Electric Current-Assisted Joining of Copper Plates Using Silver Formed by In-Situ Decomposition of Ag2C2O4
Abstract
1. Introduction
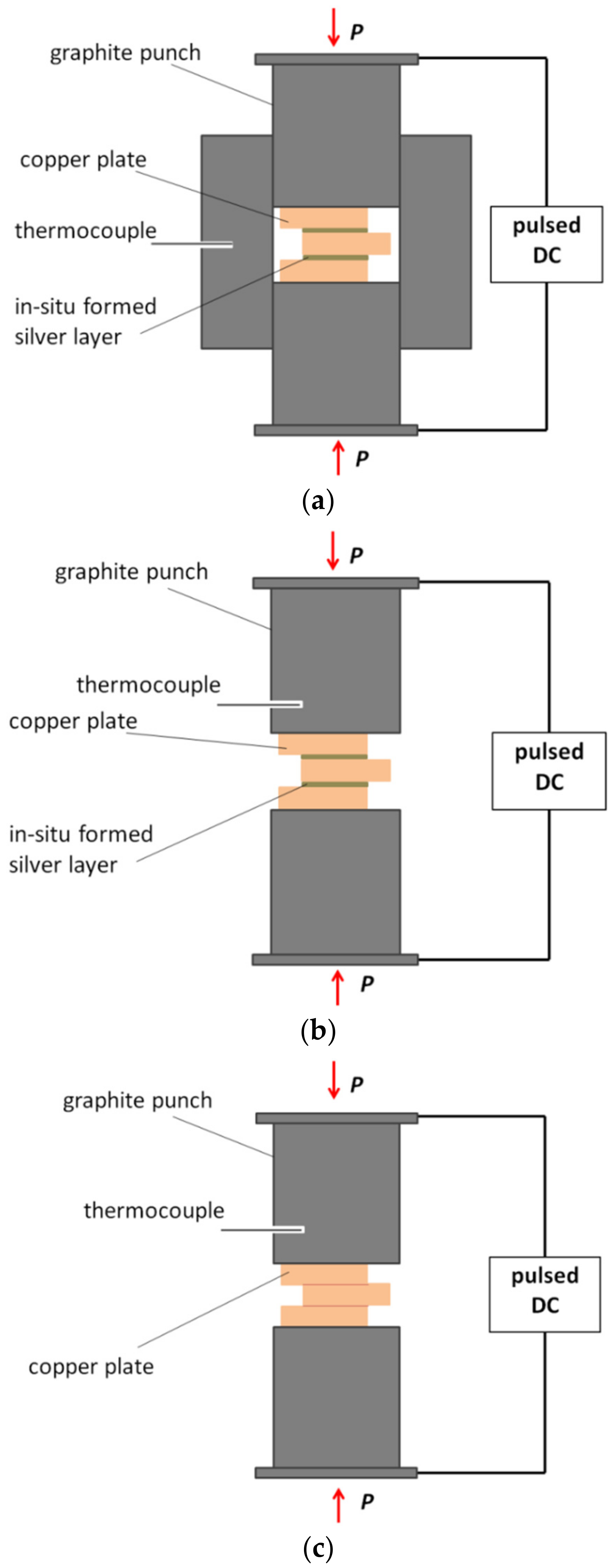
2. Materials and Methods
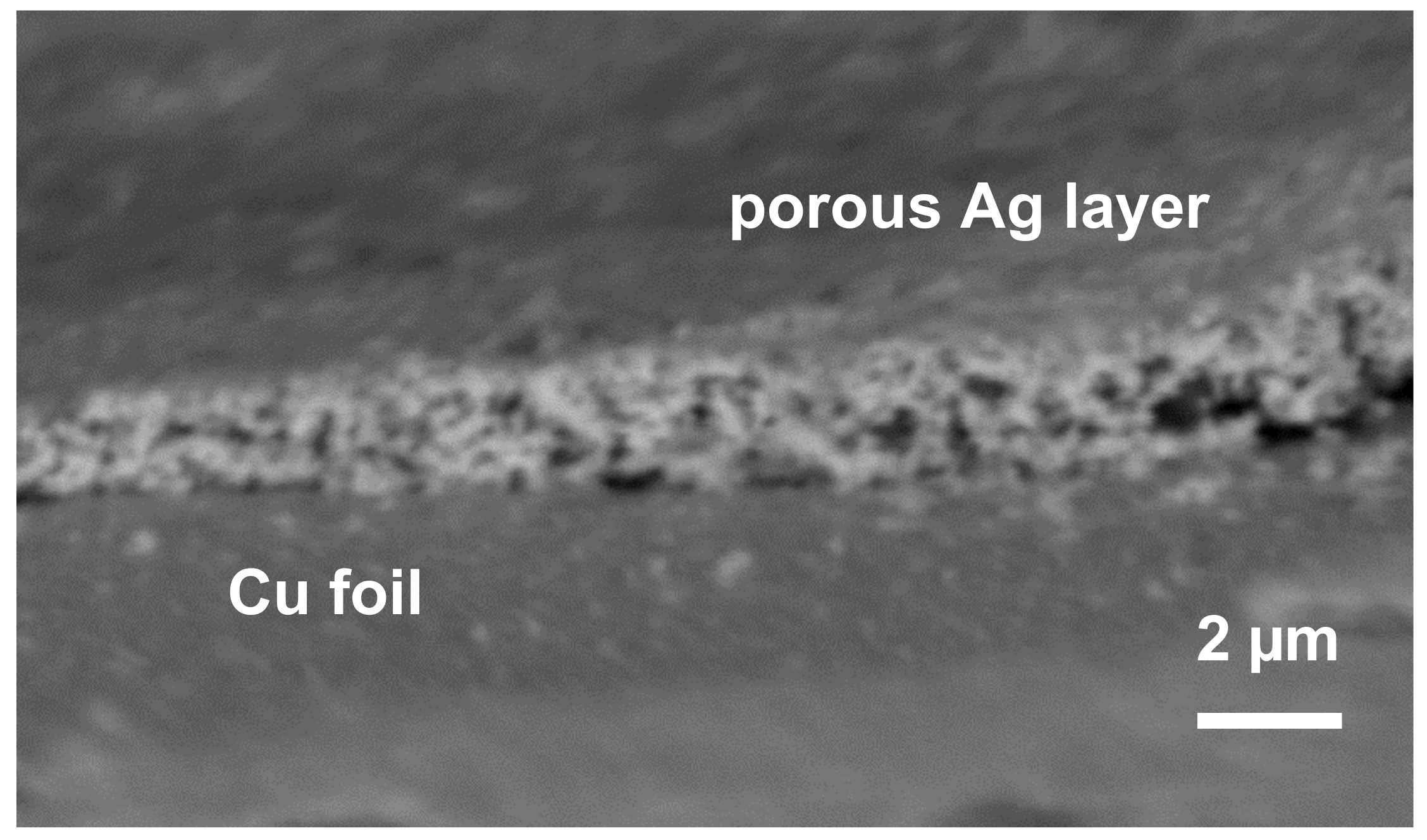
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Olevsky, E.A.; Dudina, D.V. Field-Assisted Sintering: Science and Applications; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; in press. [Google Scholar]
- Tokita, M. Spark Plasma Sintering (SPS) method, systems and applications. In Handbook of Advanced Ceramics: Materials, Applications, Processing and Properties, 2nd ed.; Somiya, S., Ed.; Academic Press: Cambridge, MA, USA, 2013; pp. 1149–1178. [Google Scholar]
- Mulukutla, M.; Singh, A.; Harimkar, S. Spark Plasma Sintering for multi-scale surface engineering of materials. JOM 2010, 62, 65–71. [Google Scholar] [CrossRef]
- Peng, P.; Hu, A.; Gerlich, A.P.; Zou, G.; Liu, L.; Zhou, Y.N. Joining of silver nanomaterials at low temperatures: Processes, properties, and applications, ACS Appl. Mater. Interfaces 2015, 7, 12597–12618. [Google Scholar] [CrossRef] [PubMed]
- Siow, K.S. Are sintered silver joints ready for use as interconnect material in microelectronic packaging? J. Electron. Mater. 2014, 43, 947–961. [Google Scholar] [CrossRef]
- Fu, Q.; Cui, Z.; Xue, Y.; Duan, H. Research of size- and shape-dependent thermodynamic properties of the actual melting process of nanoparticles. J. Phys. Chem. C 2018. [Google Scholar] [CrossRef]
- Kiryukhina, K.; Le Trong, H.; Tailhades, Ph.; Lacaze, J.; Baco, V.; Gougeon, M.; Courtade, F.; Dareys, S.; Vendier, O.; Raynaud, L. Silver oxalate-based solders: New materials for high thermal conductivity microjoining. Scr. Mater. 2013, 68, 623–626. [Google Scholar] [CrossRef]
- Boldyrev, V.V. Thermal decomposition of silver oxalate. Thermochim. Acta 2002, 388, 63–90. [Google Scholar] [CrossRef]
- Feng, S.T.; Mei, Y.H.; Chen, G.; Li, X.; Lu, G.Q. Characterizations of rapid sintered nanosilver joint for attaching power chips. Materials 2016, 9, 564. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.Q.; Shearwood, C.; Xu, B.; Yu, L.G.; Khor, K.A. Characterization of spark plasma sintered Ag nanopowders. Nanotechnology 2010, 21, 115707. [Google Scholar] [CrossRef] [PubMed]
- Dudina, D.V.; Matvienko, A.A.; Sidelnikov, A.A.; Legan, M.A.; Mali, V.I.; Esikov, M.A.; Gribov, P.A.; Boldyrev, V.V. Decomposition of Ag2C2O4 in a Spark Plasma Sintering apparatus: Morphological study and application for materials joining. Mater. Today Proc. 2018. under review. [Google Scholar]
- Savel’ev, G.G.; Greben, V.P. Effect of electric and magnetic fields on the thermal decomposition of Ag2C2O4, AgN3, and BaN6. Sov. Phys. J. 1967, 10, 20–22. [Google Scholar] [CrossRef]
- Holland, T.B.; Anselmi-Tamburini, U.; Quach, D.V.; Tran, T.B.; Mukherjee, A.K. Local field strengths during early stage field assisted sintering (FAST) of dielectric materials. J. Eur. Ceram. Soc. 2012, 32, 3659–3666. [Google Scholar] [CrossRef]
- Zou, G.; Yan, J.; Mu, F.; Wu, A.; Ren, J.; Hu, A.; Zhou, Y.N. Low temperature bonding of Cu metal through sintering of Ag nanoparticles for high temperature electronic application. Open Surf. Sci. J. 2011, 3, 70–75. [Google Scholar] [CrossRef]
- Akada, Y.; Tatsumi, H.; Yamaguchi, T.; Hirose, A.; Morita, T.; Ide, E. Interfacial bonding mechanism using silver metallo-organic nanoparticles to bulk metals and observation of sintering behavior. Mater. Trans. 2008, 49, 1537–1545. [Google Scholar] [CrossRef]


| Joint | Tooling Configuration | Shear Strength, MPa |
|---|---|---|
| Cu/Ag/Cu | die-assisted | 45 |
| Cu/Ag/Cu | without a die | 41 |
| Cu/Cu | without a die | 31 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dudina, D.V.; Matvienko, A.A.; Sidelnikov, A.A.; Legan, M.A.; Mali, V.I.; Esikov, M.A.; Anisimov, A.G.; Gribov, P.A.; Boldyrev, V.V. Electric Current-Assisted Joining of Copper Plates Using Silver Formed by In-Situ Decomposition of Ag2C2O4. Metals 2018, 8, 538. https://doi.org/10.3390/met8070538
Dudina DV, Matvienko AA, Sidelnikov AA, Legan MA, Mali VI, Esikov MA, Anisimov AG, Gribov PA, Boldyrev VV. Electric Current-Assisted Joining of Copper Plates Using Silver Formed by In-Situ Decomposition of Ag2C2O4. Metals. 2018; 8(7):538. https://doi.org/10.3390/met8070538
Chicago/Turabian StyleDudina, Dina V., Alexander A. Matvienko, Anatoly A. Sidelnikov, Mikhail A. Legan, Vyacheslav I. Mali, Maksim A. Esikov, Alexander G. Anisimov, Pavel A. Gribov, and Vladimir V. Boldyrev. 2018. "Electric Current-Assisted Joining of Copper Plates Using Silver Formed by In-Situ Decomposition of Ag2C2O4" Metals 8, no. 7: 538. https://doi.org/10.3390/met8070538
APA StyleDudina, D. V., Matvienko, A. A., Sidelnikov, A. A., Legan, M. A., Mali, V. I., Esikov, M. A., Anisimov, A. G., Gribov, P. A., & Boldyrev, V. V. (2018). Electric Current-Assisted Joining of Copper Plates Using Silver Formed by In-Situ Decomposition of Ag2C2O4. Metals, 8(7), 538. https://doi.org/10.3390/met8070538






