Leaching and Recovery of Rare-Earth Elements from Neodymium Magnet Waste Using Organic Acids
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Characterization
2.2. Leaching Kinetics
2.3. Solid to Liquid Ratio Study
2.4. Solvent Extraction
3. Results and Discussion
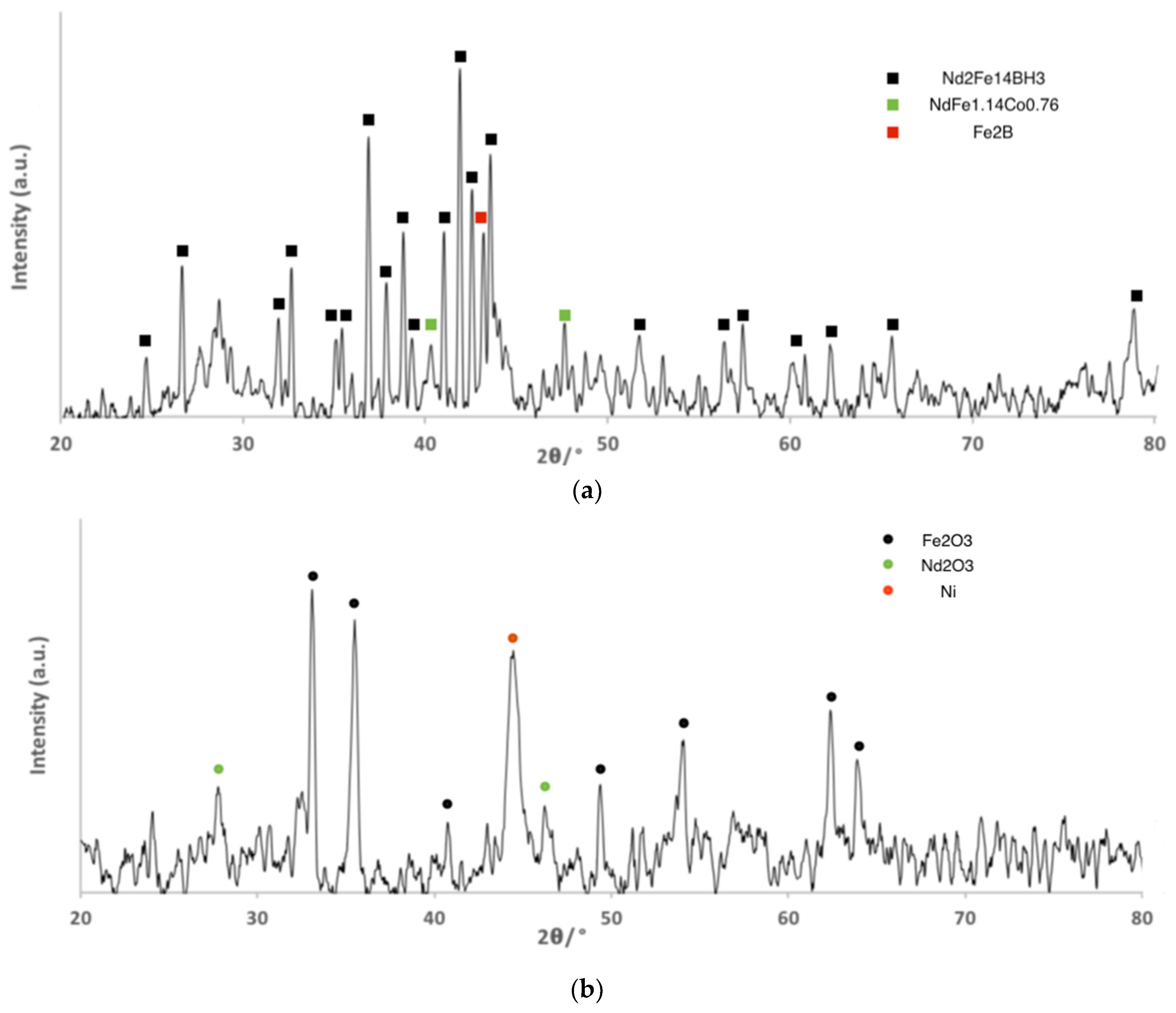
3.1. Material Characterization
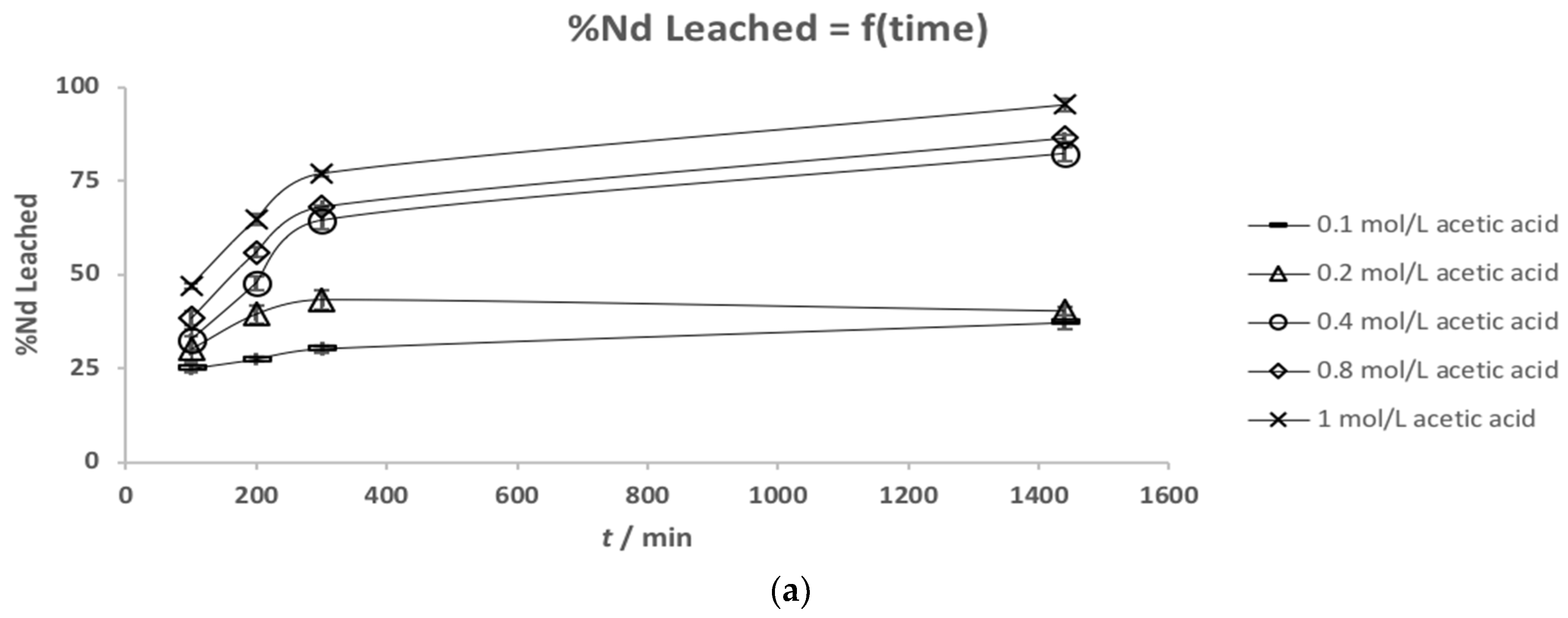
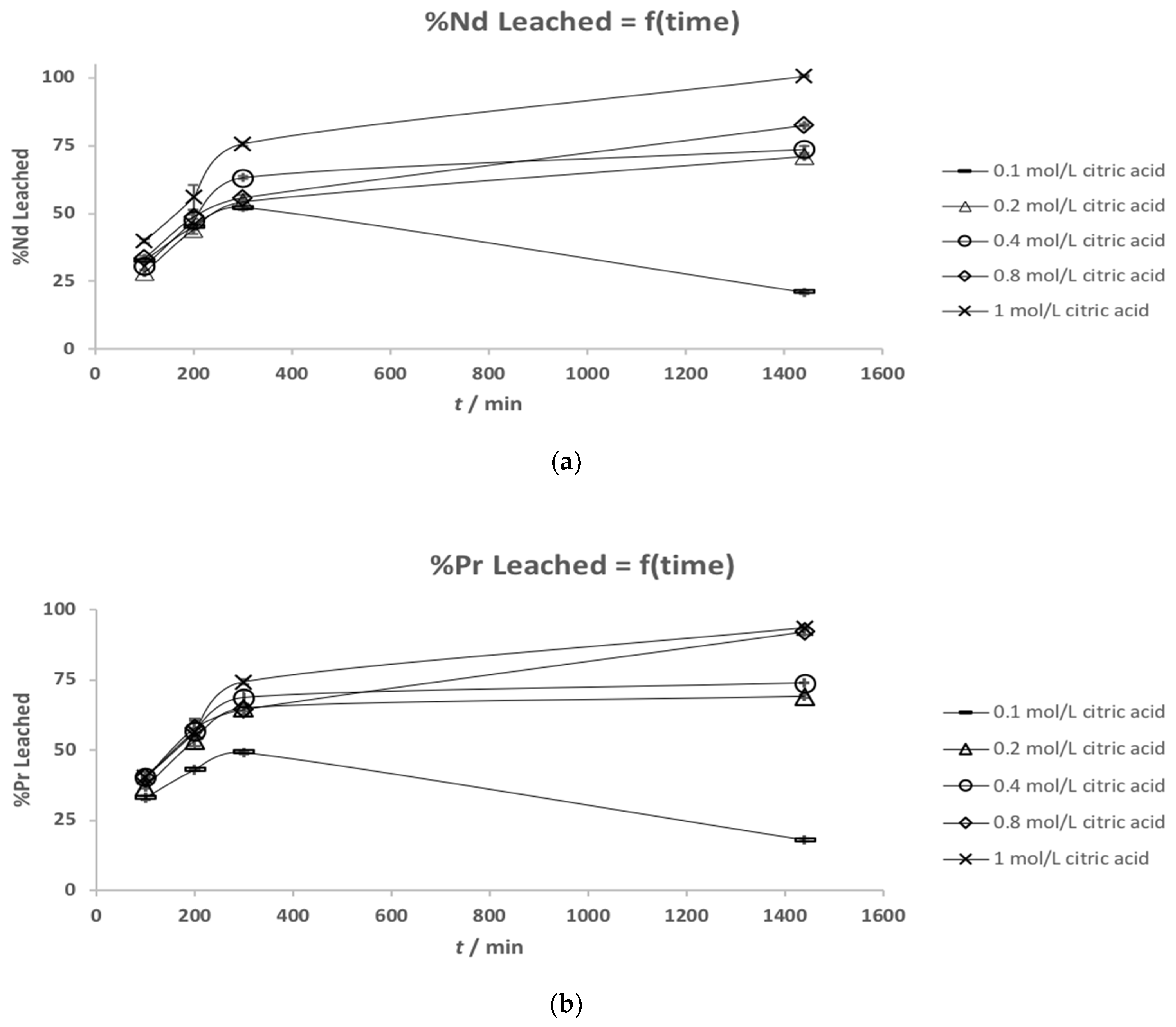
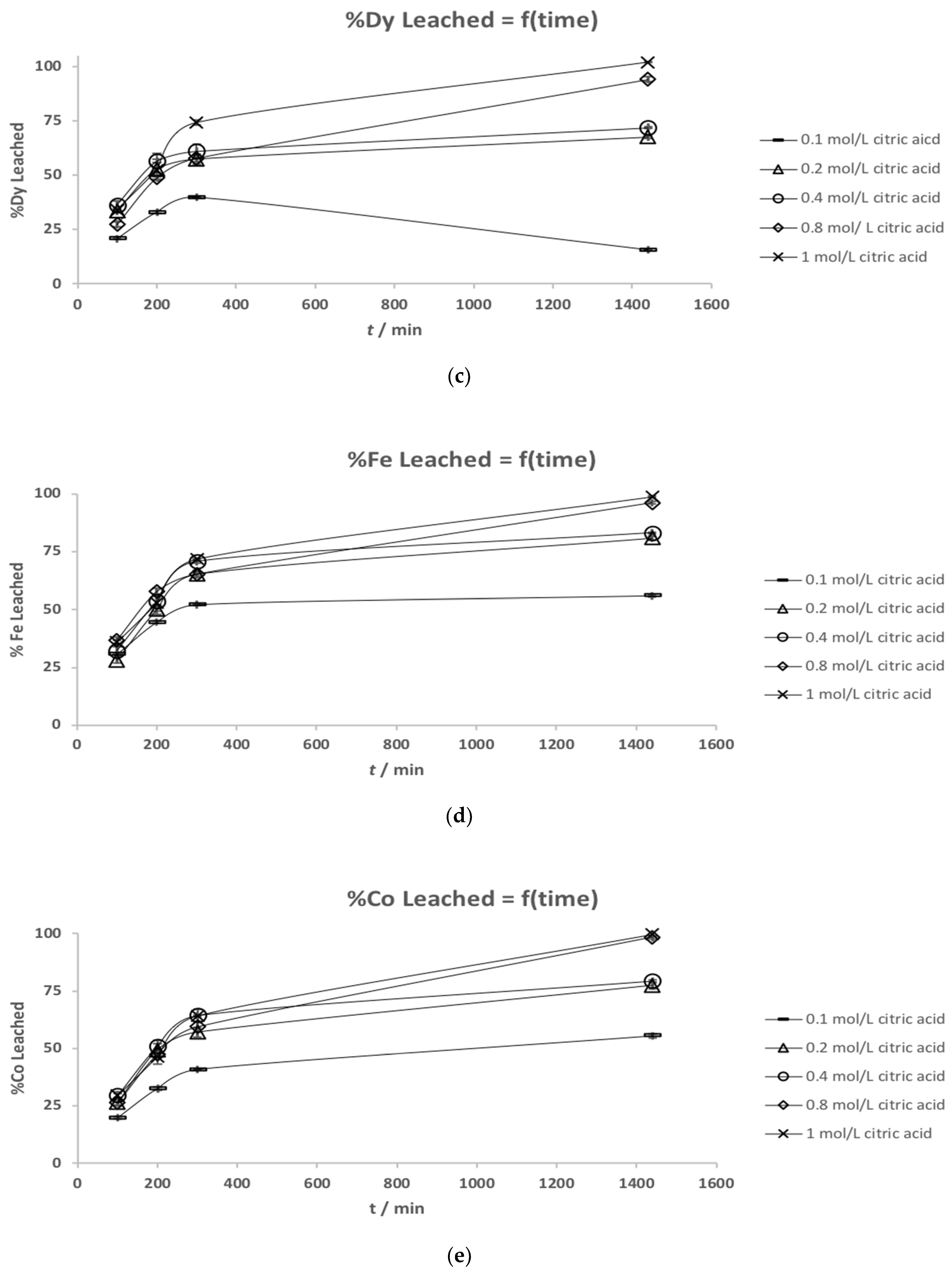
3.2. Leaching Kinetics
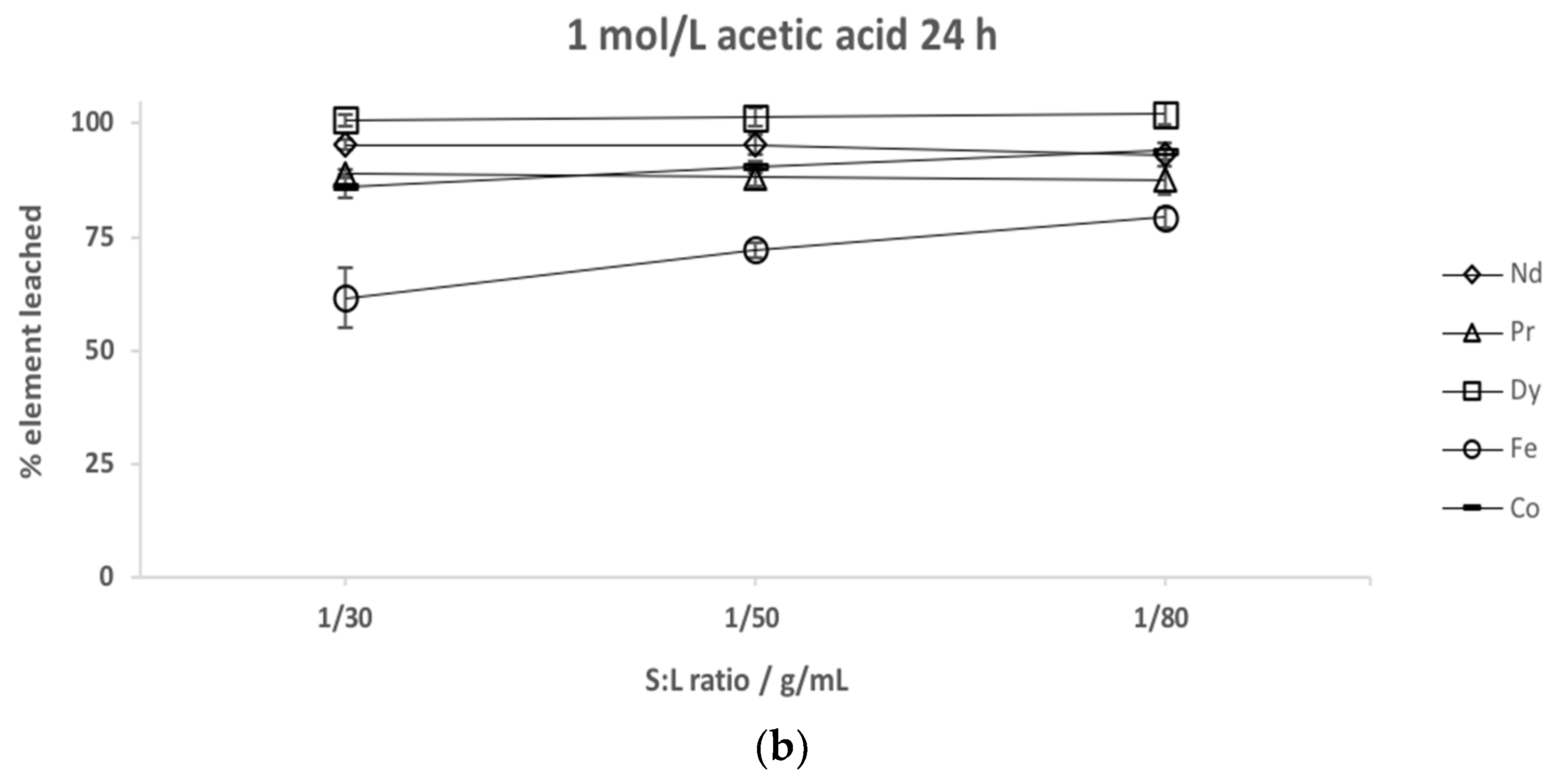
3.3. Solid to Liquid Ratio Study
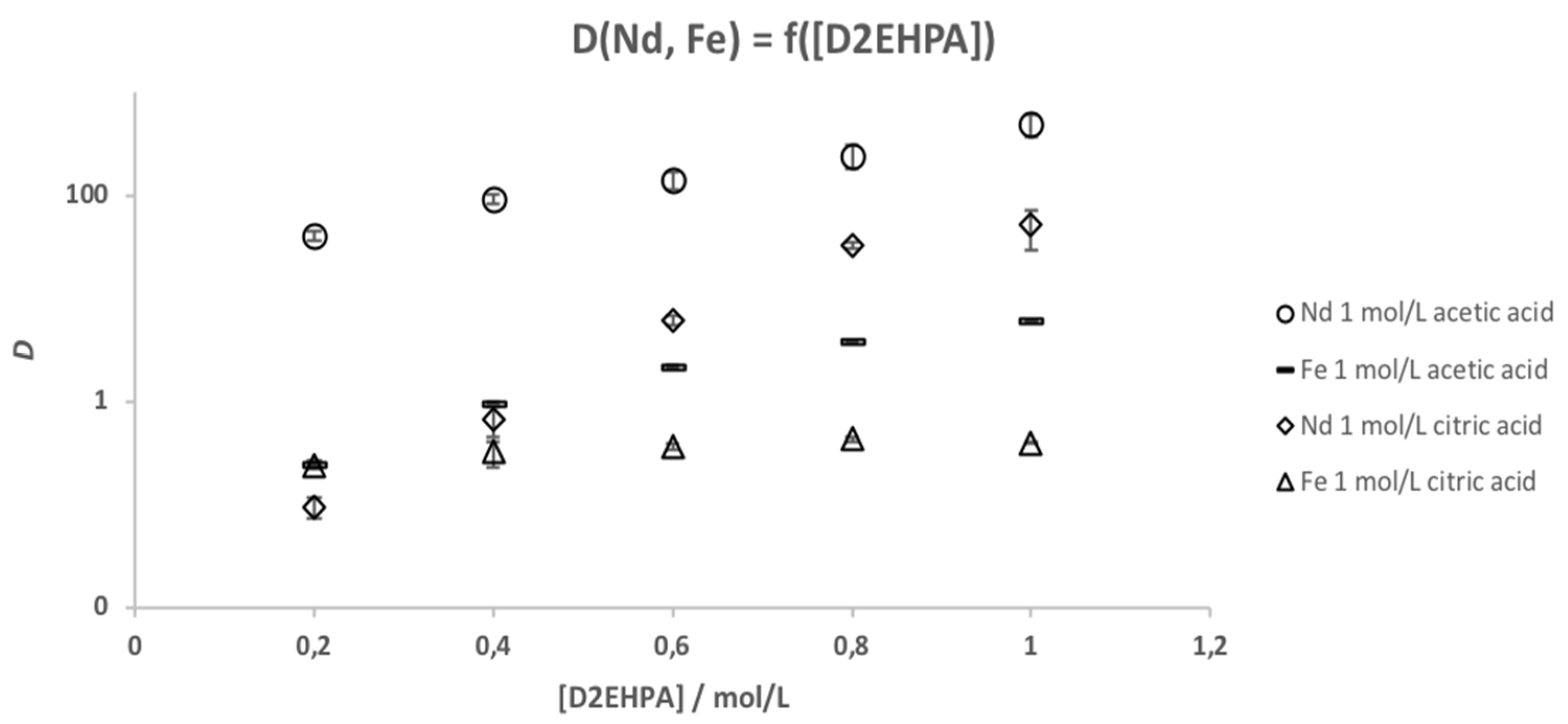
3.4. Solvent Extraction
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Firdaus, M.; Rhamdhani, M.A.; Durandet, Y.; Rankin, W.J.; McGregor, K. Review of High-Temperature Recovery of Rare Earth (Nd/Dy) from Magnet Waste. J. Sustain. Metall. 2016, 2, 276–295. [Google Scholar] [CrossRef]
- European Commission. Critical Raw Materials for the EU; Report of the Ad-hoc Working Group on Defining Critical Raw Materials: Brussels, Belgium, 2018. [Google Scholar]
- Binnemans, K.; Jones, P.T.; Blanpain, B.; Van Gerven, T.; Yang, Y.; Walton, A.; Buchert, M. Recycling of rare earths: A critical review. J. Clean. Prod. 2013, 51, 1–22. [Google Scholar] [CrossRef]
- Guyonnet, D.; Planchon, M.; Rollat, A.; Escalon, V.; Tuduri, J.; Charles, N.; Vaxelaire, S.; Dubois, D.; Fargier, H. Material flow analysis applied to rare earth elements in Europe. J. Clean. Prod. 2015, 107, 215–228. [Google Scholar] [CrossRef]
- Rollat, A.; Guyonnet, D.; Planchon, M.; Tuduri, J. Prospective analysis of the flows of certain rare earths in Europe at the 2020 horizon. Waste Manag. 2016, 49, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.S.; Parhi, P.K. Leaching kinetics study of neodymium from the scrap magnet using acetic acid. Sep. Purif. Technol. 2016, 160, 59–66. [Google Scholar] [CrossRef]
- Yang, Y.; Walton, A.; Sheridan, R.; Güth, K.; Gauß, R.; Gutfleisch, O.; Buchert, M.; Steenari, B.M.; Gerven, T.V.; Jones, P.T.; et al. REE Recovery from End-of-Life NdFeB Permanent Magnet Scrap: A Critical Review. J. Sustain. Metall. 2017, 3, 122–149. [Google Scholar] [CrossRef]
- Alonso, E.; Sherman, A.M.; Wallington, T.J.; Everson, M.P.; Field, F.R.; Roth, R.; Kirchain, R.E. Evaluating Rare Earth Element Availability: A Case with Revolutionary Demand from Clean Technologies. Environ. Sci. Technol. 2012, 46, 3406–3414. [Google Scholar] [CrossRef] [PubMed]
- Önal, M.A.R.; Borra, C.R.; Guo, M.; Blanpain, B.; Van Gerven, T. Recycling of NdFeB Magnets Using Sulfation, Selective Roasting, and Water Leaching. J. Sustain. Metall. 2015, 1, 199–215. [Google Scholar] [CrossRef]
- Yoon, H.-S.; Kim, C.; Chung, K.W.; Lee, S.-J.; Joe, A.-R.; Shin, Y.-H.; Lee, S.; Yoo, S.-J.; Kim, J.-G. Leaching kinetics of neodymium in sulfuric acid from E-scrap of NdFeB permanent magnet. Korean J. Chem. Eng. 2014, 31, 706–711. [Google Scholar] [CrossRef]
- Lee, C.; Chen, Y.; Liao, C.; Popuri, S.R.; Tsai, S.; Hung, C. Selective Leaching Process for Neodymium Recovery from Scrap Nd-Fe-B Magnet. Metall. Mater. Trans. 2013, 44, 5825–5833. [Google Scholar] [CrossRef]
- Lyman, J.W.; Palmer, G.R. Scrap Treatment Method for Rare Earth Transition Metal Alloys. U.S. Patent 5,129,945, 14 July 1992. [Google Scholar]
- Gergoric, M.; Ekberg, C.; Foreman, M.R.J.; Steenari, B.M.; Retegan, T. Characterization and Leaching of Neodymium Magnet Waste and Solvent Extraction of the Rare-Earth Elements Using TODGA. J. Sustain. Metall. 2017, 3, 638–645. [Google Scholar] [CrossRef]
- Parhi, P.K.; Sethy, T.R.; Rout, P.C.; Saranji, K. Separation and recovery of neodymium and praseodymium from permanent magnet scrap through the hydrometallurgical route. Sep. Sci. Technol. 2016, 51, 2232–2241. [Google Scholar] [CrossRef]
- Önal, M.A.R.; Chenna, E.A.; Borra, R.; Blanpain, B.; Gerven, T.V.; Guo, M. Recycling of NdFeB magnets using nitration, calcination and water leaching for REE recovery. Hydrometallurgy 2017, 167, 115–123. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, T.A.; Dreisinger, D.; Doyle, F. A critical review on solvent extraction of rare earths from aqueous solutions. Miner. Eng. 2014, 56, 10–28. [Google Scholar] [CrossRef]
- Riano, S.; Binnemans, K. Extration and separation of neodymium and dysprosium from used NdFeB magnets: an application of ionic liquids in solvent extraction towards the recycling of magnets. Green Chem. 2015, 17, 2931–2942. [Google Scholar] [CrossRef]
- Shabani, M.A.; Irannajad, M.; Azadmehr, A.R. Investigation on leaching of malachite by citric acid. Int. J. Miner. Metall. Mater. 2012, 19, 782–786. [Google Scholar] [CrossRef]
- Astuti, W.; Hirajima, T.H.; Sasaki, K.; Okibe, N. Comparison of effectiveness of citric acid and other acids in leaching of low-grade Indonesian saprolitic ores. Miner. Eng. 2016, 85, 1–16. [Google Scholar] [CrossRef]
- Park, H.; Jung, K.; Alorro, R.D.; Yoo, K. Leaching Behavior of Copper, Zinc and Lead from Contaminated Soil with Citric Acid. Mater. Trans. 2013, 54, 1220–1223. [Google Scholar] [CrossRef]
- Josso, P.; Roberts, S.; Teagle, D.A.H.; Pourret, O.; Herrington, R.; Albarran, P.L. Extraction and separation of rare earth elements from hydrothermal metalliferous sediments. Miner. Eng. 2018, 118, 106–121. [Google Scholar] [CrossRef]
- Fujimoto, J.; Tanaka, K.; Watanabe, N.; Takahashi, Y. Simultaneous recovery and separation of rare earth elements in ferromanganese nodules by using Shewanella putrefaciens. Hydrometallurgy 2016, 166, 80–86. [Google Scholar] [CrossRef]
- Astuti, W.; Hriajima, T.; Sasaki, K.; Okibe, N. Comparison of atmospheric citric acid leaching kinetics of nickel from different Indonesian saprolitic ores. Hydrometallurgy 2016, 161, 138–151. [Google Scholar] [CrossRef]
- Vandenberghe, L.P.S.; Soccol, S.R.; Pandey, A.; Lebeault, J.M. Microbial production of citric acid. Braz. Arch. Biol. Technol. 1999, 42, 263–276. [Google Scholar] [CrossRef]
- McDonald, R.G.; Whittington, B.I. Atmospheric acid leaching of nickel laterites review: Part I. Sulphuric acid technologies. Hydrometallurgy 2008, 91, 35–55. [Google Scholar] [CrossRef]
- Brown, M.A.; Kropf, A.J.; Paulenova, A.; Gelis, A.V. Aqueous complexation of citrate with neodymium(iii) and americium(iii): A study by potentiometry, absorption spectrophotometry, microcalorimetry, and XAFS. Dalton Trans. 2014, 43, 6446–6454. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.S.; Kim, A.R.; Kim, D.M.; Chang, T.S.; Kim, B.S.; Bae, J.W. Novel heterogeneous Rh-incorporated graphitic-carbon nitride for liquid-phase carbonylation of methanol to acetic acid. Catal. Commun. 2017, 99, 141–145. [Google Scholar] [CrossRef]
- Zanonato, P.L.; Bernardo, P.; Bismondo, A.; Rao, L.; Choppin, G.R. Thermodynamic Studies of the Complexation between Neodymium and Acetate at Elevated Temperatures. J. Solut. Chem. 2001, 30, 1–18. [Google Scholar] [CrossRef]
- Cotton, S. Lanthanide and Actinide Chemistry; John Wiley & Sons: Chichester, UK, 2006; ISBN 9780470010082. [Google Scholar]
- Nash, K.L.; Jensen, M.P. Analytical-scale separations of the lanthanides: A review of techniques and fundamentals. Separ. Sci. Technol. 2001, 36, 1257–1282. [Google Scholar] [CrossRef]
- Geist, A.; Nitsch, W.; Kim, J. On the kinetics of rare-earth extraction into D2EHPA. Chem. Eng. Sci. 1999, 54, 1903–1907. [Google Scholar] [CrossRef]
- Tunsu, C.; Petranikova, M.; Gergorić, M.; Ekberg, C.; Retegan, T. Reclaiming rare earth elements from end-of-life products: A review of the perspectives for urban mining using hydrometallurgical unit operations. Hydrometallurgy 2015, 156, 239–258. [Google Scholar] [CrossRef]
- Awais, M.; Coelho, F.; Degri, M.; Herraiz, E.; Walton, A.; Rowson, N. Hydrocyclone Separation of Hydrogen Decrepitated NdFeB. Recycling 2017, 2, 22. [Google Scholar] [CrossRef]
- Swain, B.; Cho, S.S.; Lee, G.H.; Lee, C.G.; Uhm, S. Extraction/Separation of Cobalt by Solvent Extraction: A Review. Korean Soc. Ind. Eng. Chem. 2015, 26, 631–639. [Google Scholar]
- Karakaplan, M.; Tural, S.; Tural, B.; Turgut, Y.; Hosgoren, H. The Solvent Extraction of Boron with Synthesized Aliphatic 1,3-Diols: Stripping and Extraction Behavior of Boron by 2,2,5-Trimethyl-1,3-hexanediol. Solvent Extr. Ion Exch. 2004, 22, 897–911. [Google Scholar] [CrossRef]
- Gergoric, M.; Ekberg, C.; Steenari, B.M.; Retegan, T. Separation of Heavy Rare-Earth Elements from Light Rare-Earth Elements Via Solvent Extraction from a Neodymium Magnet Leachate and the Effects of Diluents. J. Sustain. Metall. 2017, 3, 601–610. [Google Scholar] [CrossRef]
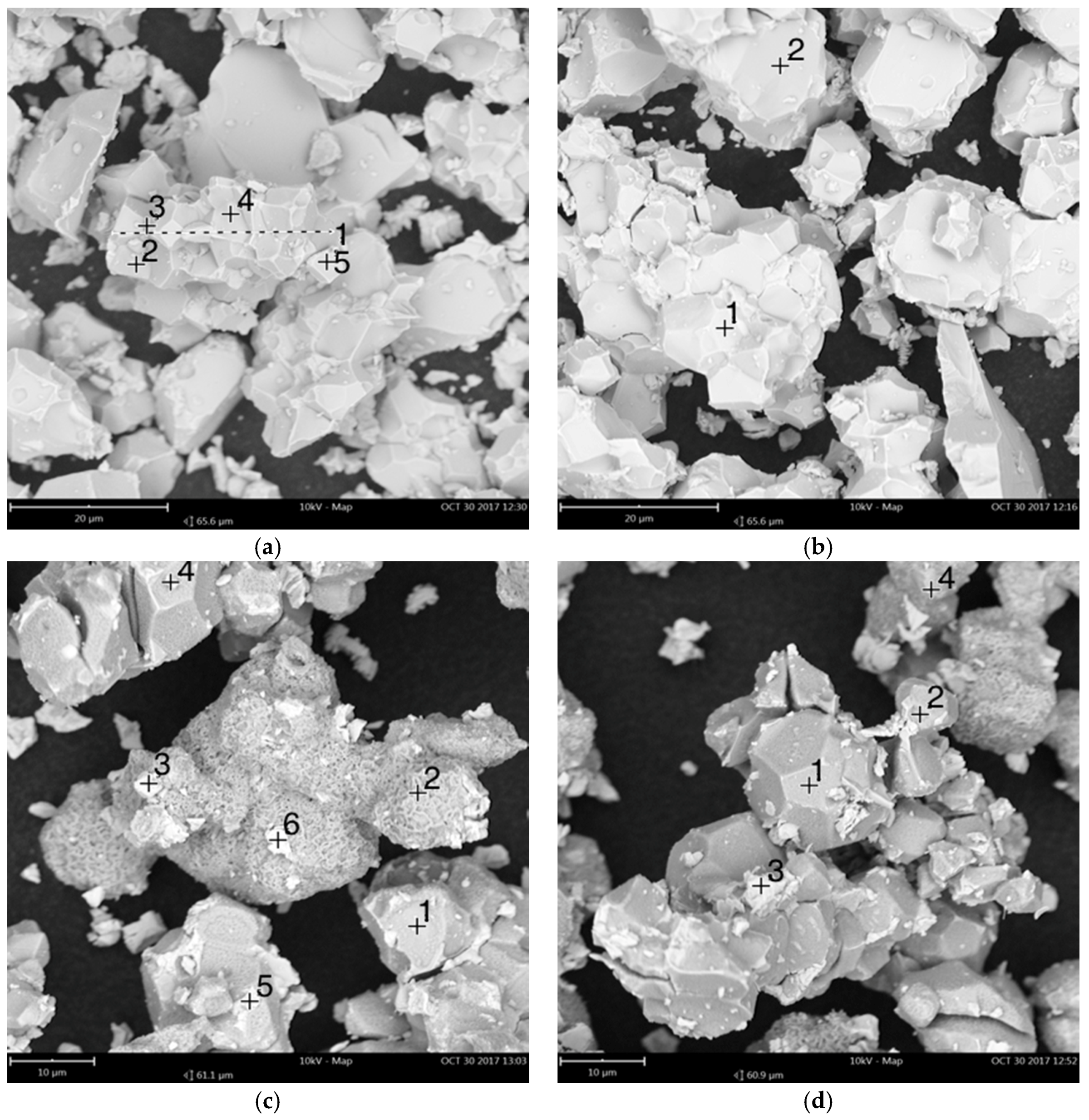


| Element | (a) Mass Fraction in HD NdFeB Magnet Powder/wt % [13] | (b) Mass Fraction in Roasted NdFeB Magnet Powder/wt % |
|---|---|---|
| Fe | 61.1 ± 1.0 | 56.4 ± 0.3 |
| Nd | 25.3 ± 0.7 | 22.7 ± 0.0 |
| Pr | 2.62 ± 0.17 | 2.73 ± 0.05 |
| Dy | 1.08 ± 0.27 | 0.77 ± 0.01 |
| Co | 1.42 ± 0.07 | 1.25 ± 0.03 |
| B | 1.00 ± 0.02 | 0.74 ± 0.01 |
| c (D2EHPA)/mol/L | SFNd/Fe (1 mol/L Acetic Acid) | SFNd/Fe (1 mol/L Citric Acid) |
|---|---|---|
| 0.2 | 161.9 ± 21.0 | 0.39 ± 0.10 |
| 0.4 | 94.9 ± 9.4 | 2.07 ± 0.86 |
| 0.6 | 64.5 ± 13.4 | 17.0 ± 2.4 |
| 0.8 | 64.1 ± 16.0 | 76.7 ± 5.9 |
| 1 | 82.2 ± 21.0 | 129 ± 55 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gergoric, M.; Ravaux, C.; Steenari, B.-M.; Espegren, F.; Retegan, T. Leaching and Recovery of Rare-Earth Elements from Neodymium Magnet Waste Using Organic Acids. Metals 2018, 8, 721. https://doi.org/10.3390/met8090721
Gergoric M, Ravaux C, Steenari B-M, Espegren F, Retegan T. Leaching and Recovery of Rare-Earth Elements from Neodymium Magnet Waste Using Organic Acids. Metals. 2018; 8(9):721. https://doi.org/10.3390/met8090721
Chicago/Turabian StyleGergoric, Marino, Christophe Ravaux, Britt-Marie Steenari, Fredrik Espegren, and Teodora Retegan. 2018. "Leaching and Recovery of Rare-Earth Elements from Neodymium Magnet Waste Using Organic Acids" Metals 8, no. 9: 721. https://doi.org/10.3390/met8090721
APA StyleGergoric, M., Ravaux, C., Steenari, B.-M., Espegren, F., & Retegan, T. (2018). Leaching and Recovery of Rare-Earth Elements from Neodymium Magnet Waste Using Organic Acids. Metals, 8(9), 721. https://doi.org/10.3390/met8090721






