Evaluation of Surface Characteristics and Hemocompatibility on the Oxygen Plasma-Modified Biomedical Titanium
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Plasma-Oxidized Samples
2.2. Surface Characterization Analysis of the Plasma-Oxidized Samples
2.3. Microstructure Identification of the Plasma-Oxidized Samples
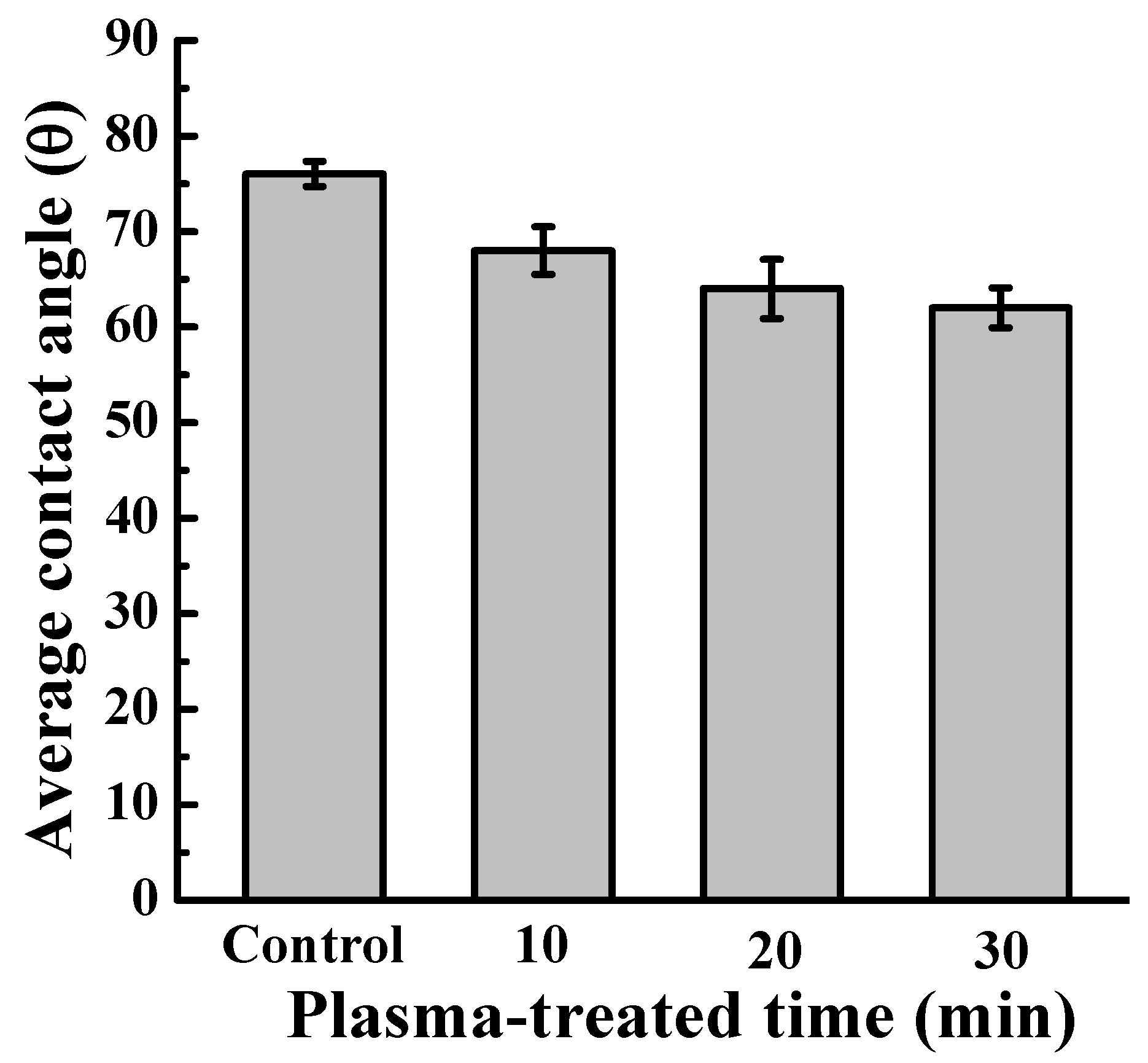
2.4. Wettability Evaluation of the Plasma-Oxidized Samples
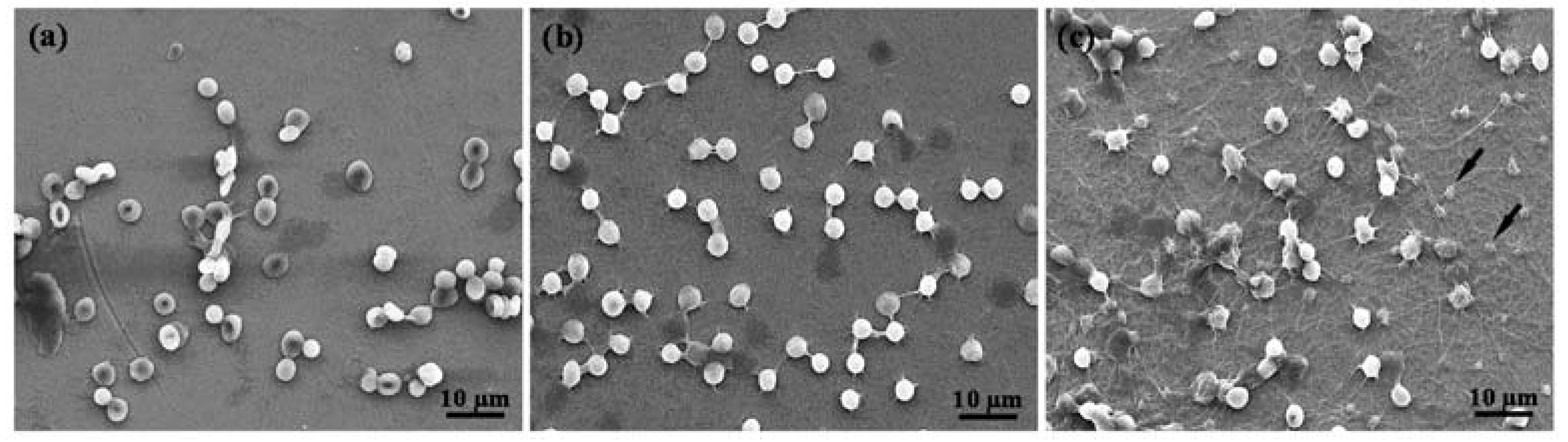
2.5. Hemocompatibility of the Plasma-Oxidized Samples
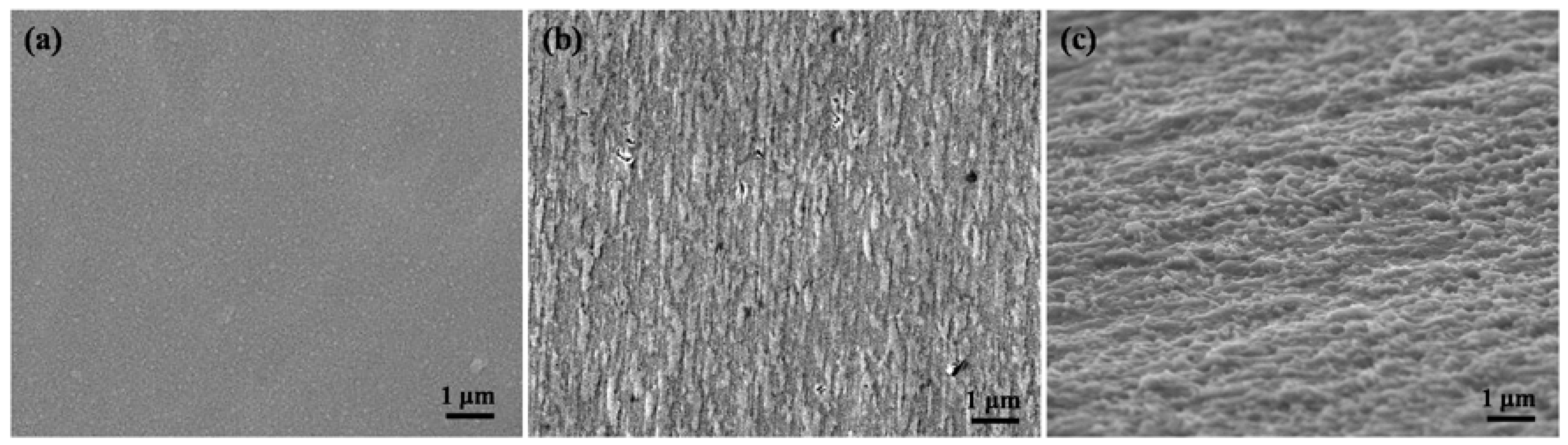
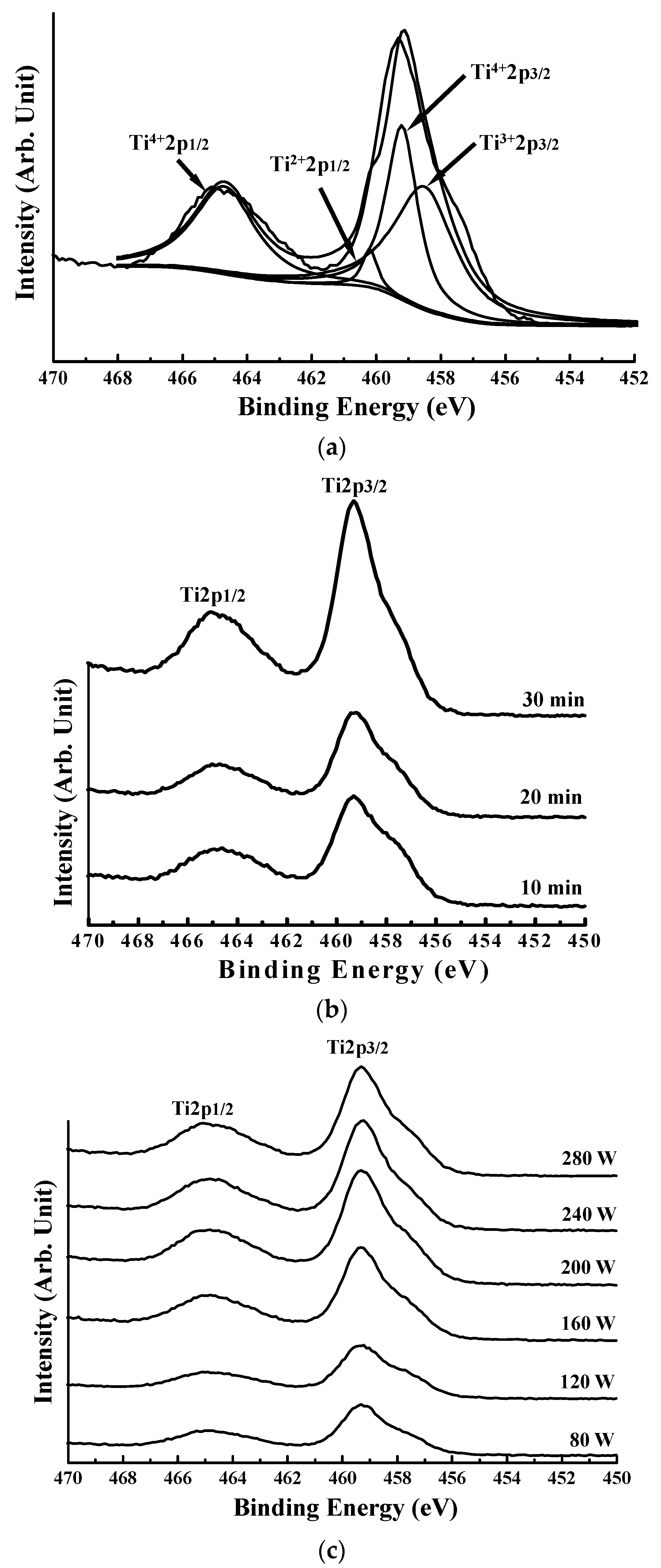
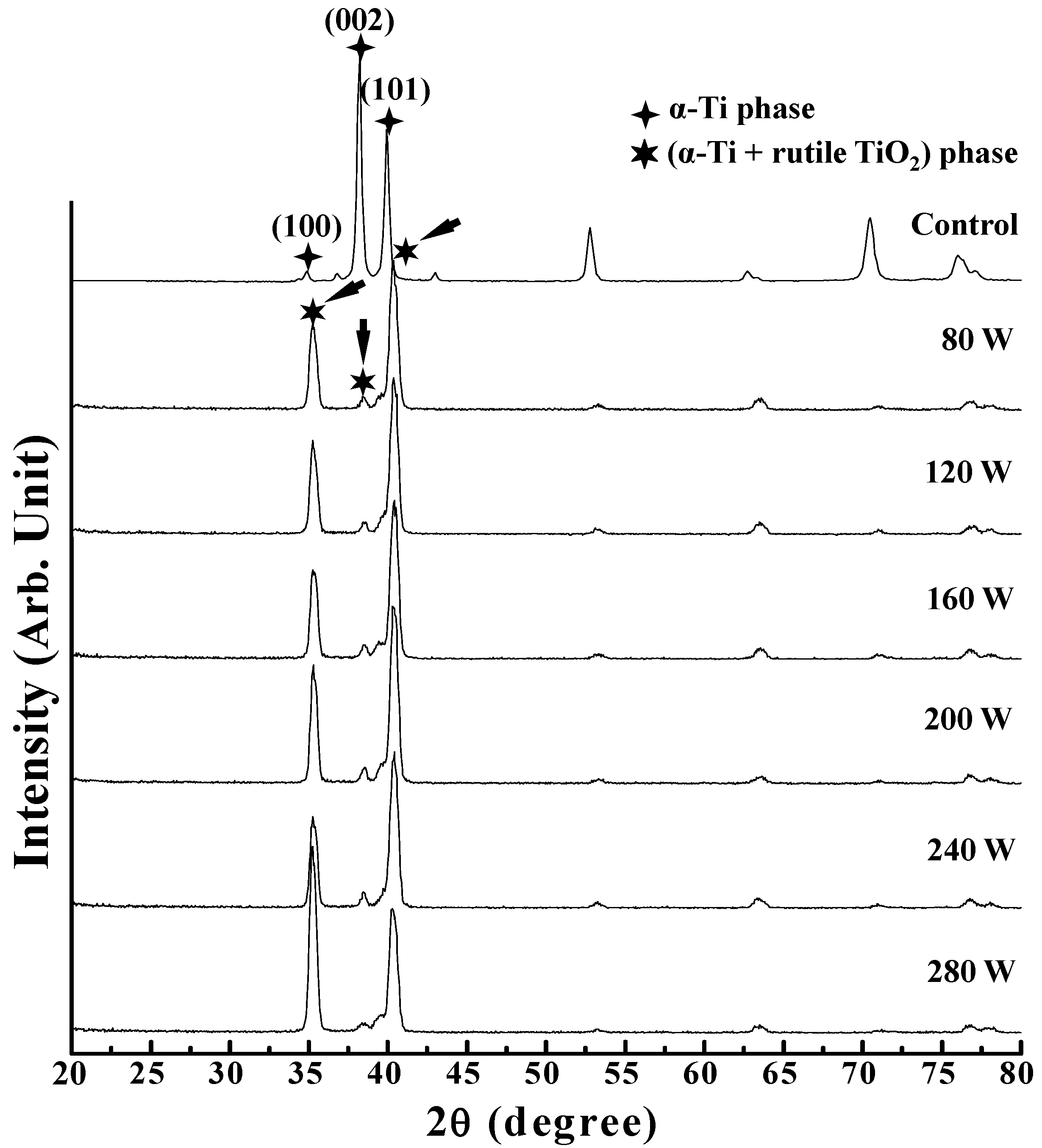
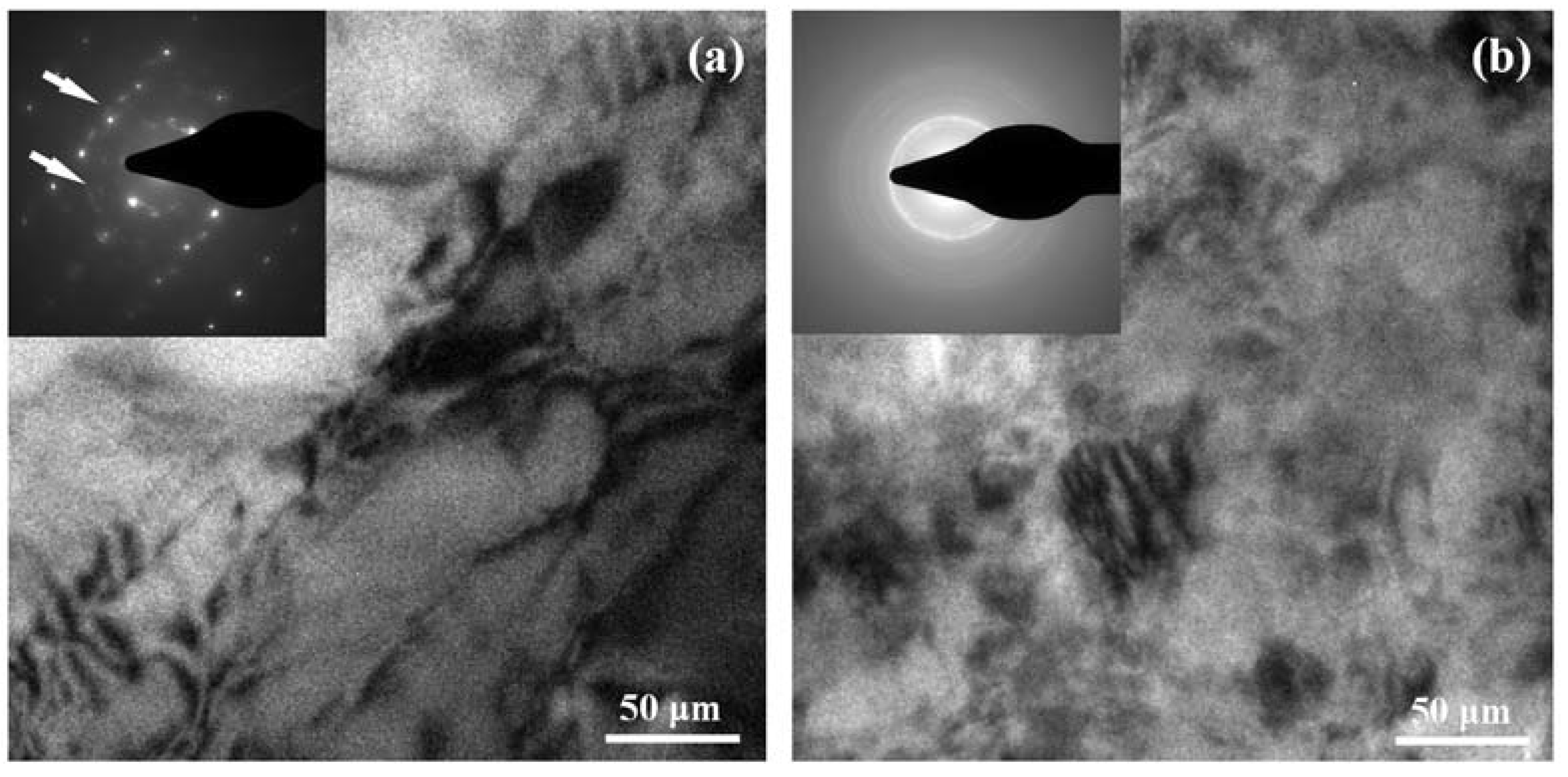
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kammerer, P.W.; Schiegnitz, E.; Palarie, V.; Dau, M.; Frerich, B.; Al-Nawas, B. Influence of platelet-derived growth factor on osseous remodeling properties of a variable-thread tapered dental implant in vivo. Clin. Oral Implant. Res. 2017, 28, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.E.; Yun, Y.P.; Lee, J.Y.; Shim, J.S.; Park, K.; Huh, J.B. Co-delivery of platelet-derived growth factor (PDGF-BB) and bone morphogenic protein (BMP-2) coated onto heparinized titanium for improving osteoblast function and osteointegration. J. Tissue Eng. Regen. Med. 2015, 9, E219–E228. [Google Scholar] [CrossRef] [PubMed]
- Al-Hezaimi, K.; Nevins, M.; Kim, S.W.; Fateh, A.; Kim, D.M. Efficacy of growth factor in promoting early osseointegration. J. Oral Implantol. 2014, 40, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Haimov, H.; Yosupov, N.; Pinchasov, G.; Juodzbalys, G. Bone Morphogenetic Protein Coating on Titanium Implant Surface: A Systematic Review. J. Oral Maxillofac. Res. 2017, 8, e1. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Lee, Y.; Seo, S.J.; Lim, J.H.; Kim, Y.G. Effects of Escherichia Coli-derived Recombinant Human Bone Morphogenetic Protein-2 Loaded Porous Hydroxyaptite-based Ceramics on Calvarial Defect in Rabbits. J. Bone Metab. 2017, 24, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Biao, M.N.; Chen, Y.M.; Xiong, S.B.; Wu, B.Y.; Yang, B.C. Synergistic effects of fibronectin and bone morphogenetic protein on the bioactivity of titanium metal. J. Biomed. Mater. Res. 2017, 105, 2485–2498. [Google Scholar] [CrossRef] [PubMed]
- Bafail, A.S.; Alamri, A.M.; Spivakovsky, S. Effect of antibiotics on implant failure and postoperative infection. Evid. Based Dent. 2014, 15, 58. [Google Scholar] [CrossRef] [PubMed]
- Rimondini, L.; Fini, M.; Giardino, R. The microbial infection of biomaterials: A challenge for clinicians and researchers. A short review. J. Appl. Biomater. Biomech. 2005, 3, 1–10. [Google Scholar] [PubMed]
- Huang, C.F.; Chiang, H.J.; Lan, W.C.; Chou, H.H.; Ou, K.L.; Yu, C.H. Development of silver-containing austenite antibacterial stainless steels for biomedical applications part I: Microstructure characteristics, mechanical properties and antibacterial mechanisms. Biofouling 2011, 27, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Lee, F.P.; Wang, D.J.; Chen, L.K.; Kung, C.M.; Wu, Y.C.; Ou, K.L.; Yu, C.H. Antibacterial nanostructured composite films for biomedical applications: Microstructural characteristics, biocompatibility, and antibacterial mechanisms. Biofouling 2013, 29, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Olivares-Navarrete, R.; Baier, R.E.; Meyer, A.E.; Tannenbaum, R.; Boyan, B.D.; Schwartz, Z. Effect of cleaning and sterilization on titanium implant surface properties and cellular response. Acta Biomater. 2012, 8, 1966–1975. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, K.I.; Sangha, N.; Genheimer, C.W.; Basu, J.; Ludlow, J.W. Migration assay to evaluate cellular interactions with biomaterials for tissue engineering/regenerative medicine applications. Methods Mol. Biol. 2013, 1001, 189–196. [Google Scholar] [PubMed]
- Echeverry-Rendon, M.; Galvis, O.; Aguirre, R.; Robledo, S.; Castano, J.G.; Echeverria, F. Modification of titanium alloys surface properties by plasma electrolytic oxidation (PEO) and influence on biological response. J. Mater. Sci. Mater. Med. 2017, 28, 169. [Google Scholar] [CrossRef] [PubMed]
- Hou, P.J.; Ou, K.L.; Wang, C.C.; Huang, C.F.; Ruslin, M.; Sugiatno, E.; Yang, T.S.; Chou, H.H. Hybrid micro/nanostructural surface offering improved stress distribution and enhanced osseointegration properties of the biomedical titanium implant. J. Mech. Behav. Biomed. Mater. 2017, 79, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Guojiang, W.; Maitz, M.F.; Yifeng, L.; Nan, H.; Hong, S. Characterization and mechanical investigation of Ti-O2−x film prepared by plasma immersion ion implantation and deposition for cardiovascular stents surface modification. Beam Interact. Mater. Atoms 2012, 289, 91–96. [Google Scholar]
- Tsai, M.H.; Haung, C.F.; Shyu, S.S.; Chou, Y.R.; Lin, M.H.; Peng, P.W.; Ou, K.L.; Yu, C.H. Surface modification induced phase transformation and structure variation on the rapidly solidified recast layer of titanium. Mater. Charact. 2015, 106, 463–469. [Google Scholar] [CrossRef]
- Lu, T.; Qiao, Y.; Liu, X. Surface modification of biomaterials using plasma immersion ion implantation and deposition. Interface Focus 2012, 2, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Gottlicher, M.; Rohnke, M.; Kunz, A.; Thomas, J.; Henning, R.A.; Leichtweiss, T.; Gemming, T.; Janek, J. Anodization of titanium in radio frequency oxygen discharge—Microstructure, kinetics & transport mechanism. Solid State Ion. 2016, 290, 130–139. [Google Scholar]
- Hung, W.C.; Chang, F.M.; Yang, T.S.; Ou, K.L.; Lin, C.T.; Peng, P.W. Oxygen-implanted induced formation of oxide layer enhances blood compatibility on titanium for biomedical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 68, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Maitz, M.F.; Pham, M.T.; Wieser, E.; Tsyganov, I. Blood compatibility of titanium oxides with various crystal structure and element doping. J. Biomater. Appl. 2003, 17, 303–319. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.C.; Paulose, M.; Grimes, C.A. The effect of TiO2 nanotubes in the enhancement of blood clotting for the control of hemorrhage. Biomaterials 2007, 28, 4667–4672. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, C.; Lausmaa, J.; Nygren, H. Interactions between human whole blood and modified TiO2-surfaces: Influence of surface topography and oxide thickness on leukocyte adhesion and activation. Biomaterials 2001, 22, 1987–1996. [Google Scholar] [CrossRef]
- Baier, R.E.; Carter, J.M.; Sorensen, S.E.; Meyer, A.E.; McGowan, B.D.; Kasprzak, S.A. Radiofrequency gas plasma (glow discharge) disinfection of dental operative instruments, including handpieces. J. Oral Implantol. 1992, 18, 236–242. [Google Scholar] [PubMed]
- Ou, K.L.; Shih, Y.H.; Huang, C.F.; Chen, C.C.; Liu, C.M. Preparation of bioactive amorphous-like titanium oxide layer on titanium by plasma oxidation treatment. Appl. Surf. Sci. 2008, 255, 2046–2051. [Google Scholar] [CrossRef]
- Shen, J.W.; Chen, Y.; Yang, G.L.; Wang, X.X.; He, F.M.; Wang, H.M. Effects of storage medium and UV photofunctionalization on time-related changes of titanium surface characteristics and biocompatibility. J. Biomed. Mater. Res. B 2016, 104, 932–940. [Google Scholar] [CrossRef] [PubMed]
- Stevens, N.; Priest, C.I.; Sedev, R.; Ralston, J. Wettability of photoresponsive titanium dioxide surfaces. Langmuir 2003, 19, 3272–3275. [Google Scholar] [CrossRef]
- Ou, S.F.; Chou, H.H.; Lin, C.S.; Shih, C.J.; Wang, K.K.; Pan, Y.N. Effects of anodic oxidation and hydrothermal treatment on surface characteristics and biocompatibility of Ti-30Nb-1Fe-1Hf alloy. Appl. Surf. Sci. 2012, 258, 6190–6198. [Google Scholar] [CrossRef]
- Wu, W.F.; Ou, K.L.; Chou, C.P.; Wu, C.C. Effects of nitrogen plasma treatment on tantalum diffusion barriers in copper metallization. J. Electrochem. Soc. 2003, 150, G83–G89. [Google Scholar] [CrossRef]
- Park, J.Y.; Davies, J.E. Red blood cell and platelet interactions with titanium implant surfaces. Clin. Oral Implant. Res. 2000, 11, 530–539. [Google Scholar] [CrossRef]
- Park, J.Y.; Gemmell, C.H.; Davies, J.E. Platelet interactions with titanium: Modulation of platelet activity by surface topography. Biomaterials 2001, 22, 2671–2682. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiang, H.-J.; Chou, H.-H.; Ou, K.-L.; Sugiatno, E.; Ruslin, M.; Waris, R.A.; Huang, C.-F.; Liu, C.-M.; Peng, P.-W. Evaluation of Surface Characteristics and Hemocompatibility on the Oxygen Plasma-Modified Biomedical Titanium. Metals 2018, 8, 513. https://doi.org/10.3390/met8070513
Chiang H-J, Chou H-H, Ou K-L, Sugiatno E, Ruslin M, Waris RA, Huang C-F, Liu C-M, Peng P-W. Evaluation of Surface Characteristics and Hemocompatibility on the Oxygen Plasma-Modified Biomedical Titanium. Metals. 2018; 8(7):513. https://doi.org/10.3390/met8070513
Chicago/Turabian StyleChiang, Hsi-Jen, Hsin-Hua Chou, Keng-Liang Ou, Erwan Sugiatno, Muhammad Ruslin, Rahmat Abd Waris, Chiung-Fang Huang, Chung-Ming Liu, and Pei-Wen Peng. 2018. "Evaluation of Surface Characteristics and Hemocompatibility on the Oxygen Plasma-Modified Biomedical Titanium" Metals 8, no. 7: 513. https://doi.org/10.3390/met8070513
APA StyleChiang, H.-J., Chou, H.-H., Ou, K.-L., Sugiatno, E., Ruslin, M., Waris, R. A., Huang, C.-F., Liu, C.-M., & Peng, P.-W. (2018). Evaluation of Surface Characteristics and Hemocompatibility on the Oxygen Plasma-Modified Biomedical Titanium. Metals, 8(7), 513. https://doi.org/10.3390/met8070513




