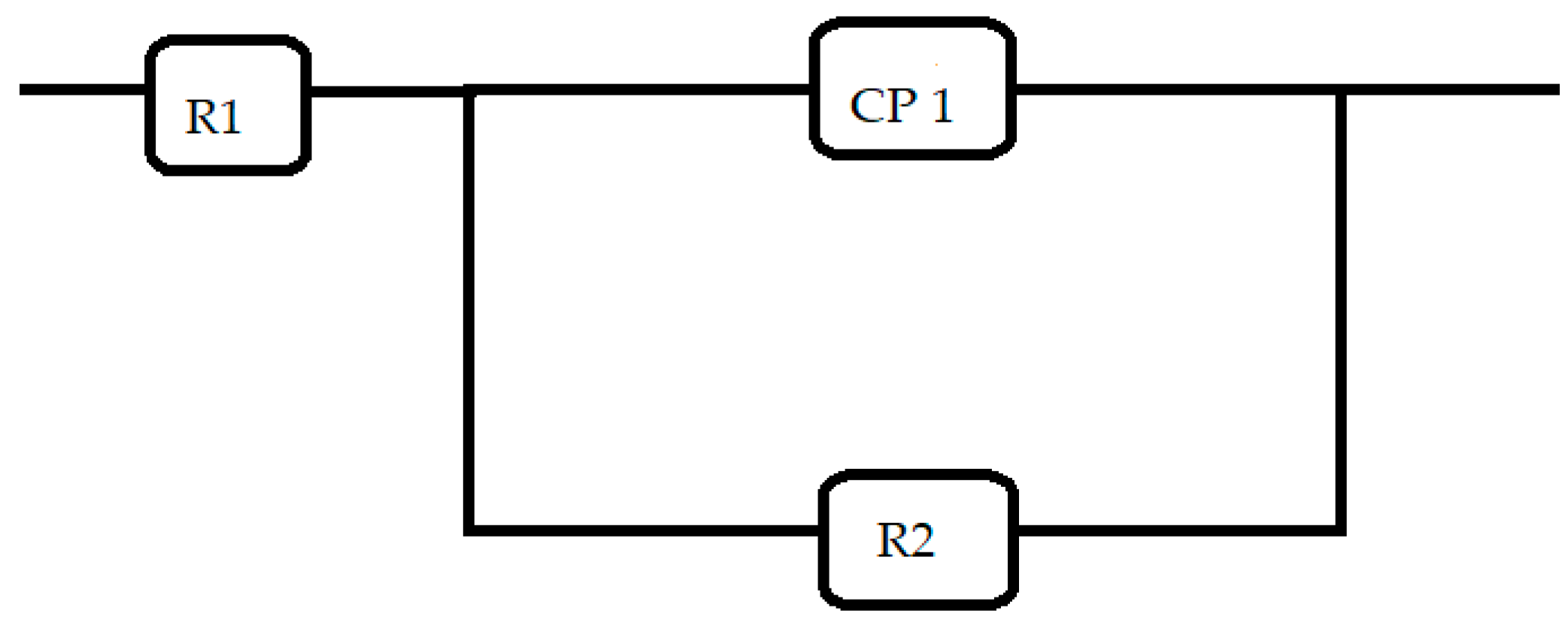
- Cryogenic treatment resulted in better corrosion resistance when compared to the conventional annealing. The Jcorr values decreased up to 41 times for the cryo-treated specimens, and the corrosion rate of the weld zone was as low as 0.003 × 10−6 mm/yr, resulting in a 97% reduction in corrosion rate. The electrical equivalent circuit selected matches with the phase angle plot.
- Among the zones identified for dissimilar welds, corrosion rate is lowest for weld zone and highest for SS 316L HAZ. The corrosion rate for Monel base, Monel HAZ, and SS 316L base are in between them and are almost in the same range as observed in both polarization and electro chemical impedance analyses.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BM | Base metal zone |
| CT | Cryo-treated specimen |
| DCEN | Direct Current Electrode Negative |
| DCT | Deep cryogenic treatment |
| DMW | Dissimilar metal welding |
| EDS | Energy dispersion X-ray Spectroscopy |
| EIS | Electrochemical impedance spectroscopy |
| GTAW | Gas Tungsten Arc Welding |
| HAZ | Heat Affected Zone |
| HT | Heat-treated specimen |
| LPM | Liters per minute |
| SMAW | Shielded metal arc welding |
| SS | Stainless steel |
| WM | Weld metal zone |
References
- Mhetre, R.N.; Jadhav, S.G. Finite element analysis of welded joints. Int. J. Instrum. Control Autom. 2012, 1, 116–120. [Google Scholar]
- Narayanan, S.; Devendranath Ramkumar, K.; Arivazhagan, N. High temperature corrosion behavior of PCGTA weldments of Monel 400 and AISI 304 exposed in the molten salt environment at 600 °C. Int. J. Chem. Technol. Res. 2014, 6, 1775–1780. [Google Scholar]
- Mohandas, T.; Satyanarayana, V.V.; Madhusudhan Reddy, G. Dissimilar metal friction welding of austenitic–ferritic stainless steels. J. Mater. Process. Technol. 2005, 160, 128–137. [Google Scholar]
- Ramkumar, K.D.; Arivazhagan, N.; Narayanan, S.; Mishra, D. Hot corrosion behavior of monel 400 and AISI 304 dissimilar weldments exposed in the molten salt environment containing Na2SO4 + 60% V2O5 at 600 °C. Mater. Res. 2014, 17, 1273–1284. [Google Scholar] [CrossRef]
- Ukadgaonker, V.G.; Bhat, S.; Jha, M.; Deasai, P.B. Fatigue crack growth towards the weld interface of alloy and maraging steels. Int. J. Fatigue 2008, 30, 689–705. [Google Scholar] [CrossRef]
- Sadek, A.A.; Abass, M.; Zaghloul, B.; Elrefaey, A.; Ushio, M. Investigation of dissimilar Joints between Low Carbon steel and Monel 400. Trans. JWRI 2000, 29, 21–28. [Google Scholar]
- Kim, Y.H.; Frankel, G.S.; Lippold, J.C.; Guaytima, G. Development of a chromium-free consumable for austenitic stainless steels. Part 1: Monel (Alloy 400) filler metal. Corrosion 2006, 62, 44–53. [Google Scholar] [CrossRef]
- Devendranath Ramkumar, K.; Arivazhagan, N.; Narayanan, S. Effect of filler materials on the performance of Gas Tungsten arc welded AISI 304 and Monel 400. Mater. Des. 2012, 40, 70–79. [Google Scholar] [CrossRef]
- Ram, G.D.J.; Reddy, A.V.; Rao, K.P.; Reddy, G.M.; Rao, A.S. Effect of magnetic arc oscillation on microstructure and properties of Inconel 718 GTA welds. Trans. Indian Inst. Met. 2006, 59, 85–97. [Google Scholar]
- Al-Quran, F.M.F.; Al-Itawi, H.I. Effects of the Heat Treatment on Corrosion Resistance and Microhardness of Alloy Steel. Eur. J. Sci. Res. 2010, 39, 251–256. [Google Scholar]
- PrietoW, G.; Tuckart, R. Influence of Cryogenic Treatments on the Wear Behavior of AISI 420 Martensitic Stainless Steel. J. Mater. Eng. Perform. 2017, 26, 5262–5271. [Google Scholar] [CrossRef]
- Uygur, I.; Gereng, H.; Arslan, Y.; Kurtay, M. The Effects of Cryogenic Treatment on the Corrosion of AISI D3 Steel. Mat. Res. 2015, 18, 569–574. [Google Scholar] [CrossRef]
- Xu, L.-N.; Zhu, J.-Y.; Lu, M.-X. Electrochemical impedance spectroscopy study on the corrosion of the weld zone of 3Cr steel welded joints in CO2 environments. Int. J. Miner. Metall. Mater. 2015, 22, 500–508. [Google Scholar] [CrossRef]
- Sendooran, S.; Raja, P. Metallurgical investigation on Cryogenic treated HSS tool. Int. J. Eng. Sci. Technol. 2011, 3, 5. [Google Scholar]
- Prieto, G.; PerezIpiña, J.E.; Tuckart, W.R. Cryogenic treatments on AISI 420 stainless steel: Microstructure and mechanical properties. Mater. Sci. Eng. A 2014, 605, 236–243. [Google Scholar] [CrossRef]
- Kianersi, D.; Mostafaei, A.; Amadeh, A.A. Resistance spot welding joints of AISI 316L austenitic stainless-steel sheets: Phase transformations, mechanical properties and microstructure characterizations. Mater. Des. 2015, 61, 251–263. [Google Scholar] [CrossRef]
- Wong, L.L.; Martin, S.I.; Rebak, R.B. Methods To Calculate Corrosion Rates For Alloy 22 From Polarization Resistance Experiments. In Proceedings of the 2006 ASME Pressure Vessels And Piping Division Conference, Vancouver, BC, Canada, 23–27 July 2006. [Google Scholar]
- ASTM G5-14e1. Standard Reference Test Method for Making Potentio-Dynamic Anodic Polarization Measurements; ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- El-Taib Heakal, F.; Tantawy, N.S.; Shehata, O.S. Influence of cerium (III) ions on corrosion and hydrogen evolution of carbon steel in acid solutions. Int. J. Hydrog. Energy 2012, 37, 19219–19230. [Google Scholar] [CrossRef]
- Govindasam, R.; Ayappan, S. Study of Corrosion Inhibition Properties of Novel Semi carbazones on mild steel in acidic solutions. Chil. Chem. Soc. 2015, 60. [Google Scholar] [CrossRef]
- Duncan, J.B. Corrosion Study for the Effluent Treatment Facility for Chrome (VI) Reductant Solution Using 304 and 316L Stainless Steel; CH2M HILL Hanford Group, Inc.: Richland, WA, USA, 2007; Available online: https://digital.library.unt.edu/ark:/67531/metadc896721 (accessed on 7 October 2019).
- Ramkumar, K.D.; Krishnan, S.R.; Ramanand, R.; Logesh, S. Structure–property relationships of PCGTA welds of Inconel X750 in as-welded and post-weld heat treated conditions—A comparative study. J. Manuf. Process. 2015, 20, 1–14. [Google Scholar] [CrossRef]
- Farahani, F.; Shamanian, F.; Ashrafizadeh, A. Comparative study on direct and pulsed current gas tungsten arc welding of Alloy 617. AMAE Int. Manuf. Mater. Sci. 2012, 2, 1–6. [Google Scholar]
- Sahlaoui, H.; Makhlouf, K. Effects of ageing conditions on the precipitates evolution, chromium depletion and intergranular corrosion susceptibility of AISI 316L: Experimental and modeling results. Mater. Sci. Eng. A 2004, 372, 98–108. [Google Scholar] [CrossRef]
- Mylaudy, S.R.; Rajadurai; Naveen, S.; Afnas, M.; Arun, T.; Nirmal, K.; Surendhar, S. Methods to Avoid Material Sensitization During Welding for Developing Corrosion Resistant Exhaust System. Int. J. Recent Dev. Eng. Technol. 2015, 4. Available online: https://www.google.com/search?client=firefox-b-d&q=Methods+to+Avoid+Material+Sensitization+During+Welding+for+Developing+Corrosion+Resistant+Exhaust+System (accessed on 7 October 2019).
- López, B.; Rodriguez-Ibabe, J.M. Recrystallisation and grain growth in hot working of steels. In Microstructure Evolution in Metal Forming Processes; Woodhead Publishing Limited: Sawston, UK, 2012; pp. 67–113. [Google Scholar] [CrossRef]
- Atanda, P.O.; Fatudimu, A.; Oluwole, O. Sensitization Study of Normalized 316L Stainless Steel. J. Miner. Mater. Charact. Eng. 2010, 9, 13–23. [Google Scholar]
- Bansoda, A.V.; Patil, A.P.; Verma, J.; Shukla, S. Microstructure, Mechanical and Electrochemical Evaluation of Dissimilar low Ni SS and using Different Filler Materials. Mater. Res. 2019, 22. [Google Scholar] [CrossRef]
- Alqarni, N.D.; Wysocka, J.; El-Bagoury, N.; Ryl, J.; Amin, M.A.; Boukherroub, R. Effect of cobalt addition on the corrosion behavior of near equi atomic NiTi shape memory alloy in normal saline solution. RSC Adv. 2018, 34, 19289. [Google Scholar] [CrossRef]
- Anbarasan, N.; Jerome, S.; Arivazhagan, N. Argon and Argon-Hydrogen Shielding Gas effects on the Laves Phase Formation and corrosion behavior of Inconel 718 Gas Tungsten Arc Welds. J. Mater. Process. Technol. 2018, 263. [Google Scholar] [CrossRef]
- Li, L.; Dong, C.F.; Xiao, K. Effect of pH on pitting corrosion of stainless steel welds in alkaline salt Water. Constr. Build. Mater. 2014, 68, 709–715. [Google Scholar] [CrossRef]
- Verma, J.; Taiwade, R.V.; Kataria, R.; Kumar, A. Welding and electrochemical behavior of ferritic AISI 430 and austeno ferritic UNS 32205 dissimilar welds. J. Manuf. Process. 2018, 34, 292–302. [Google Scholar] [CrossRef]
- Brytan, Z.; Niagaj, J.; Reiman, L. Corrosion studies using potentiodynamic and EIS electrochemical techniques of welded lean duplex stainless steel UNS S82441. Appl. Surf. Sci. 2016, 388, 160–168. [Google Scholar] [CrossRef]
- ASM International. Corrosion of Weldments (05182G); ASM International: Materials Park, OH, USA, 2006; Available online: https://www.asminternational.org/documents/10192/1849770/ACFAB85.pdf (accessed on 16 January 2019).
- Kangazian, J.; Shamanian, M.; Ashrafi, A. Dissimilar welding between SAF 2507 stainless steel and Incoloy 825 Ni-based alloy: The role of microstructure on corrosion behavior of the weld metals Jalal. J. Manuf. Process. 2017, 29, 376–388. [Google Scholar] [CrossRef]
- Mani, C.; Karthikeyan, R.; Vincent, S. A study on corrosion resistance of dissimilar welds between Monel 400 and 316L austenitic stainless steel. IOP Conf. Ser. Mater. Sci. Eng. 2018, 346, 012025. [Google Scholar] [CrossRef]
- Mohd Touseef Nauman, S.; Mohideen, R.; Kaleem, N. Material characterization of 316L stainless steel after being subjected to cryogenic treatment. Int. J. Mech. Ind. Eng. 2012, 2, 2231–6477. [Google Scholar]
- Barron, R.F.; Thompson, R.H. Effect of cryogenic treatment on corrosion resistance. In Proceedings of the 8th International Cryogenic Materials Conference, Los Angeles, CA, USA, 24–28 July 1989. [Google Scholar]
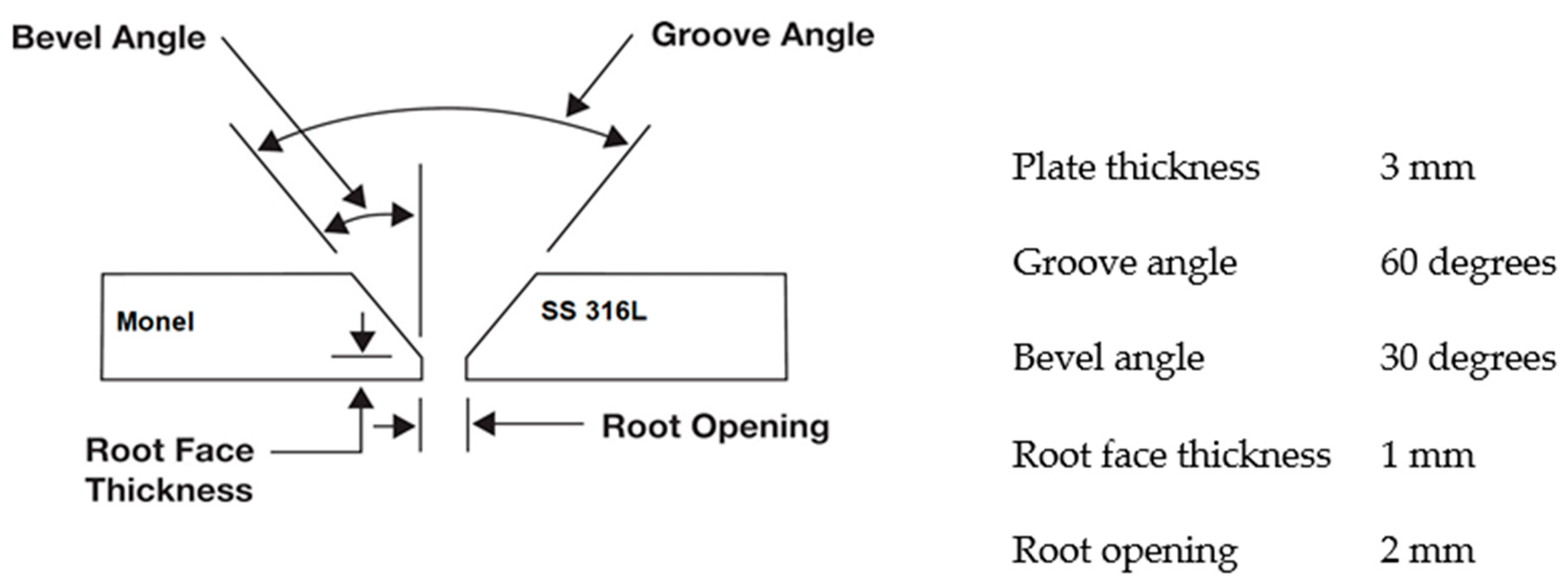


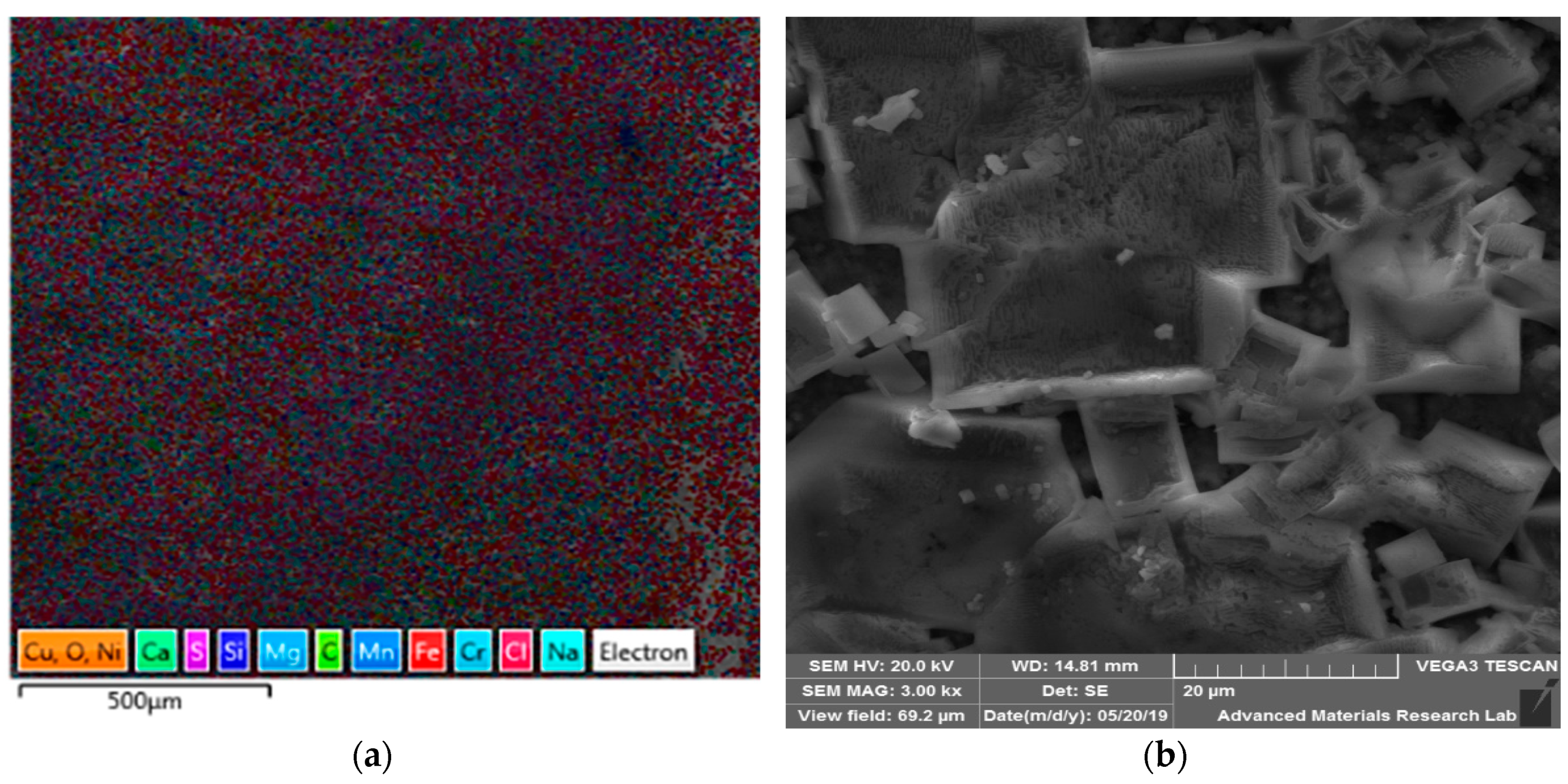
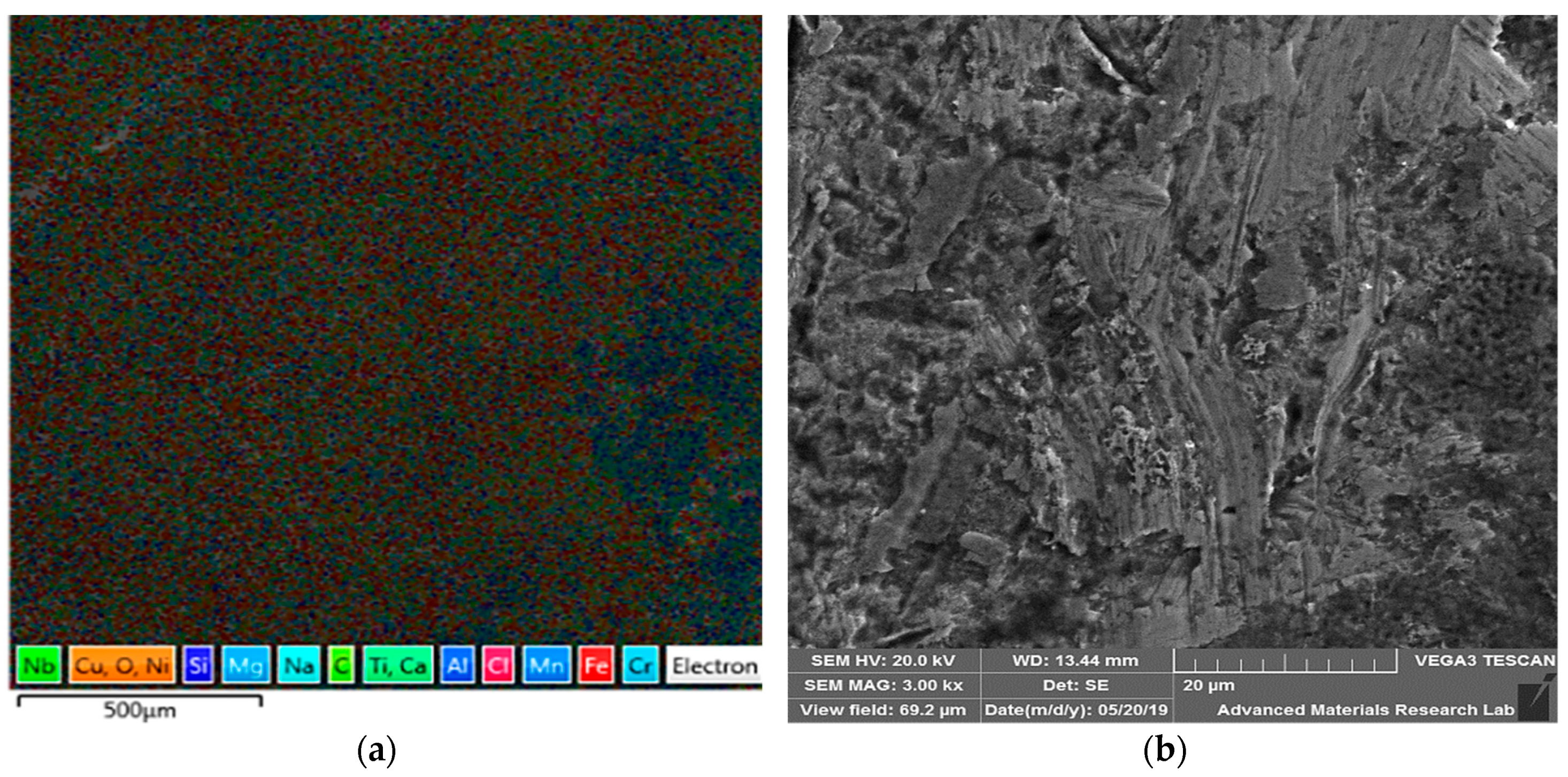


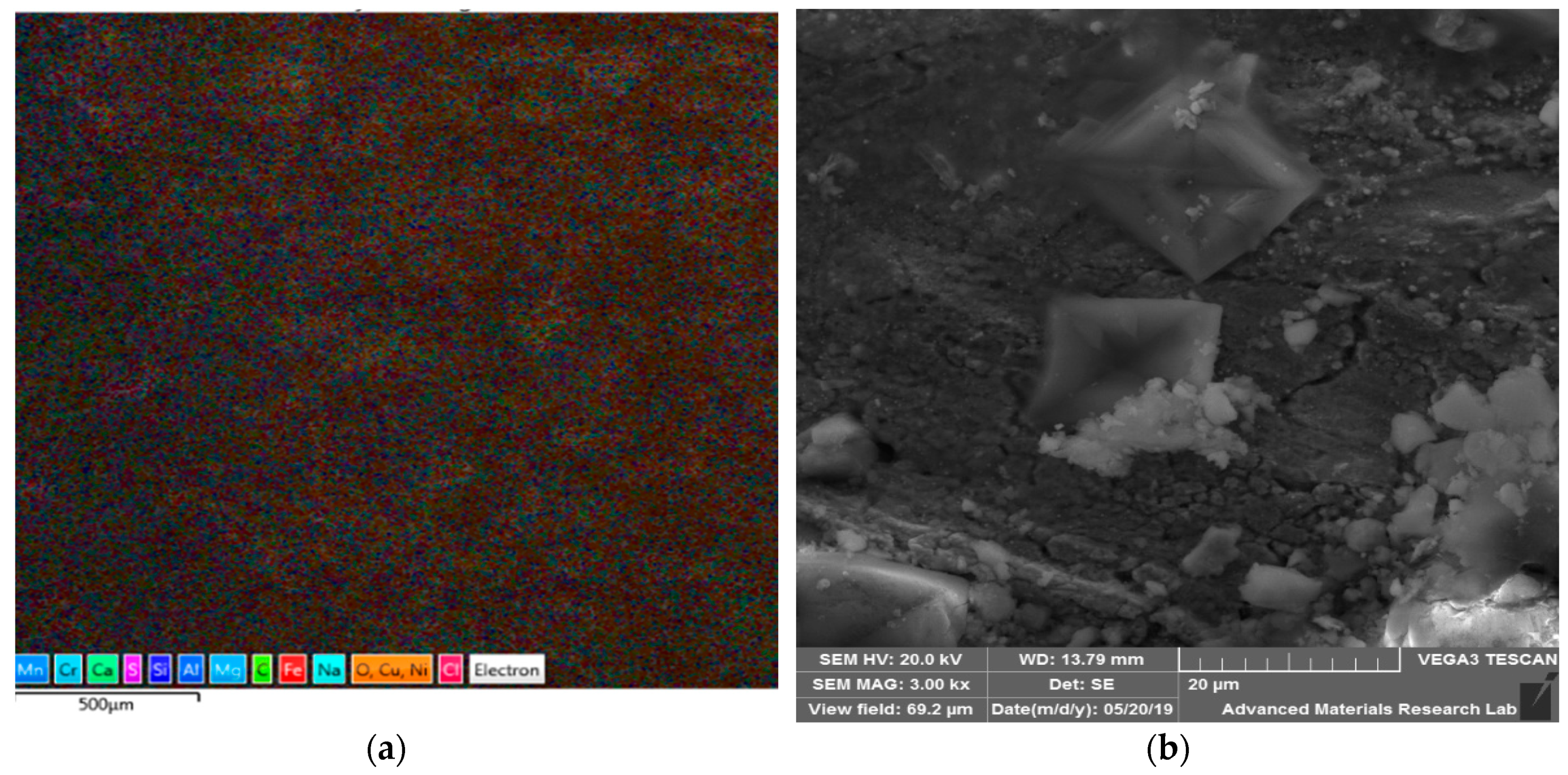
| Material | C | Cr | Fe | Mn | Ni | P | S | Si | Mo | Cu | Ti | Co | Nb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SS 316L | 0.03 | 17 | 65.64 | 2 | 12 | 0.045 | 0.03 | 0.75 | 2.5 | 0 | - | - | - |
| Monel 400 | 0.3 | - | 2.5 | 2 | 63 | - | 0.024 | 0.5 | - | 31.676 | - | - | - |
| ENiCrFe-5 | 0.04 | 14–17 | 6–10 | 1.0 | 70 (min) | 0.03 | 0.015 | 0.35 | - | 0.5 | 0.12 | 1.5 |
| wt % | SS HAZ after Corrosion | Weld after Corrosion | Monel HAZ after Corrosion | |||
|---|---|---|---|---|---|---|
| Component | CT | HT | CT | HT | CT | HT |
| Cr | 5.6 | 4.8 | 10.6 | 11.5 | - | 0.7 |
| Fe | 19.7 | 12.1 | 9.1 | 11 | - | 2.4 |
| Mn | 1.9 | 2.5 | 1.5 | 2.9 | - | 1.2 |
| Ni | 3.9 | 4.4 | 34.5 | 22.1 | 21.86 | 28.2 |
| Mo | - | - | - | 0.5 | - | - |
| Cu | 1.2 | 0.8 | 4 | 2.9 | 10.48 | 12.7 |
| Ti | - | - | 1.4 | 0.6 | - | - |
| Cl | 16.2 | 26.2 | - | 6.5 | 1.81 | 10.5 |
| Nb | - | - | 1.7 | 1.9 | - | - |
| Na | 18.5 | 24.6 | 2.3 | 11.8 | 6.79 | 16.4 |
| Specimen | Zones | Area | Ecorr | Jcorr | Icorr | Tafel Slope | Corrosion Rate | Polarization Resistance | |
|---|---|---|---|---|---|---|---|---|---|
| cm2 | V | µA/cm2 | µA | βa | βc | (mm/yr) × 10−6 | (M ohm) | ||
| Specimen CT | SS 316L Base | 0.48 | 0.165 | 7.53 | 6.85 | 0.053 | −0.166 | 0.079 | 2.54 |
| SS 316L HAZ | 0.94 | 0.104 | 56.49 | 51.36 | 0.096 | −0.146 | 0.589 | 0.49 | |
| Weld Zone | 1.02 | 0.090 | 0.32 | 0.29 | 0.296 | −0.692 | 0.003 | 313.10 | |
| Monel HAZ | 1.80 | 0.111 | 2.05 | 1.86 | 0.151 | −0.098 | 0.020 | 13.80 | |
| Monel 400 Base | 0.90 | 0.099 | 1.48 | 1.34 | 0.066 | −0.073 | 0.014 | 11.20 | |
| Specimen HT | SS 316L Base | 1.04 | 0.182 | 257.04 | 233.67 | 0.028 | −0.175 | 2.679 | 0.04 |
| SS 316L HAZ | 1.03 | 0.183 | 562.34 | 511.22 | 0.098 | −0.134 | 5.861 | 0.05 | |
| Weld Zone | 1.07 | 0.062 | 13.55 | 12.32 | 0.095 | −0.257 | 0.141 | 2.45 | |
| Monel HAZ | 1.09 | 0.135 | 39.08 | 35.53 | 0.105 | −0.277 | 0.378 | 0.93 | |
| Monel 400 Base | 1.01 | 0.094 | 19.05 | 17.32 | 0.049 | −0.190 | 0.184 | 0.99 | |
| Specimen | SPECIMEN CT | SPECIMEN HT | ||||
|---|---|---|---|---|---|---|
| Zones | R1 | R2 | P1 | R1 | R2 | P1 |
| Ohm | Ohm | Farads | Ohm | Ohm | Farads | |
| Weld | 6.99 | 1915.5 | 0.0000621 | 6 | 1188 | 0.00012 |
| Monel Base | 5.64 | 990.1 | 0.00027 | 4.25 | 742 | 0.00023 |
| Monel HAZ | 4.03 | 741.29 | 0.00025 | 5.54 | 704 | 0.00029 |
| SS Base | 5.54 | 704.28 | 0.00029 | 9.84 | 610 | 0.00015 |
| SS HAZ | 1.98 | 375 | 0.0015 | 4.9 | 555 | 0.0009 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mani, C.; Karthikeyan, R.; Kannan, S. Electrochemical Impedance Analysis on Cryogenically Treated Dissimilar Metal Welding of 316L Stainless Steel and Monel 400 Alloy Using GTAW. Metals 2019, 9, 1088. https://doi.org/10.3390/met9101088
Mani C, Karthikeyan R, Kannan S. Electrochemical Impedance Analysis on Cryogenically Treated Dissimilar Metal Welding of 316L Stainless Steel and Monel 400 Alloy Using GTAW. Metals. 2019; 9(10):1088. https://doi.org/10.3390/met9101088
Chicago/Turabian StyleMani, Cherish, R Karthikeyan, and Sathish Kannan. 2019. "Electrochemical Impedance Analysis on Cryogenically Treated Dissimilar Metal Welding of 316L Stainless Steel and Monel 400 Alloy Using GTAW" Metals 9, no. 10: 1088. https://doi.org/10.3390/met9101088
APA StyleMani, C., Karthikeyan, R., & Kannan, S. (2019). Electrochemical Impedance Analysis on Cryogenically Treated Dissimilar Metal Welding of 316L Stainless Steel and Monel 400 Alloy Using GTAW. Metals, 9(10), 1088. https://doi.org/10.3390/met9101088




