Electrochemical Behaviour of Chalcopyrite in Chloride Solutions
Abstract
1. Introduction
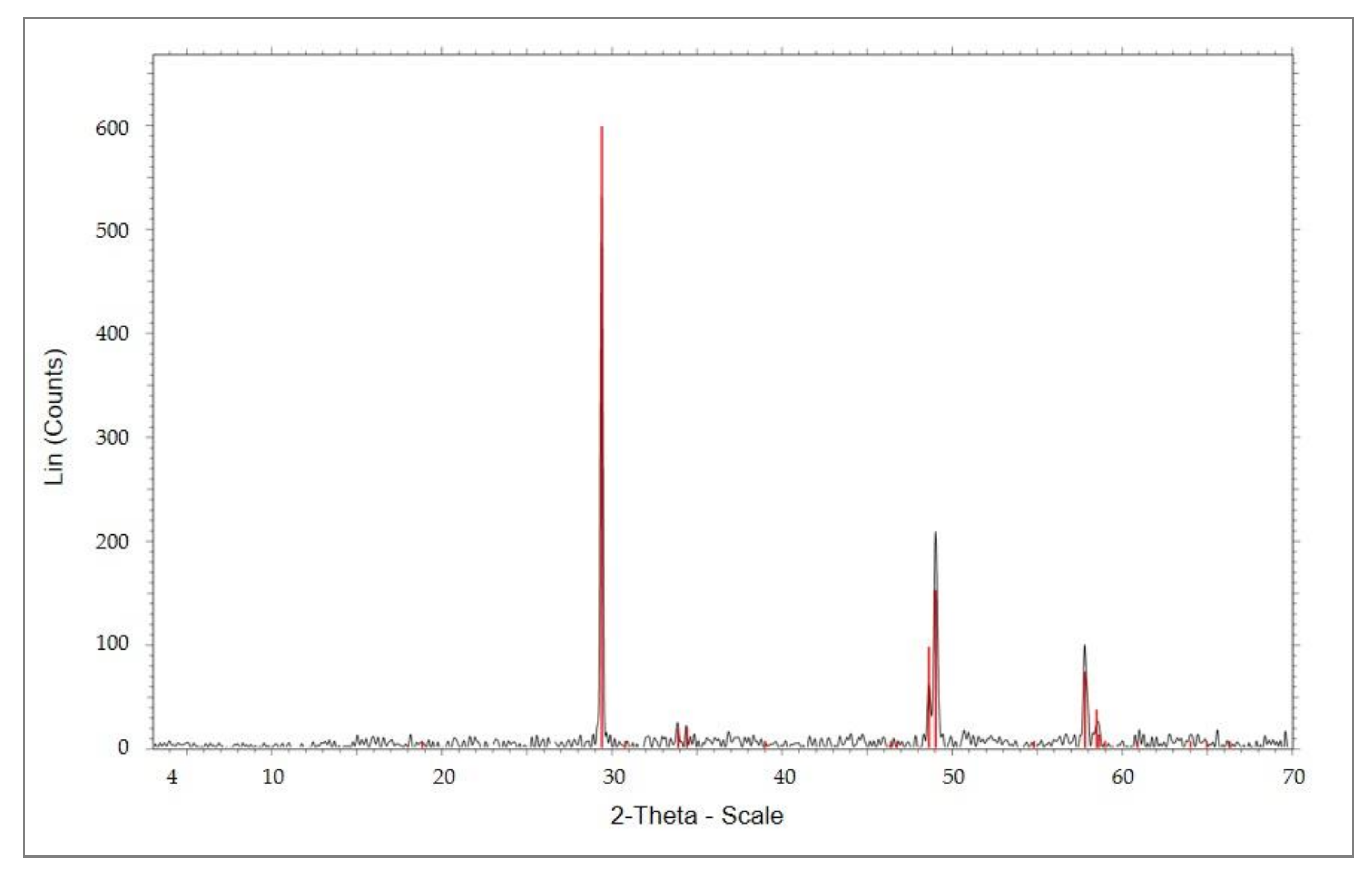
2. Materials and Methods
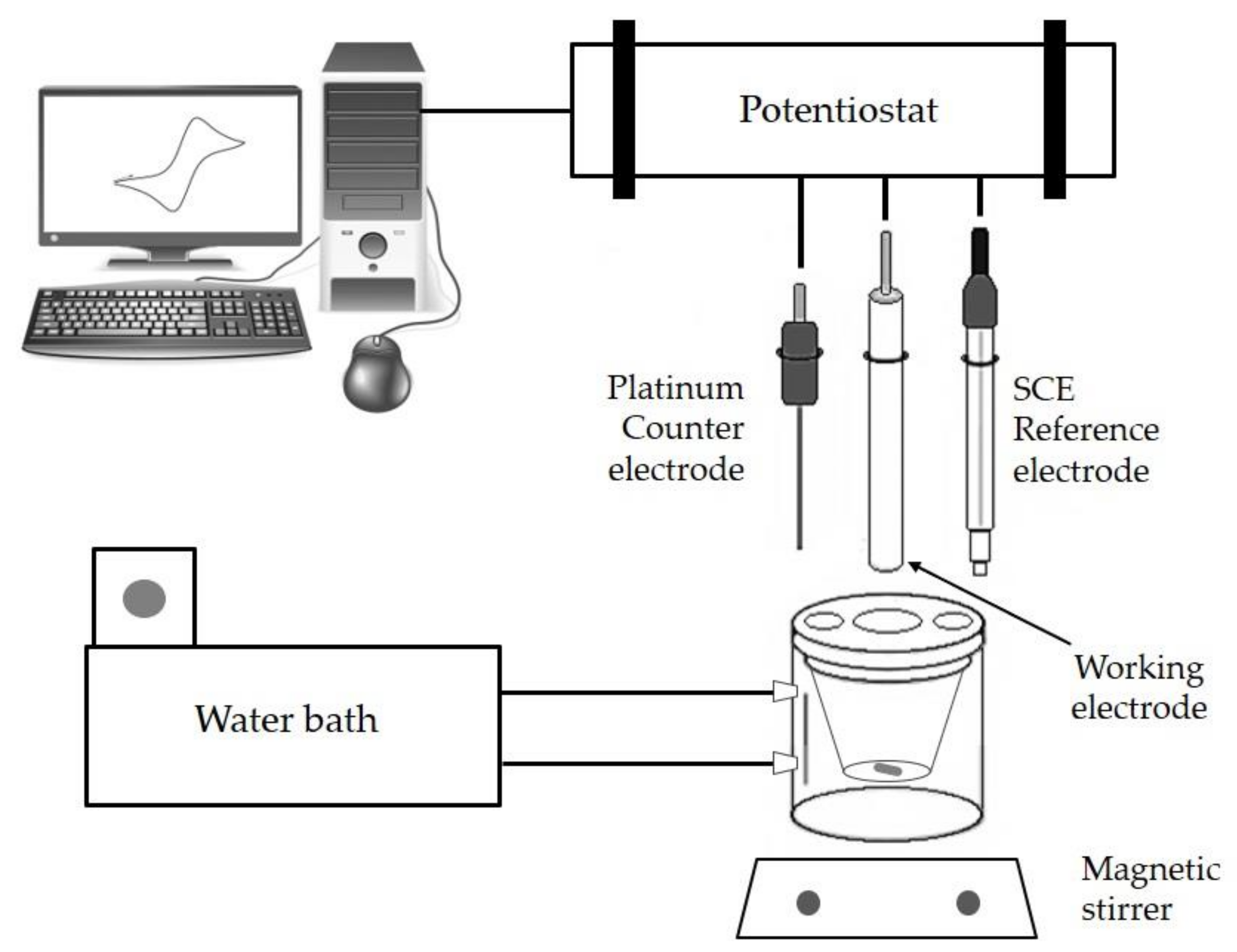
2.1. Instrumentation Used
2.2. Methodology
2.3. Parameters Assessed
- Deionised water (DW) at pH 1
- DW at pH 1 and 20 g/L [Cl−] (from NaCl)
- DW with concentrations of 20 g/L [Cl−], 0.5 g/L [Cu2+], 1 g/L [Fe2+] at pH 1
- Seawater with concentrations of 0.5 g/L [Cu2+], 1 g/L [Fe2+] at pH 1
3. Results and Discussions
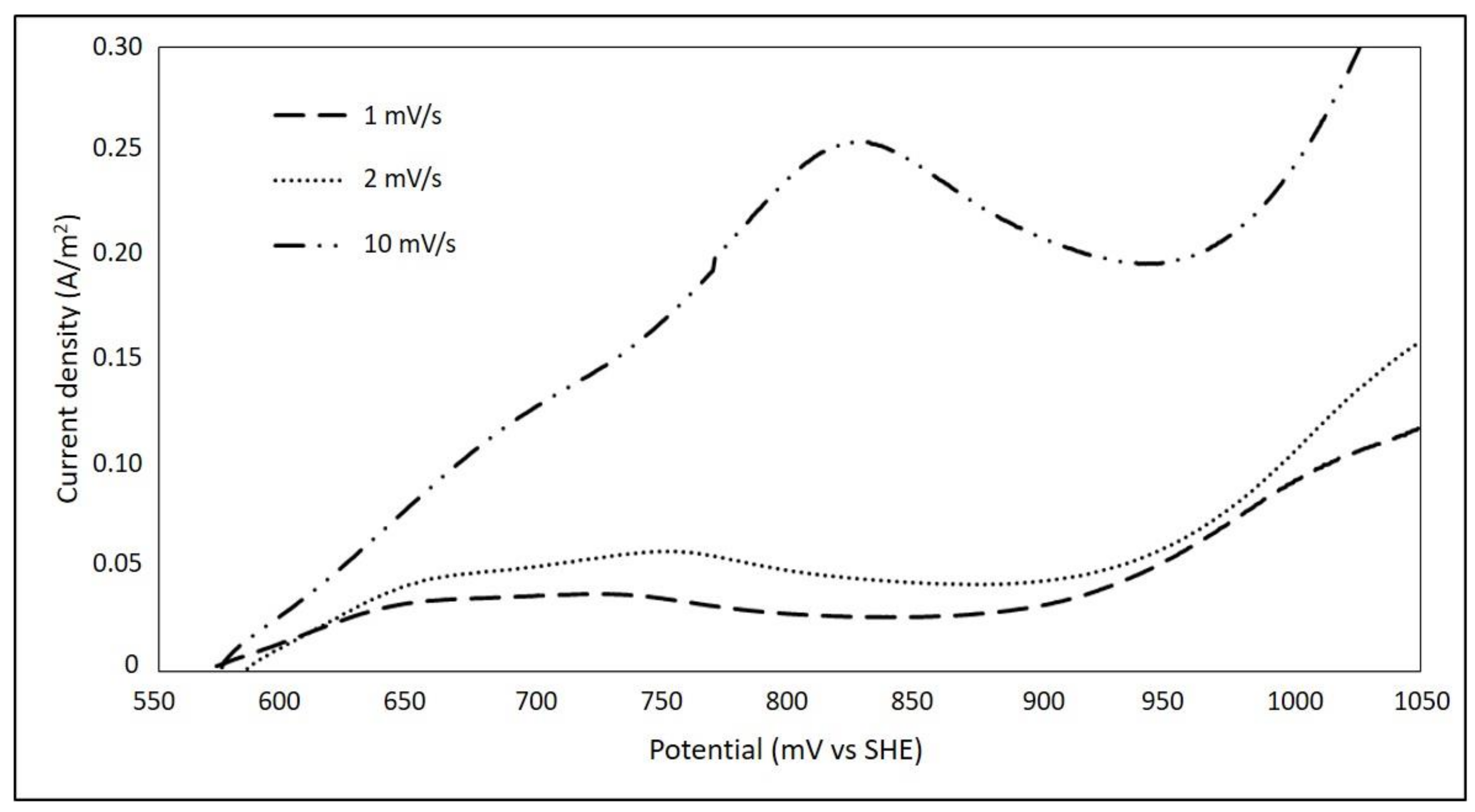
3.1. Effect of Sweep Speed
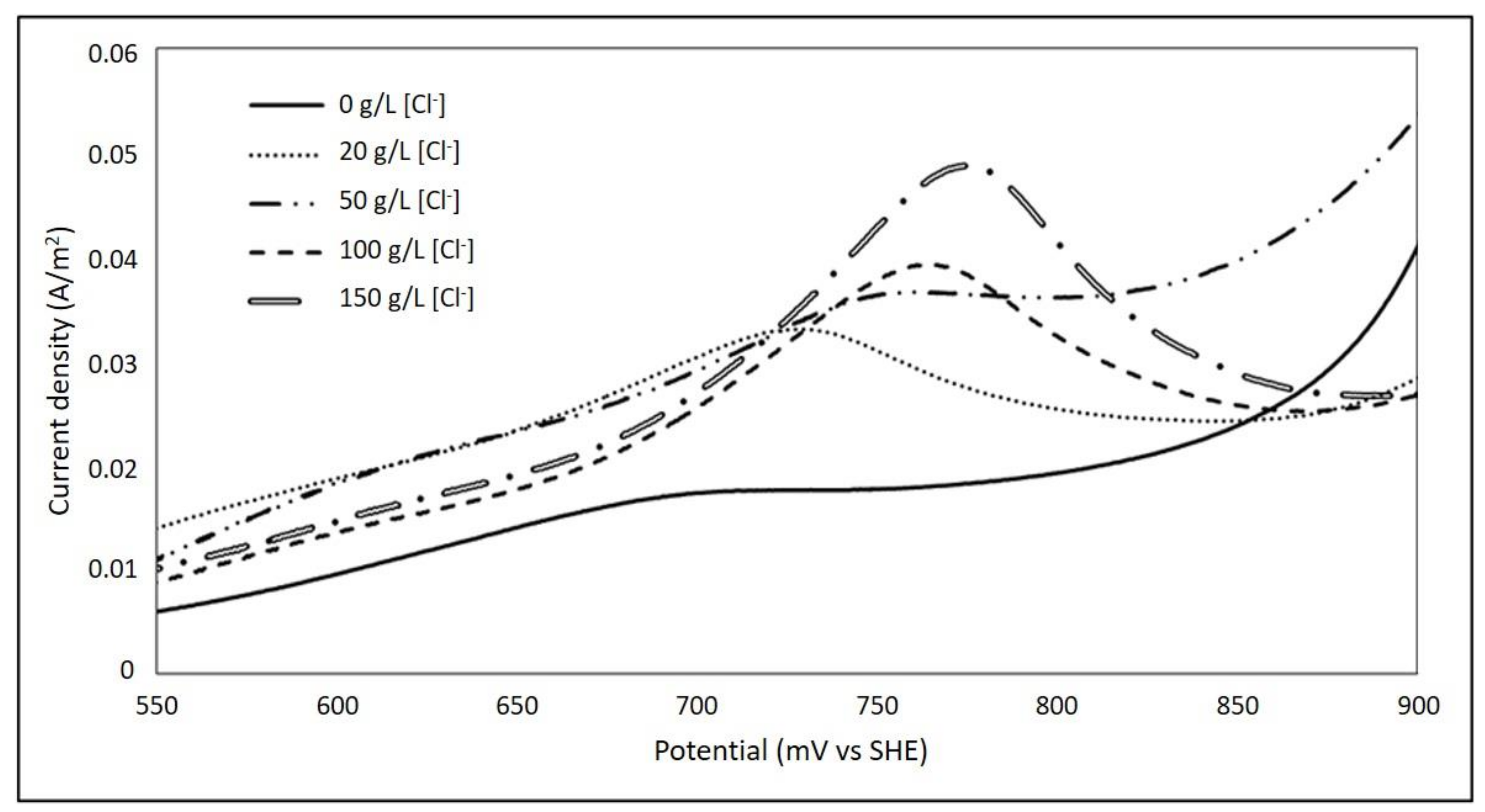
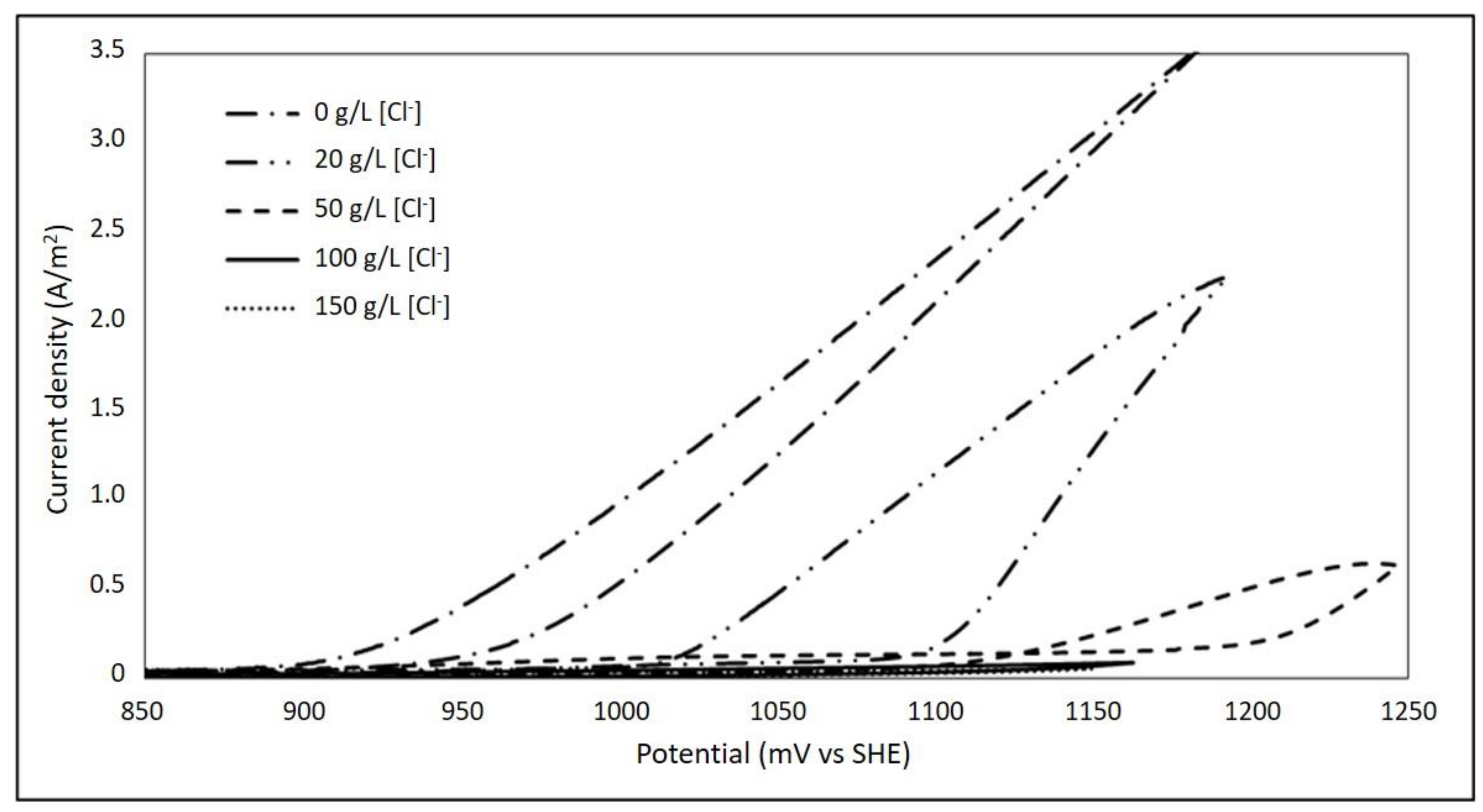
3.2. Effect of Chloride Ions
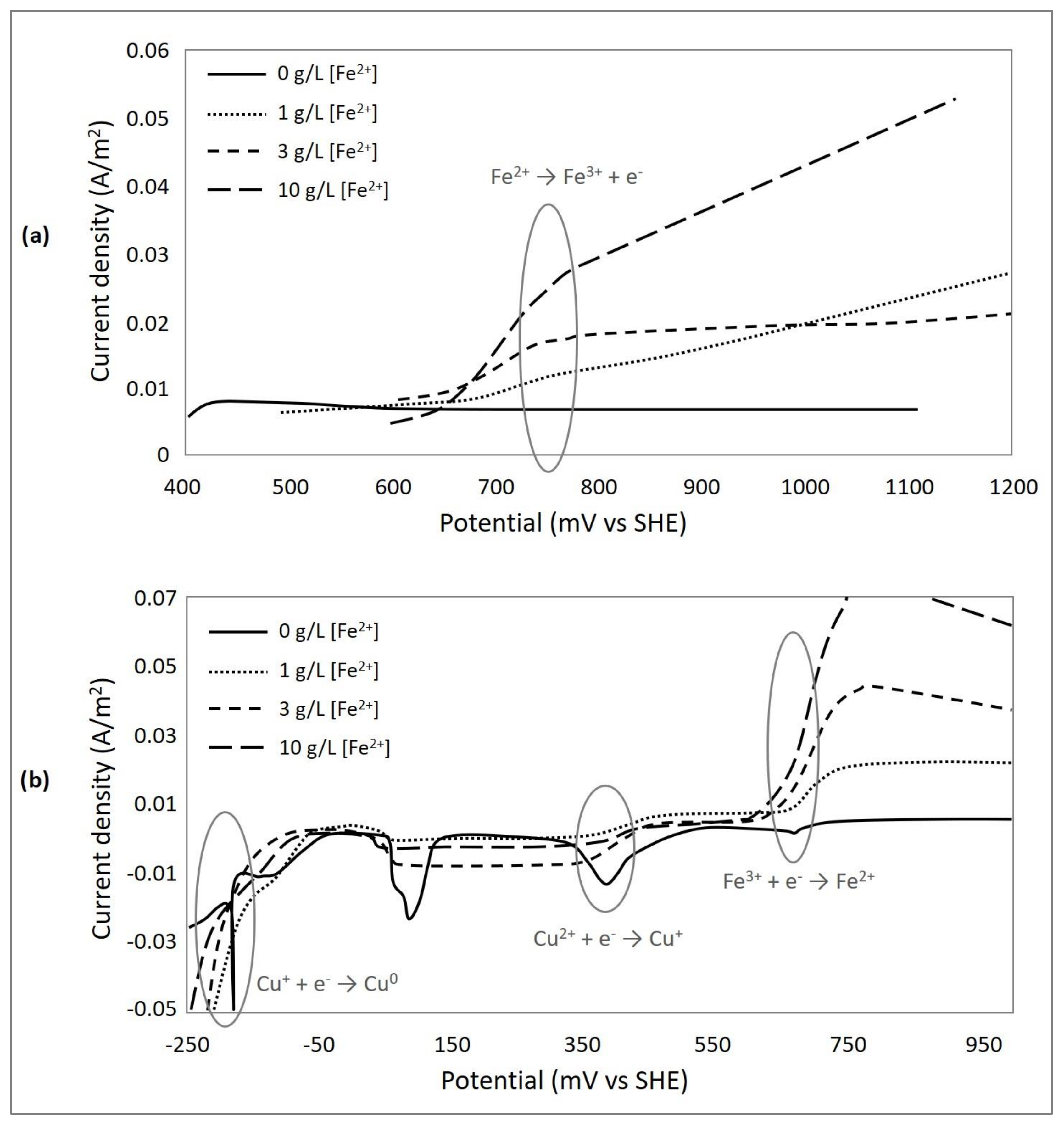
3.3. Effect of Ferrous Ions
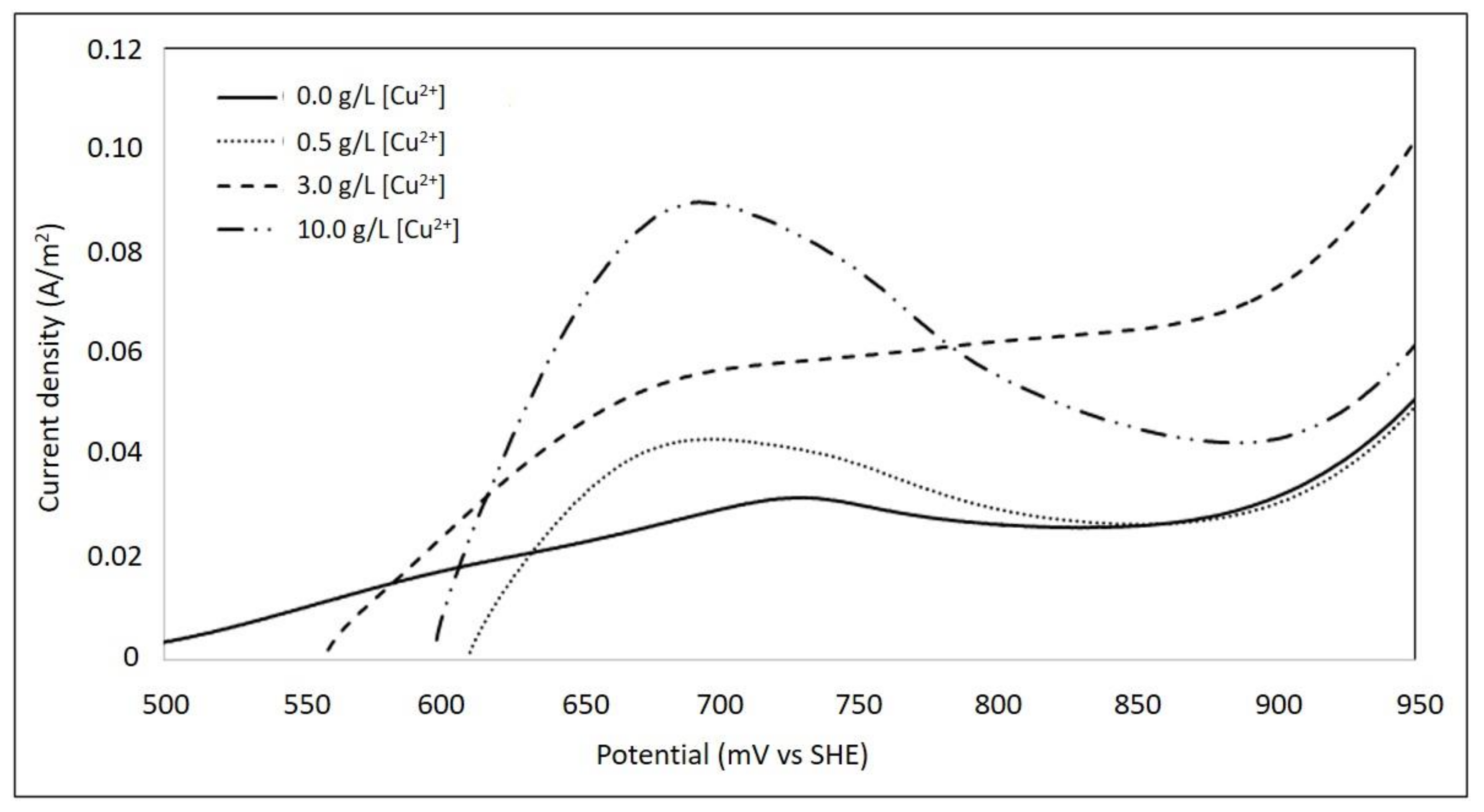
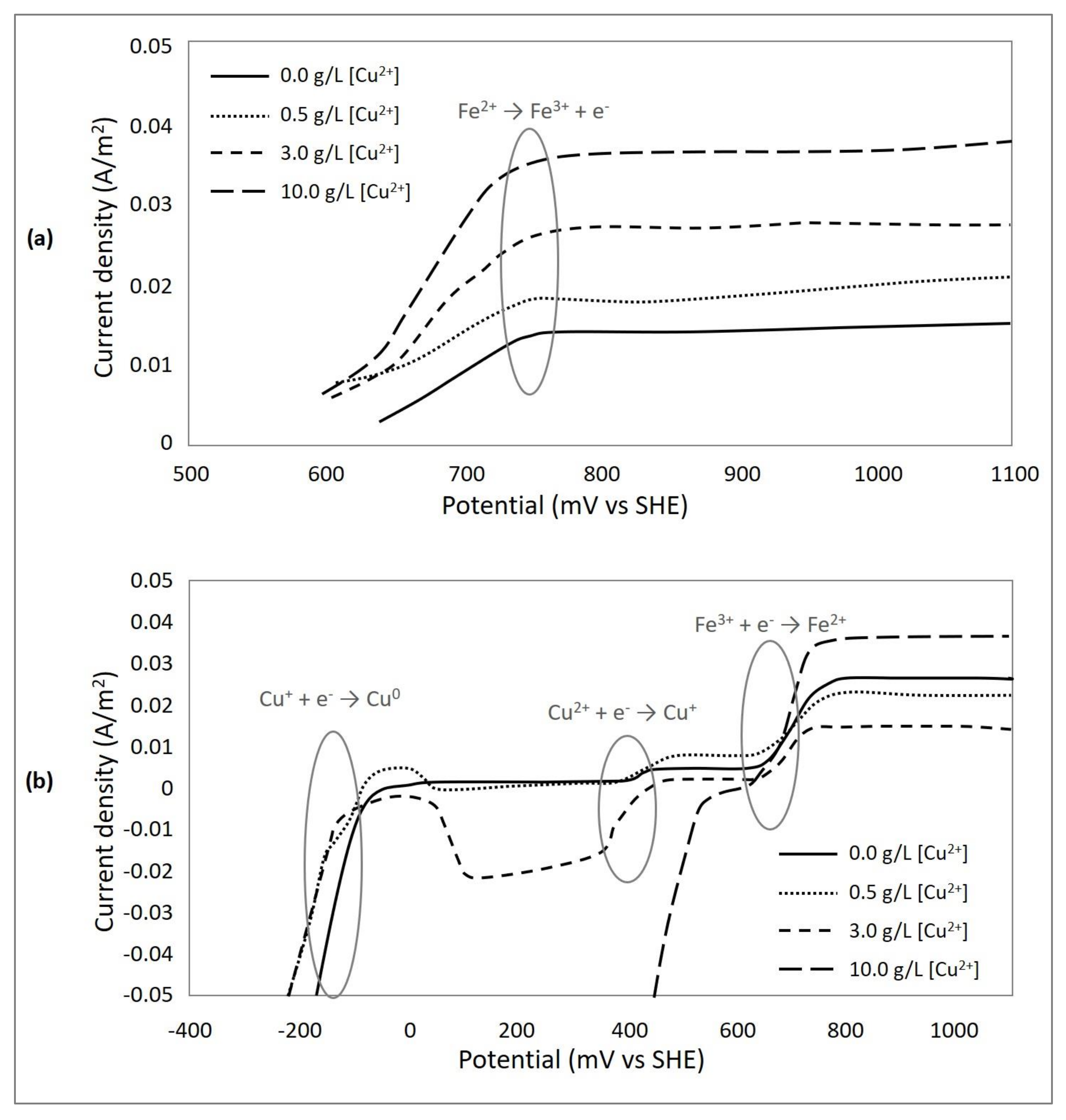
3.4. Effect of Cupric Ions
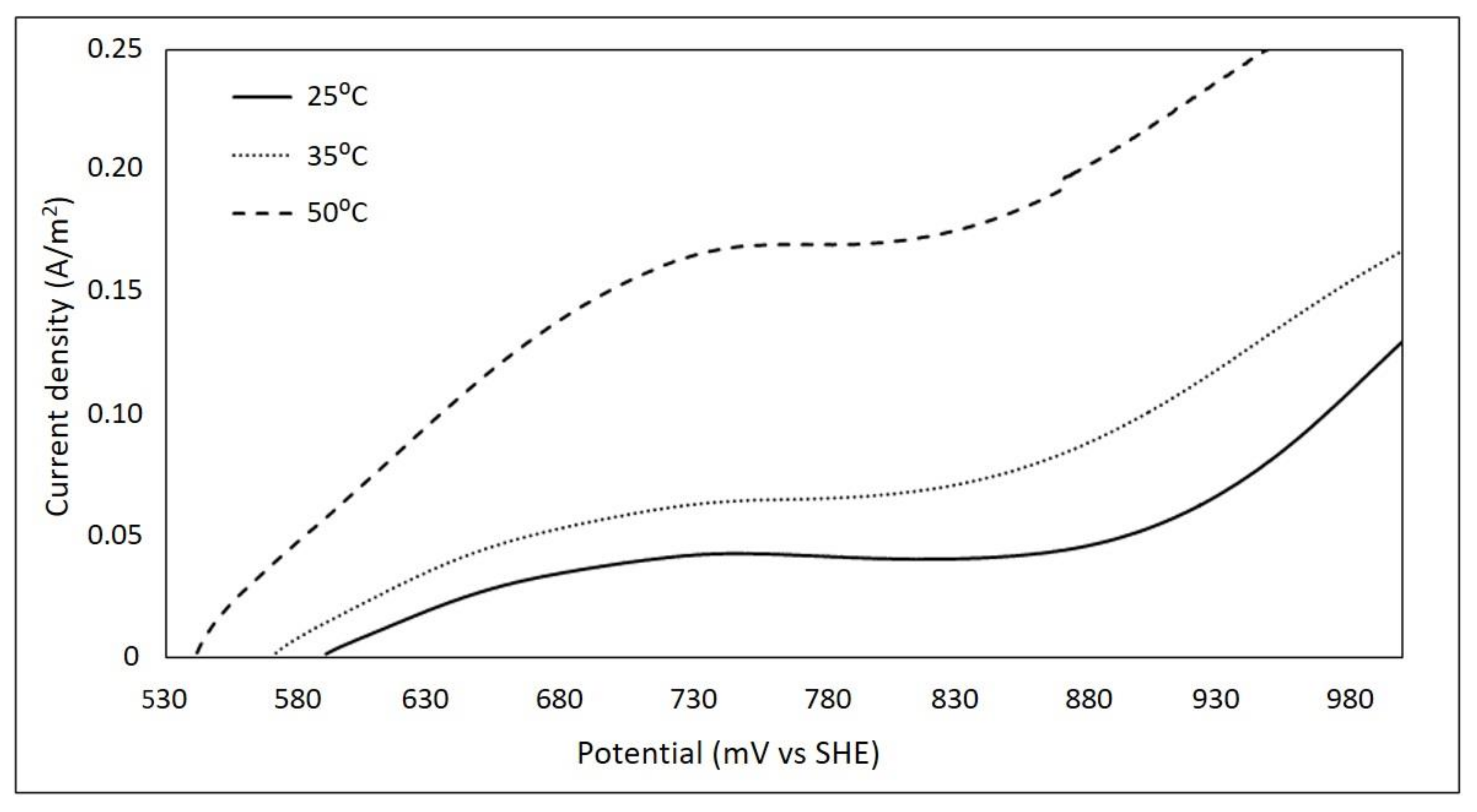
3.5. Effect of Temperature
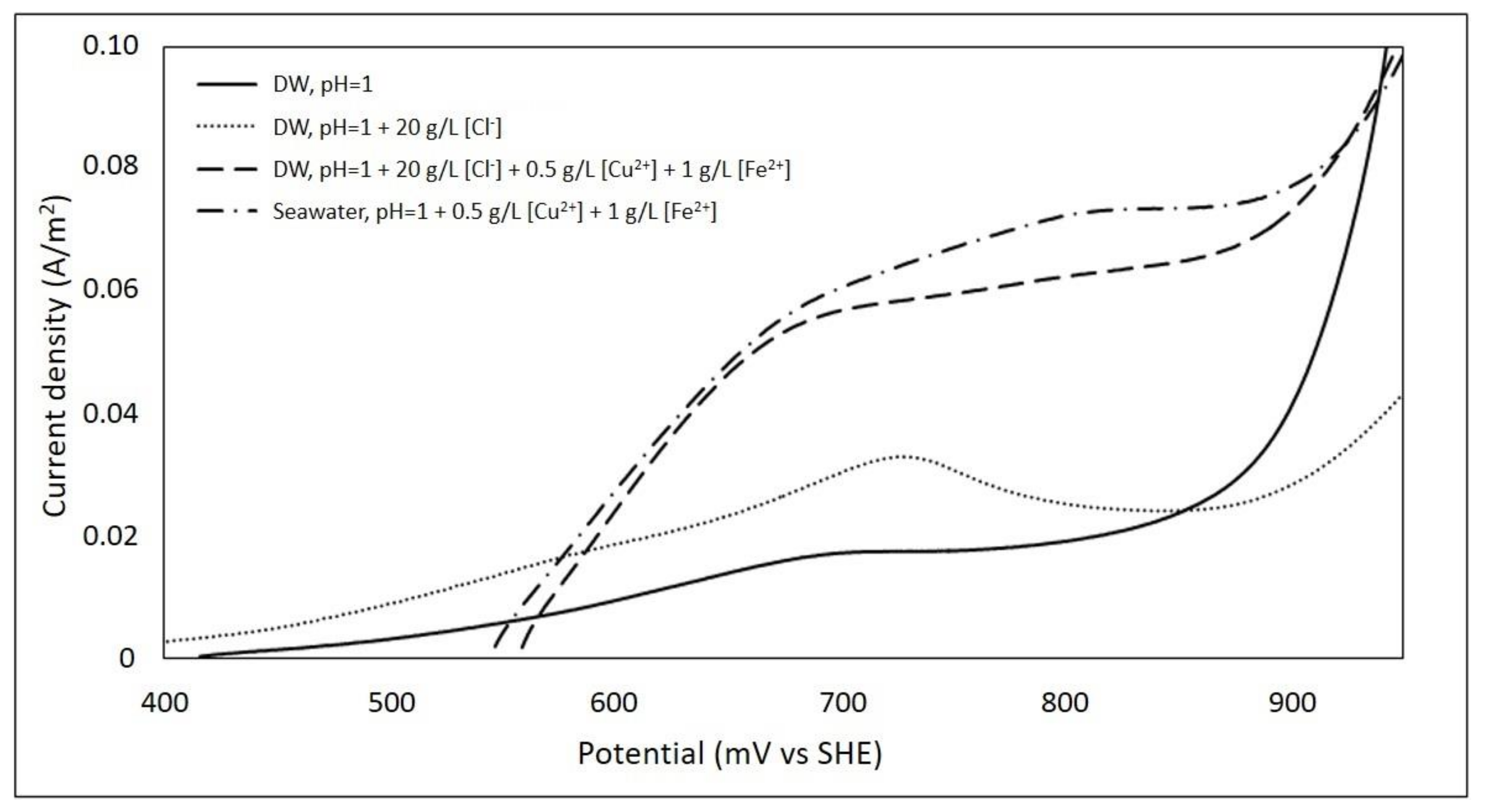
3.6. Effect of Seawater
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cifuentes, G.; Vargas, C.; Simpson, J. Análisis de las principales variables de proceso que influyen en el rechazo de los cátodos durante el electrorrefino del cobre. Rev. Metal. 2009, 45, 228–236. [Google Scholar] [CrossRef]
- Dreisinger, D. Copper leaching from primary sulfides: Options for biological and chemical extraction of copper. Hydrometallurgy 2006, 83, 10–20. [Google Scholar] [CrossRef]
- Elsherief, A.E. The influence of cathodic reduction, Fe2+ and Cu2+ ions on the electrochemical dissolution of chalcopyrite in acidic solution. Miner. Eng. 2002, 15, 215–223. [Google Scholar] [CrossRef]
- Velásquez-Yévenes, L.; Nicol, M.; Miki, H. The dissolution of chalcopyrite in chloride solutions: Part 1. the effect of solution potential. Hydrometallurgy 2010, 103, 108–113. [Google Scholar] [CrossRef]
- Lu, Z.; Jeffrey, M.; Lawson, F. An electrochemical study of the effect of chloride ions on the dissolution of chalcopyrite in acidic solutions. Hydrometallurgy 2000, 56, 145–155. [Google Scholar] [CrossRef]
- Yévenes, L.V.; Miki, H.; Nicol, M. The dissolution of chalcopyrite in chloride solutions: Part 2: Effect of various parameters on the rate. Hydrometallurgy 2010, 103, 80–85. [Google Scholar] [CrossRef]
- Córdoba, E.M.; Muñoz, J.A.; Blázquez, M.L.; González, F.; Ballester, A. Leaching of chalcopyrite with ferric ion. Part I: General aspects. Hydrometallurgy 2008, 93, 81–87. [Google Scholar] [CrossRef]
- Nicol, M.; Zhang, S. The anodic behaviour of chalcopyrite in chloride solutions: Potentiostatic measurements. Hydrometallurgy 2017, 167, 72–80. [Google Scholar] [CrossRef]
- Carneiro, M.F.C.; Leão, V.A. The role of sodium chloride on surface properties of chalcopyrite leached with ferric sulphate. Hydrometallurgy 2007, 87, 73–82. [Google Scholar] [CrossRef]
- Córdoba, E.M.; Muñoz, J.A.; Blázquez, M.L.; González, F.; Ballester, A. Leaching of chalcopyrite with ferric ion. Part II: Effect of redox potential. Hydrometallurgy 2008, 93, 88–96. [Google Scholar] [CrossRef]
- Lu, Z.Y.; Jeffrey, M.I.; Lawson, F. Effect of chloride ions on the dissolution of chalcopyrite in acidic solutions. Hydrometallurgy 2000, 56, 189–202. [Google Scholar] [CrossRef]
- Hackl, R.P.; Dreisinger, D.B.; Peters, E.; King, J.A. Passivation of chalcopyrite during oxidative leaching in sulfate media. Hydrometallurgy 1995, 39, 25–48. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, M.; Yang, Y. The effect of chloride ions on the electrochemical dissolution of chalcopyrite in sulfuric acid solutions. Electrochim. Acta 2017, 253, 257–267. [Google Scholar] [CrossRef]
- Senanayake, G. A review of chloride assisted copper sulfide leaching by oxygenated sulfuric acid and mechanistic considerations. Hydrometallurgy 2009, 98, 21–32. [Google Scholar] [CrossRef]
- Almeida, T.D.C.; Garcia, E.M.; Da Silva, H.W.A.; Matencio, T.; Lins, V.D.F.C. Electrochemical study of chalcopyrite dissolution in sulfuric, nitric and hydrochloric acid solutions. Int. J. Miner. Process. 2016, 149, 25–33. [Google Scholar] [CrossRef]
- Nicol, M.; Miki, H.; Zhang, S. The anodic behaviour of chalcopyrite in chloride solutions: Voltammetry. Hydrometallurgy 2017, 171, 198–205. [Google Scholar] [CrossRef]
- COCHILCO. Proyección de Consumo de Agua en la Minería del Cobre 2017–2028; COCHILCO: Santiago, Chile, 2017. [Google Scholar]
- Baeza Reyes, A.; Mendoza, A.G. Principios de Electroquímica Analítica; Universidad Nacional Autónoma de México: Ciudad de México, Mexico, 2011. [Google Scholar]
- Viramontes-Gamboa, G.; Rivera-Vasquez, B.F.; Dixon, D.G. The Active-Passive Behavior of Chalcopyrite. J. Electrochem. Soc. 2007, 154, C299. [Google Scholar] [CrossRef]
- Nicol, M.J. The anodic behaviour of chalcopyrite in chloride solutions: Overall features and comparison with sulfate solutions. Hydrometallurgy 2017, 169, 321–329. [Google Scholar] [CrossRef]
- Beltrán, E.C.; Frisch, G.; Velasquez, L. Chalcopyrite Dissolution using Electrochemical Tests. In Proceedings of the Sustainable Hydrometallurgical Extraction of Metals, SAIMM, Cape Town, South Africa, 1–3 August 2016; pp. 157–165. [Google Scholar]
- Hiroyoshi, N.; Miki, H.; Hirajima, T.; Tsunekawa, M. Enhancement of chalcopyrite leaching by ferrous ions in acidic ferric sulfate solutions. Hydrometallurgy 2001, 60, 185–197. [Google Scholar] [CrossRef]
- Hiroyoshi, N.; Miki, H.; Hirajima, T.; Tsunekawa, M. Model for ferrous-promoted chalcopyrite leaching. Hydrometallurgy 2000, 57, 31–38. [Google Scholar] [CrossRef]
- Hiroyoshi, N.; Kuroiwa, S.; Miki, H.; Tsunekawa, M.; Hirajima, T. Synergistic effect of cupric and ferrous ions on active-passive behavior in anodic dissolution of chalcopyrite in sulfuric acid solutions. Hydrometallurgy 2004, 74, 103–116. [Google Scholar] [CrossRef]
- Veloso, T.C.; Peixoto, J.J.M.; Pereira, M.S.; Leao, V.A. Kinetics of chalcopyrite leaching in either ferric sulphate or cupric sulphate media in the presence of NaCl. Int. J. Miner. Process. 2016, 148, 147–154. [Google Scholar] [CrossRef]
- Senanayake, G. Chloride assisted leaching of chalcocite by oxygenated sulphuric acid via Cu(II)-OH-Cl. Miner. Eng. 2007, 20, 1075–1088. [Google Scholar] [CrossRef]
- Velásquez Yévenes, L. The Kinetics of the Dissolution of Chalcopyrite in Chloride Media. Ph.D. Thesis, Murdoch University, Perth, Australia, 2009. [Google Scholar]
- Ibáñez, T.; Velásquez, L. Lixiviación de la calcopirita en medios clorurados. Rev. Metal. 2013, 49, 131–144. [Google Scholar] [CrossRef]
- Zierenberg, R.A.; Schiffmant, P. Microbial control of silver mineralization at a sea-floor hydrothermal site on the northern Gorda Ridge. Nature 1990, 348, 155–157. [Google Scholar] [CrossRef]
- Velásquez-Yévenes, L.; Quezada-Reyes, V. Influence of seawater and discard brine on the dissolution of copper ore and copper concentrate. Hydrometallurgy 2018, 180, 88–95. [Google Scholar] [CrossRef]
- Torres, C.M.; Taboada, M.E.; Graber, T.A.; Herreros, O.O.; Ghorbani, Y.; Watling, H.R. The effect of seawater based media on copper dissolution from low-grade copper ore. Miner. Eng. 2015, 71, 139–145. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beiza, L.; Quezada, V.; Melo, E.; Valenzuela, G. Electrochemical Behaviour of Chalcopyrite in Chloride Solutions. Metals 2019, 9, 67. https://doi.org/10.3390/met9010067
Beiza L, Quezada V, Melo E, Valenzuela G. Electrochemical Behaviour of Chalcopyrite in Chloride Solutions. Metals. 2019; 9(1):67. https://doi.org/10.3390/met9010067
Chicago/Turabian StyleBeiza, Luis, Víctor Quezada, Evelyn Melo, and Gonzalo Valenzuela. 2019. "Electrochemical Behaviour of Chalcopyrite in Chloride Solutions" Metals 9, no. 1: 67. https://doi.org/10.3390/met9010067
APA StyleBeiza, L., Quezada, V., Melo, E., & Valenzuela, G. (2019). Electrochemical Behaviour of Chalcopyrite in Chloride Solutions. Metals, 9(1), 67. https://doi.org/10.3390/met9010067





