An Overview of Key Challenges in the Fabrication of Metal Matrix Nanocomposites Reinforced by Graphene Nanoplatelets
Abstract
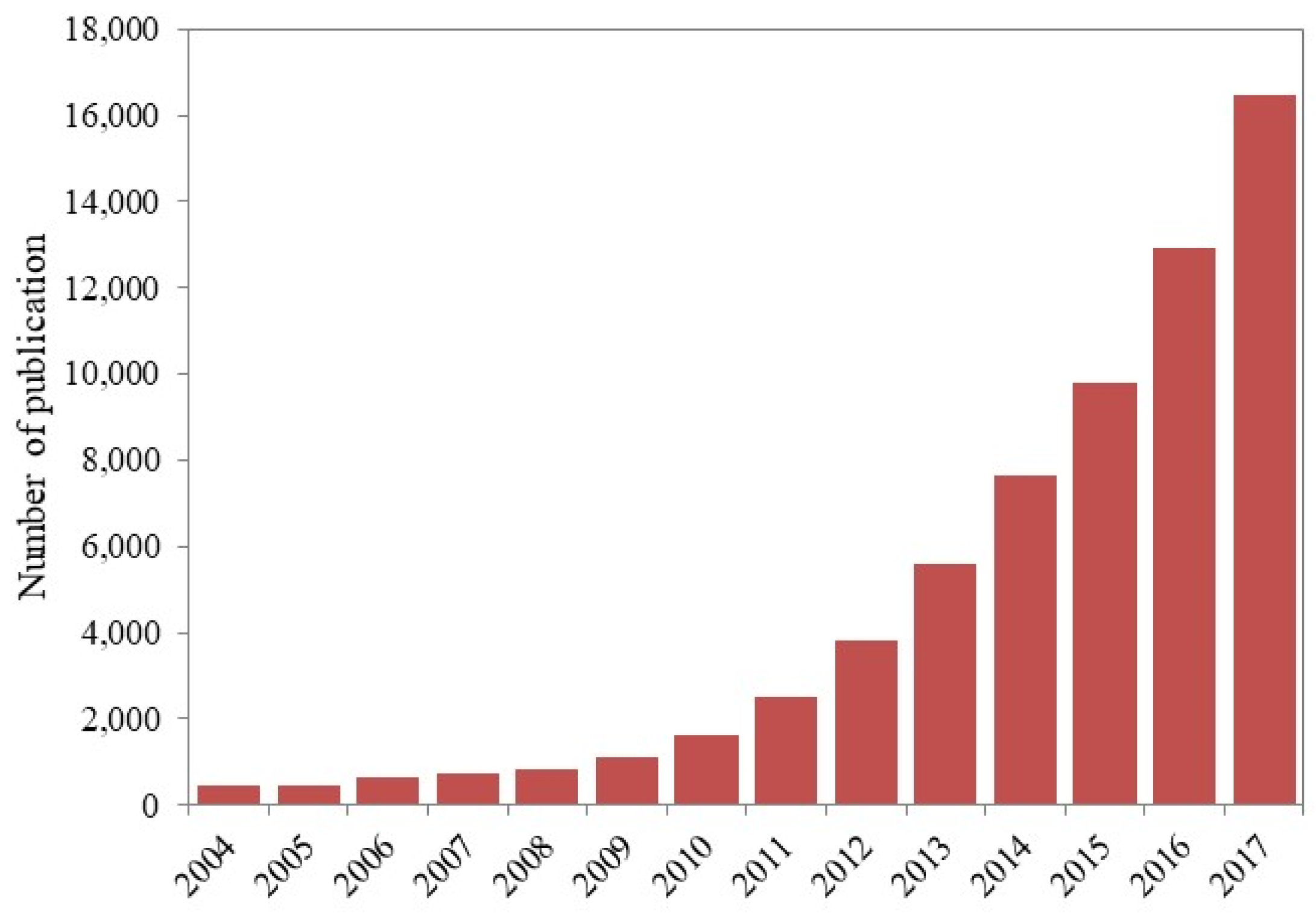
:1. Introduction
2. Metal Matrix Nanocomposites
- Higher mechanical properties
- Lower specific gravity
- Improved elevated temperature properties
- Better thermal expansion coefficient
- Higher thermal conductivity
- Higher wear resistance
- Improved damping capabilities
3. Reinforcement (First Challenge)
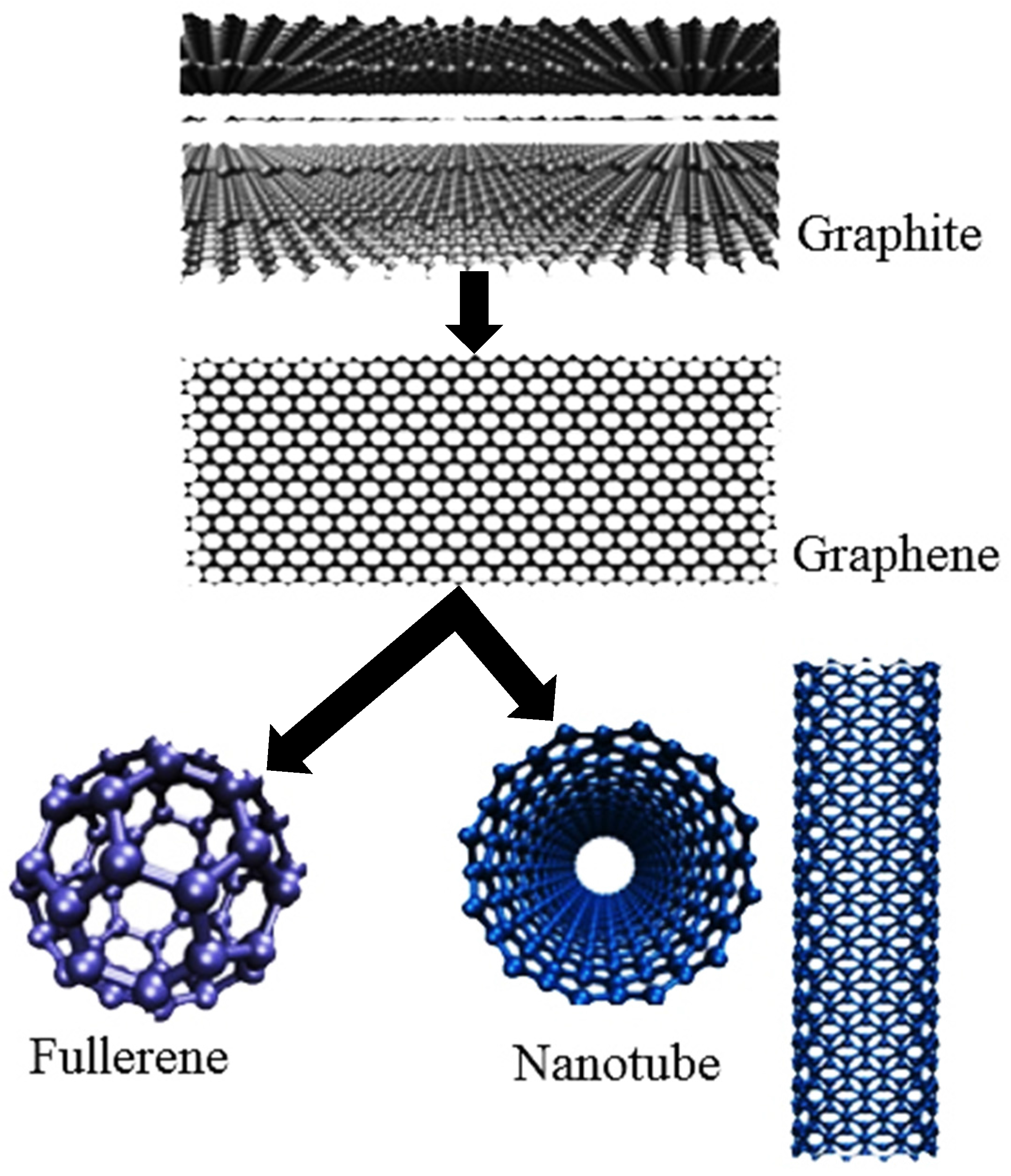
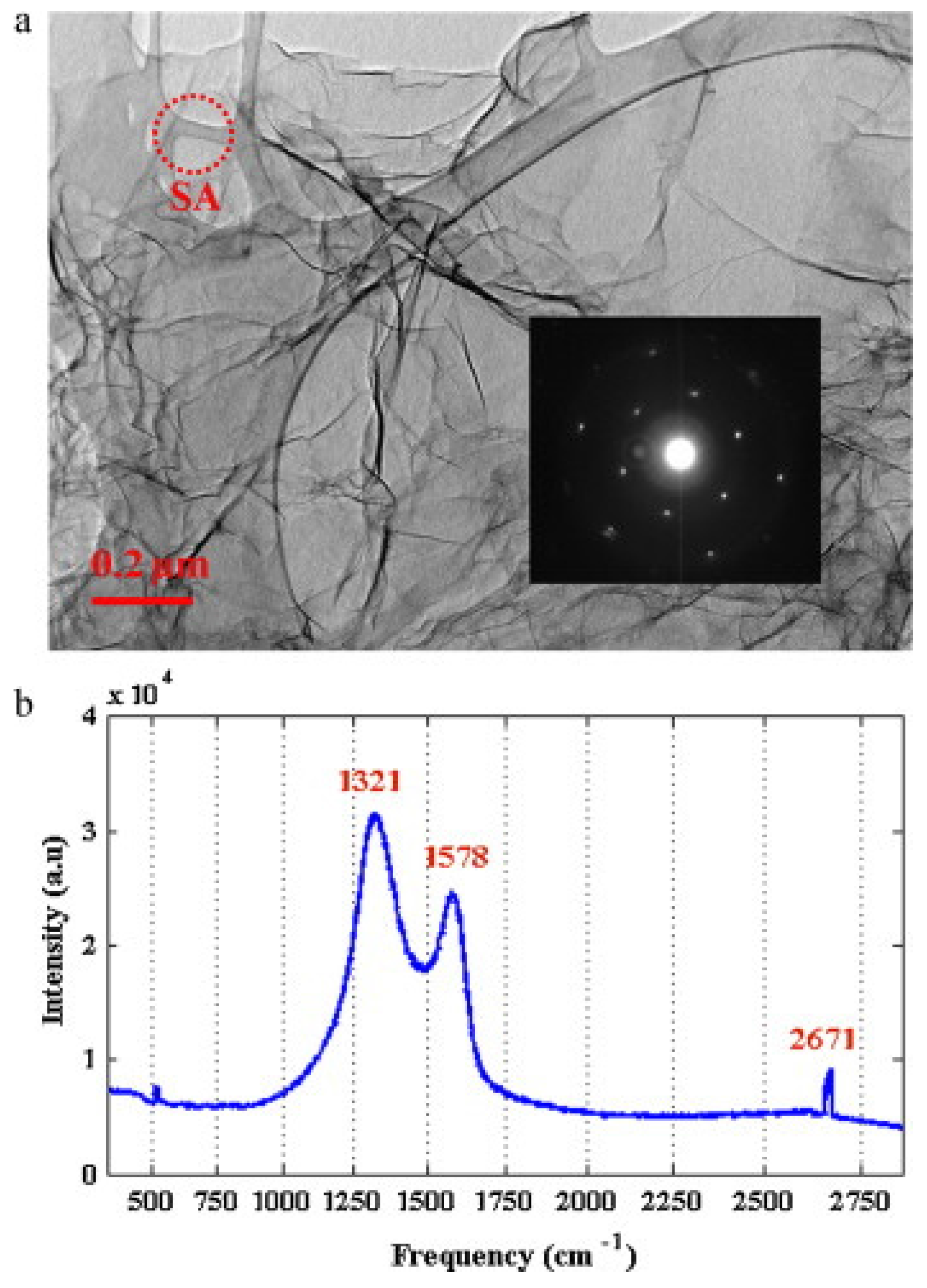
Graphene Nanoplatelets
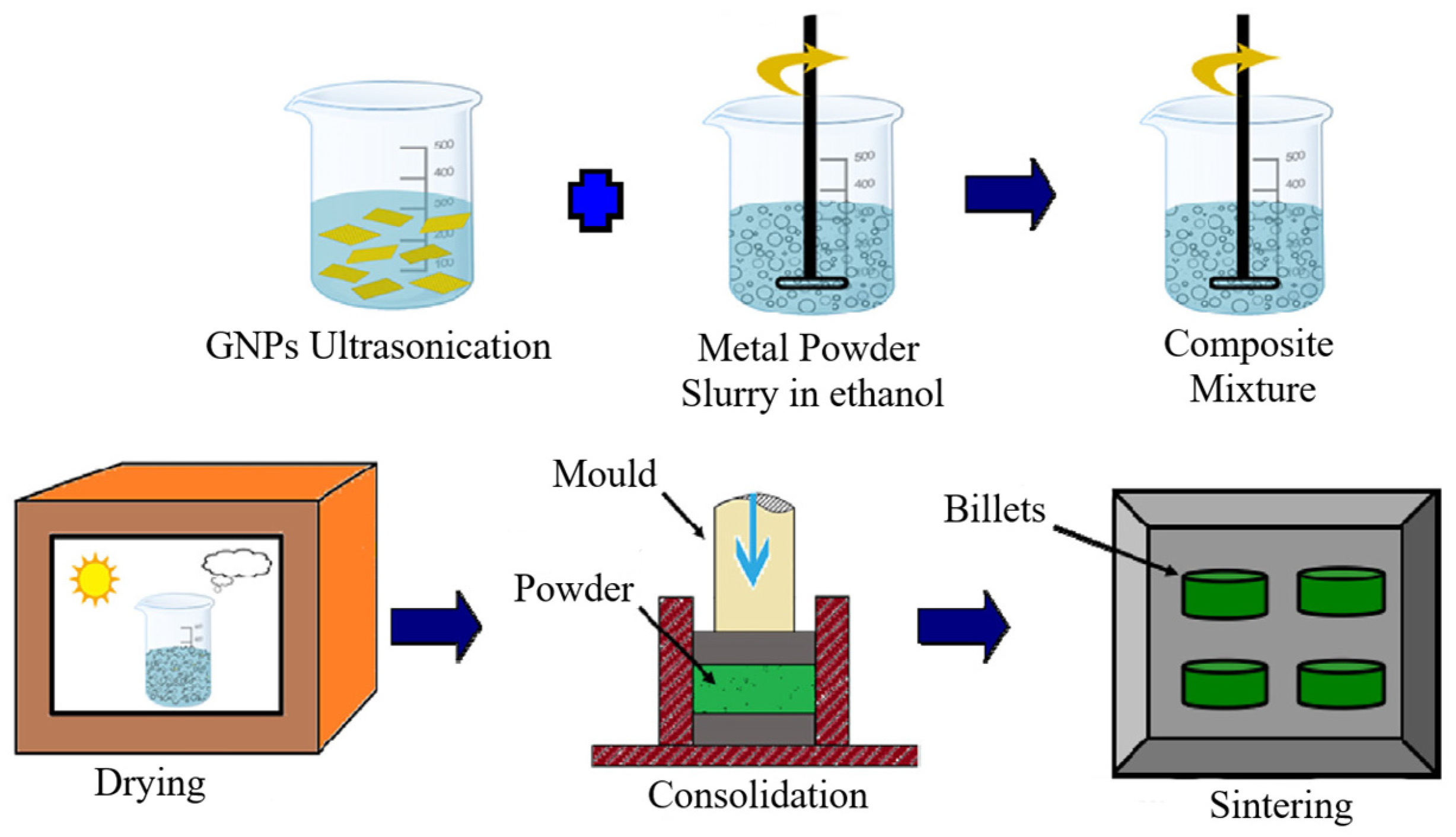
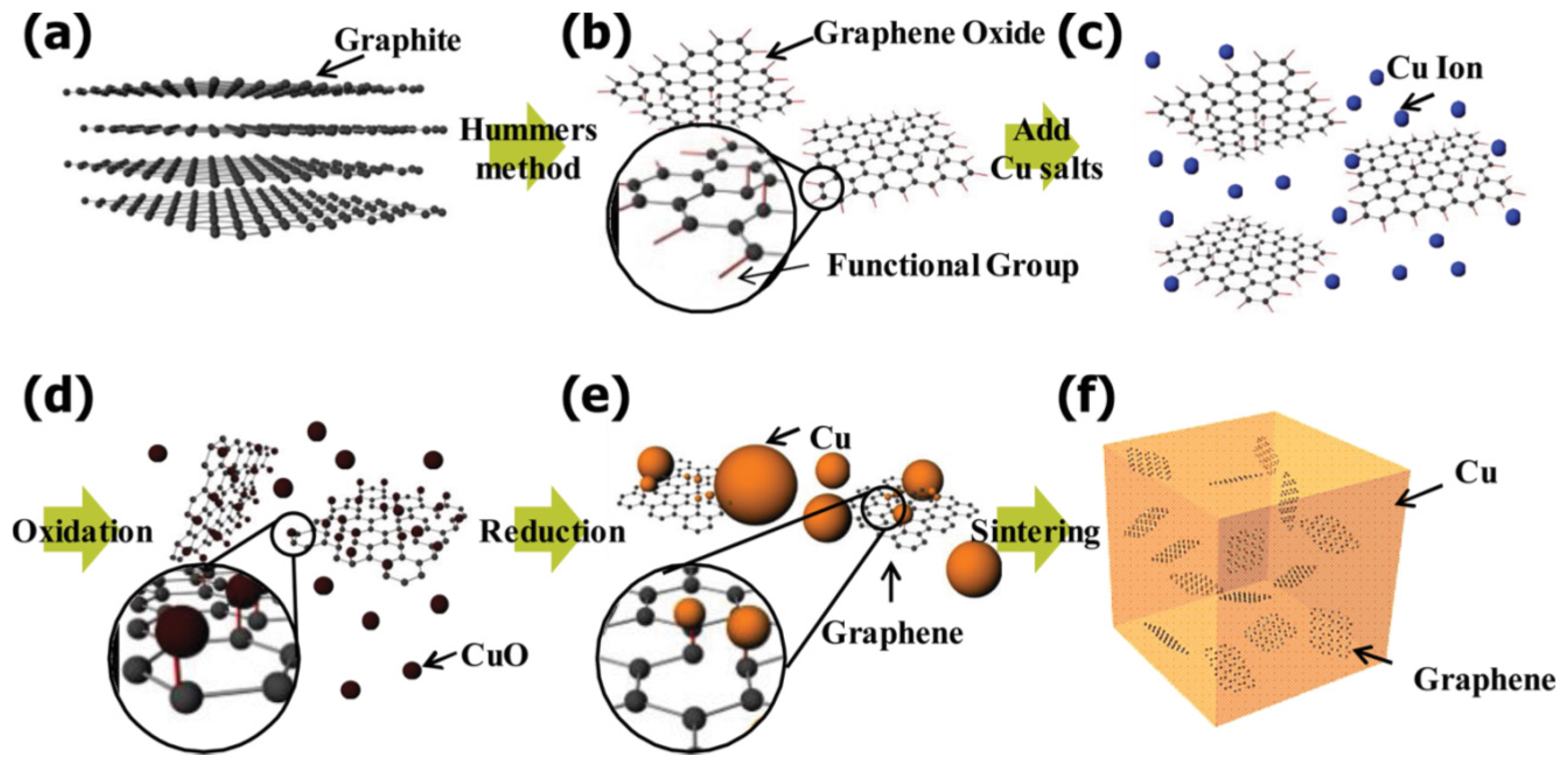
4. Preparation of MMNCs Reinforced by GNPs
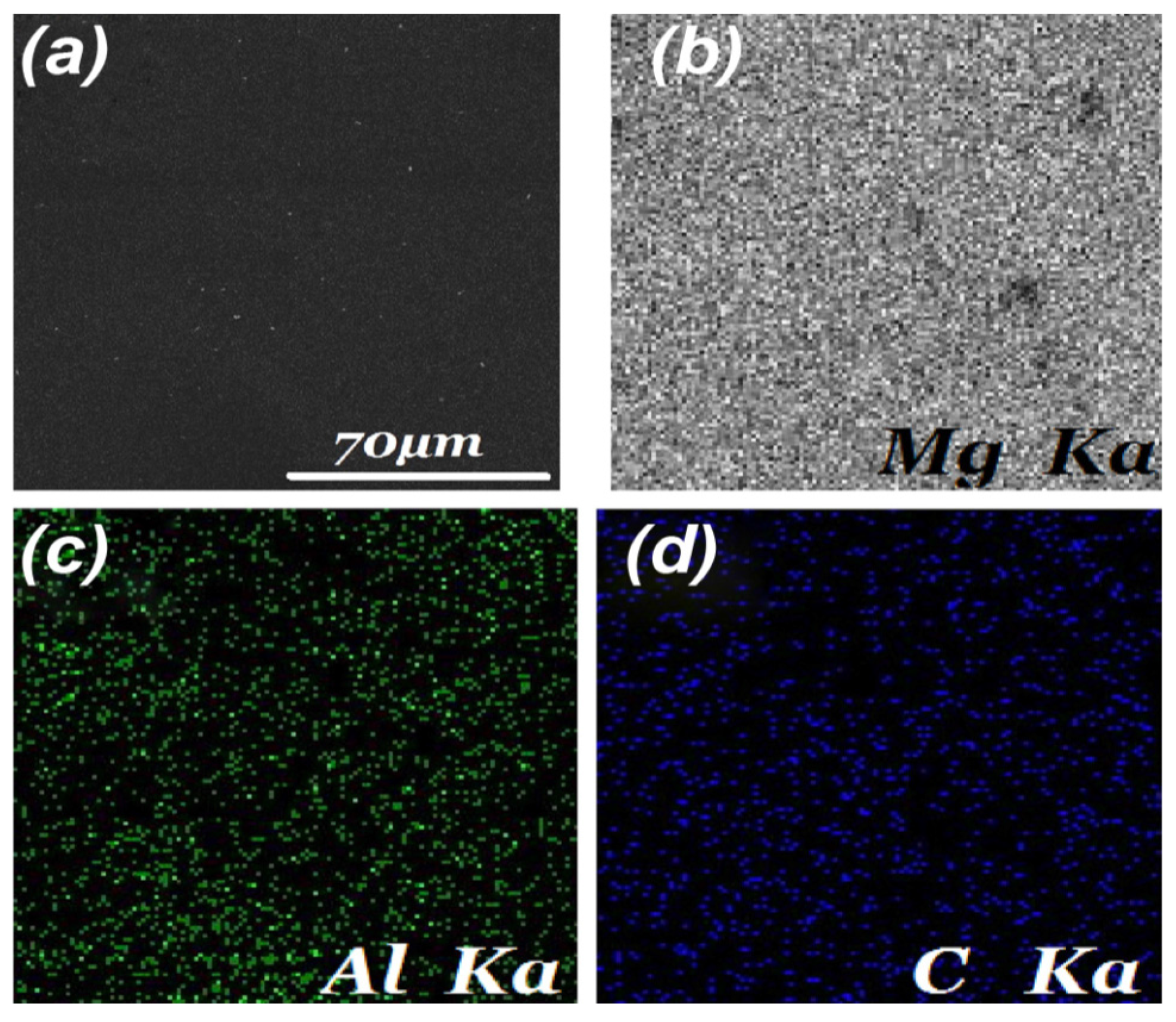
4.1. Dispersion of GNPs (Second Challenge)
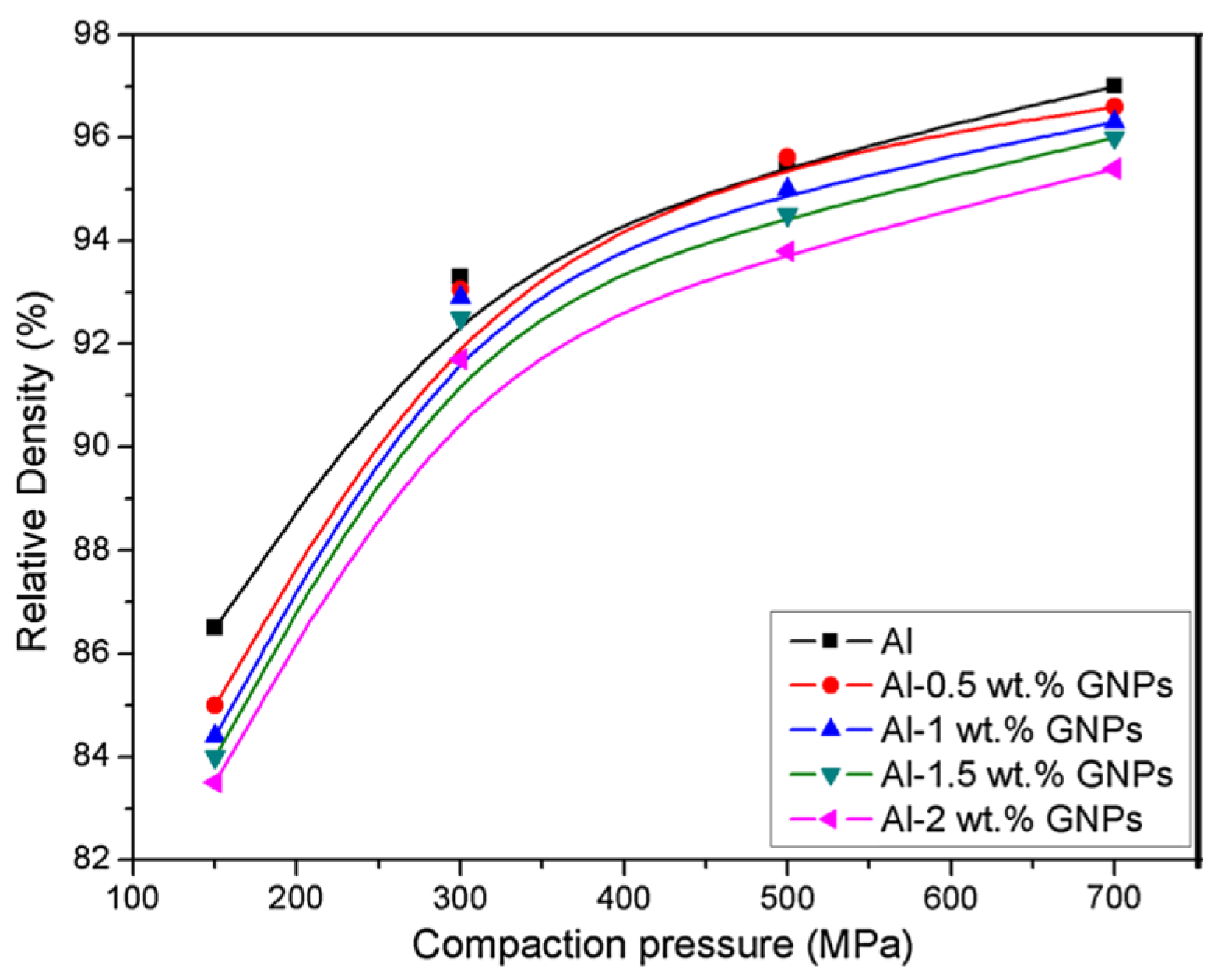
4.2. Consolidation and Sintering (Third Challenge)
4.3. Reactivity and Interface Design (Fourth and Fifth Challenge)
5. Properties of MMNCs Reinforced by GNPs
5.1. Density and Vickers Hardness
5.2. Electrical Conductivity
5.3. Tribological Performance
6. Conclusions
Author Contributions
Conflicts of Interest
References
- Rashad, M.; Pan, F.; Asif, M.; Tang, A. Powder metallurgy of Mg–1%Al–1%Sn alloy reinforced with low content of graphene nanoplatelets (GNPs). J. Ind. Eng. Chem. 2014, 20, 4250–4255. [Google Scholar] [CrossRef]
- Wang, X.; Li, J.; Wang, Y. Improved high temperature strength of copper-graphene composite material. Mater. Lett. 2016, 181, 309–312. [Google Scholar] [CrossRef]
- Wang, J.; Li, Z.; Fan, G.; Pan, H.; Zhang, D. Reinforcement with graphene nanosheets in aluminum matrix composites. Sci. Mater. 2012, 66, 594–597. [Google Scholar] [CrossRef]
- Jeon, C.-H.; Jeong, Y.-H.; Seo, J.-J.; Tien, H.N.; Hong, S.-T.; Yum, Y.-J.; Hur, S.-H.; Lee, K.-J. Material properties of graphene/aluminum metal matrix composites fabricated by friction stir processing. Int. J. Precis. Eng. Manuf. 2014, 15, 1235–1239. [Google Scholar] [CrossRef]
- Perez-Bustamante, R.; Bolanos-Morales, D.; Bonilla-Maetinez, J.; Estrada-Guel, I. Microstructural and hardness behavior of graphene-nanoplatelets/aluminum composites synthesized by mechanical alloying. J. Alloy. Compd. 2014, 615, S578–S582. [Google Scholar] [CrossRef]
- Saboori, A.; Padovano, E.; Pavese, M.; Badini, C. Novel Magnesium Elektron21-AlN Nanocomposites Produced by Ultrasound-Assisted Casting; Microstructure, Thermal and Electrical Conductivity. Materials 2018, 11, 27. [Google Scholar] [CrossRef] [PubMed]
- Tabandeh-Khorshid, M.; Omrani, E.; Menezes, P.L.; Rohatgi, P.K. Tribological performance of self-lubricating aluminum matrix nanocomposites: Role of graphene nanoplatelets. Eng. Sci. Technol. Int. J. 2016, 19, 463–469. [Google Scholar] [CrossRef]
- Saboori, A.; Pavese, M.; Badini, C.; Fino, P. A Novel Approach to Enhance the Mechanical Strength and Electrical and Thermal Conductivity of Cu-GNP Nanocomposites. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2018, 49. [Google Scholar] [CrossRef]
- Moghadam, A.D.; Omrani, E.; Menezes, P.L.; Rohatgi, P.K. Mechanical and tribological properties of self-lubricating metal matrix nanocomposites reinforced by carbon nanotubes (CNTs) and graphene—A review. Compos. Part B Eng. 2015, 77, 402–420. [Google Scholar] [CrossRef]
- Saboori, A.; Padovano, E.; Pavese, M.; Dieringa, H.; Badini, C. Effect of Solution Treatment on Precipitation Behaviors, Age Hardening Response and Creep Properties of Elektron21 Alloy Reinforced by AlN Nanoparticles. Materials 2017, 10, 1380. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, E.; Kitzmantel, M.; Hulman, M.; Angerer, P. Potential and challenges of metal-matrix-composites reinforced with carbon nanofibers and carbon nanotubes. Compos. Sci. Technol. 2010, 70, 2228–2236. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, C.; Xiao, W.; Ameyama, K.; Ma, C. Enhanced mechanical properties of Al5083 alloy with graphene nanoplates prepared by ball milling and hot extrusion. Mater. Sci. Eng. A 2016, 658, 8–15. [Google Scholar] [CrossRef]
- Dieringa, H. Properties of magnesium alloys reinforced with nanoparticles and carbon nanotubes: A review. J. Mater. Sci. 2011, 46, 289–306. [Google Scholar] [CrossRef]
- Ben-hamu, G.; Eliezer, D.; Shin, K.S.; Cohen, S. The relation between microstructure and corrosion behavior of Mg–Y–RE–Zr alloys. J. Alloy. Compd. 2007, 431, 269–276. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, X.; Liu, E.; He, C.; Han, Y.; Li, Q.; Nash, P.; Zhao, N. Fabrication of three-dimensional graphene/Cu composite by in-situ CVD and its strengthening mechanism. J. Alloy. Compd. 2016, 688, 69–76. [Google Scholar] [CrossRef]
- Yolshina, L.A.; Muradymov, R.V.; Korsun, I.V.; Yakovlev, G.A.; Smirnov, S.V. Novel aluminum-graphene and aluminum-graphite metallic composite materials: Synthesis and properties. J. Alloy. Compd. 2016, 663, 449–459. [Google Scholar] [CrossRef]
- Chen, F.; Ying, J.; Wang, Y.; Du, S.; Liu, Z.; Huang, Q. Effects of graphene content on the microstructure and properties of copper matrix composites. Carbon 2016, 96, 836–842. [Google Scholar] [CrossRef]
- Yue, H.; Yao, L.; Gao, X.; Zhang, S.; Guo, E.; Zhang, H.; Lin, X.; Wang, B. Effect of ball-milling and graphene contents on the mechanical properties and fracture mechanisms of graphene nanosheets reinforced copper matrix composites. J. Alloy. Compd. 2017, 691, 755–762. [Google Scholar] [CrossRef]
- Xue, C.; Bai, H.; Tao, P.F.; Wang, J.W.; Jiang, N. Thermal conductivity and mechanical properties of flake graphite/Al composite with a SiC nano-layer on graphite surface. Mater. Des. 2016, 108, 250–258. [Google Scholar] [CrossRef]
- Gao, X.; Yue, H.; Guo, E.; Zhang, H.; Lin, X.; Yao, L.; Wang, B. Preparation and tensile properties of homogeneously dispersed graphene reinforced aluminum matrix composites. Mater. Des. 2016, 94, 54–60. [Google Scholar] [CrossRef]
- Tian, W.; Li, S.; Wang, B.; Chen, X.; Liu, J.; Yu, M. Graphene-reinforced aluminum matrix composites prepared by spark plasma sintering. Int. J. Miner. Met. Mater. 2016, 23, 723–729. [Google Scholar] [CrossRef]
- Lavagna, L.; Massella, D.; Pavese, M. Preparation of hierarchical material by chemical grafting of carbon nanotubes onto carbon fibers. Diam. Relat. Mater. 2017, 80, 118–124. [Google Scholar] [CrossRef]
- Prasad, S.V.; Asthana, R. Aluminum metal-matrix composites for automotive applications: Tribological considerations. Tribol. Lett. 2004, 17, 445–453. [Google Scholar] [CrossRef]
- Musfirah, A.H.; Jaharah, A.G. Magnesium and Aluminum Alloys in Automotive Industry. J. Appl. Sci. Res. 2012, 8, 4865–4875. [Google Scholar]
- Kulekci, M.K. Magnesium and its alloys applications in automotive industry. Int. J. Adv. Manuf. Technol. 2008, 39, 851–865. [Google Scholar] [CrossRef]
- Saboori, A.; Gallo, D.; Biamino, S.; Fino, P.; Lombardi, M. An Overview of Additive Manufacturing of Titanium Components by Directed Energy Deposition: Microstructure and Mechanical Properties. Appl. Sci. 2017, 7, 883. [Google Scholar] [CrossRef]
- Hu, H.; Yu, A.; Li, N.; Allison, J.E. Potential Magnesium Alloys for High Temperature Die Cast Automotive Applications: A Review. Mater. Manuf. Process. 2003, 18, 687–717. [Google Scholar] [CrossRef]
- Macke, A.; Schultz, B.F. Metal Matrix Composites Offer the Automotive Industry and Opportunity to Reduce Vehicle Weight, Improve Performance. Adv. Mater. Process. 2012, 170, 19–23. [Google Scholar]
- Saboori, A.; Pavese, M.; Badini, C.; Eivani, A.R. Studying the age hardening kinetics of A357 aluminum alloys through the Johnson–Mehl–Avrami theory. Met. Powder Rep. 2017, 72, 420–424. [Google Scholar] [CrossRef]
- Rajkumar, K.; Aravindan, S. Tribological behavior of microwave processed copper–nanographite composites. Tribiol. Int. 2013, 57, 282–296. [Google Scholar] [CrossRef]
- Metal Matrix Composite. 2013. Available online: https://en.wikipedia.org/wiki/Metal_matrix_composite (accessed on 25 November 2017).
- Kopeliovich, D. Metal Matrix Composites (Introduction). 2013. Available online: http://www.substech.com/dokuwiki/doku.php?id=metal_matrix_composites_introduction (accessed on 25 November 2017).
- Casati, R.; Vedani, M. Metal Matrix Composites Reinforced by Nano-Particles—A Review. Metals 2014, 4, 65–83. [Google Scholar] [CrossRef]
- Chen, Z.-C.; Takeda, T.; Ikeda, K. Microstructural evolution of reactive-sintered aluminum matrix composites. Compos. Sci. Technol. 2008, 68, 2245–2253. [Google Scholar] [CrossRef]
- Kopeliovich, D. Polymer Matrix Composites (Introduction). 2013. Available online: http://www.substech.com/dokuwiki/doku.php?id=polymer_matrix_composites_introduction (accessed on 11 September 2017).
- Kopeliovich, D. Ceramic Matrix Composites (Introduction). 2013. Available online: http://www.substech.com/dokuwiki/doku.php?id=ceramic_matrix_composites_introduction (accessed on 11 September 2017).
- Callister, W.D. Materials Science and Engineering—An Introduction; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; ISBN 9780471736967. [Google Scholar]
- Tang, Y.; Liu, L.; Li, W.; Shen, B.; Hu, W. Interface characteristics and mechanical properties of short carbon fibers/Al composites with different coatings. Appl. Surf. Sci. 2009, 255, 4393–4400. [Google Scholar] [CrossRef]
- Włodarczyk-Fligier, A.; Dobrzański, L.A.; Kremzer, M.; Adamiak, M. Manufacturing of aluminium matrix composite materials reinforced by Al2O3 particles. J. Achiev. Mater. Manuf. Eng. 2008, 27, 99–102. [Google Scholar]
- Huber, T.; Degischer, H.P.; Lefrance, G.; Schmitt, T. Thermal expansion studies on aluminium-matrix composites with different reinforcement architecture of SiC particles. Compos. Sci. Technol. 2006, 66, 2206–2217. [Google Scholar] [CrossRef]
- Huu, T.; Requena, G.; Degischer, P. Thermal expansion behaviour of aluminum matrix composites with densely packed SiC particles. Compos. Part A Appl. Sci. Manuf. 2008, 39, 856–865. [Google Scholar] [CrossRef]
- Jeyasimman, D.; Sivaprasad, K.; Sivasankaran, S.; Narayanasamy, R. Fabrication and consolidation behavior of Al 6061 nanocomposite powders reinforced by multi-walled carbon nanotubes. Powder Technol. 2014, 258, 189–197. [Google Scholar] [CrossRef]
- Rashad, M.; Pan, F.; Tang, A.; Asif, M.; Aamir, M. Synergetic effect of graphene nanoplatelets (GNPs) and multi-walled carbon nanotube (MW-CNTs) on mechanical properties of pure magnesium. J. Alloy. Compd. 2014, 603, 111–118. [Google Scholar] [CrossRef]
- Nie, K.B.; Wang, X.J.; Xu, L.; Wu, K.; Hu, X.S.; Zheng, M.Y. Influence of extrusion temperature and process parameter on microstructures and tensile properties of a particulate reinforced magnesium matrix nanocomposite. Mater. Des. 2012, 36, 199–205. [Google Scholar] [CrossRef]
- Gaspera, E.D.; Tucker, R.; Star, K.; Lan, E.H.; Ju, Y.S.; Dunn, B. Copper-Based Conductive Composites with Tailored Thermal Expansion. ACS Appl. Mater. Interfaces 2013, 5, 10966–10974. [Google Scholar] [CrossRef] [PubMed]
- Varol, T.; Canakci, A. The effect of type and ratio of reinforcement on the synthesis and characterization Cu-based nanocomposites by flake powder metallurgy. J. Alloy. Compd. 2015, 649, 1066–1074. [Google Scholar] [CrossRef]
- Tang, Y.; Yang, X.; Wang, R.; Li, M. Enhancement of the mechanical properties of graphene–copper composites with graphene–nickel hybrids. Mater. Sci. Eng. A 2014, 599, 247–254. [Google Scholar] [CrossRef]
- Rana, R.S.; Purohit, R.; Das, S. Review of recent Studies in Al matrix composites. Int. J. Sci. Eng. Res. 2012, 3, 1–16. [Google Scholar]
- Hu, H.; Kong, J. Improved Thermal Performance of Diamond-Copper Composites with Boron Carbide Coating. J. Mater. Eng. Perform. 2014, 23, 651–657. [Google Scholar] [CrossRef]
- Zhang, D.; Zhan, Z. Preparation of graphene nanoplatelets-copper composites by a modified semi-powder method and their mechanical properties. J. Alloy. Compd. 2016, 658, 663–671. [Google Scholar] [CrossRef]
- Kielbus, A.; Rzychon, T.; Przeliorz, R. DSC and Microstructural Investigations of The Elektron 21 Magnesium Alloy. Mater. Sci. Forum 2010, 642, 1447–1452. [Google Scholar] [CrossRef]
- Katsarou, L.; Mounib, M.; Lefebvre, W.; Vorozhtsov, S.; Pavese, M.; Badini, C.; Molina-aldareguia, J.M.; Cepeda, C.; MariaTeresa, P.P.; Dieringa, H. Microstructure, mechanical properties and creep of magnesium alloy Elektron21 reinforced with AlN nanoparticles by ultrasound-assisted stirring. Mater. Sci. Eng. A 2016, 659, 84–92. [Google Scholar] [CrossRef]
- Chen, X. Fabrication and Properties of Particulate reinforced Aluminum Matrix Composites by Spontaneous Infiltration. Ph.D. Thesis, Politecnico di Torino, Torino TO, Italy, 2013. [Google Scholar]
- Kroto, H. Handbook of Chemistry and Physics, 84th ed.; Lide, D.R., Ed.; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Nishida, Y. Introduction to Metal Matrix Composites: Fabrication and Recycling; Springer: New York, NY, USA, 2013. [Google Scholar]
- Cardarelli, F. Materials Handbook: A Coincise Desktop Reference; Springer: New York, NY, USA, 2008. [Google Scholar]
- Srinivasan, M. Non-Oxide Materials: Applications and Engineering; Springer: Dordrecht, The Netherlands, 1996; pp. 3–42. [Google Scholar]
- Safdari, M. A Computational and Experimental Study on the Electrical and Thermal Properties of Hybrid Nanocomposites Based on Carbon Nanotubes and Graphite Nanoplatelets. Ph.D. Thesis, Virginia Polytechnic Institute and State University, Blacksburg, VA, USA, 2012. [Google Scholar]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Al, E. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Abdala, A.A.; Macosko, C.W. Graphene/Polymer Nanocomposites. Macromolecules 2010, 43, 6515–6530. [Google Scholar] [CrossRef]
- Rollings, E.; Gweon, G.-H.; Zhou, S.Y.; Mun, B.S.; McChesney, J.L.; Hussain, B.S.; Fedorov, A.V.; First, P.N.; de Heer, W.A.; Lanzara, A. Synthesis and characterization of atomically thin graphite films on a silicon carbide substrate. J. Phys. Chem. Solids 2006, 67, 2172–2177. [Google Scholar] [CrossRef]
- Wang, X.; You, H.; Liu, F.; Li, M.; Wan, L.; Li, S.; Li, Q.; Xu, Y.; Tian, R.; Yu, Z.; et al. Large-Scale Synthesis of Few-Layered Graphene using CVD. Chem. Vap. Depos. 2009, 15, 5356. [Google Scholar] [CrossRef]
- Kosynkin, D.V.; Higginbotham, A.L.; Sinitskii, A.; Lomeda, J.R.; Dimiev, A.; Price, B.K.; Tour, J.M. Longitudinal unzipping of carbon nanotubes to form graphene Nanoribbons. Nature 2009, 458, 872–876. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Hu, Y.; Li, C.; Qin, C.; Shi, M.; Ye, M. Layer-by-Layer Self-Assembly of Graphene Nanoplatelets. Langmuir 2009, 25, 6122–6128. [Google Scholar] [CrossRef] [PubMed]
- Jang, B.; Zhamu, A. Processing of nanographene platelets (NGPs) and NGP nanocomposites: A review. J. Mater. Sci. 2008, 43, 5092–5101. [Google Scholar] [CrossRef]
- Fukushima, H.; Drzal, L.; Rook, B.; Rich, M. Thermal conductivity of exfoliated graphite nanocomposites. J. Therm. Anal. Calorim. 2006, 85, 235–238. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Bartolucci, S.F.; Paras, J.; Rafiee, M.A.; Rafiee, J.; Lee, S.; Kapoor, D.; Koratkar, N. Graphene–aluminum nanocomposites. Mater. Sci. Eng. A 2011, 528, 7933–7937. [Google Scholar] [CrossRef]
- Saboori, A.; Moheimani, S.K.; Pavese, M.; Badini, C.; Fino, P. New nanocomposite materials with improved mechanical strength and tailored coefficient of thermal expansion for electro-packaging applications. Metals 2017, 7. [Google Scholar] [CrossRef]
- Saboori, A.; Pavese, M.; Badini, C.; Fino, P. Microstructure and thermal conductivity of Al-Graphene composites fabricated by powder metallurgy and hot rolling techniques. Acta Metall. Sin. Engl. Lett. 2017, 30, 675–687. [Google Scholar] [CrossRef]
- Hwang, J.; Yoon, T.; Jin, S.H.; Lee, J.; Kim, T.-S.; Hong, S.H.; Jeon, S. Enhanced Mechanical Properties of Graphene/Copper Nanocomposites Using a Molecular-Level Mixing Process. Adv. Mater. 2013, 25, 6724–6729. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Arai, S.; Endo, M. Metallization of multi-walled carbon nanotubes with copper by an electroless deposition process. Electrochem. Commun. 2004, 6, 1042–1044. [Google Scholar] [CrossRef]
- Jiang, L.; Gao, L. Modified carbon nanotubes: An effective way to selective attachment of gold nanoparticles. Carbon 2003, 41, 2923–2929. [Google Scholar] [CrossRef]
- Chin, K.C.; Gohel, A.; Chen, W.Z.; Elim, H.I.; Ji, W.; Chong, G.L.; Sow, C.H.; Wee, A.T.S. Gold and silver coated carbon nanotubes: An improved broad-band optical limiter. Chem. Phys. Lett. 2005, 409, 85–88. [Google Scholar] [CrossRef]
- Karches, M.; von Rohr, P.R. Microwave plasma characteristics of a circulating fluidized bed-plasma reactor for coating of powders. Surf. Coat. Technol. 2001, 142–144, 28–33. [Google Scholar] [CrossRef]
- Kouadri-Mostefa, S.; Serp, P.; Hémati, M.; Caussat, B. Silicon Chemical Vapor Deposition (CVD) on microporous powders in a fluidized bed. Powder Technol. 2001, 120, 82–87. [Google Scholar] [CrossRef]
- Dag, S.; Durgun, E.; Ciraci, S. High-conducting magnetic nanowires obtained from uniform titanium-covered carbon nanotubes. Phys. Rev. B 2004, 69, 121407. [Google Scholar] [CrossRef]
- Zhang, Y.; Franklin, N.W.; Chen, R.J.; Dai, H. Metal coating on suspended carbon nanotubes and its implication to metal–tube interaction. Chem. Phys. Lett. 2000, 331, 35–41. [Google Scholar] [CrossRef]
- Rashad, M.; Pan, F.; Yu, Z.; Asif, M. Investigation on microstructural, mechanical and electrochemical properties of aluminum composites reinforced with graphene nanoplatelets. Prog. Nat. Sci. Mater. Int. 2015, 25, 460–470. [Google Scholar] [CrossRef]
- Rashad, M.; Pan, F.; Tang, A.; Asif, M. Effect of Graphene Nanoplatelets addition on mechanical properties of pure aluminum using a semi-powder method. Prog. Nat. Sci. Mater. Int. 2014, 24, 101–108. [Google Scholar] [CrossRef]
- Saboori, A.; Pavese, M.; Badini, C.; Fino, P. Effect of Sample Preparation on the Microstructural Evaluation of Al–GNPs Nanocomposites. Metall. Microstruct. Anal. 2017, 6, 619–622. [Google Scholar] [CrossRef]
- Van der Zwan, J.; Siskens, C.A.M. The compaction and mechanical properties of agglomerated materials. Powder Technol. 1982, 33, 43–54. [Google Scholar] [CrossRef]
- Padmavathi, C.; Upadhyaya, A. Densification, Microstructure and Properties of Supersolidus Liquid Phase Sintered 6711Al-SiC Metal Matrix Composites. Sci. Sinter. 2010, 42, 363–382. [Google Scholar] [CrossRef]
- Balshin, M.; Metalloprom, V. Theory Compact. Vestnik Metalloprom 1938, 18, 124–137. [Google Scholar]
- Heckel, R.W. Density-Pressure Relationships in Powder Compaction. Trans. Metall. Soc. AIME 1961, 221, 671–675. [Google Scholar]
- Walker, E.E. The properties of powders. Part VI. The compressibility of powders. Trans. Faraday Soc. 1923, 19, 73–82. [Google Scholar] [CrossRef]
- Cooper, A.R.; EatonEato, L.E. Compaction behaviour of some ceramic powders. J. Am. Ceram. Soc. 1962, 45, 97–101. [Google Scholar] [CrossRef]
- Kawakita, K.; Ludde, K. Some considerations on powder compression equations. Powder Technol. 1971, 4, 61–68. [Google Scholar] [CrossRef]
- Panelli, R.; Filho, F.A. A study of a new phenomenological compacting equation. Powder Technol. 2001, 114, 255–261. [Google Scholar] [CrossRef]
- Ge, R. A new powder compaction equation. Int. J. Powder Metall. 1991, 27, 211–216. [Google Scholar]
- Doraivelu, S.M.; Gegel, H.L.; Gunasekera, J.S.; Malas, J.C.; Morgan, J.T.; Thomas, J.F., Jr. A new yield function for compressible P M materials. Int. J. Mech. Sci. 1984, 26, 527–535. [Google Scholar] [CrossRef]
- Fleck, N.A.; Kuhn, L.T.; McMeeking, R.M. Yielding of Metal Powder Bonded by Isolated Contacts. Phys. Solids 1992, 40, 1139–1162. [Google Scholar] [CrossRef]
- Hesabi, Z.R.; Hafizpour, H.R.; Simchi, A. An investigation on the compressibility of aluminum/nano-alumina composite powder prepared by blending and mechanical milling. Mater. Sci. Eng. A 2007, 454, 89–98. [Google Scholar] [CrossRef]
- Saboori, A.; Novara, C.; Pavese, M.; Badini, C.; Giorgis, F.; Fino, P. An Investigation on the Sinterability and the Compaction Behavior of Aluminum/Graphene Nanoplatelets (GNPs) Prepared by Powder Metallurgy. J. Mater. Eng. Perform. 2017, 26, 993–999. [Google Scholar] [CrossRef]
- Rashad, M.; Pan, F.; Tang, A.; Asif, M.; Hussain, S.; Gou, J.; Mao, J. Improved strength and ductility of magnesium with addition of aluminum and graphene nanoplatelets (Al + GNPs) using semi powder metallurgy method. J. Ind. Eng. Chem. 2015, 23, 243–250. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, X.; Liu, E.; He, C.; Shi, C.; Li, J.; Nash, P.; Zhao, N. Fabrication of in-situ grown graphene reinforced Cu matrix composites. Sci. Rep. 2016, 6, 19363. [Google Scholar] [CrossRef] [PubMed]
- Saboori, A.; Pavese, M.; Badini, C.; Fino, P. Development of Al- and Cu-based nanocomposites reinforced by graphene nanoplatelets: Fabrication and characterization. Front. Mater. Sci. 2017, 11, 171–181. [Google Scholar] [CrossRef]
- Laha, T.; Kuchibhatla, S.; Seal, S.; Li, W.; Agarwal, A. Interfacial phenomena in thermally sprayed multiwalled carbon nanotube reinforced aluminum nanocomposite. Acta Mater. 2007, 55, 1059–1066. [Google Scholar] [CrossRef]
- Ci, L.; Ryu, Z.; Jin-Phillipp, N.Y.; Rühle, M. Investigation of the interfacial reaction between multi-walled carbon nanotubes and aluminum. Acta Mater. 2006, 54, 5367–5375. [Google Scholar] [CrossRef]
- Kwon, H.; Estili, M.; Takagi, K.; Miyazaki, T.; Kawasaki, A. Combination of hot extrusion and spark plasma sintering for producing carbon nanotube reinforced aluminum matrix composites. Carbon 2009, 47, 570–577. [Google Scholar] [CrossRef]
- Deng, C.F.; Zhang, X.X.; Wang, D.Z.; Ma, Y.X. Calorimetric study of carbon nanotubes and aluminum. Mater. Lett. 2007, 61, 3221–3223. [Google Scholar] [CrossRef]
- Deng, C.F.; Wang, D.Z.; Zhang, X.X.; Li, A.B. Processing and properties of carbon nanotubes reinforced aluminum composites. Mater. Sci. Eng. A 2007, 444, 138–145. [Google Scholar] [CrossRef]
- Kuzumaki, T.; Miyazawa, K.; Ichinose, H.; Ito, K. Processing of Carbon Nanotube Reinforced Aluminum Composite. J. Mater. Res. 1998, 13, 2445–2449. [Google Scholar] [CrossRef]
- George, R.; Kashyap, K.T.; Rahul, R.; Yamdagni, S. Strengthening in carbon nanotube/aluminium (CNT/Al) composites. Scr. Mater. 2005, 53, 1159–1163. [Google Scholar] [CrossRef]
- Pérez-Bustamante, R.; Estrada-Guel, I.; Antúnez-Flores, W.; Miki-Yoshida, M.; Ferreira, P.J.; Martínez-Sánchez, R. Novel Al-matrix nanocomposites reinforced with multi-walled carbon nanotubes. J. Alloy. Compd. 2008, 450, 323–326. [Google Scholar] [CrossRef]
- Kwon, H.; Park, D.H.; Silvain, J.F.; Kawasaki, A. Investigation of carbon nanotube reinforced aluminum matrix composite materials. Compos. Sci. Technol. 2010, 70, 546–550. [Google Scholar] [CrossRef]
- Schniepp, H.C.; Li, J.-L.; McAllister, M.J.; Sai, H.; Herrera-Alonso, M.; Adamson, D.H.; Prud’homme, R.K.; Car, R.; Saville, D.A.; Aksay, I.A. Functionalized Single Graphene Sheets Derived from Splitting Graphite Oxide. J. Phys. Chem. B 2006, 110, 8535–8539. [Google Scholar] [CrossRef] [PubMed]
- Rafiee, M.A.; Rafiee, J.; Srivastava, I.; Wang, Z.; Song, H.; Yu, Z.-Z.; Koratkar, N. Fracture and Fatigue in Graphene Nanocomposites. Small 2010, 6, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Saboori, A. Metal Matrix Nanocomposites; Potentials, Challenges and Feasible Solutions. Ph.D. Thesis, Politecnico di Torino, Torino TO, Italy, 2017. [Google Scholar]
- Saboori, A.; Pietroluongo, M.; Pavese, M.; Badini, C.F.; Fino, P. Influence of Graphene nanoplatelets (GNPs) on compressibility and sinterability of Al matrix nanocomposites prepared by powder metallurgy. In World PM2016; EPMA: Hamburg, Germany, 2016; p. 8. [Google Scholar]
- Saboori, A.; Pavese, M.; Badini, C.; Fino, P. A Novel Cu—GNPs Nanocomposite with Improved Thermal and Mechanical Properties. Acta Metall. Sin. Engl. Lett. 2017. [Google Scholar] [CrossRef]
- Shin, S.E.; Choi, H.J.; Shin, J.H.; Bae, D.H. Strengthening behavior of few-layered graphene/aluminum composites. Carbon 2014, 82, 143–151. [Google Scholar] [CrossRef]
- Bakshi, S.R.; Agarwal, A. An analysis of the factors affecting strengthening in carbon nanotube reinforced aluminum composites. Carbon 2011, 49, 533–544. [Google Scholar] [CrossRef]
- Chawla, N.; Shen, Y. Mechanical Behavior of Particle Reinforced Metal Matrix Composites. Adv. Eng. Mater. 2001, 3, 357–370. [Google Scholar] [CrossRef]
- Song, S.G.; Shi, N.; Gray, G.T.; Roberts, J.A. Reinforcement shape effects on the fracture behavior and ductility of particulate-reinforced 6061-Al matrix composites. Metall. Mater. Trans. A 1996, 27, 3739–3746. [Google Scholar] [CrossRef]
- Akhtar, F.; Javid, S.; Ali, K.; Du, X.; Guo, S. Microstructure, mechanical properties, electrical conductivity and wear behavior of high volume TiC reinforced Cu-matrix composites. Mater. Charact. 2009, 60, 327–336. [Google Scholar] [CrossRef]
- Goli, P.; Ning, H.; Li, X.; Lu, C.Y.; Novoselov, K.S.; Balandin, A.A. Thermal Properties of Graphene–Copper–Graphene Heterogeneous Films. NANO Lett. 2014, 14, 1497–1503. [Google Scholar] [CrossRef] [PubMed]
- Arnaud, C.; Lecouturier, F.; Mesguich, D.; Ferreira, N.; Chevallier, G.; Estournès, C.; Weibel, A.; Laurent, C. High strength—High conductivity double-walled carbon nanotube—Copper composite wires. Carbon 2016, 96, 212–215. [Google Scholar] [CrossRef]
- Moustafa, S.F.; El-Badry, S.A.; Sanad, A.M.; Kieback, B. Friction and wear of copper–graphite composites made with Cu-coated and uncoated graphite powders. Wear 2002, 253, 699–710. [Google Scholar] [CrossRef]
- Moustafa, S.F.; El-Badry, S.A.; Sanad, A.M. Effect of Graphite with and Without Copper Coating on Consolidation Behaviour and Sintering of Copper–Graphite Composite. Powder Metall. 1997, 40, 201–206. [Google Scholar] [CrossRef]
- Yao, G.C.; Mei, Q.S.; Li, J.Y.; Li, C.L.; Ma, Y.; Chen, F.; Liu, M. Cu/C composites with a good combination of hardness and electrical conductivity fabricated from Cu and graphite by accumulative roll-bonding. Mater. Des. 2016, 110, 124–129. [Google Scholar] [CrossRef]
- Zhao, S.; Zheng, Z.; Huang, Z.; Dong, S.; Luo, P.; Zhang, Z.; Wang, Y. Cu matrix composites reinforced with aligned carbon nanotubes: Mechanical, electrical and thermal properties. Mater. Sci. Eng. A 2016, 675, 82–91. [Google Scholar] [CrossRef]
- Tu, J.P.; Rong, W.; Guo, S.Y.; Yang, Y.Z. Dry sliding wear behavior of in situ Cu–TiB2 nanocomposites against medium carbon steel against medium carbon steel. Wear 2003, 255, 832–835. [Google Scholar] [CrossRef]
- Nadkarni, A.V.; Samal, P.K.; Synk, J.E. Dispersion strengthened metal composites. U.S. Patent 4,752,334, 21 June 1988. [Google Scholar]
- Dorri Moghadam, A.; Schultz, B.F.; Ferguson, J.B.; Omrani, E.; Rohatgi, P.K.; Gupta, N. Functional Metal Matrix Composites: Self-lubricating, Self-healing, and Nanocomposites-An Outlook. JOM 2014, 66, 872–881. [Google Scholar] [CrossRef]



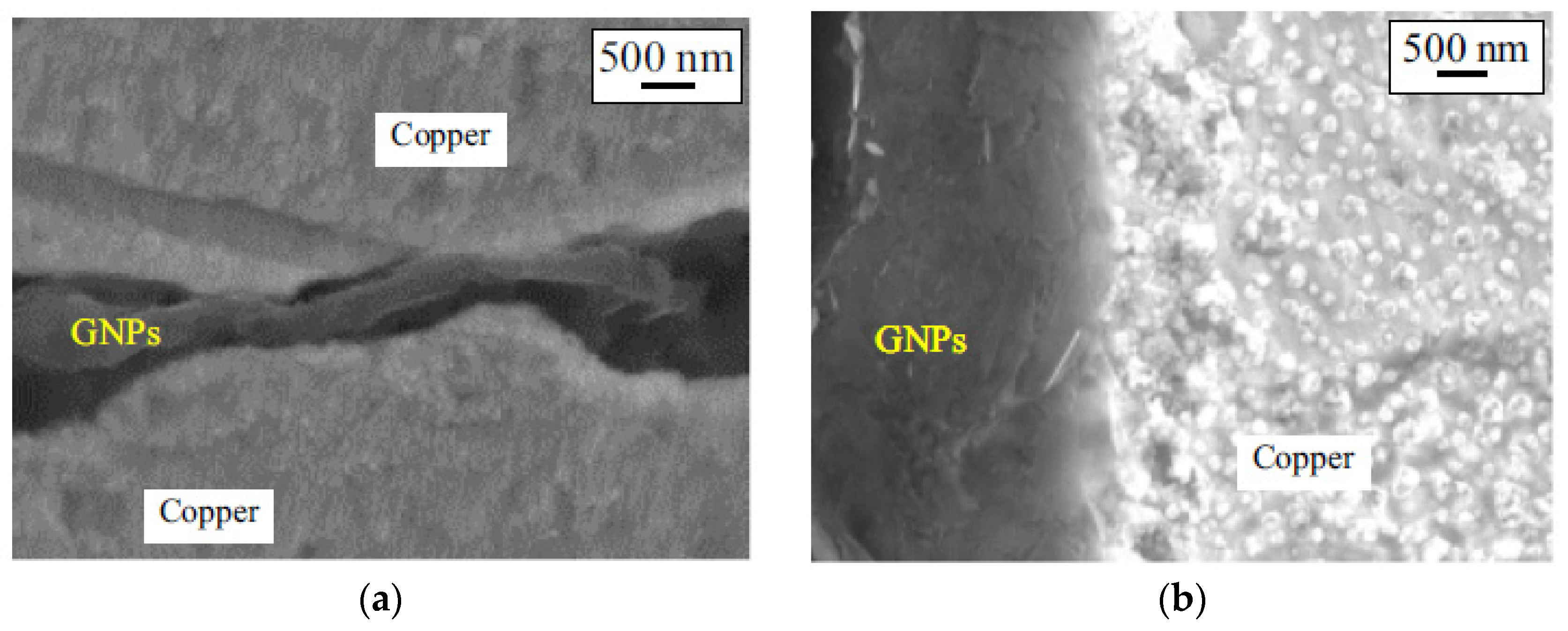
| Challenge | Source | Role | Solution(s) |
|---|---|---|---|
| Selection of high-quality GNPs | Lack of data from suppliers | Defects play key roles in the reactivity and also the final mechanical properties | Characterizing as-received GNPs in terms of residual defects and morphology |
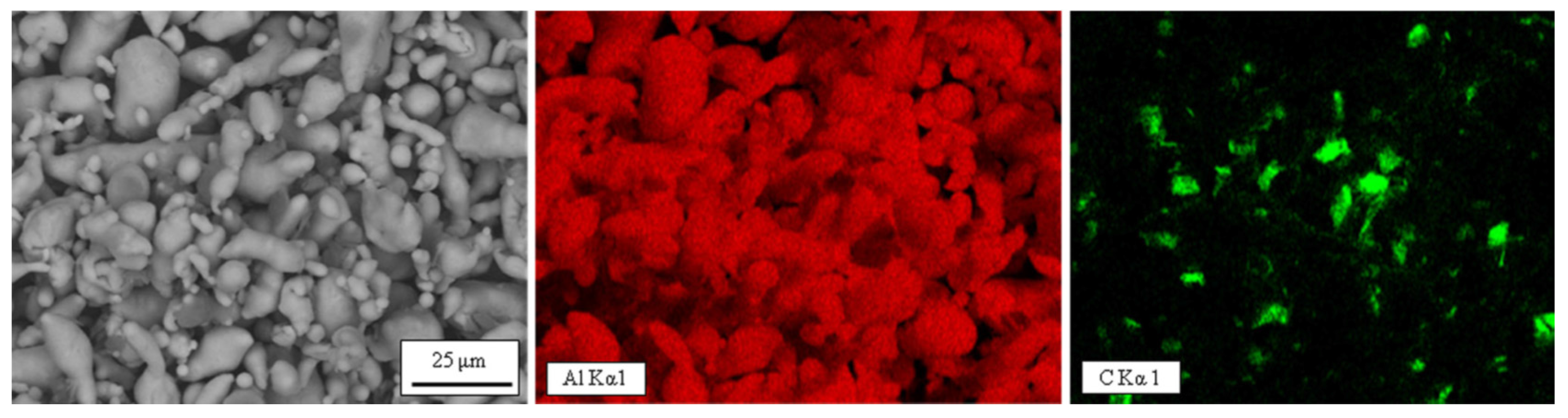
| Uniform dispersion of GNPs | A van der Waals force between the platelets | Agglomerates of GNPs leave some porosities and are deleterious for all the properties | Using a novel wet mixing technique for mixing and the powder metallurgy method for production |
| Reactivity with matrix | Residual defects in GNPs and the molten state of the matrix | Carbide formation in Al/GNPs system is deleterious for the final properties | Using a novel wet mixing method for dispersion, using GNPs with the lowest level of defects, employing powder metallurgy techniques for production |
| Interfacial bonding between metal/GNPs | Poor adhesion/wettability between metal matrix and GNPs | Poor interfacial bonding results in poor mechanical and thermophysical properties | Coating of GNPs or using post-processing techniques to remove the voids from the interface of metal/GNPs |
| Alignment/orientation of GNPs | High aspect ratio of GNPs (1:10–1:100) | The preferred orientation of GNPs results in anisotropy properties | Using hot isostatic pressing instead of unidirectional consolidation |
| Material | Density (g/cm3) | Thermal Conductivity (W/m·K) | Thermal Expansion Coefficient (106/K) | Melting Point (°C) | Vickers Hardness (HV) | Elastic Modulus (GPa) |
|---|---|---|---|---|---|---|
| α-Al2O3 | 3.95–3.98 | 35–39 | 7.1–8.4 | 2054–2072 | 1800–3000 | 365–393 |
| AlN | 3.05–3.26 | 130–180 | 2.5–5.3 | 2200–2230 | 1170–1530 | 308–346 |
| α-SiC | 3.15 | 42.5–270 | 4.3–5.8 | 2093–2400 | 2400–2500 | 386–476 |
| β-SiC | 3.16 | 135 | 4.5 | 2093 decom. | 2700 | 262–468 |
| Diamond | 3.52 | 2400 | - | 3550 | 8000 | 930 |
| Graphite | 2.25 | 25–470 | 0.6–4.3 | - | - | 4.8–27 |
| SWCNTs | 1.8 | Up to 2900 | Negligible | - | - | 1000 |
| GNPs | 1.8–2.2 | 5300 | −0.8–0.7 | - | - | 1000 |
| Composition (Cu-GNPs) | ID/IG | I2D/IG | ||
|---|---|---|---|---|
| Ball Milled | Wet Mixed | Ball Milled | Wet Mixed | |
| Before mixing | 0.112 | 0.112 | 0.511 | 0.511 |
| After mixing | 0.893 | 0.127 | 0.584 | 0.525 |
| Matrix | Graphene Content (wt. %) | Fabrication Techniques | Theoretical Density (g/cm3) | Sintered Density (g/cm3) | Ref. |
|---|---|---|---|---|---|
| Mg | 0 | Semi-PM | 1.740 | 1.738 | [95] |
| 0.18 | Semi-PM | 1.743 | 1.733 | [95] | |
| Al | 0 | Conventional PM | 2.700 | 2.646 | [110] |
| 0.5 | Conventional PM | 2.695 | 2.628 | [110] | |
| 1.0 | Conventional PM | 2.690 | 2.556 | [110] | |
| Al | 0 | Hot rolling | 2.700 | 2.557 | [70] |
| 0.5 | Hot rolling | 2.695 | 2.526 | [70] | |
| 1.0 | Hot rolling | 2.690 | 2.441 | [70] | |
| Cu | 0 | Conventional PM | 8.961 | 8.781 | [111] |
| 1.0 | Conventional PM | 8.661 | 8.532 | [111] | |
| 2.0 | Conventional PM | 8.391 | 8.143 | [111] | |
| Cu | 0 | Conventional PM + Repressed + annealed | 8.960 | 8.821 | [8] |
| 1.0 | Conventional PM+ Repressed + annealed | 8.660 | 8.562 | [8] | |
| 2.0 | Conventional PM + Repressed + annealed | 8.390 | 8.231 | [8] | |
| Cu | 0 | Conventional PM + HIP | 8.960 | 8.951 | [8] |
| 1.0 | Conventional PM + HIP | 8.660 | 8.652 | [8] | |
| 2.0 | Conventional PM + HIP | 8.390 | 8.371 | [8] | |
| Al | 0 | Hot extrusion | 2.700 | 2.699 | [79] |
| 0.25 | Hot extrusion | 2.699 | 2.698 | [79] | |
| 0.5 | Hot extrusion | 2.697 | 2.696 | [79] | |
| 1.0 | Hot extrusion | 2.694 | 2.691 | [79] |
| Matrix | Graphene Content (wt. %) | Fabrication Techniques | Vickers Hardness (HV) | Ref. |
|---|---|---|---|---|
| Al | 0 | Hot extrusion | 76 ± 4.0 | [79] |
| 0.25 | Hot extrusion | 80 ± 5.0 | [79] | |
| 0.5 | Hot extrusion | 85 ± 4.0 | [79] | |
| 1.0 | Hot extrusion | 90 ± 4.0 | [79] | |
| Al | 0 | Conventional PM | 44 ± 2.1 | [110] |
| 0.5 | Conventional PM | 51 ± 1.4 | [110] | |
| 1.0 | Conventional PM | 57 ± 4.2 | [110] | |
| Al | 0 | Hot rolling | 42 ± 1.1 | [70] |
| 0.5 | Hot rolling | 43 ± 1.5 | [70] | |
| 1.0 | Hot rolling | 42 ± 2.3 | [70] | |
| Cu | 0 | Conventional PM | 42.3 ± 2.1 | [111] |
| 1.0 | Conventional PM | 45.1 ± 3.2 | [111] | |
| 2.0 | Conventional PM | 48.6 ± 5.0 | [111] | |
| Cu | 0 | Conventional PM + Repressed + annealed | 45.2 ± 1.5 | [8] |
| 1.0 | Conventional PM + Repressed + annealed | 51.6 ± 2.2 | [8] | |
| 2.0 | Conventional PM + Repressed + annealed | 55.8 ± 3.6 | [8] | |
| Cu | 0 | Conventional PM + HIP | 50.4 ± 0.9 | [8] |
| 1.0 | Conventional PM + HIP | 57.5 ± 1.6 | [8] | |
| 2.0 | Conventional PM + HIP | 62.3 ± 1.2 | [8] | |
| Mg | 0 | Semi-PM | 41 ± 4.0 | [95] |
| 0.18 | Semi-PM | 55 ± 2.0 | [95] |
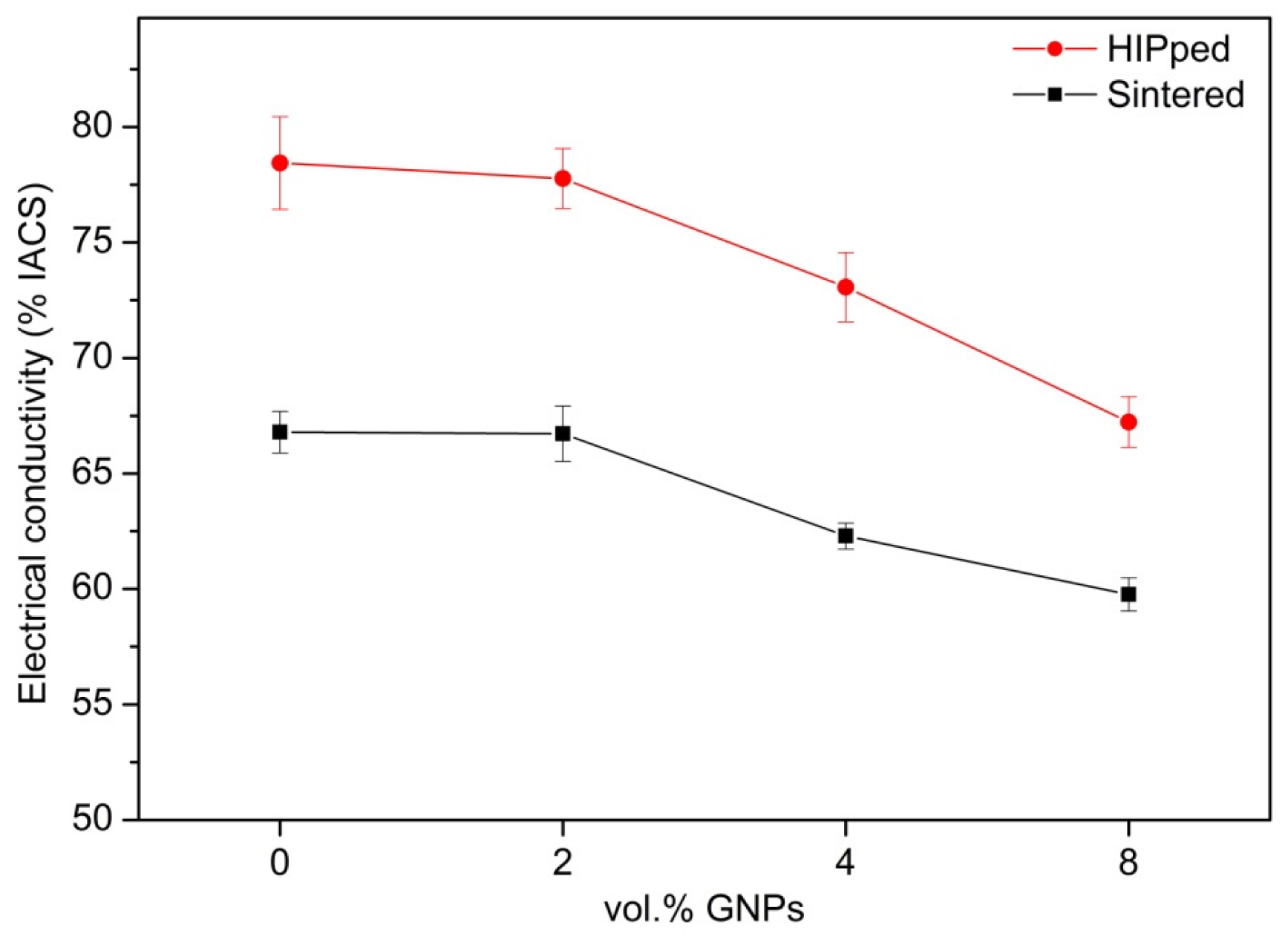
| Reinforcement | Content | Electrical Conductivity (% IACS) | Fabrication Method | Ref. |
|---|---|---|---|---|
| CNTs | 0.5 vol % | 91.2 | SPS + wire drawing | [118] |
| GNPs | 0.6 vol % | 88 | Molecular level mixing + SPS | [17] |
| Graphite | 8.0 wt % | 55 | Cu coating of graphene + sintering | [119,120] |
| Graphite | 0.1 vol % | 90 | Roll bonding | [121] |
| - | - | 52.3 | Sintering + Forging | [122] |
| SWCNTs | 5.0 vol % | 44.5 | Sintering + Forging | |
| - | - | 78 | Sintering + HIPing | [8] |
| GNPs | 2.0 vol % | 77 | Sintering + HIPing | [8] |
| GNPs | 4.0 vol % | 72.5 | Sintering + HIPing | [8] |
| GNPs | 8.0 vol % | 67.5 | Sintering + HIPing | [8] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saboori, A.; Moheimani, S.K.; Dadkhah, M.; Pavese, M.; Badini, C.; Fino, P. An Overview of Key Challenges in the Fabrication of Metal Matrix Nanocomposites Reinforced by Graphene Nanoplatelets. Metals 2018, 8, 172. https://doi.org/10.3390/met8030172
Saboori A, Moheimani SK, Dadkhah M, Pavese M, Badini C, Fino P. An Overview of Key Challenges in the Fabrication of Metal Matrix Nanocomposites Reinforced by Graphene Nanoplatelets. Metals. 2018; 8(3):172. https://doi.org/10.3390/met8030172
Chicago/Turabian StyleSaboori, Abdollah, Seyed Kiomars Moheimani, Mehran Dadkhah, Matteo Pavese, Claudio Badini, and Paolo Fino. 2018. "An Overview of Key Challenges in the Fabrication of Metal Matrix Nanocomposites Reinforced by Graphene Nanoplatelets" Metals 8, no. 3: 172. https://doi.org/10.3390/met8030172
APA StyleSaboori, A., Moheimani, S. K., Dadkhah, M., Pavese, M., Badini, C., & Fino, P. (2018). An Overview of Key Challenges in the Fabrication of Metal Matrix Nanocomposites Reinforced by Graphene Nanoplatelets. Metals, 8(3), 172. https://doi.org/10.3390/met8030172









