Dissolution of Scrap in Hot Metal under Linz–Donawitz (LD) Steelmaking Conditions
Abstract
1. Introduction
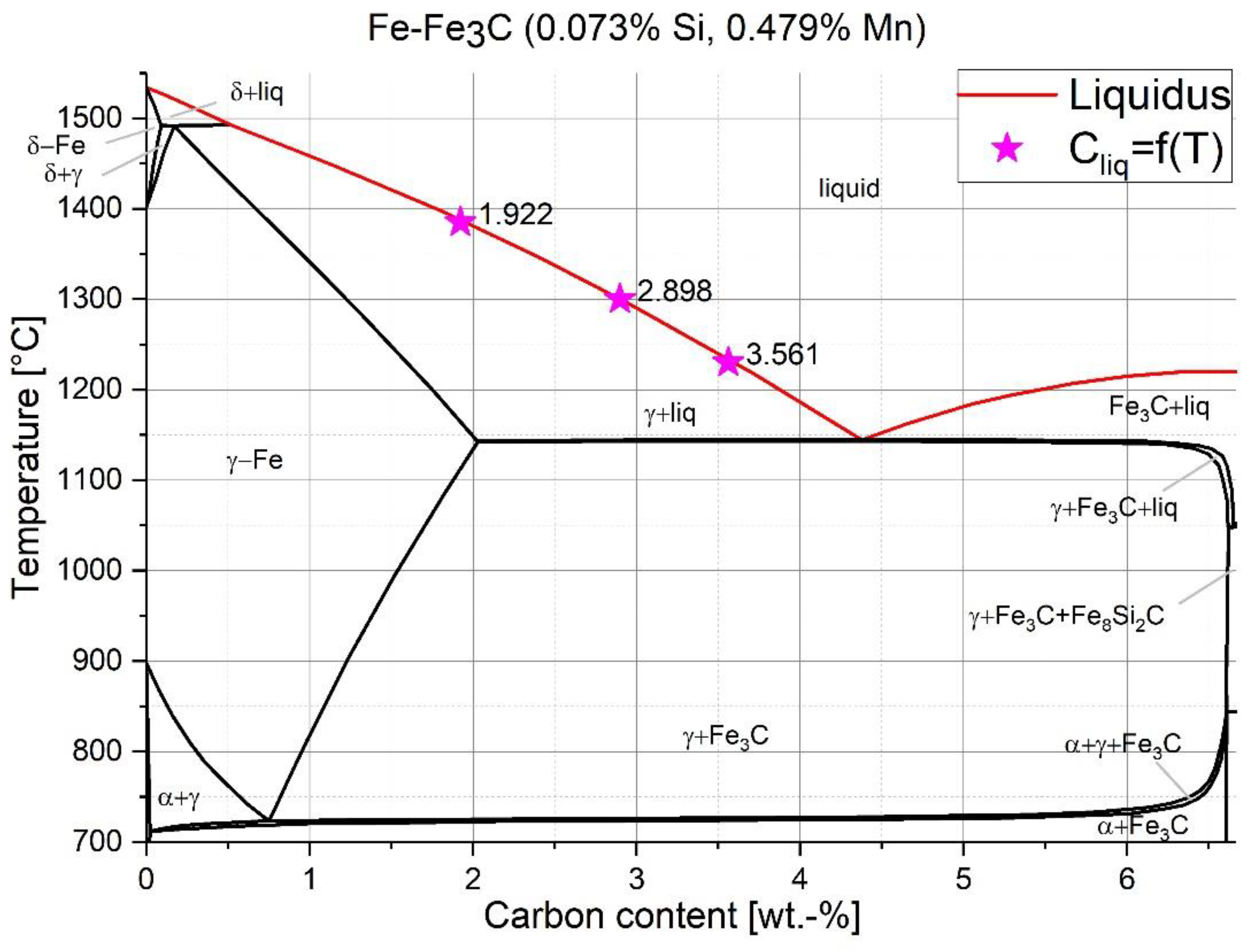
2. Background of Diffusive Scrap Melting
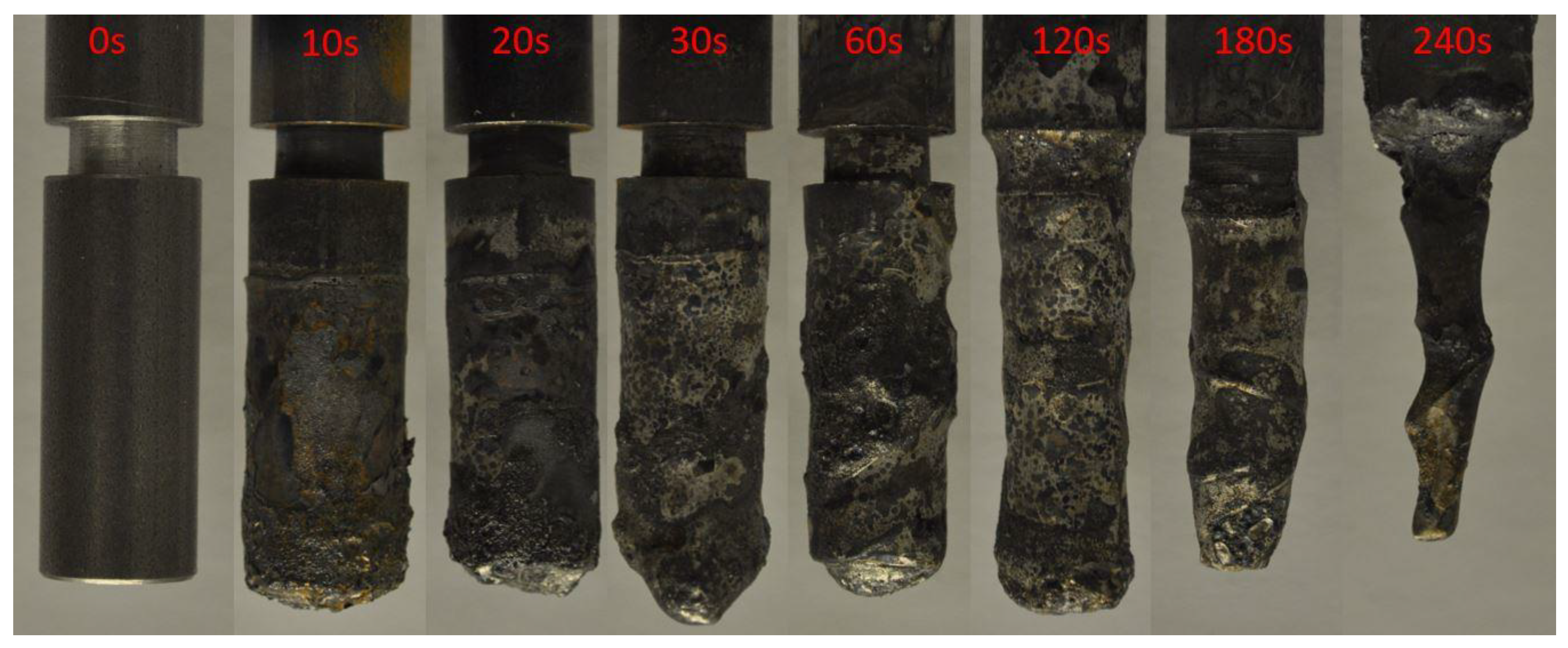
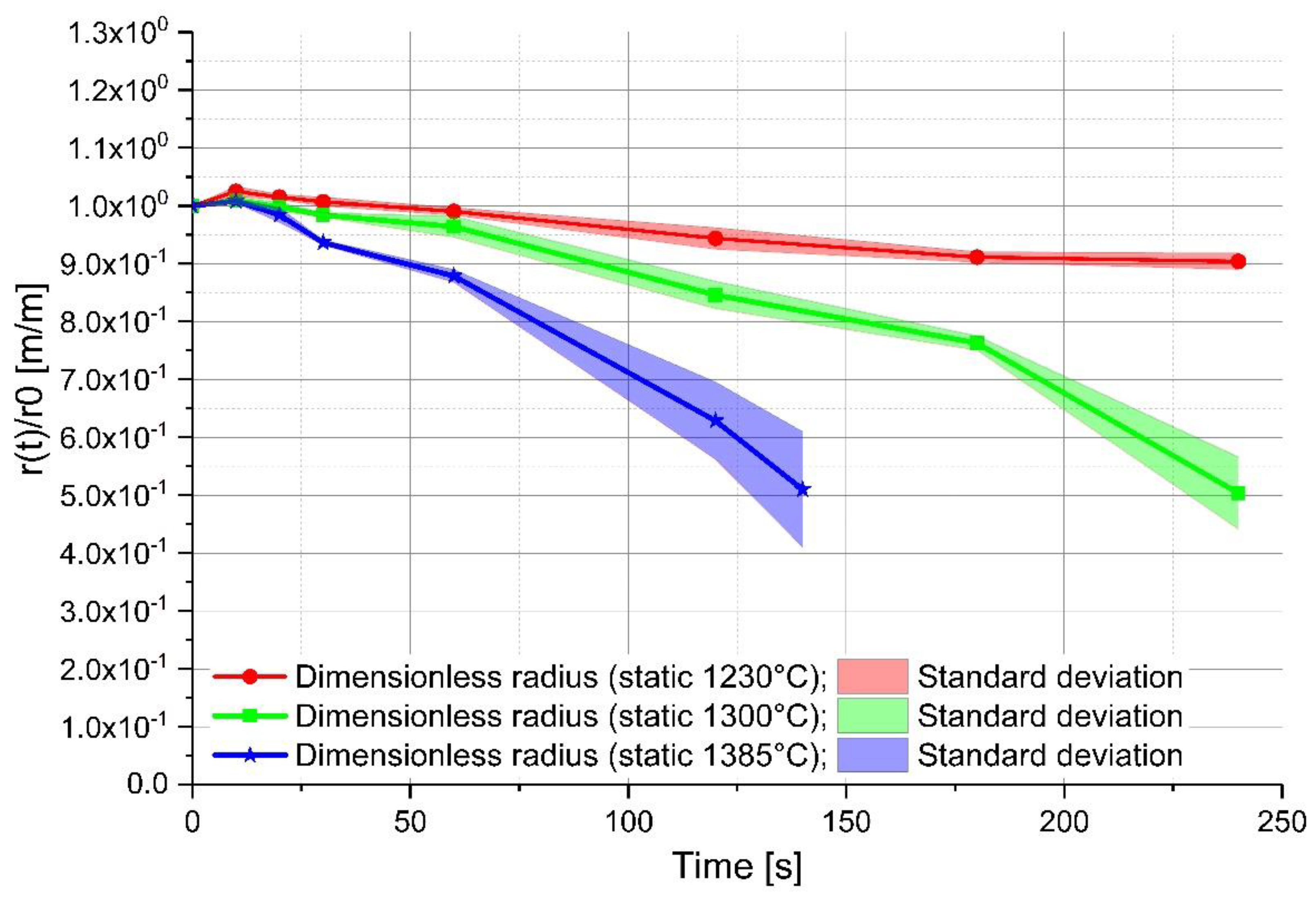
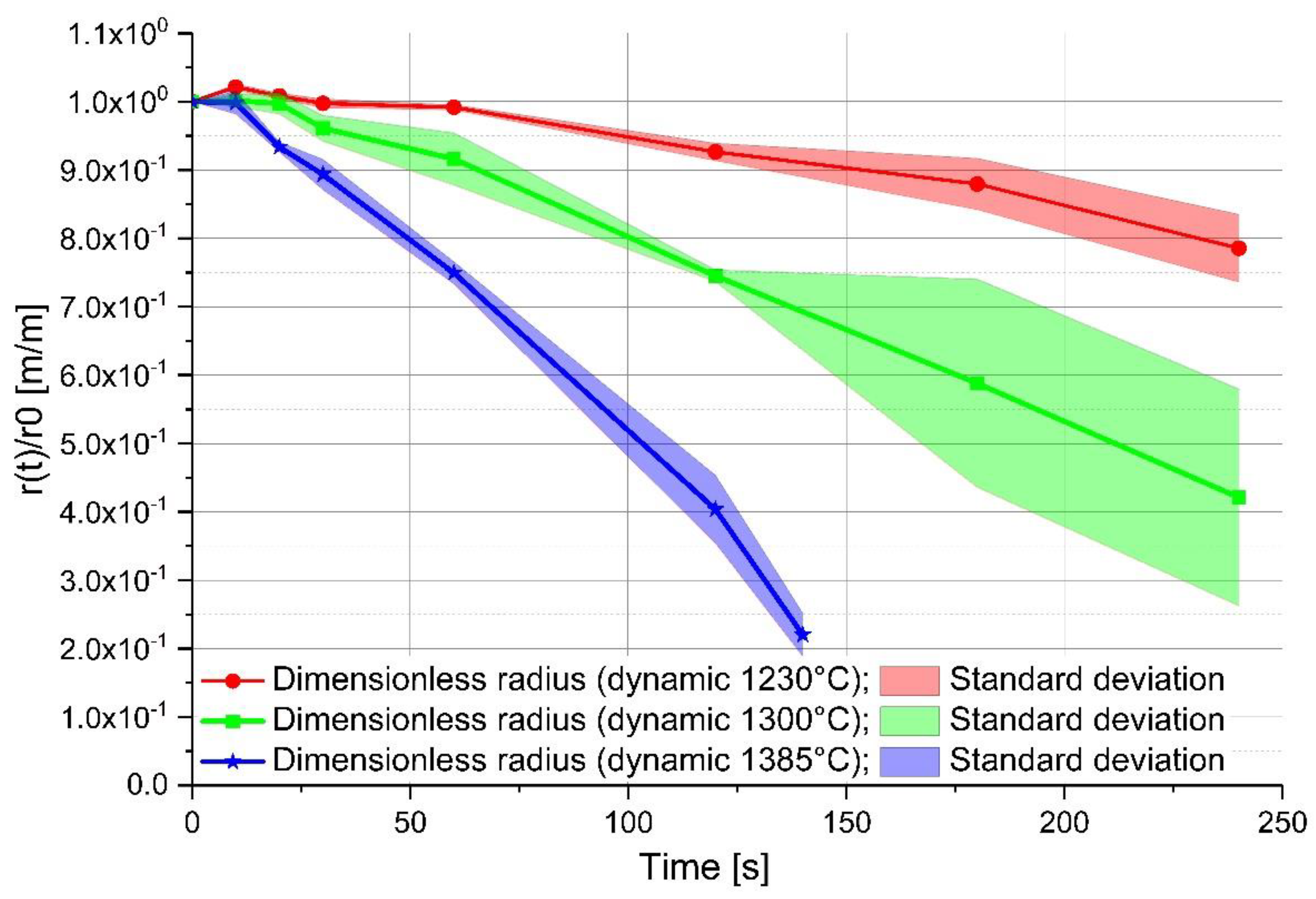
3. Description of the Experiment
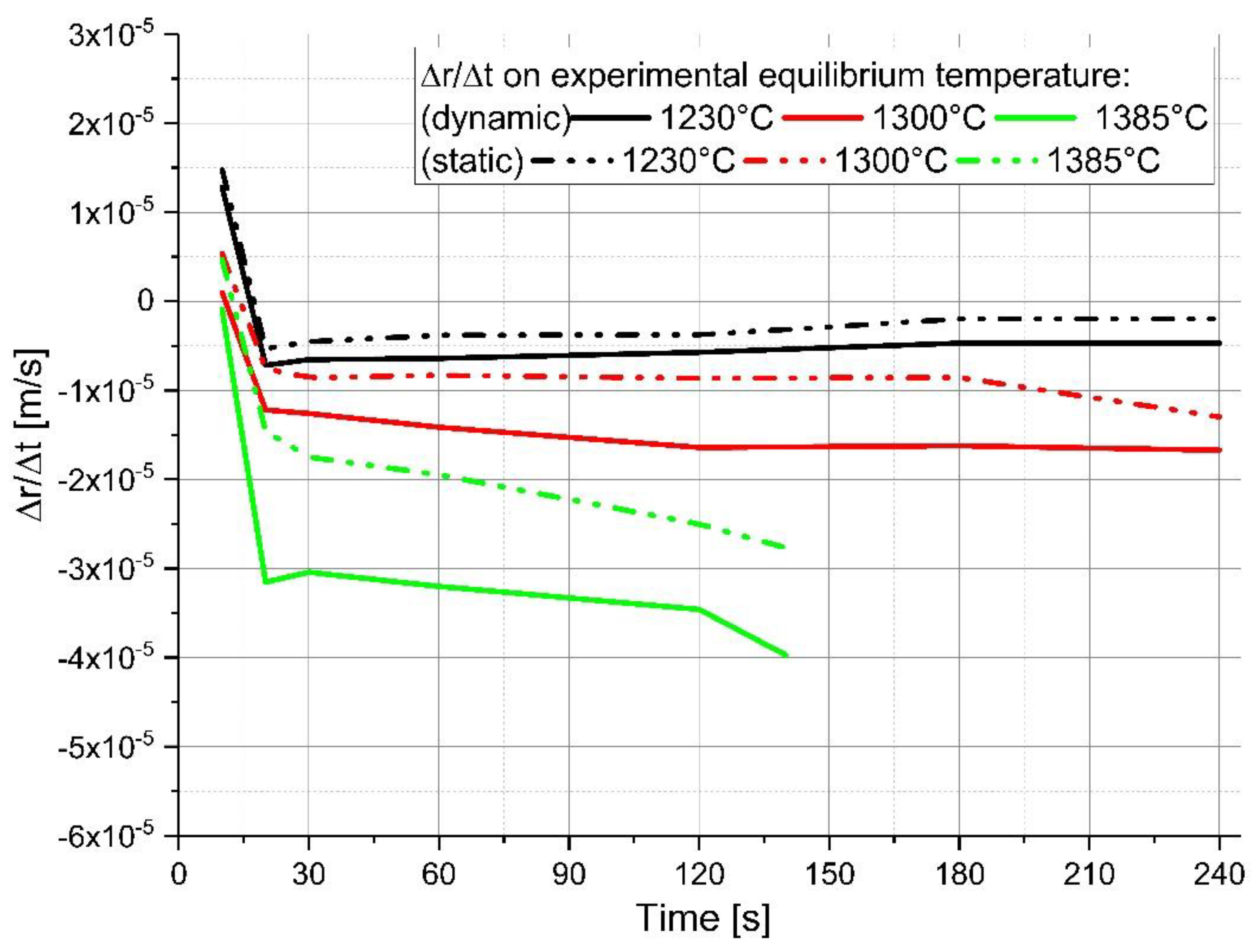
4. Discussion of the Experimental Results
5. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Turkdogan, E.T. Fundamentals of Steelmaking; The Institute of Materials: London, UK, 1996; pp. 209–244. [Google Scholar]
- Ghosh, A.; Chatterjee, A. Ironmaking and Steelmaking Theory and Practice; PHI Learning Private Limited: Delhi, India, 2015; pp. 285–292. [Google Scholar]
- Asai, S.; Muchi, I. Effect of scrap melting on the process variables in LD converter caused by the change of operating conditions. Trans. ISIJ 1971, 11, 107–115. [Google Scholar]
- Gaye, H.; Wanin, M.; Gugliermina, P.; Schittly, P. Kinetics of scrap dissolution in the converter. Theoretical model and plant experimentation. In Proceedings of the 68th Steelmaking Conference, AIME, Detroit, MI, USA, 14–17 April 1985; pp. 91–103. [Google Scholar]
- Isobe, K.; Maede, H.; Ozawa, K.; Umezawa, K.; Saito, C. Analysis of the scrap melting rate in high carbon molten iron. ISIJ 1990, 76, 2033–2040. [Google Scholar]
- Zhang, L.; Oeters, F. Schmelzen und Mischen von Legierungsstoffen in Stahlschmelzen; Verlag Stahleisen GmbH: Düsseldorf, Germany, 2012; pp. 38–40. [Google Scholar]
- Szekely, J.; Chuang, Y.K.; Hlinka, J.W. The melting and dissolution of low-carbon steels in iron-carbon melts. Metall. Trans. 1972, 3, 2825–2833. [Google Scholar] [CrossRef]
- Shukla, A.K.; Deo, B.; Robertson, D.G.C. Scrap Dissolution in Molten Iron Containing Carbon for the Case of Coupled Heat and Mass Transfer Control. Metall. Mater. Trans. B 2013, 44, 1407–1427. [Google Scholar] [CrossRef]
- Den Hartog, H.W.; Kreyger, P.J.; Snoeijer, A.B. Dynamic model of the dissolution of scrap in BOF process. CRM Rep. 1973, 37, 13–22. [Google Scholar]
- Kawakami, M.; Takatani, K.; Brabie, L.C. Heat and Mass Transfer Analysis of Scrap Melting in Steel Bath. Tetsu-to-Hagané 1999, 85, 658–665. [Google Scholar] [CrossRef]
- Kruskopf, A.; Holappa, L. Scrap melting model for steel converter founded on interfacial solid/liquid phenomena. Metall. Res. Technol. 2018, 115, 201–208. [Google Scholar] [CrossRef]
- Sethi, G.; Shukla, A.K.; Das, P.C.; Chandra, P.; Deo, B. Theoretical Aspects of Scrap Dissolution in Oxygen Steelmaking Converters. In Proceedings of the AISTech 2004 Proceedings Volume II, Nashville, TN, USA, 15–17 September 2014; pp. 915–926. [Google Scholar]
- Medzhibozhskiy, M.Y. Basis of Thermodynamic and Kinetic of Steelmaking; Vischa shkola: Kyiv, Ukraine, 1979; p. 229. [Google Scholar]
- Lytvynyuk, Y.; Schenk, J.; Hiebler, M.; Sormann, A. Thermodynamic and Kinetic Model of the Converter Steelmaking Process. Part 1: The Description of the BOF Model. Steel Res. Int. 2014, 85, 537–543. [Google Scholar] [CrossRef]
- Penz, F.M.; Bundschuh, P.; Schenk, J.; Panhofer, H.; Pastucha, K.; Paul, A. Effect of Scrap Composition on the Thermodynamics of Kinetic Modelling of BOF Converter. In Proceedings of the 2nd VDEh-ISIJ-JK Symposium, Stockholm, Sweden, 12–13 June 2017; pp. 124–135. [Google Scholar]
- Zarl, M. Development and Evaluation of a BOF Pre-Processor Model. Master’s Thesis, Montanuniversität Leoben, Leoben, Austria, 2017. [Google Scholar]
- Esser, A.; Grossmann, S. Analytic expression for Taylor-Couette stability boundary. Phys. Fluids 1996, 8, 1814–1819. [Google Scholar] [CrossRef]
- Racina, A. Vermischung in Taylor-Couette Strömung; Universitätsverlag: Karlsruhe, Germany, 2009; pp. 29–40. [Google Scholar]
- Penz, F.M.; Schenk, J.; Ammer, R.; Maunz, B.; Pastucha, K. Dissolution behavior of ULC steel in carbon saturated hot metal. In Proceedings of the 2nd International Congress on Science and Technology of Steelmaking, Venice/Mestre, Italy, 12–13 June 2018. CD-ROM. [Google Scholar]
- Miettinen, J. Calculation of solidification-related thermophysical properties for steels. Metall. Mater. Trans. B 2018, 28 B, 281–297. [Google Scholar] [CrossRef]
- Chapra, S.C.; Canale, R.P. Métodos Numéricos para Engenharia, 4th ed.; McGraw-Hill International do Brasil Ltda: São Paulo, Brazil, 2008; p. 549. [Google Scholar]

| Definition | Hot metal | Scrap |
|---|---|---|
| Carbon content [wt%] | 4.58 | 0.1 |
| Silicon content [wt%] | 0.37 | 0.0733 |
| Manganese content [wt%] | 0.63 | 0.479 |
| Phosphorus content [wt%] | 0.07 | 0.01 |
| Mass [g] | 330 | 26.3 |
| Initial temperature [°C] | 1305/1370/1450 | 25 |
| Equilibrium temperature [°C] | 1230/1300/1385 | 1230/1300/1385 |
| Equilibrium temperature [°C] | 1230 | 1230 | 1300 | 1300 | 1385 | 1385 |
| Experimental condition | static | dynamic | static | dynamic | static | dynamic |
| Ablation rate [(m/s) × 10−6] | −1.98 to −5.30 | −4.68 to −7.19 | −7.5 to −12.9 | −12.1 to −16.7 | −14.6 to −27.7 | −30.4 to −39.7 |
| Mass transfer coefficient [(m/s) × 10−6] | 7.39–20.4 | 18.5–27.0 | 16.1–26.8 | 25.5–36.5 | 18.2–31.1 | 33.7–44.4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Penz, F.M.; Schenk, J.; Ammer, R.; Klösch, G.; Pastucha, K. Dissolution of Scrap in Hot Metal under Linz–Donawitz (LD) Steelmaking Conditions. Metals 2018, 8, 1078. https://doi.org/10.3390/met8121078
Penz FM, Schenk J, Ammer R, Klösch G, Pastucha K. Dissolution of Scrap in Hot Metal under Linz–Donawitz (LD) Steelmaking Conditions. Metals. 2018; 8(12):1078. https://doi.org/10.3390/met8121078
Chicago/Turabian StylePenz, Florian Markus, Johannes Schenk, Rainer Ammer, Gerald Klösch, and Krzysztof Pastucha. 2018. "Dissolution of Scrap in Hot Metal under Linz–Donawitz (LD) Steelmaking Conditions" Metals 8, no. 12: 1078. https://doi.org/10.3390/met8121078
APA StylePenz, F. M., Schenk, J., Ammer, R., Klösch, G., & Pastucha, K. (2018). Dissolution of Scrap in Hot Metal under Linz–Donawitz (LD) Steelmaking Conditions. Metals, 8(12), 1078. https://doi.org/10.3390/met8121078





