A Kinetic Study on the Preparation of AlNi Alloys by Aluminothermic Reduction of NiO Powders
Abstract
1. Introduction
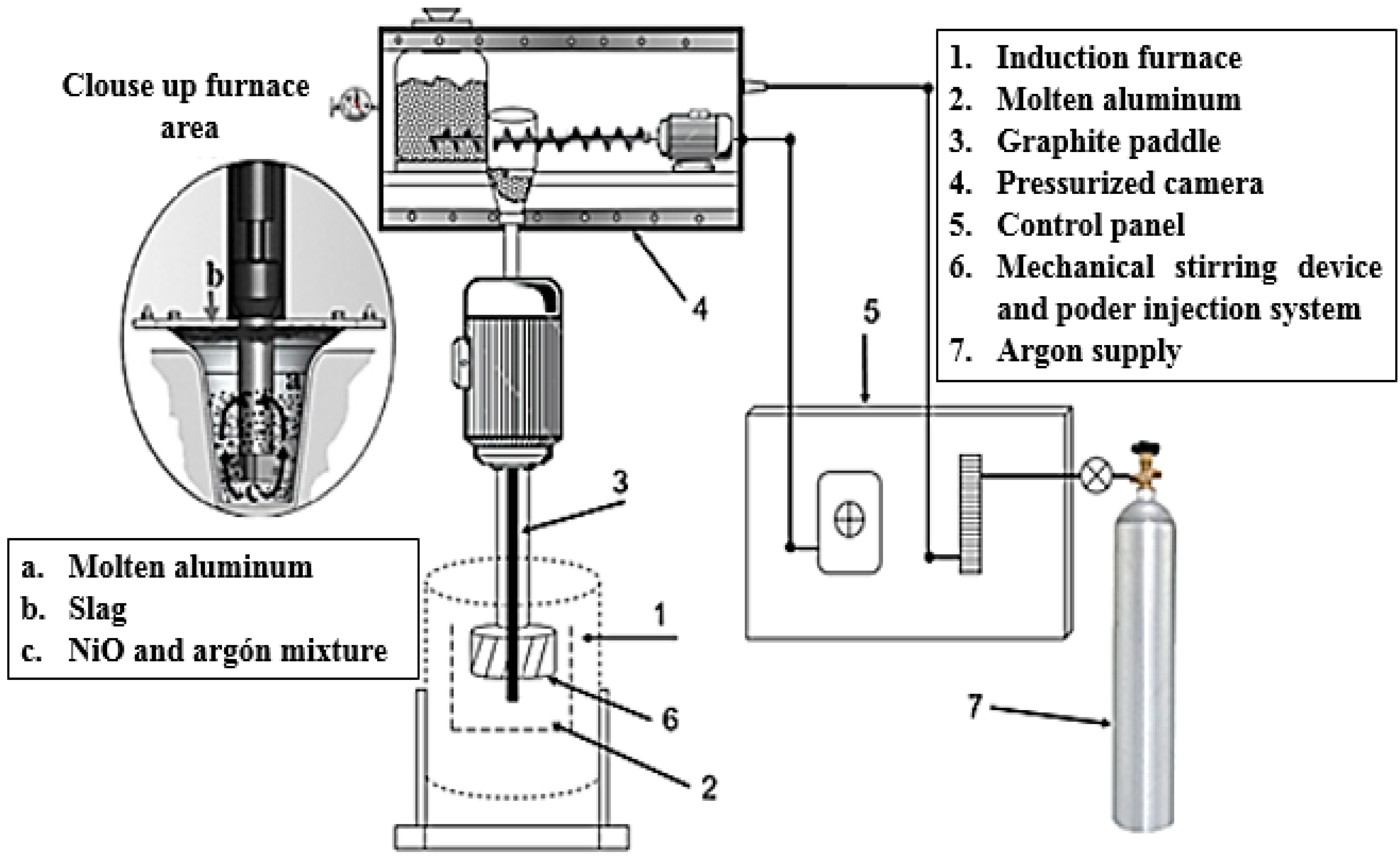
2. Materials and Methods
3. Results and Discussion
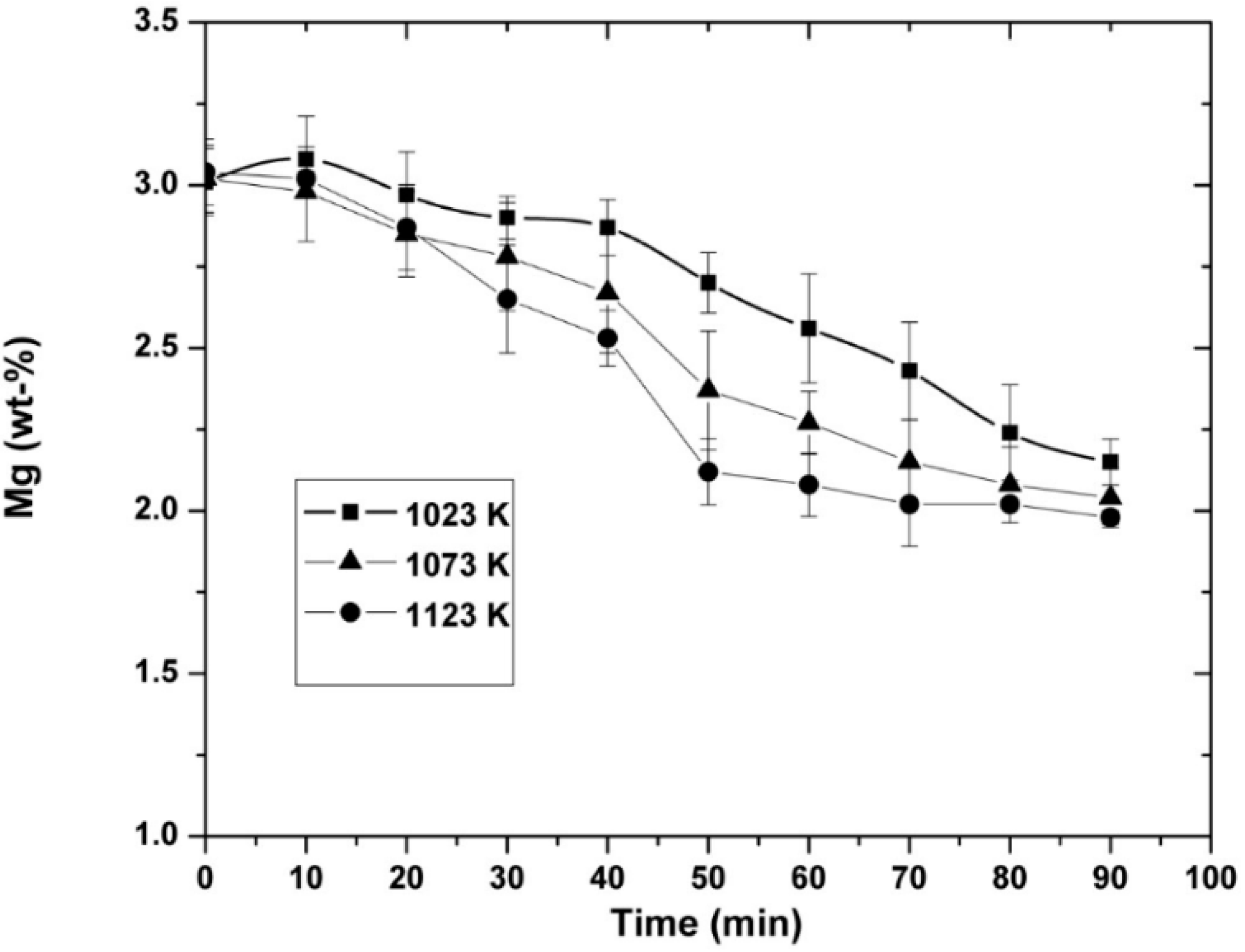
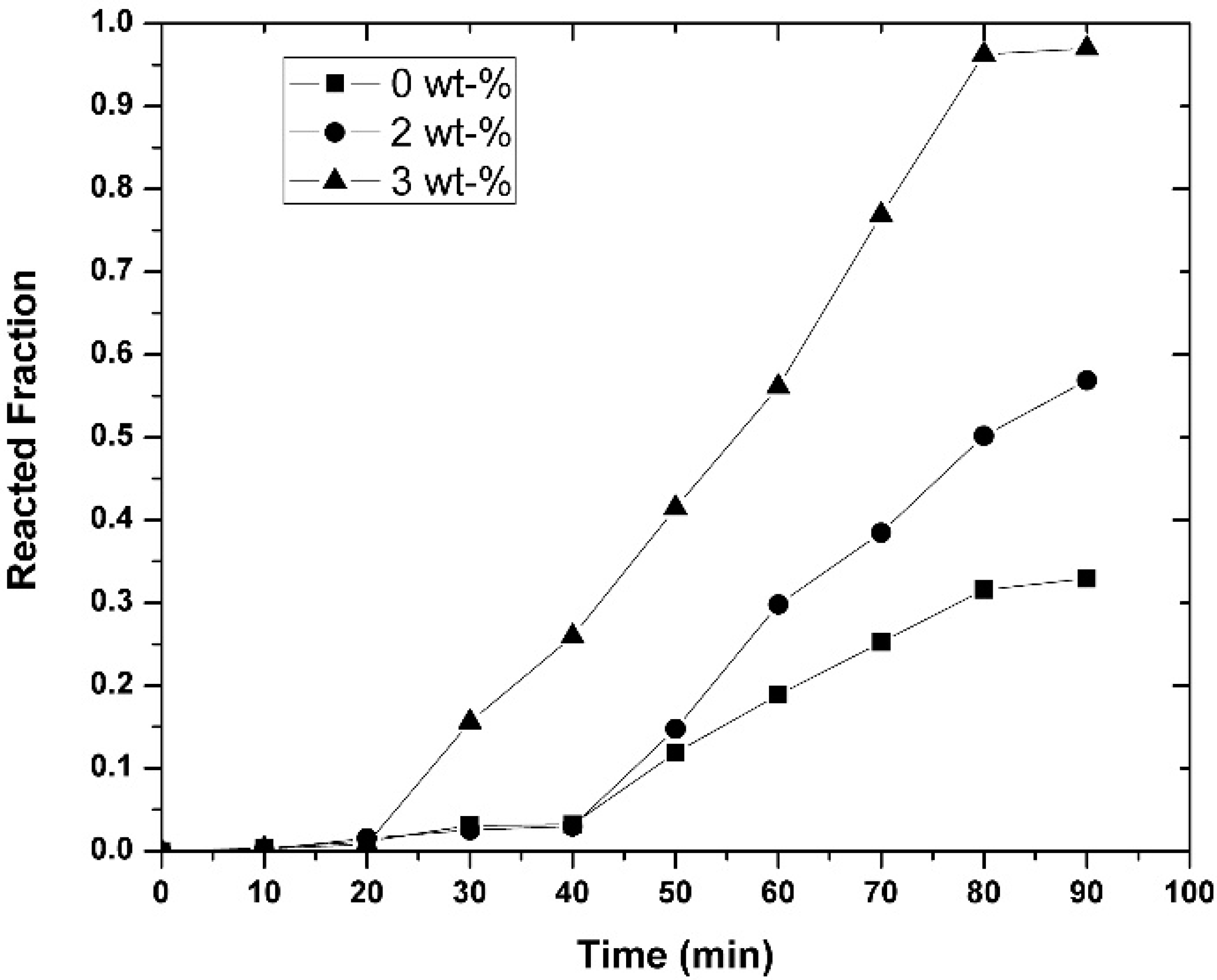
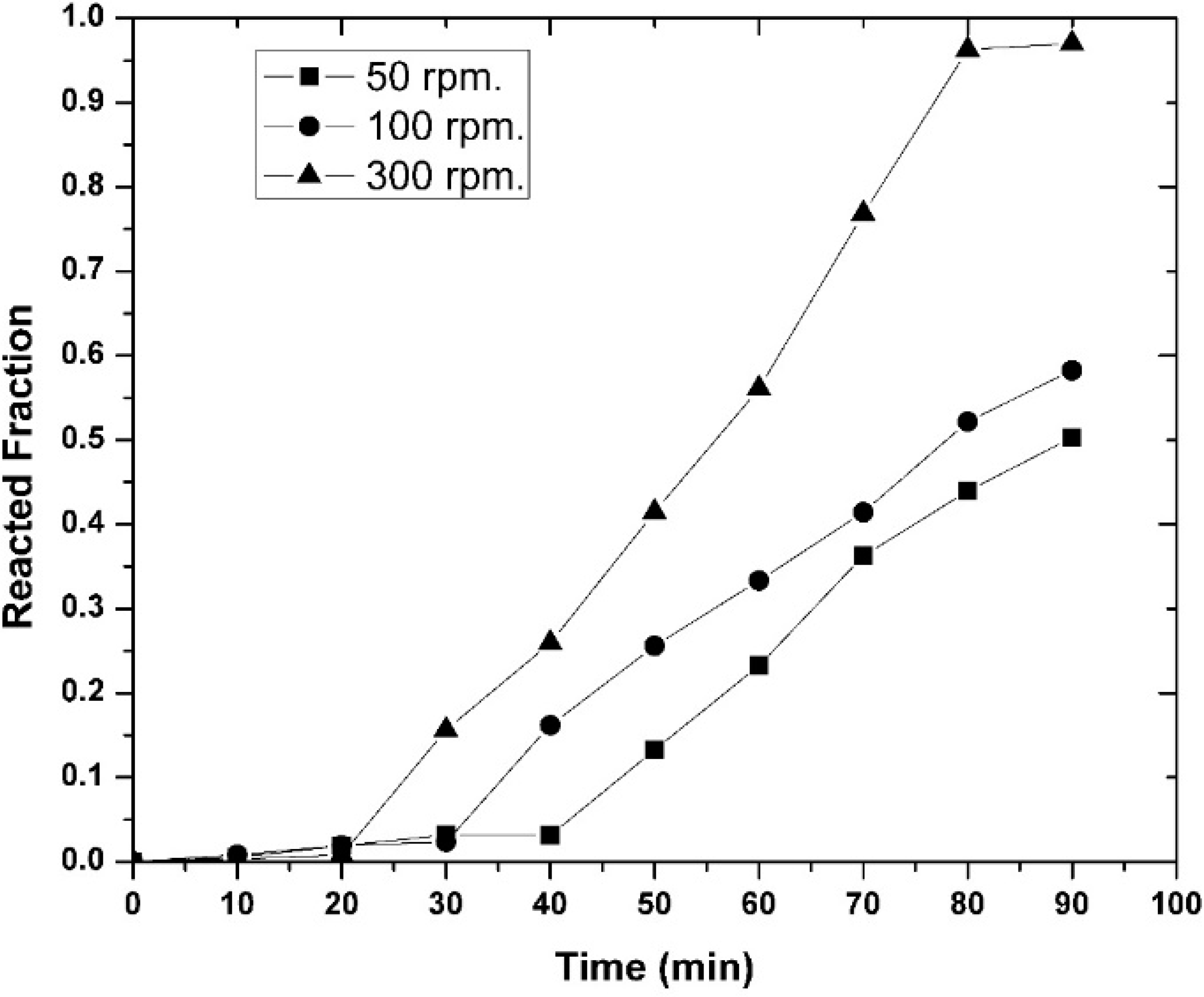
3.1. Experimental Results
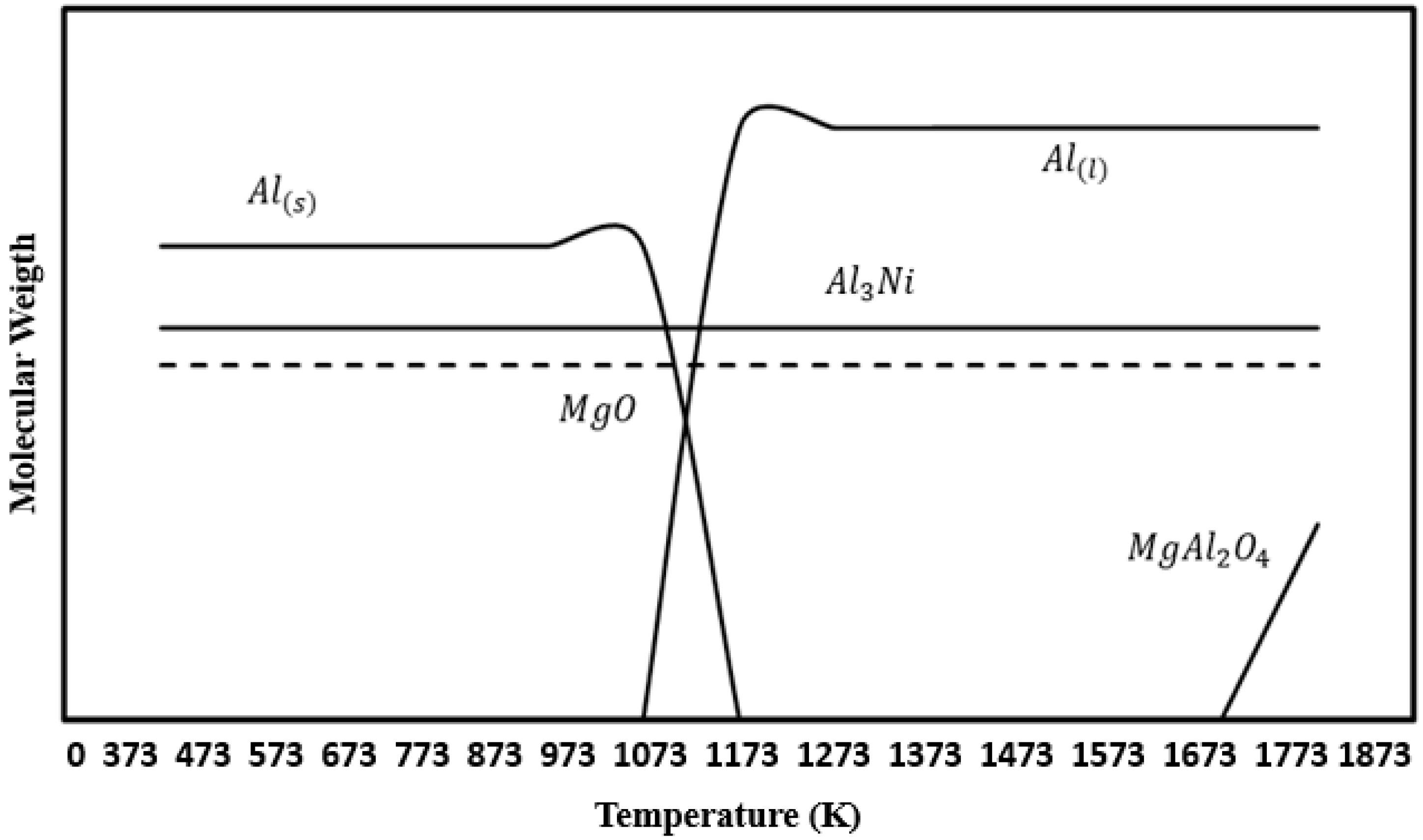
3.2. Thermodynamic Consideration for the Al-NiO-Mg System
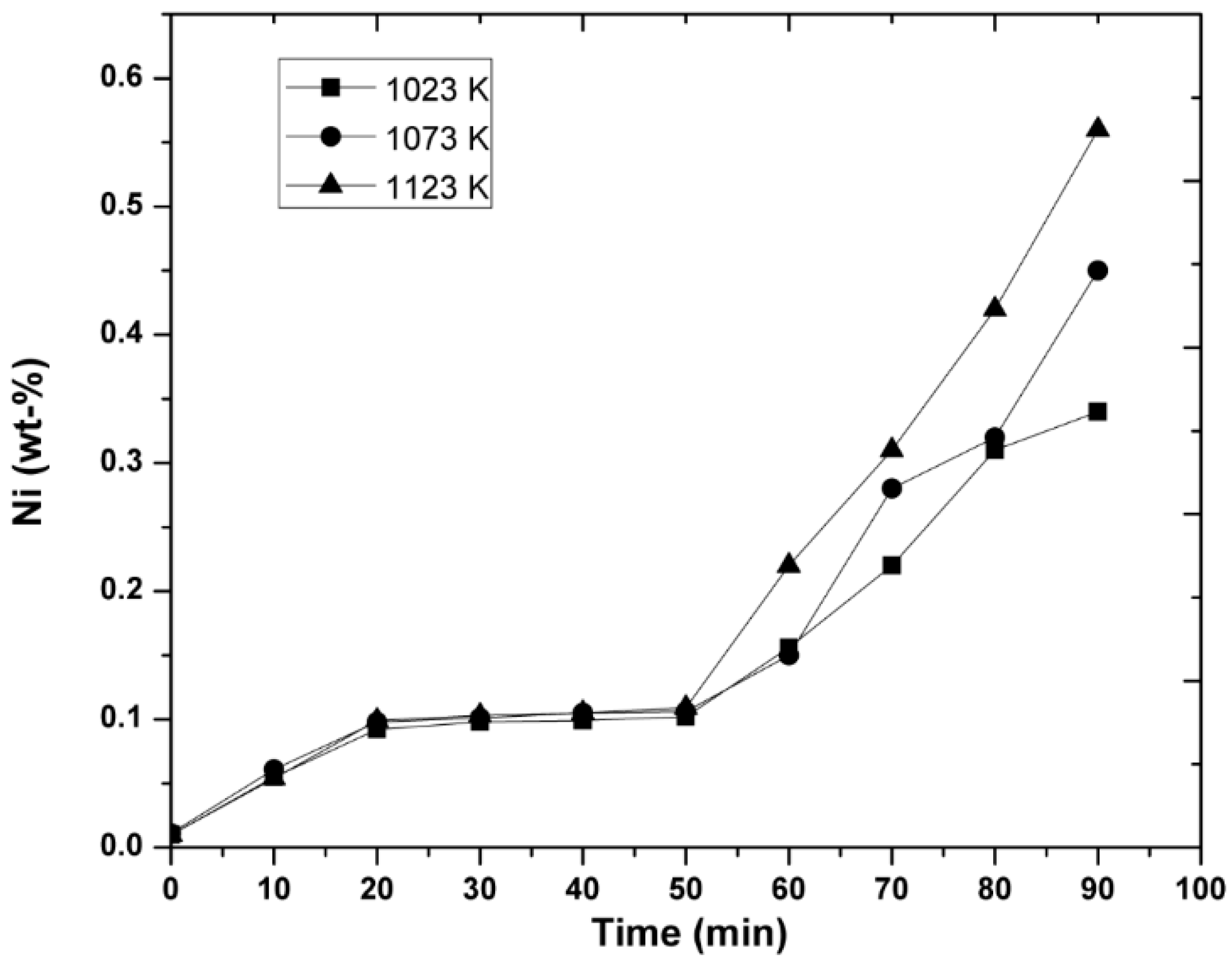
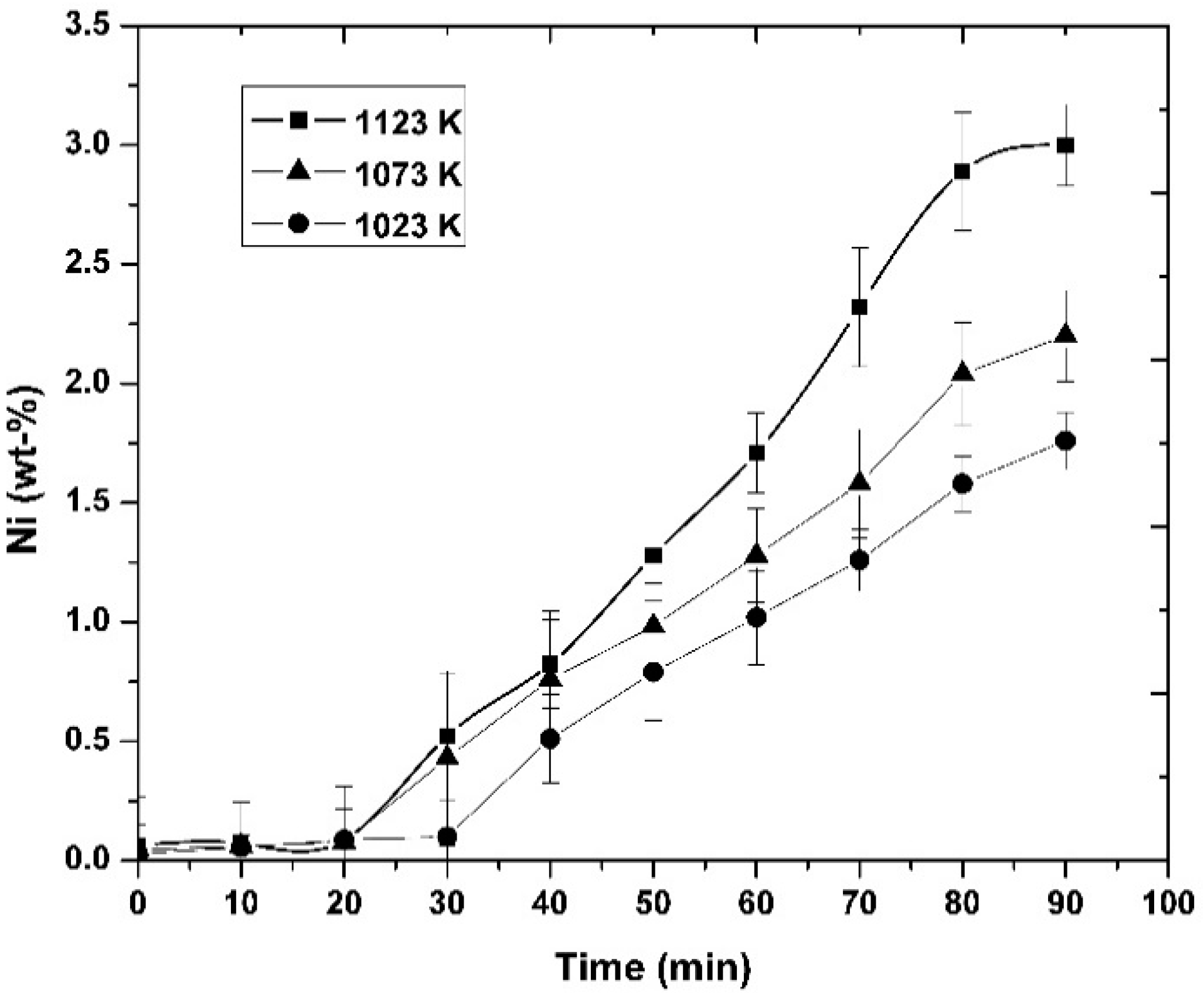
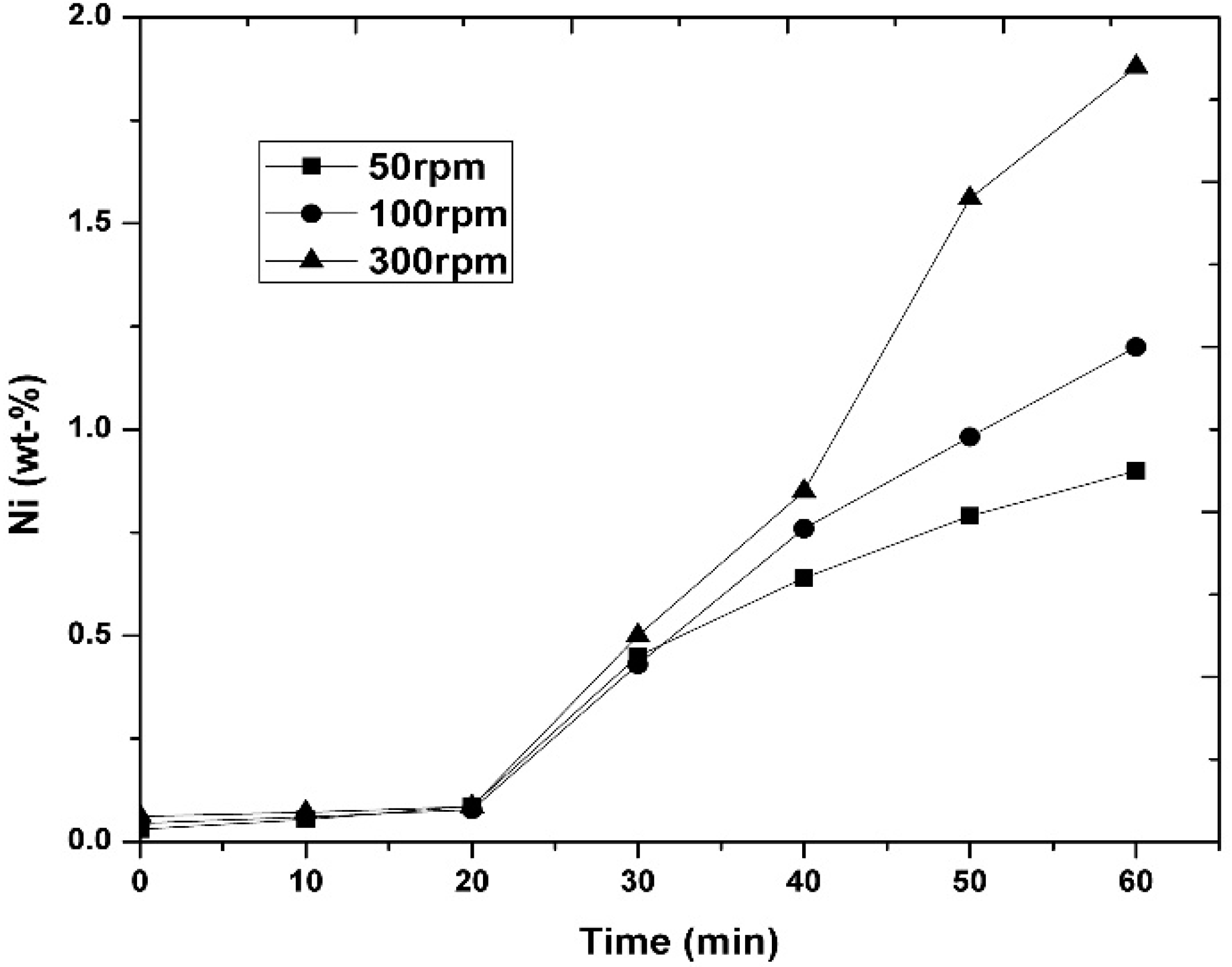
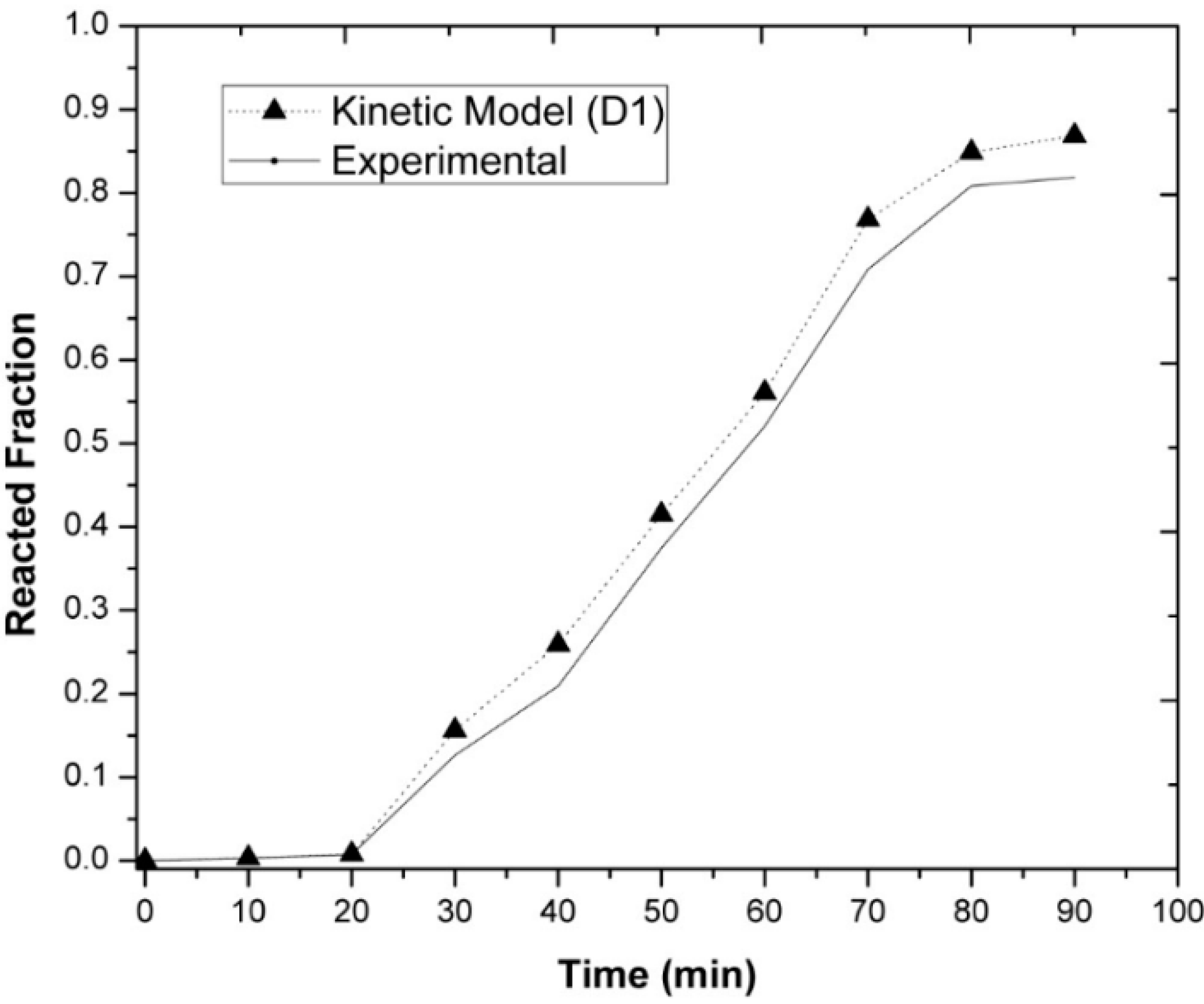
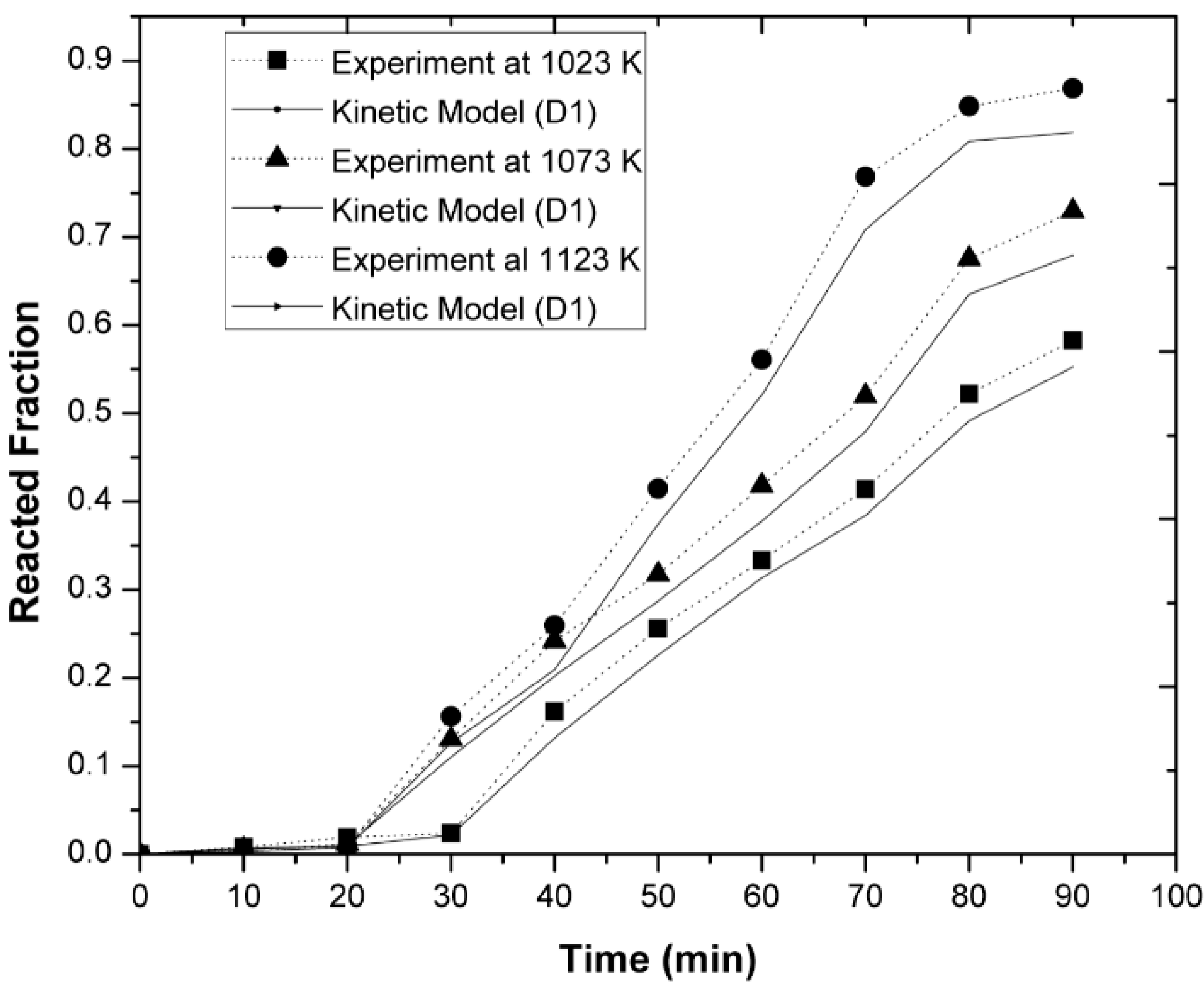
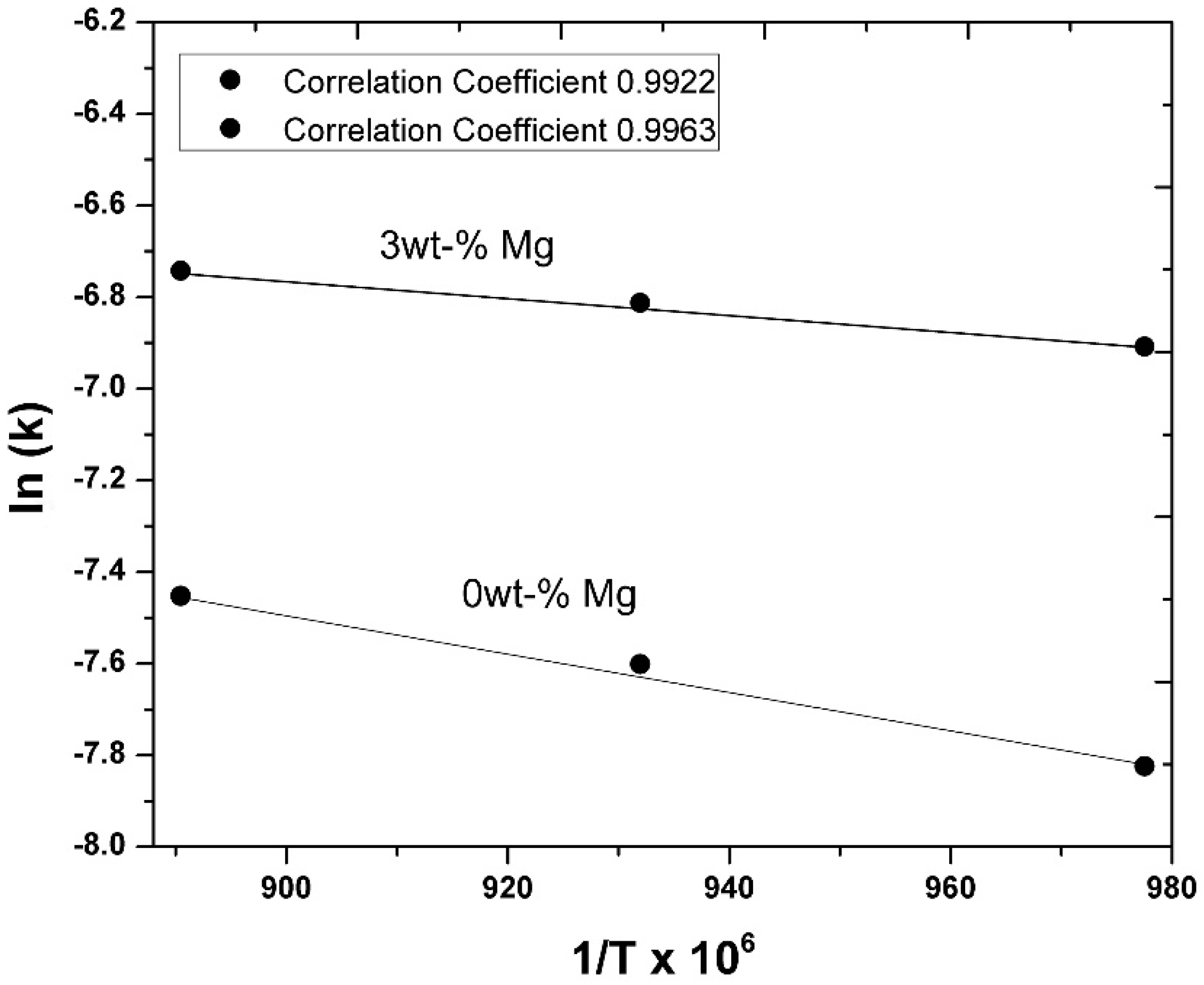
3.3. Kinetic Measurements
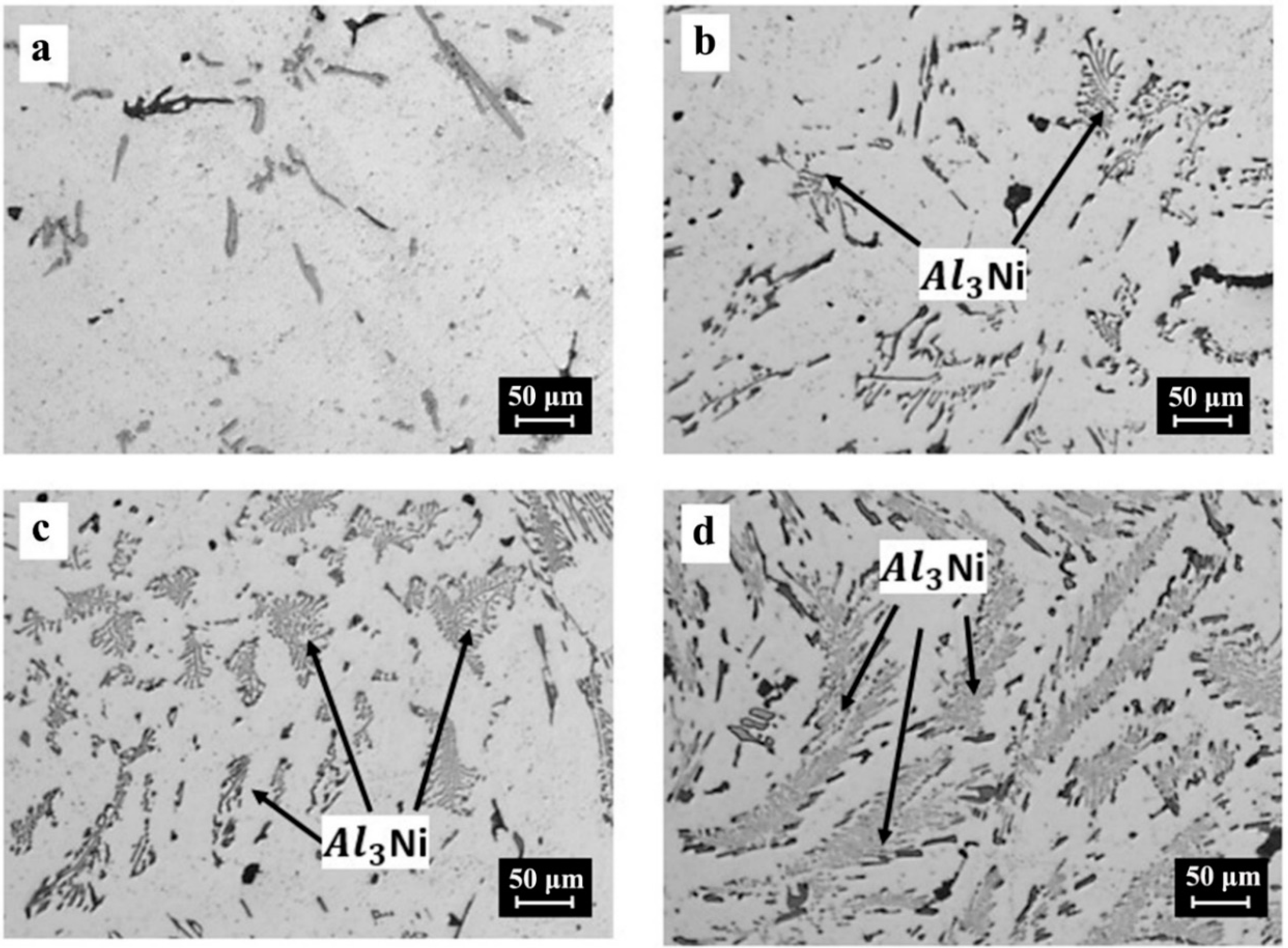
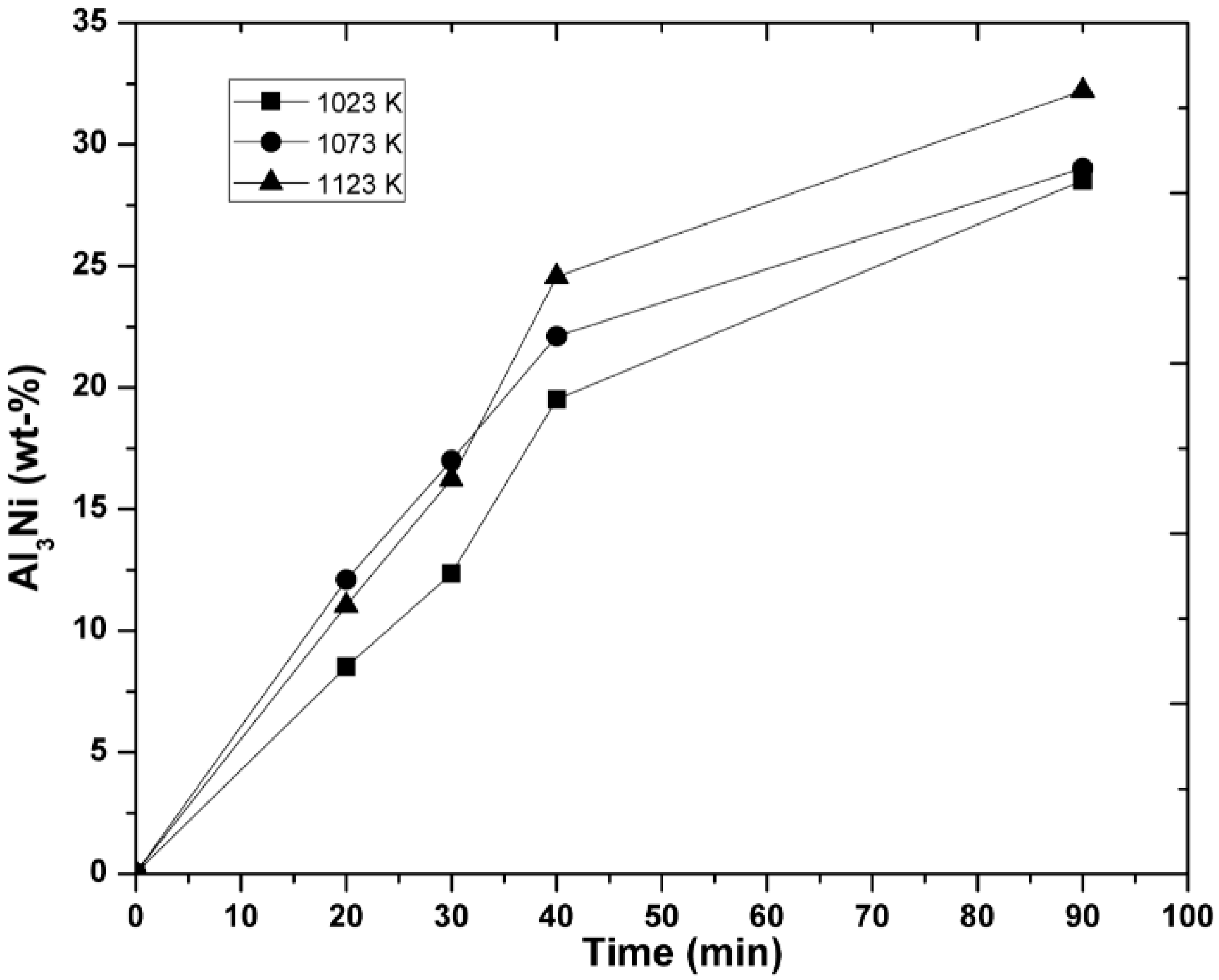
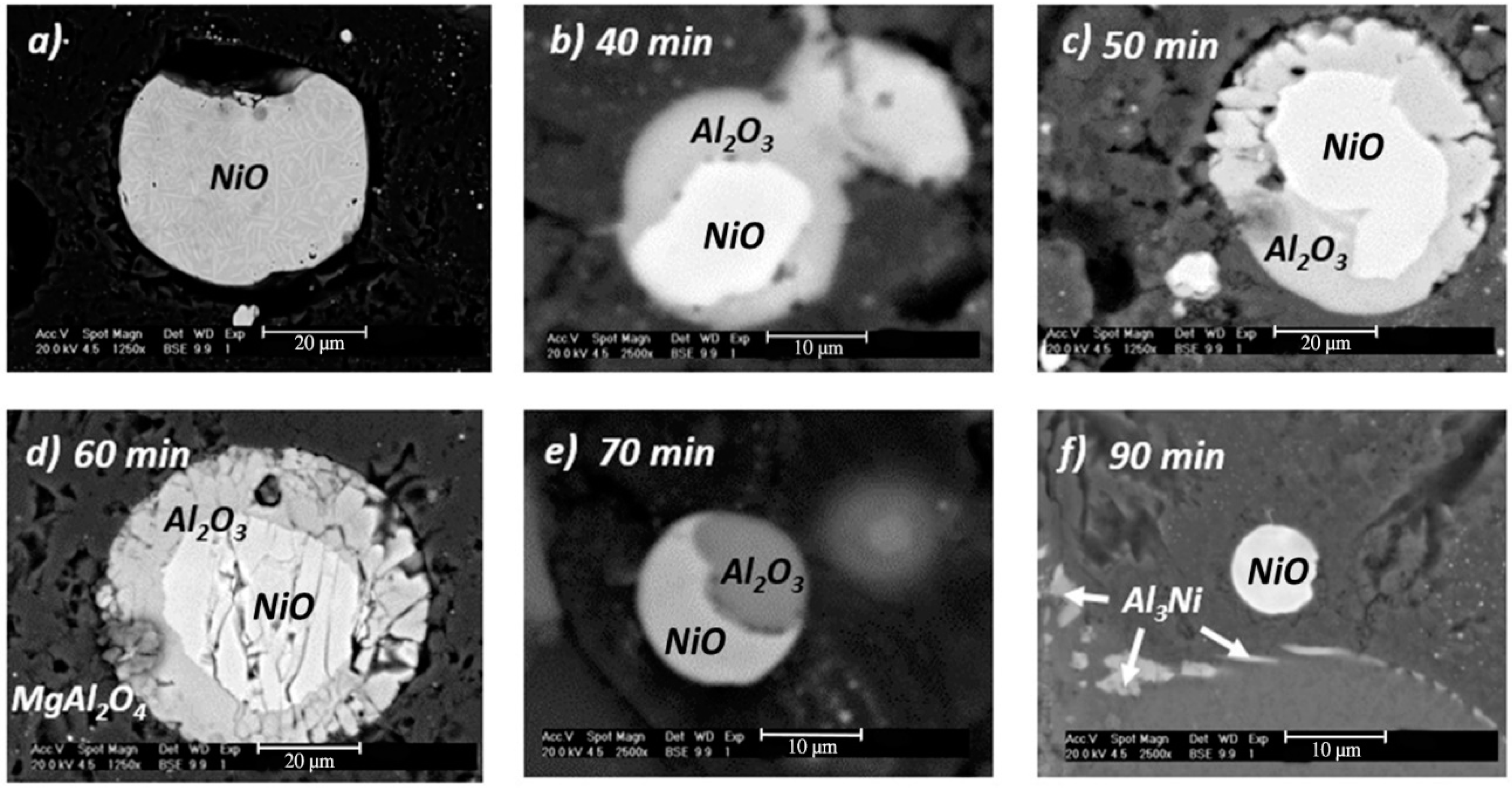
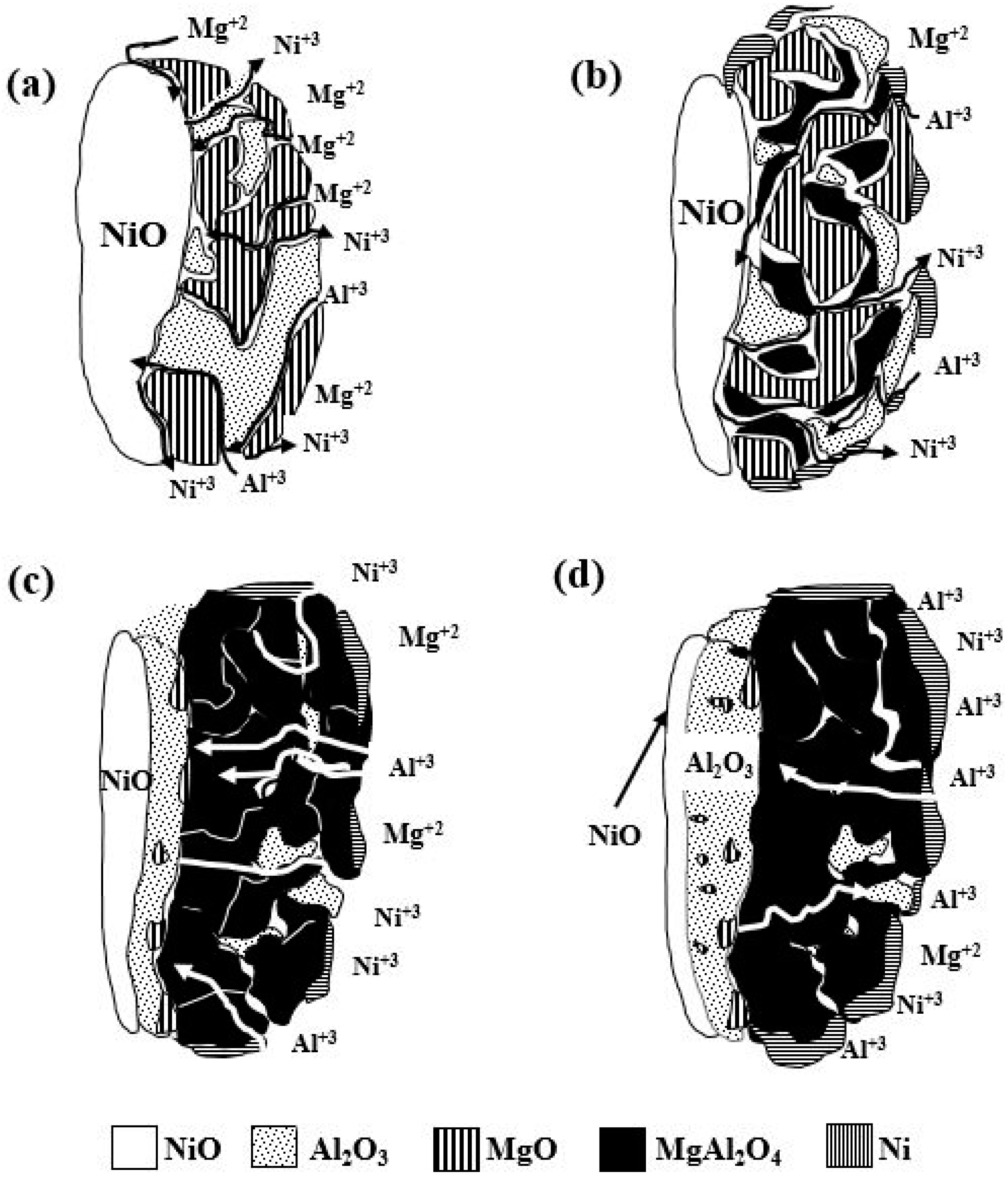
3.4. Mechanism of Reaction
4. Conclusions
- The aluminothermic reduction of NiO was studied at a laboratory scale by means of the powder injection technique, assisted by mechanical agitation, and achieved an increase in the nickel concentration in the solidified alloys of up to 3 wt-% for some of the experiments carried out.
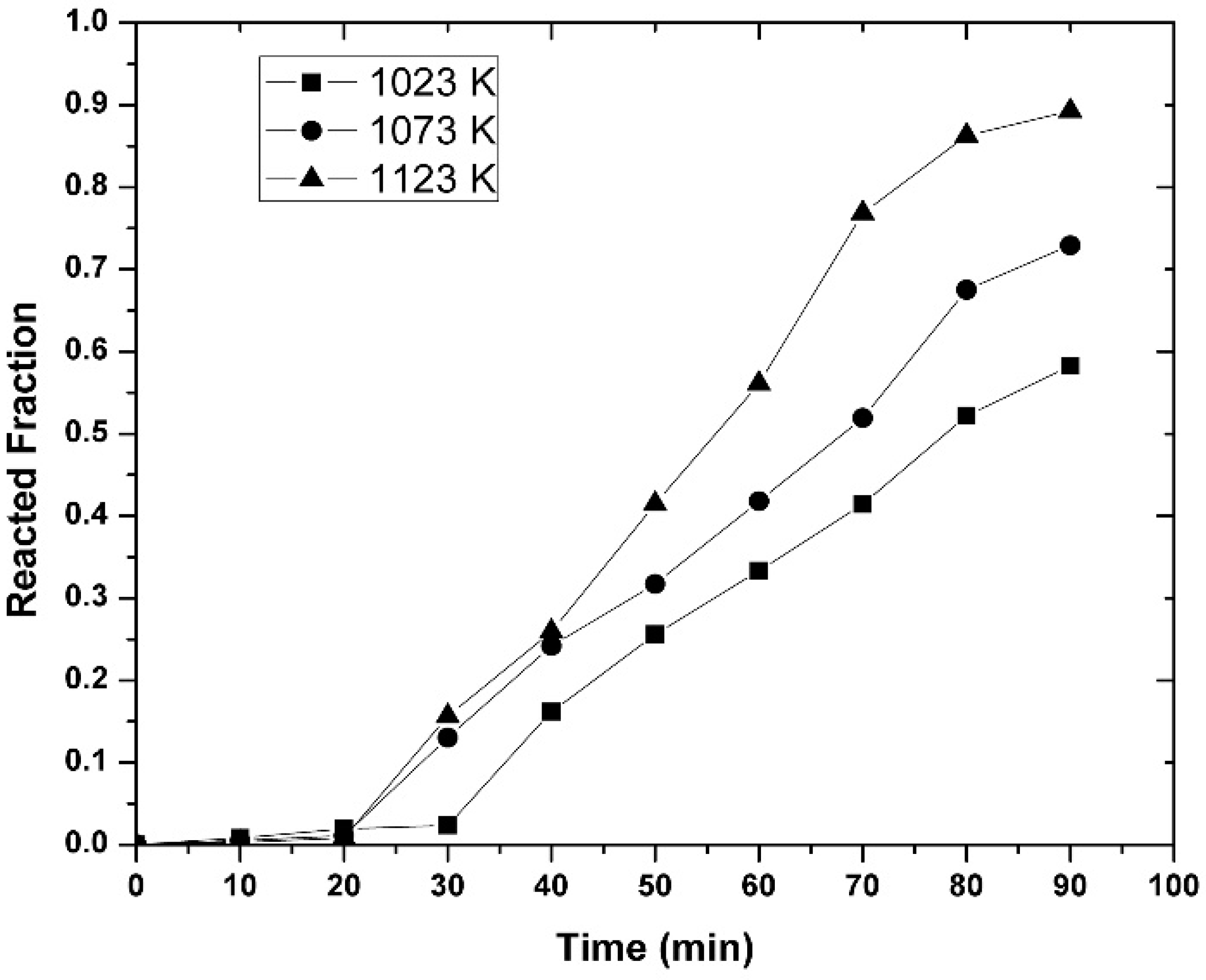
- It was observed that increasing the temperature favored the increase in the nickel concentration, because the mechanisms that govern the kinetics of the process, diffusion to the boundary layer, diffusion inside the layers of reaction products, and chemical reaction, were thermally activated. Increasing the mixing conditions by increasing the stirring speed of the molten bath using mechanical agitators at the velocity of 300 rpm promoted a greater agitation, therefore improving the efficiency of the reaction.
- Increasing the initial magnesium concentration in the molten alloy allowed the Ni concentration to increase in the alloys, as was thermodynamically and experimentally demonstrated.
- The experimentally obtained values of the Ni concentration as a function of time for different values of the parameters investigated (temperature, agitation speed, or initial Mg concentration) were adjusted to the kinetic equation of the diffusion model, which adjusted reasonably well. This allowed us to determine the values of some kinetic parameters of interest. On the other hand, the activation energy of the processes based on the Arrhenius equation was determined to be 15.80 KJ mol−1 for alloys containing an initial amount of 3 wt-% Mg.
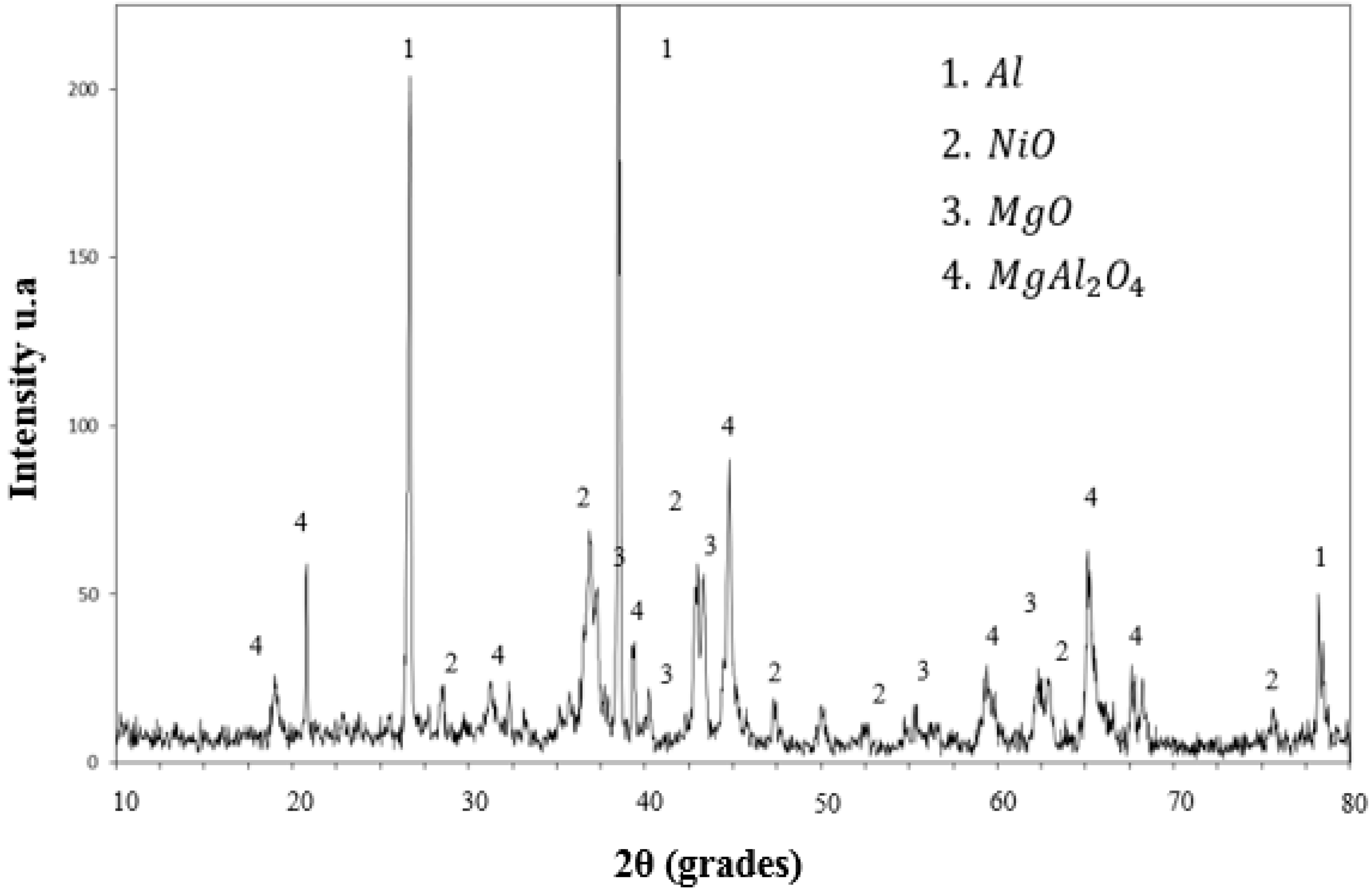
- With respect to the reaction mechanism, it was found that the step that controlled the overall chemical reaction was the diffusion of the Al and Mg atoms to the boundary layer, where they reacted with NiO particles, releasing Ni and forming Al2O3 and MgO as the reaction products. In turn, these compounds formed MgAl2O4 during cooling. The formation and breaking of MgAl2O4 into many crystals ensured the porosity required for the diffusion of the chemical species involved.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- López, L.N.; Fernandes, H.; Gutiérrez, A.; Sánchez, J.A. Turning of thick thermal spray coatings. J. Therm. Spray Technol. 2001, 10, 249–254. [Google Scholar]
- Beranoagirre, A.; Lopez de Lacalle, L.N. Optimising the milling of titanium aluminide alloys. Int. J. Math. Math. Sci. 2010, 3, 425–436. [Google Scholar] [CrossRef]
- Priarone, P.C.; Klocke, F.; Giulia, M.; Lung, D.; Settineri1, L. Tool life and surface integrity when turning titanium aluminides with PCD tools under conventional wet cutting and cryogenic cooling. Int. J. Adv. Manuf. Technol. 2016, 85, 807–816. [Google Scholar] [CrossRef]
- Beranoagirre, A.; Olvera, D.; López de Lacalle, L.N. Milling of gamma titanium–aluminum alloys. Int. J. Adv. Manuf. Technol. 2012, 62, 83–88. [Google Scholar] [CrossRef]
- Quintana, R.; Perdomo, L.; Cruz, A.; Gomez, L.; Garcia, L.L.; Cerpa, A.; Cores, A. Obtención simultanea de ferroaleación multicomponente y escoria a partir de arenas negras, para el desarrollo de consumibles de soldadura por arco eléctrico. Rev. Metal. 2004, 40, 294–303. [Google Scholar] [CrossRef]
- Ai, D.; Liu, K.; Lu, Z.; Zou, M.; Zeng, D.; Jun, M. Aluminothermal synthesis and characterization Li3V2-xAlx(PO4)3 cathode materials for lithium ion batteries. Electrochim. Acta 2011, 56, 2823–2827. [Google Scholar] [CrossRef]
- Muñiz, R.; Flores, A.; Torres, J. A kinetic study of the strontium extraction by metallothermic reduction using submerged SrO powders injection. Mater. Lett. 2008, 62, 637–640. [Google Scholar] [CrossRef]
- Luna, S.; Flores, A.; Muñiz, R.; Fernández, A.; Torres, J.; Rodriguez, N.; Ortíz, J.C.; Orozco, P. Cerium extraction by metallothermic reduction using cerium oxide powder injection. J. Rare Earths 2011, 29, 74–77. [Google Scholar] [CrossRef]
- Flores, A.; Juárez, R.; Torrres, J.; Ayala, Z. A kinetic study on the aluminothermic reduction of ZrO2. J. Eng. Technol. 2012, 2, 17–22. [Google Scholar]
- Guía, J.C. Estudio de la Reducción Metalotérmica de Una Mezcla de Fe3O4-Cr2O3 para la Obtención de Fe-C. Master’s Thesis, CINVESTAV-Saltillo, Ramos Arizpe, Mexico, 2010. [Google Scholar]
- Ochoa, R.M. Utilización de Electrodos de Baterías Alcalinas Descargadas como Materia Prima para la Elaboración de Aleaciones Al-Zn-Mg y Al-Mn’. Master’s Thesis, CINVESTAV Saltillo, Ramos Arizpe, Mexico, 2009. [Google Scholar]
- Udhayabanu, V.; Singh, N.; Murty, B.S. Mechanical activation of aluminothermic reduction of NiO by high energy ball milling. J. Alloys Compd. 2010, 497, 142–146. [Google Scholar] [CrossRef]
- López, F.J. Estudio Termodinámico y Cinético de la Reducción de SrCO3 por Al Bajo Condiciones de Vacío. Master’s Thesis, CINVESTAV Saltillo, Ramos Arizpe, Mexico, 2006. [Google Scholar]
- Muñiz, C.R. Estudio del Proceso de Elaboración de Aleaciones Maestras Al–Si–Sr Mediante la Inyección de Polvos de SrCO3. Ph.D. Thesis, CINVESTAV Saltillo, Ramos Arizpe, Mexico, 2008. [Google Scholar]
- Zhao, K.; Feng, N.; Wang, Y. Fabrication of Ti-Al intermetallics by a two-stage aluminothermic reduction process using Na2TiF6. Intermetallics 2017, 85, 156–162. [Google Scholar] [CrossRef]
- La, P.; Li, Z.; Li, C.; Hu, S.; Lu, X.; Wei, Y.; Wei, F. Effect of substrates on microstructure and mechanical properties of nano-eutectic 1080 steel produced by aluminothermic reaction. Mater. Charact. 2014, 92, 84–90. [Google Scholar] [CrossRef]
- Mishra, K.; Zheng, J.; Patel, R.; Estevez, L.; Jia, H.; Luo, L.; Khoury, P.E.; Li, X.; Zhou, X.D.; Zhang, J.G. High performance Si@C anodes synthesized by low temperature aluminothermic reaction. Electrochim. Acta 2018, 269, 509–516. [Google Scholar] [CrossRef]
- Liu, F.G.; Wen, C.D.; Hu, X.W.; Gao, B.L.; Shi, Z.N.; Wang, Z.W. Preparation of aluminum-zirconium master alloy by aluminothermic reduction in cryolite melt. JOM 2017, 69, 2644–2647. [Google Scholar] [CrossRef]
- Malekia, A.; Hosseini, N.; Niroumand, B. A review on aluminothermic reaction of Al/ZnO system. Ceram. Int. 2018, 44, 10–23. [Google Scholar] [CrossRef]
- Ochoa, R.; Flores, A.; Torres, J. Effect of magnesium on the aluminothermic reduction rate of zinc oxide obtained from spent alkaline battery anodes for the preparation of Al-Z-Mg alloys. J. Int. Miner. Metall. Mater. 2016, 23, 458–465. [Google Scholar] [CrossRef]
- HSC Chemistry Software, Version 6.12. Outotec. 2007. Available online: https://www.outotec.com (accessed on 9 July 2018).
- Thermochemical Software and Data Base System, Version 6.1. FactSage. 2009. Available online: http://www.factsage.com/ (accessed on 9 July 2018).
- Guedes, M.; Ferreira, J.M.; Ferro, A.C. A study on CuO-Al2O3 infiltration by aluminium. Mater. Sci. Forum 2010, 636–637, 571–577. [Google Scholar] [CrossRef]
- Langlais, J.; Harris, R. Strontium extraction by aluminothermic reduction. Can. Metall. Q 1991, 31, 127–131. [Google Scholar] [CrossRef]
- Pai, B.C.; Ray, S. Fabrication of aluminum–alumina (magnesia) particulate composites in foundries using magnesium additions to melts. Mater. Sci. Eng. A 1976, 24, 31–44. [Google Scholar] [CrossRef]
- Gul, F.; Karakulak, E.; Yamanoglu, R.; Zeren, M. Mechanical properties of Al-Ni Cast alloys. In Proceedings of the 23rd International Conference on Metallurgy and Materials, Brno, Czech Republic, 21–23 May 2014; pp. 1283–1287. [Google Scholar]
- Bergsmark, E.; Simensen, C.J.; Kofstad, P. The oxidation of molten aluminum. Mater. Sci. Eng. A 1989, 120–121, 91–95. [Google Scholar] [CrossRef]
- Ochoa, R. Relación Microestructura—Propiedades Mecánicas de las Aleaciones Al-Zn-Mg-Cu y Al-Zn-Mg-Cu-1% Li Elaboradas por Reducción Aluminotérmica del Ánodo de Pilas Alcalinas Descargadas y Latas para Bebidas. Ph.D. Thesis, CINVESTAV Saltillo, Ramos Arizpe, Mexico, 2016. [Google Scholar]
- Allaire, C. Furnaces: Improving low cement castable by non-wetting additives. JOM 2001, 53, 24–27. [Google Scholar]
- Brown, M.E.; Gallagher, P.K. Handbook of Thermal Analysis and Calorimetry; Elsevier: London, UK, 2008; pp. 148–149. [Google Scholar]
- Zhong, W.M.; L’Espérance, G.L.; Suery, M. Interfacial reactions in Al-Mg (5083)/SiCp composites during fabrication and remelting. Metall. Mater. Trans. A 1995, 26, 2625–2635. [Google Scholar] [CrossRef]
- Mcleod, A.D.; Gabryel, C.M. Kinetics of the growth of spinel, MgAl2O4, on alumina particulate in aluminum alloys containing magnesium. Metall. Mater. Trans. A 1992, 23, 1279–1283. [Google Scholar] [CrossRef]
- Molins, R.; Bartout, J.D.; Bienvenu, Y. Microstructural and analytical characterization of Al2O3-(Al-Mg) composite interfaces. Mater. Sci. Eng. A 1991, 135, 111–117. [Google Scholar] [CrossRef]
- Dimotakis, P.E. The mixing transition in turbulent flows. J. Fluid. Mech. 2000, 409, 69–98. [Google Scholar] [CrossRef]
| Factors | Level | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| Temperature (K) | 1023 | 1073 | 1123 |
| Mg (wt-%) | 0 | 2 | 3 |
| Agitation (rpm) | 50 | 100 | 300 |
| E1 1023,0,50 | E2 1023,0,100 | E3 1023,0,300 | E4 1073,2,50 | E5 1073,2,100 |
| E6 1073,2,300 | E7 1073,3,50 | E8 1073,3,100 | E9 1073,3,300 | E10 1073,0,50 |
| E11 1073,0,100 | E12 1073,0,300 | E13 1073,2,50 | E14 1073,2,100 | E15 1073,2,300 |
| E16 1073,3,50 | E17 1073,3,100 | E18 1073,3,300 | E19 1123,0,50 | E20 1123,0,100 |
| E21 1123,0,300 | E22 1123,2,50 | E23 1123,2,100 | E24 1123,2,300 | E25 1123,3,50 |
| E26 1123,3,100 | E27 1123,3,30 |
| Element | Si | Fe | Cu | Mn | Mg | Cr | Ni | Zn | Sn | Ti | Al |
|---|---|---|---|---|---|---|---|---|---|---|---|
| wt-% | 0.013 | 0.48 | 0.025 | 0.012 | 0.089 | 0.0012 | 0.0003 | 0.021 | 0.013 | 0.0012 | 99.34 |
| Element | Ni | Co | Fe | Cu | Zn | Mn | Mg | Ca | Na | S | O |
|---|---|---|---|---|---|---|---|---|---|---|---|
| wt-% | 76.39 | 0.02 | 0.005 | 0.001 | 0.007 | 0.02 | 0.01 | 0.009 | 0.004 | 0.06 | 23.45 |
| Particle | wt-% | |||
|---|---|---|---|---|
| Ni | O | Al | Mg | |
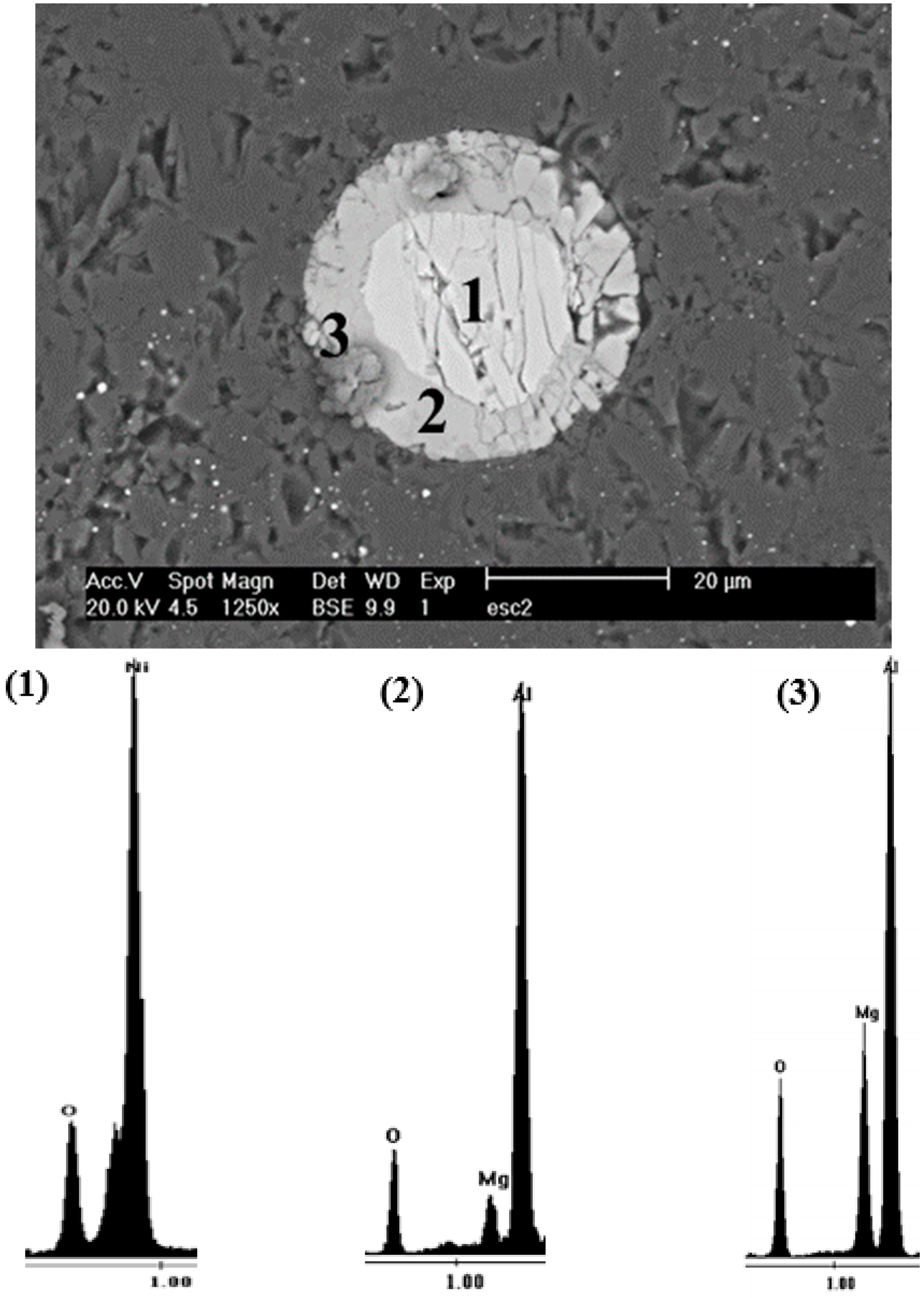
| 1 | 67.51 | 32.49 | 0 | 0 |
| 2 | 0 | 25.76 | 72.14 | 2.1 |
| 3 | 0 | 31.45 | 49.79 | 18.76 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beltran, C.S.; Valdes, A.F.; Torres, J.T.; Palacios, R.O. A Kinetic Study on the Preparation of AlNi Alloys by Aluminothermic Reduction of NiO Powders. Metals 2018, 8, 675. https://doi.org/10.3390/met8090675
Beltran CS, Valdes AF, Torres JT, Palacios RO. A Kinetic Study on the Preparation of AlNi Alloys by Aluminothermic Reduction of NiO Powders. Metals. 2018; 8(9):675. https://doi.org/10.3390/met8090675
Chicago/Turabian StyleBeltran, Cesar Silva, Alfredo Flores Valdes, Jesús Torres Torres, and Rocio Ochoa Palacios. 2018. "A Kinetic Study on the Preparation of AlNi Alloys by Aluminothermic Reduction of NiO Powders" Metals 8, no. 9: 675. https://doi.org/10.3390/met8090675
APA StyleBeltran, C. S., Valdes, A. F., Torres, J. T., & Palacios, R. O. (2018). A Kinetic Study on the Preparation of AlNi Alloys by Aluminothermic Reduction of NiO Powders. Metals, 8(9), 675. https://doi.org/10.3390/met8090675




