Carbon Dissolution Using Waste Biomass—A Sustainable Approach for Iron-Carbon Alloy Production
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Pyrolysis of Macadamia Shell Waste
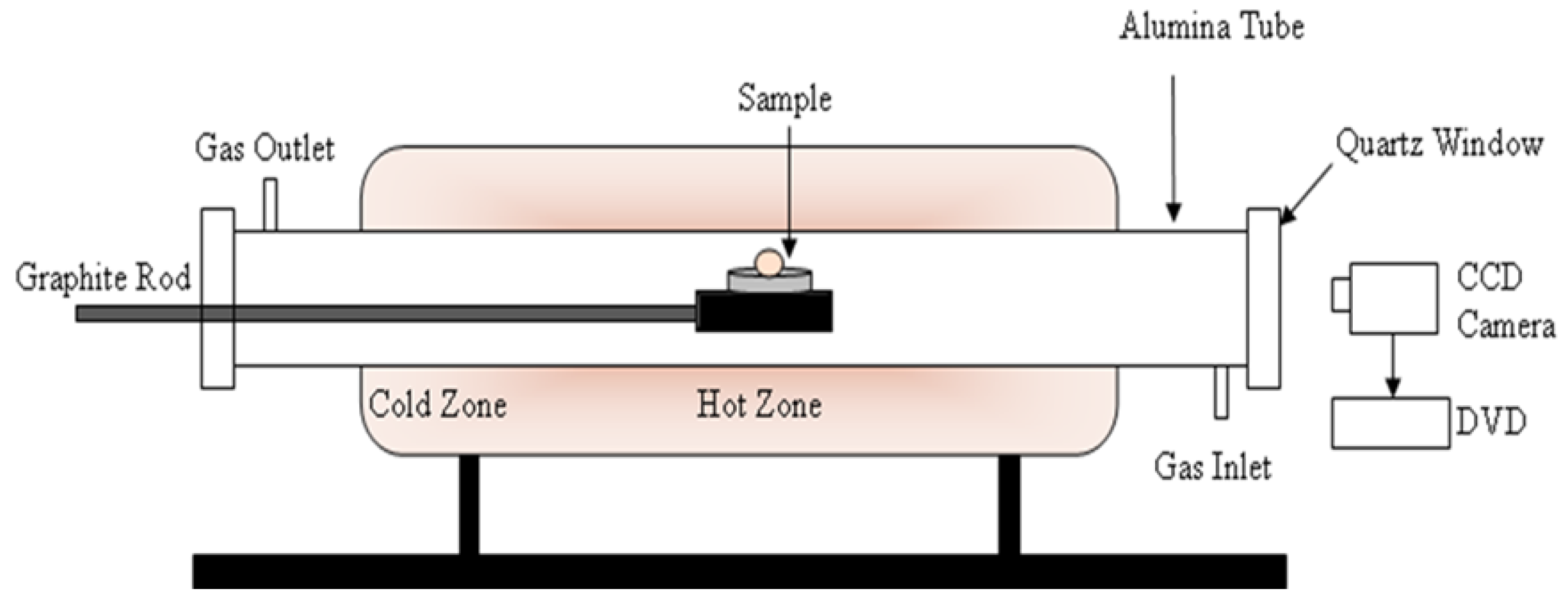
2.3. Formation of Iron-Carbon Alloy
3. Results and Discussion
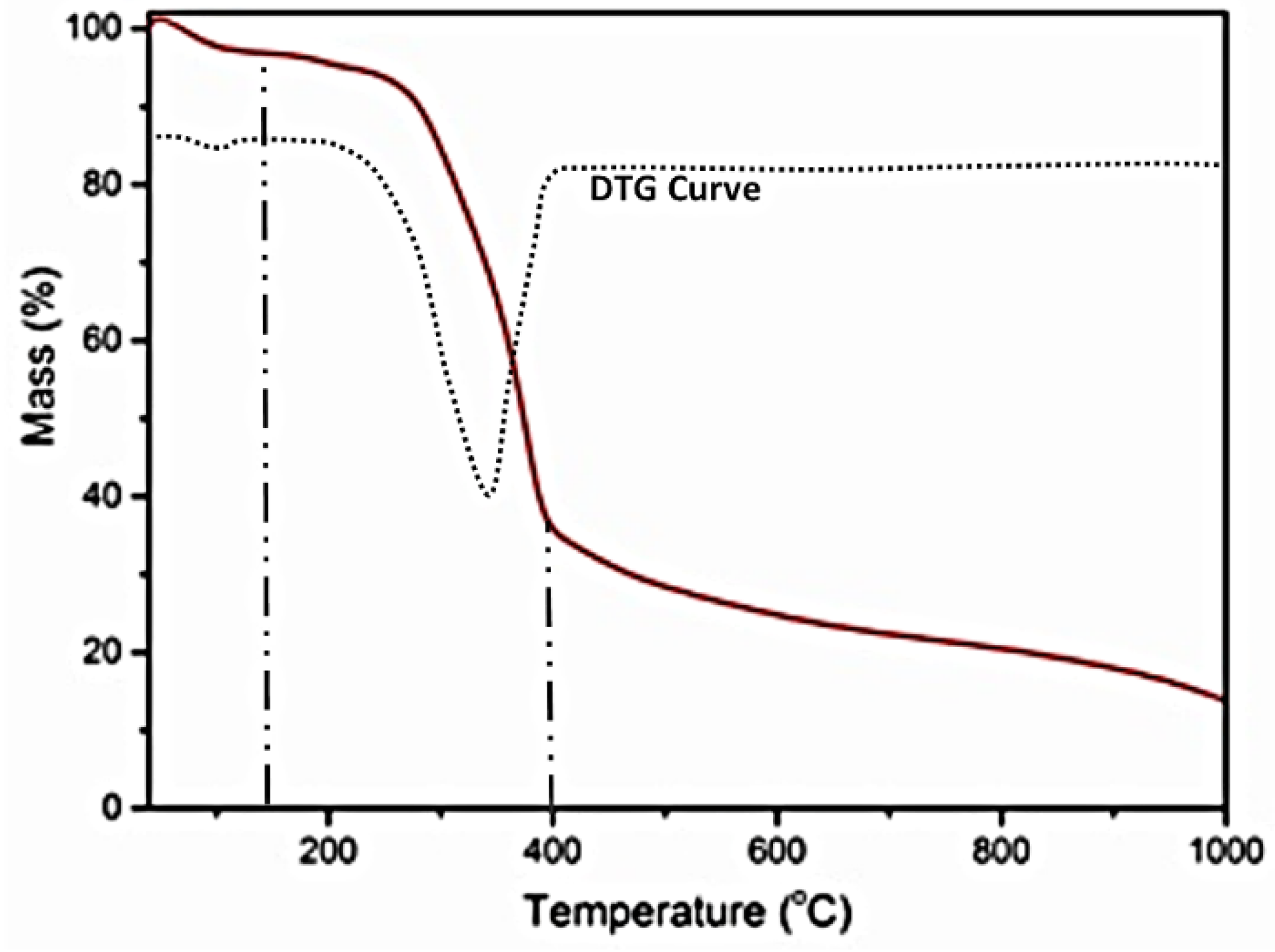
3.1. Elemental Analysis and TGA
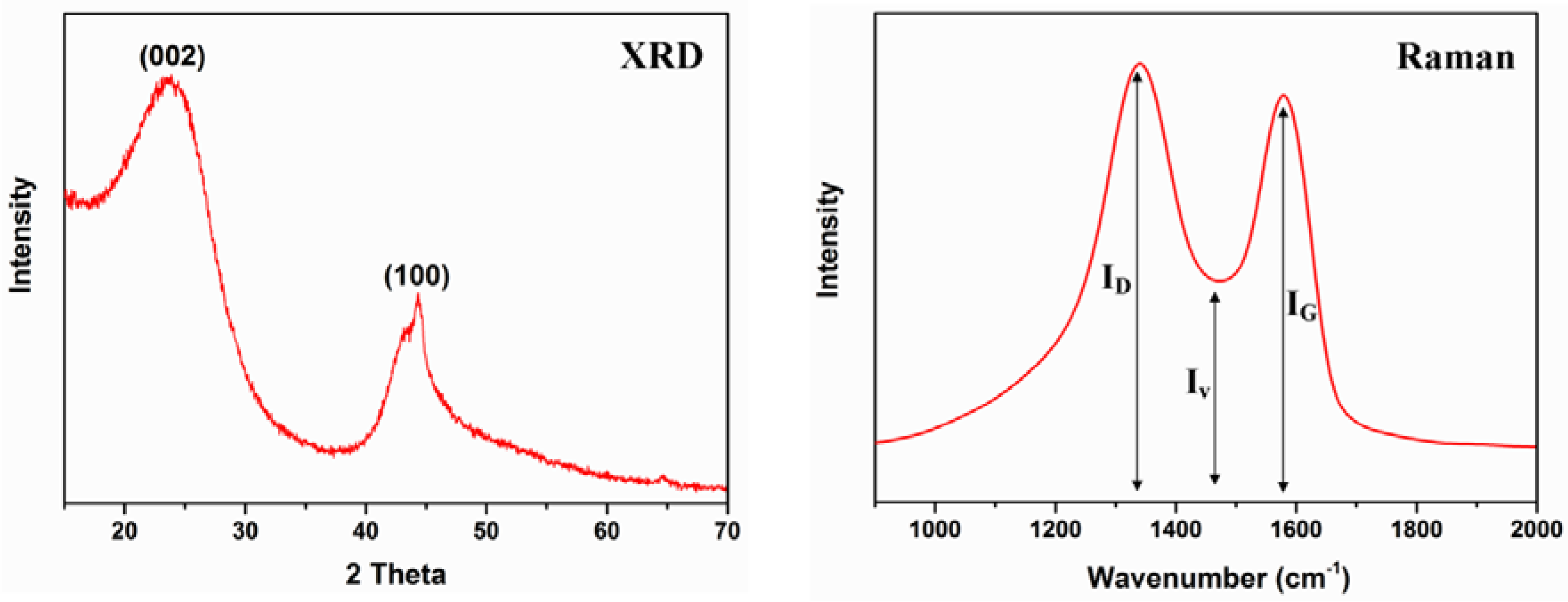
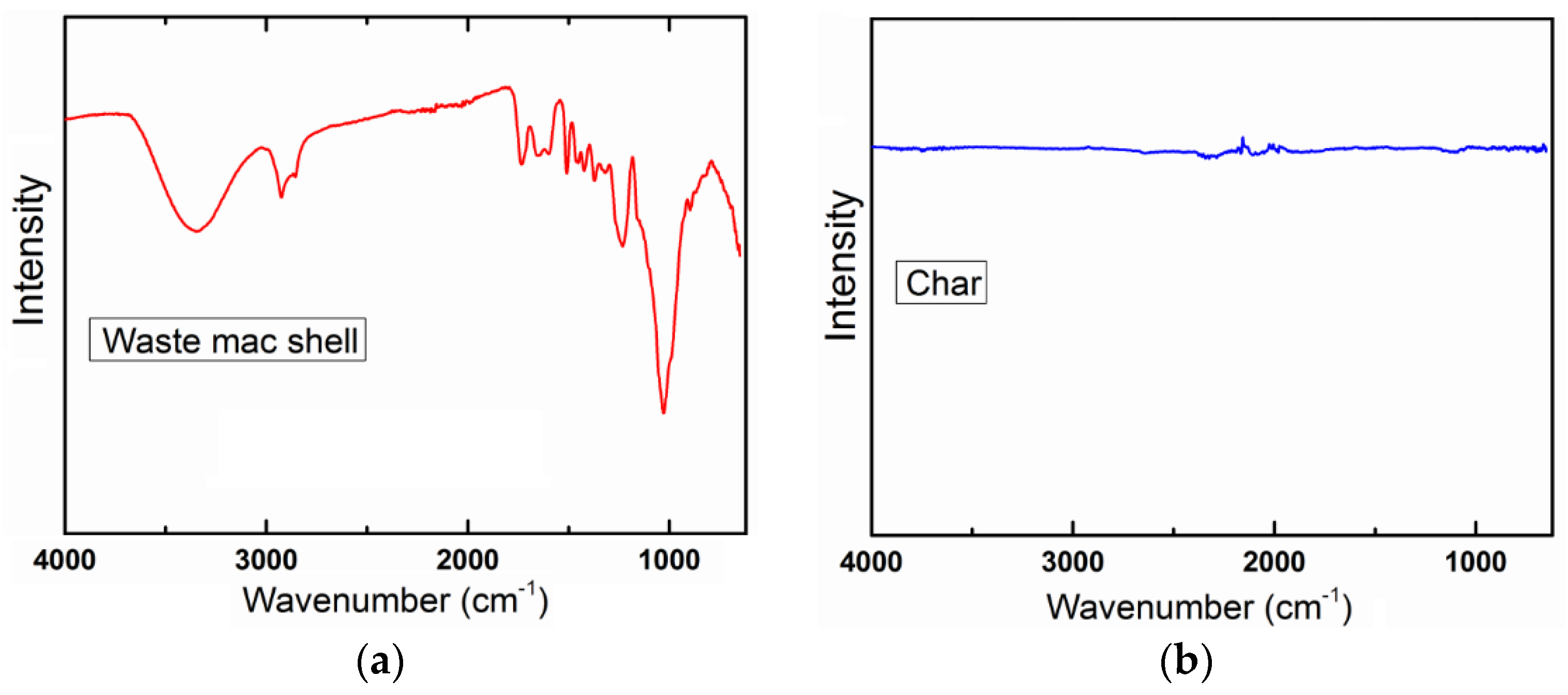
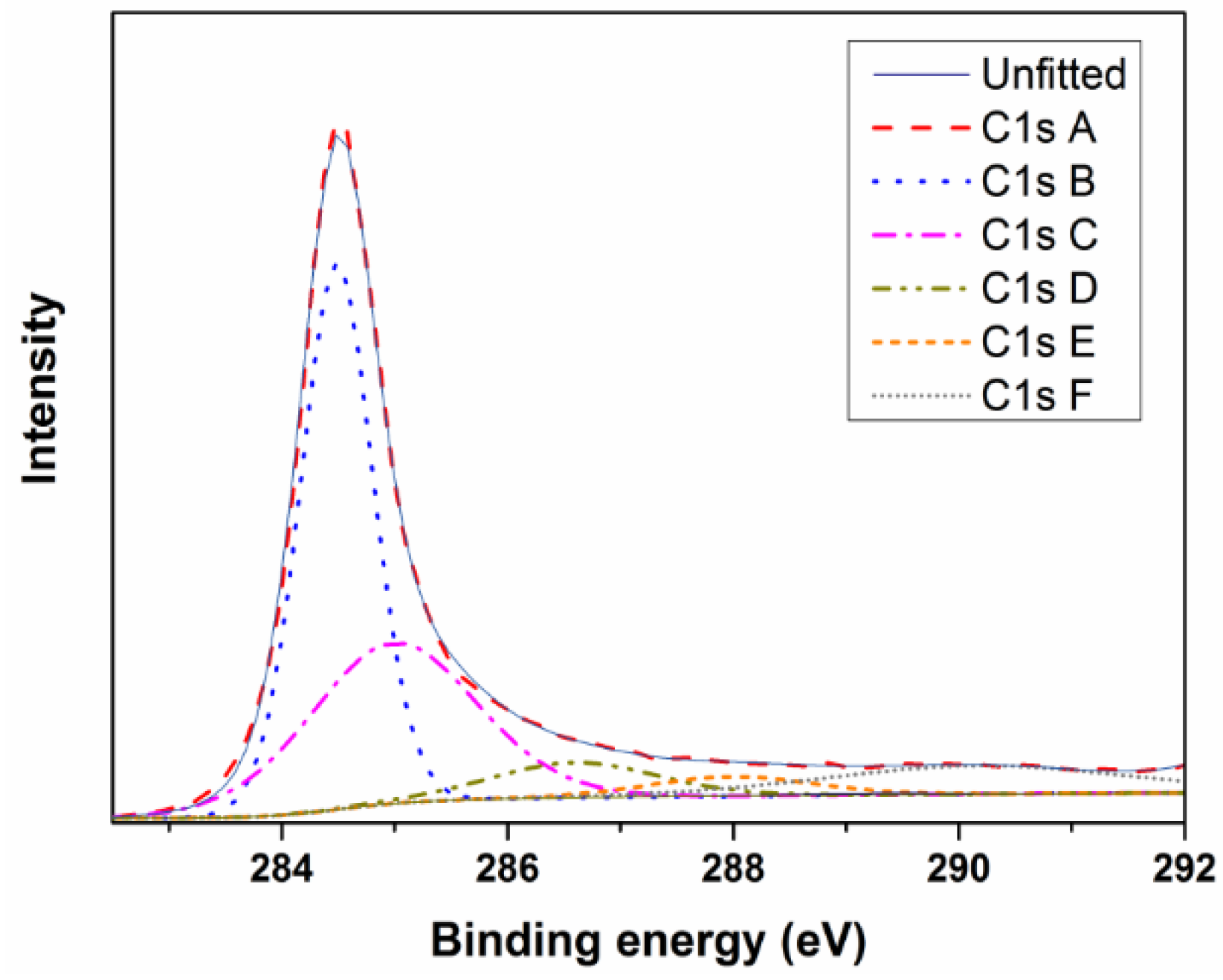
3.2. Characterisation of Char
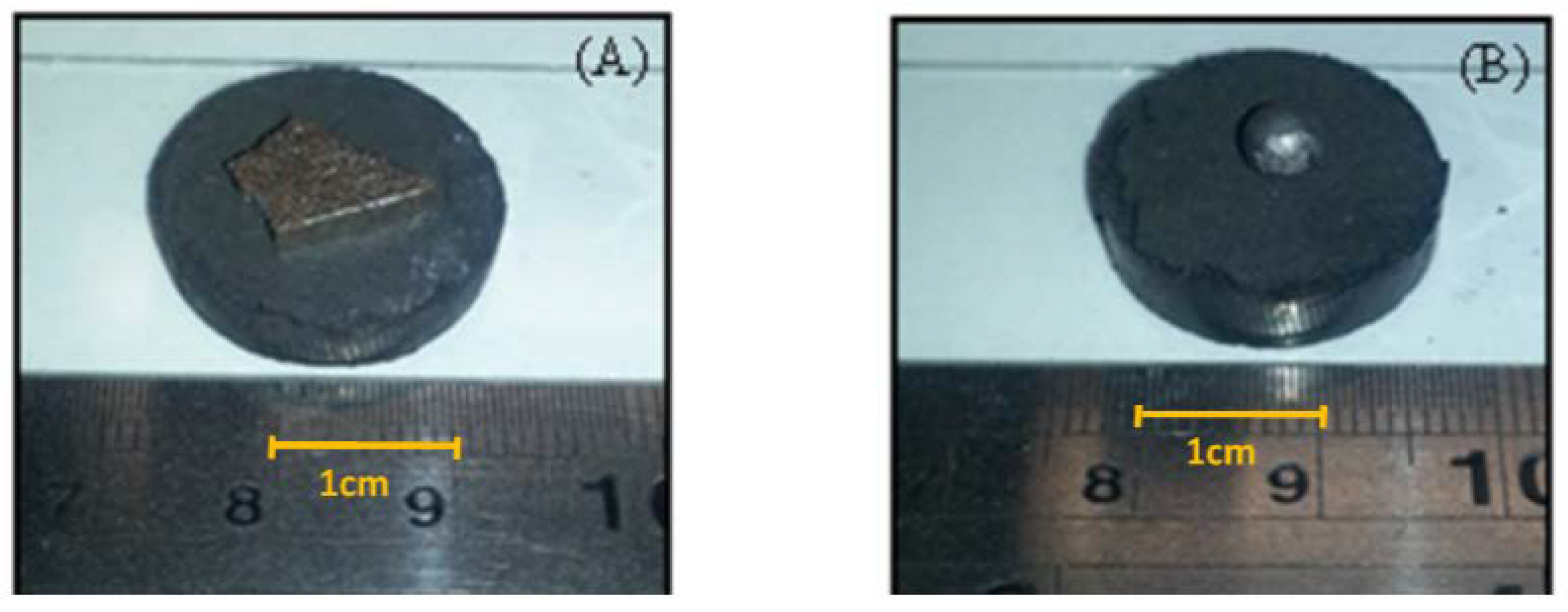
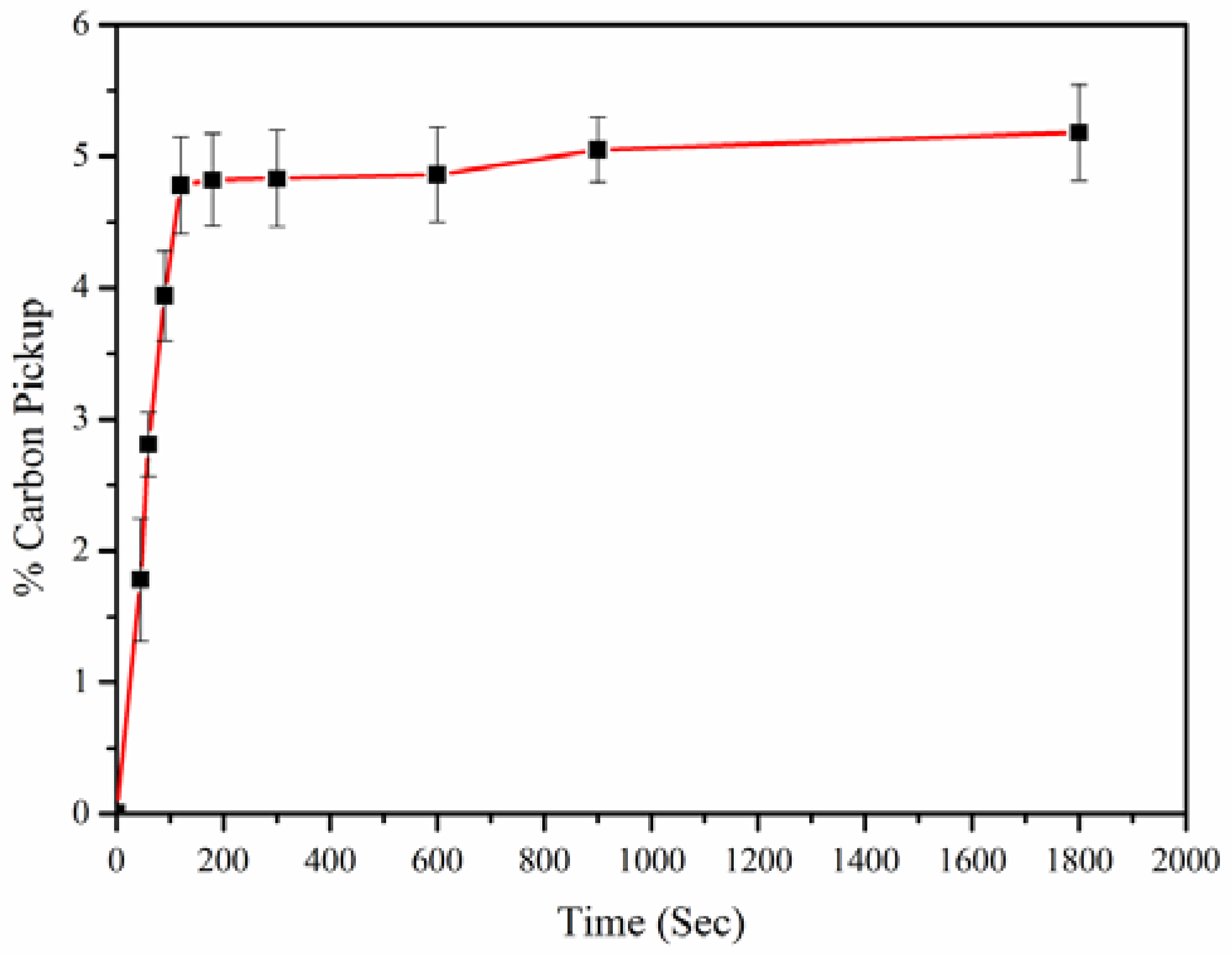
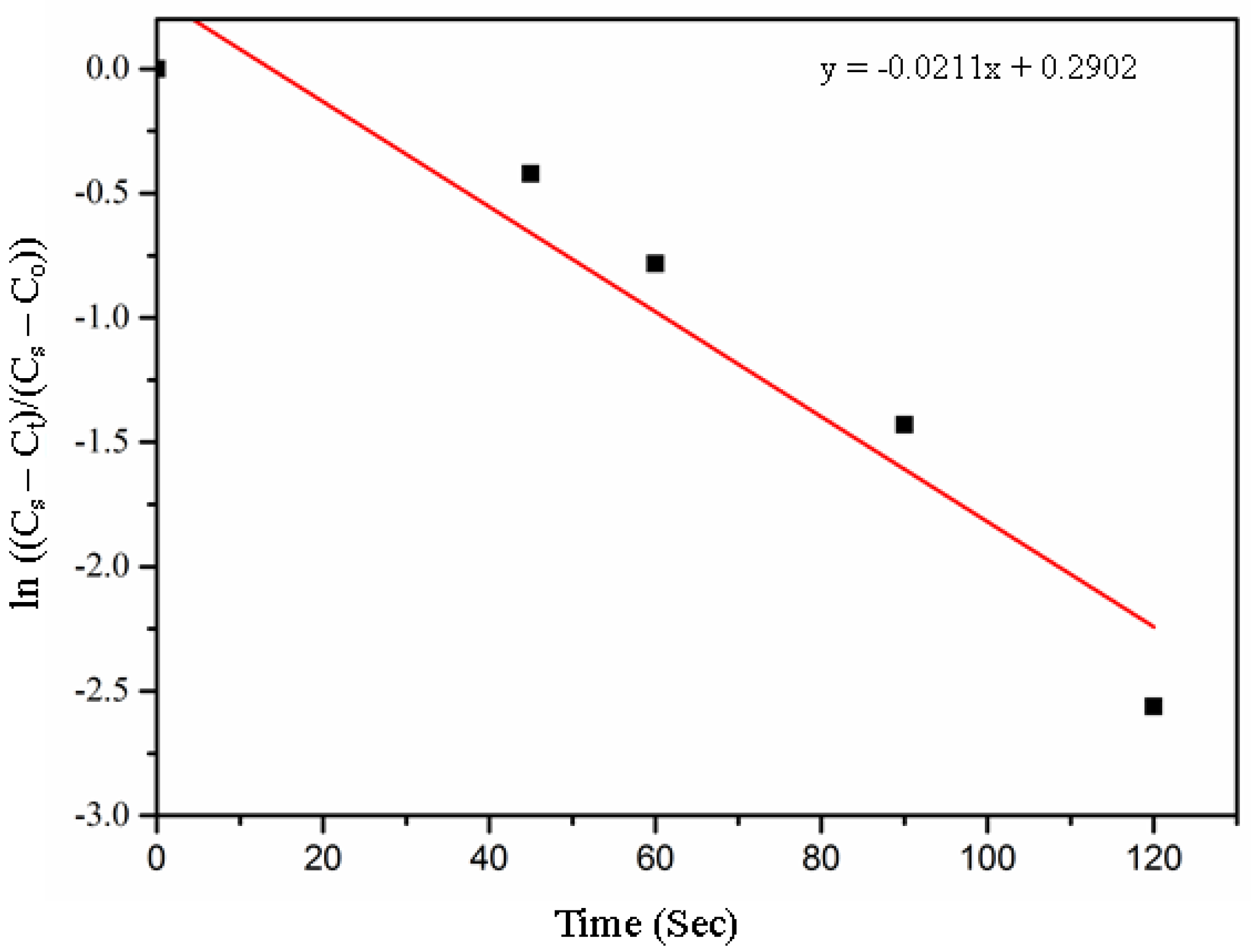
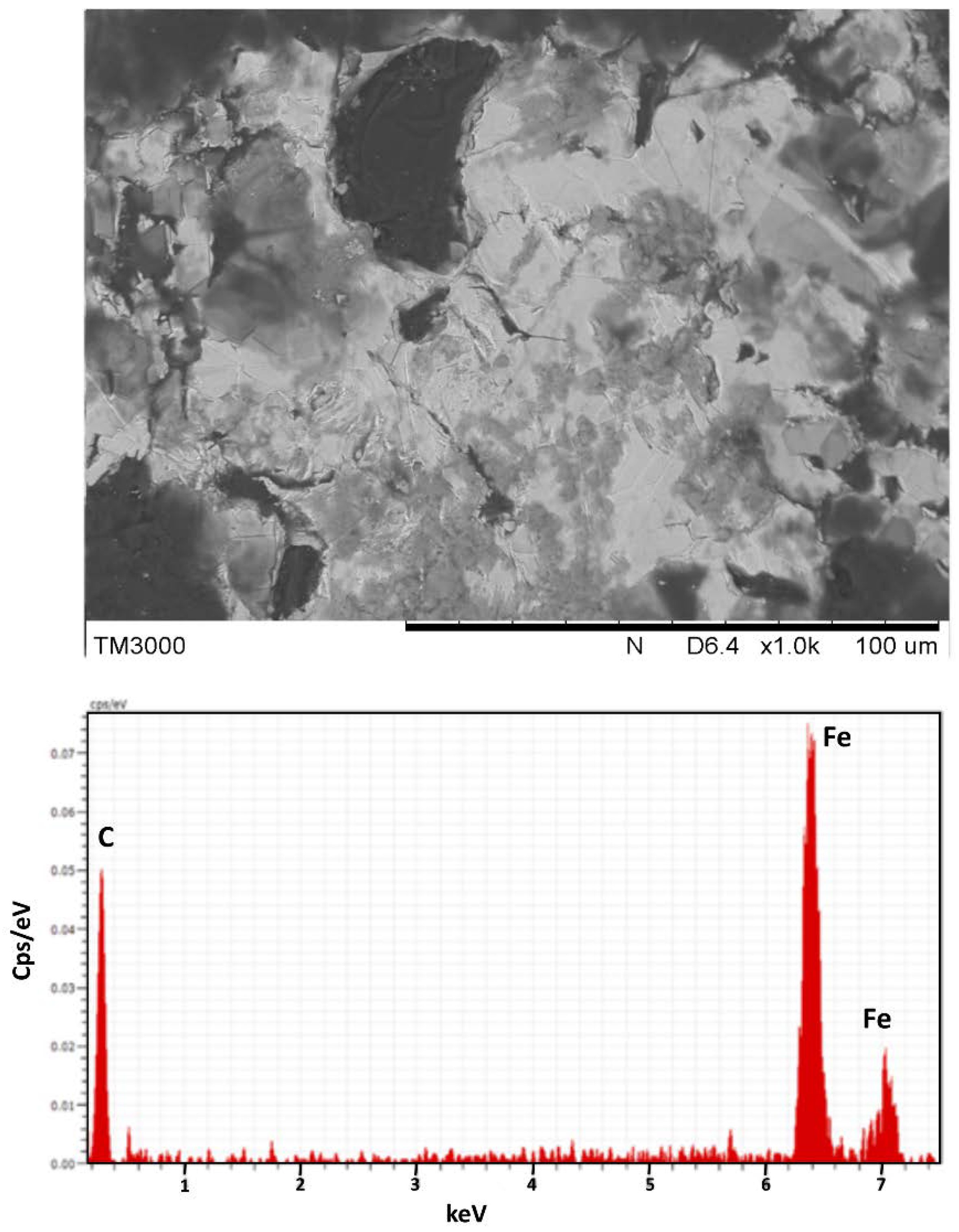
3.3. Iron-Carbon Alloy
4. Conclusions
- High temperature pyrolysis of waste macadamia shell yield 22 wt % char residue with rich in carbon content of ~98 wt % C and negligible amount of ash impurities.
- Less ash, high char yield, high fixed carbon and aromatic contents makes char as ideal carbon precursor for Iron-carbon alloy synthesis.
- The high rate of carbon dissolution into molten iron was observed using macadamia shell char as a carbon source reaching to 5.2 wt % of C in Iron-carbon alloy.
- Carbon dissolution rate using macadamia char was comparatively higher than other carbonaceous materials such as metallurgical coke, coal chars and waste CD char due to high % of carbon and low ash content.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Steel Association. 2014. Available online: https://www.worldsteel.org/en/dam/jcr:f07b864c-908e-4229-9f92-669f1c3abf4c/fact_energy_2018_.pdf (accessed on 16 April 2018).
- World Steel Association. Fact Sheet: Energy Use in the Steel Industry; World Steel Association: Brussels, Belgium, 2018. [Google Scholar]
- Manojlović, V.; Kamberović, Z.; Sokić, M.; Gavrilovski, M.; Korać, M. Designing of synergistic waste mixtures for multiphase reactive smelting. Metals 2016, 6, 138. [Google Scholar] [CrossRef]
- Haykiri-Acma, H.; Yaman, S. Effect of co-combustion on the burnout of lignite/biomass blends: A Turkish case study. Waste Manag. 2008, 28, 2077–2084. [Google Scholar] [CrossRef] [PubMed]
- Huggins, T.; Wang, H.; Kearns, J.; Jenkins, P.; Ren, Z.J. Biochar as a sustainable electrode material for electricity production in microbial fuel cells. Bioresour. Technol. 2014, 157, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Iakovou, E.; Karagiannidis, A.; Vlachos, D.; Toka, A.; Malamakis, A. Waste biomass-to-energy supply chain management: A critical synthesis. Waste Manag. 2010, 30, 1860–1870. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, V.A.; Cavallaro, C.G.; Stefana, G.L.; Parisi, M.F. Coffee grounds as filler for pectin: Green composites with competitive performances dependent on the UV irradiation. Carbohydr. Polym. 2017, 170, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Coletti, A.; Valerio, A.; Vismara, E. Posidonia oceanica as a renewable lignocellulosic biomass for the synthesis of cellulose acetate and glycidyl methacrylate grafted cellulose. Materials 2013, 6, 2043–2058. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, G.; Lazzara, G.; Konnova, S.; Fakhrullin, R.; Lvov, Y. Composite films of natural clay nanotubes with cellulose and chitosan. Green Mater. 2014, 2, 232–242. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, M.; Wang, G.; Dai, B.; Yu, F.; Tian, Z.; Guo, X. Enhanced oxygen reduction reaction by in situ anchoring Fe2N nanoparticles on nitrogen-doped pomelo peel-derived carbon. Nanomaterials 2017, 7, 404. [Google Scholar] [CrossRef] [PubMed]
- Caicedo, M.; Barros, J.; Ordás, B. Redefining agricultural residues as bioenergy feedstocks. Materials 2016, 9, 635. [Google Scholar] [CrossRef] [PubMed]
- Demirbas, A. Combustion characteristics of different biomass fuels. Prog. Energy Combust. Sci. 2004, 30, 219–230. [Google Scholar] [CrossRef]
- Demirbas, A. Potential applications of renewable energy sources, biomass combustion problems in boiler power systems and combustion related environmental issues. Prog. Energy Combust. Sci. 2005, 31, 171–192. [Google Scholar] [CrossRef]
- Strezov, V.; Patterson, M.; Zymla, V.; Fisher, K.; Evans, T.; Nelson, P.F. Fundamental aspects of biomass carbonisation. J. Anal. Appl. Pyrolysis 2007, 79, 91–100. [Google Scholar] [CrossRef]
- Bae, J.-S.; Su, S. Macadamia nut shell-derived carbon composites for post combustion CO2 capture. Int. J. Greenh. Gas Control 2013, 19, 174–182. [Google Scholar] [CrossRef]
- Poinern, G.E.; Senanayake, G.; Shah, N.; Thi-Le, X.N.; Parkinson, G.M.; Fawcett, D. Adsorption of the aurocyanide, Au (CN) 2-complex on granular activated carbons derived from macadamia nut shells—A preliminary study. Miner. Eng. 2011, 24, 1694–1702. [Google Scholar] [CrossRef]
- Nath, D.C.; Mansuri, I.A.; Zaharia, M.; Chaudhury, N.S.; Sahajwalla, V. Recycling of end-of-life Melamine at 1600 °C for Carbon Dissolution into Liquid Iron. ISIJ Int. 2012, 52, 922–927. [Google Scholar] [CrossRef]
- Dhunna, R.; Khanna, R.; Mansuri, I.; Sahajwalla, V. Recycling waste bakelite as an alternative carbon resource for ironmaking applications. ISIJ Int. 2014, 54, 613–619. [Google Scholar] [CrossRef]
- Mansuri, I.; Khanna, R.; Rajarao, R.; Sahajwalla, V. Recycling Waste CDs as a Carbon Resource: Dissolution of Carbon into Molten Iron at 1 550 °C. ISIJ Int. 2013, 53, 2259–2265. [Google Scholar] [CrossRef]
- Yin, S.; Rajarao, R.; Pahlevani, F.; Sahajwalla, V. Sustainable Steel Carburization by Using Snack Packaging Plastic Waste as Carbon Resources. Metals 2018, 8, 78. [Google Scholar] [CrossRef]
- Wu, C.; Wiblen, R.; Sahajwalla, V. Influence of ash on mass transfer and interfacial reaction between natural graphite and liquid iron. Metall. Mater. Trans. B 2000, 31, 1099–1104. [Google Scholar] [CrossRef]
- Antal, M.J.; Grønli, M. The art, science, and technology of charcoal production. Ind. Eng. Chem. Res. 2003, 42, 1619–1640. [Google Scholar] [CrossRef]
- Plaza, M.G.; Pevida, C.; Arias, B.; Fermoso, J.; Casal, M.D.; Martín, C.F.; Rubiera, F.; Pis, J.J. Development of low-cost biomass-based adsorbents for postcombustion CO2 capture. Fuel 2009, 88, 2442–2447. [Google Scholar] [CrossRef]
- Okutucu, C.; Duman, G.; Ucar, S.; Yasa, I.; Yanik, J. Production of fungicidal oil and activated carbon from pistachio shell. J. Anal. Appl. Pyrolysis 2011, 91, 140–146. [Google Scholar] [CrossRef]
- Lu, L.; Kong, C.; Sahajwalla, V.; Harris, D. Char structural ordering during pyrolysis and combustion and its influence on char reactivity. Fuel 2002, 81, 1215–1225. [Google Scholar] [CrossRef]
- Kawakami, M.; Kanba, H.; Sato, K.; Takenaka, T.; Gupta, S.; Chandratilleke, R.; Sahajwalla, V. Characterization of thermal annealing effects on the evolution of coke carbon structure using Raman spectroscopy and X-ray diffraction. ISIJ Int. 2006, 46, 1165–1170. [Google Scholar] [CrossRef]
- Rajarao, R.; Mansuri, I.; Dhunna, R.; Khanna, R.; Sahajwalla, V. Study of structural evolution of chars during rapid pyrolysis of waste CDs at different temperatures. Fuel 2014, 134, 17–25. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- McCarthy, F.; Sahajwalla, V.; Hart, J.; Saha-Chaudhury, N. Influence of ash on interfacial reactions between coke and liquid iron. Metall. Mater. Trans. B 2003, 34, 573–580. [Google Scholar] [CrossRef]
- Kongkarat, S.; Khanna, R.; Koshy, P.; O’Kane, P.; Sahajwalla, V. Use of waste bakelite as a raw material resource for recarburization in steelmaking processes. Steel Res. Int. 2011, 82, 1228–1239. [Google Scholar] [CrossRef]

| Proximate Analysis (wt % as Received) | |
|---|---|
| Moisture | 5.5 |
| Ash | 0.2 |
| Volatile Matter | 73.5 |
| Fixed carbon | 20.8 |
| Ultimate Analysis (wt % as Received) | |
| C | 48.39 |
| O | 40.31 |
| N | 0.333 |
| Elemental Analysis (X-ray Fluorescence Studies) | |
| Analyte | Concentration (%) |
| Na | 0.0298 |
| Mg | 0.0450 |
| Al | 0.0620 |
| Si | 0.0770 |
| P | 0.0140 |
| S | 0.0400 |
| Cl | 0.0008 |
| K | 0.1550 |
| Ca | 0.0350 |
| Cr | 0.0008 |
| Mn | 0.0047 |
| Fe | 0.0113 |
| Co | 0.0001 |
| Cu | 0.0015 |
| Zn | 0.0005 |
| Se | 0.0004 |
| Br | 0.0004 |
| Rb | 0.0001 |
| Cd | 0.0001 |
| Pb | 0.0002 |
| Name | Start BE | Peak BE | End BE | FWHM (eV) | Area (CPS eV) | At % |
|---|---|---|---|---|---|---|
| C1s A | 298.48 | 284.49 | 281.38 | 0.76 | 20,714.98 | 40.23 |
| C1s B | 298.48 | 284.99 | 281.38 | 1.73 | 13,978.33 | 27.15 |
| C1s C | 298.48 | 286.59 | 281.38 | 1.73 | 3051.44 | 5.93 |
| C1s D | 298.48 | 287.99 | 281.38 | 1.73 | 1671.52 | 3.25 |
| C1s E | 298.48 | 289.19 | 281.38 | 1.73 | 147.05 | 0.29 |
| C1s F | 298.48 | 290.16 | 281.38 | 3.2 | 4575.57 | 8.89 |
| Material | Overall Rate Constant K × 10+3 s−1 | References |
|---|---|---|
| Macadamia Char | 21.1 | This research |
| Synthetic Graphite | 24 | Wu et al. [21] |
| Waste CD char | 19.2 | Mansuri et al. [19] |
| Coal char 1 | 0.1 | McCarthy et al. [29] |
| Coal char 4 | 0.3 | McCarthy et al. [29] |
| Coke | 0.003 | Kongkarat et al. [30] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mansuri, I.; Farzana, R.; Rajarao, R.; Sahajwalla, V. Carbon Dissolution Using Waste Biomass—A Sustainable Approach for Iron-Carbon Alloy Production. Metals 2018, 8, 290. https://doi.org/10.3390/met8040290
Mansuri I, Farzana R, Rajarao R, Sahajwalla V. Carbon Dissolution Using Waste Biomass—A Sustainable Approach for Iron-Carbon Alloy Production. Metals. 2018; 8(4):290. https://doi.org/10.3390/met8040290
Chicago/Turabian StyleMansuri, Irshad, Rifat Farzana, Ravindra Rajarao, and Veena Sahajwalla. 2018. "Carbon Dissolution Using Waste Biomass—A Sustainable Approach for Iron-Carbon Alloy Production" Metals 8, no. 4: 290. https://doi.org/10.3390/met8040290
APA StyleMansuri, I., Farzana, R., Rajarao, R., & Sahajwalla, V. (2018). Carbon Dissolution Using Waste Biomass—A Sustainable Approach for Iron-Carbon Alloy Production. Metals, 8(4), 290. https://doi.org/10.3390/met8040290






