Efficiency of Sulfuric Acid on Selective Scandium Leachability from Bauxite Residue †
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Characterization
2.2. Leaching Experiments
2.3. Analytical Methods—Instrumentation
3. Results
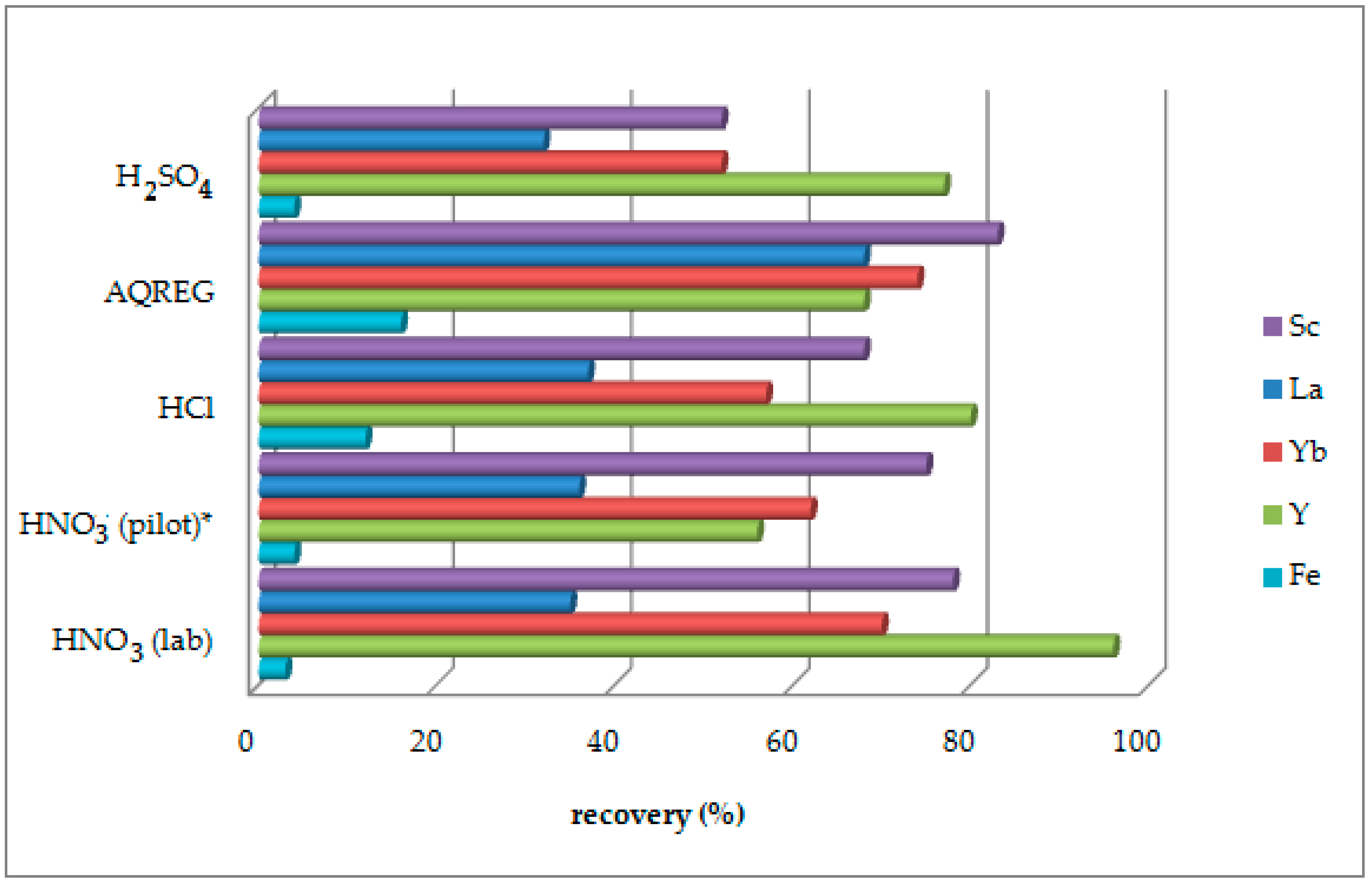
3.1. Selection of Leaching Agent
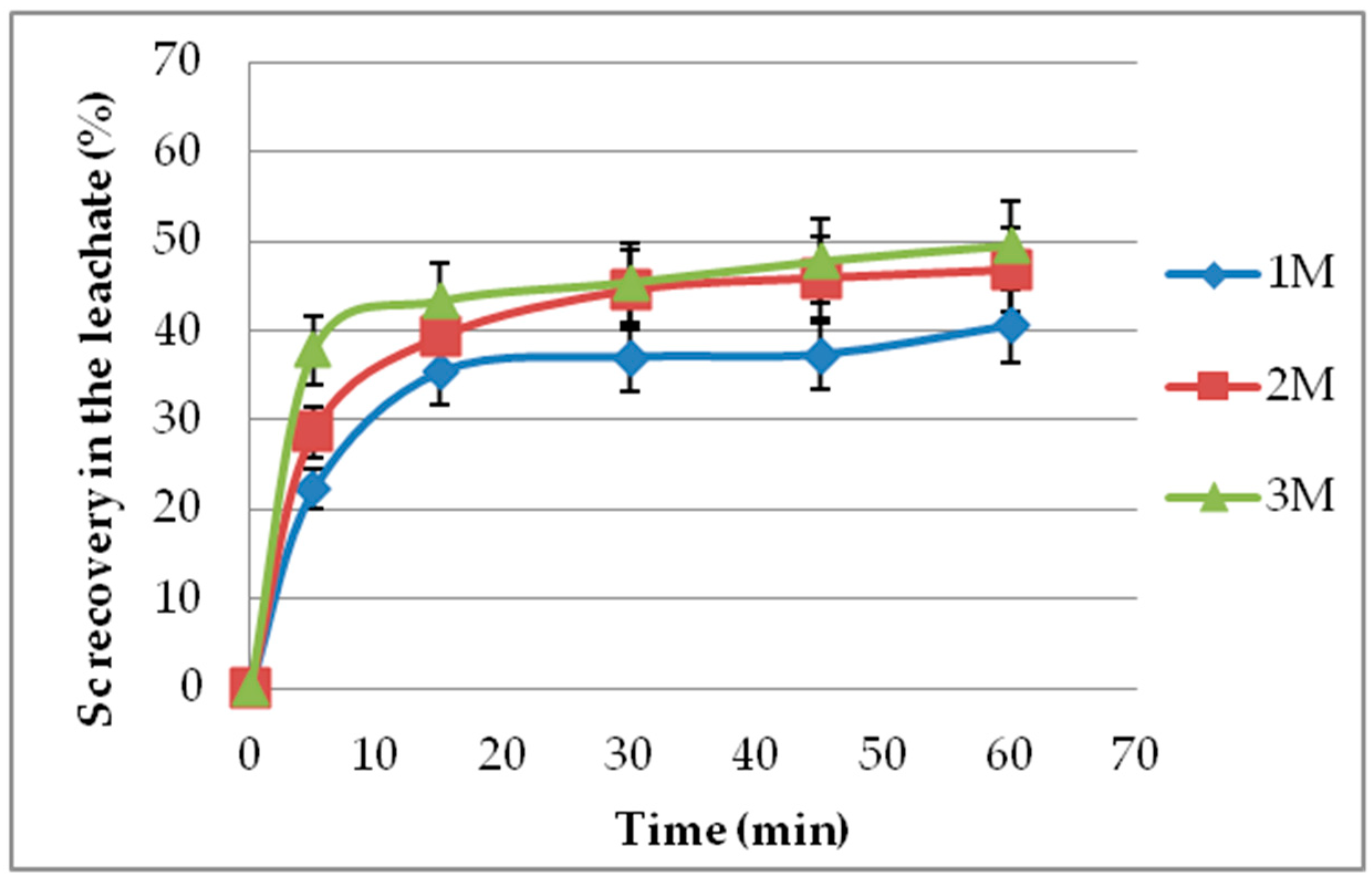
3.2. Effect of Leaching Time
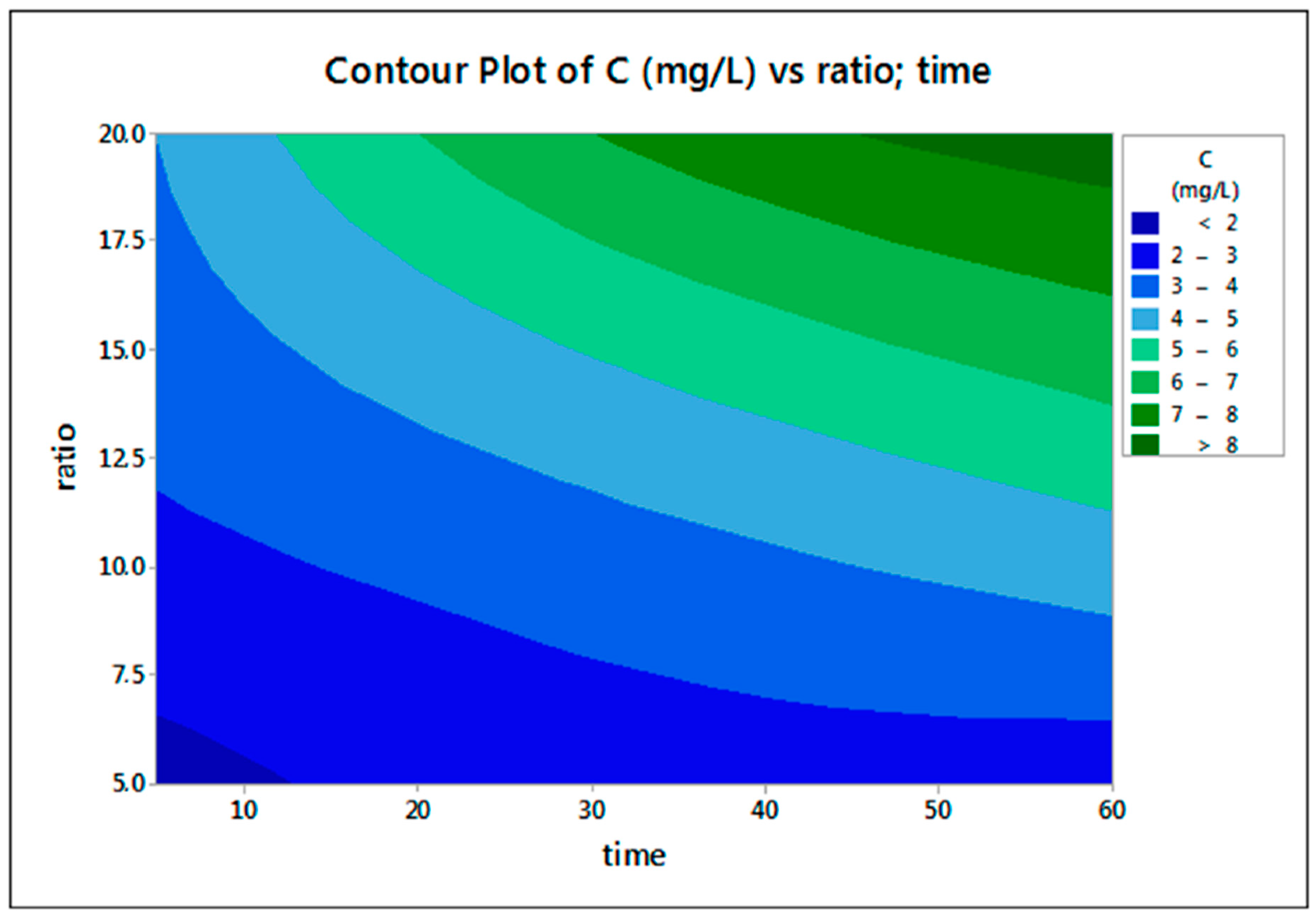
3.3. Effect of Acid Molarity
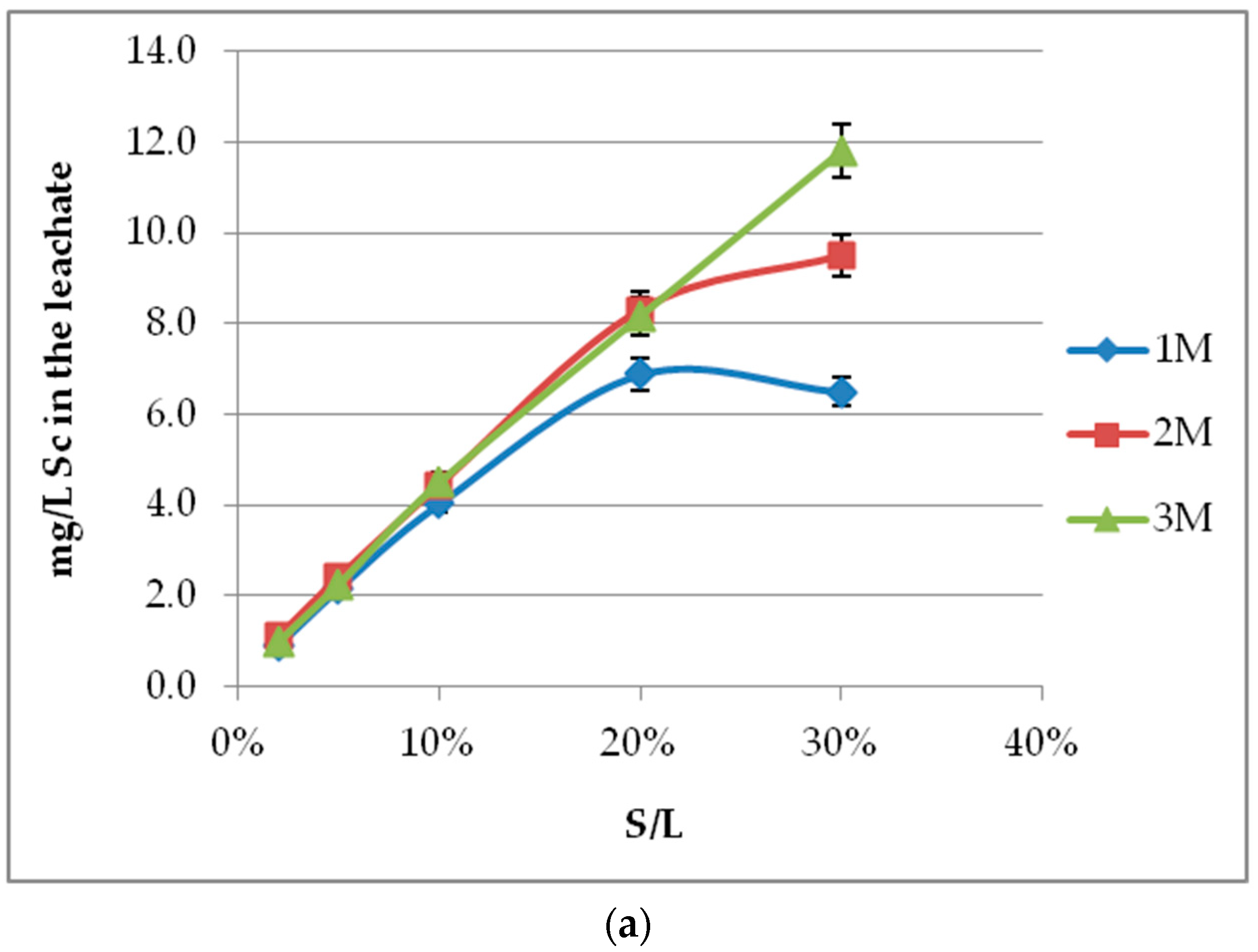
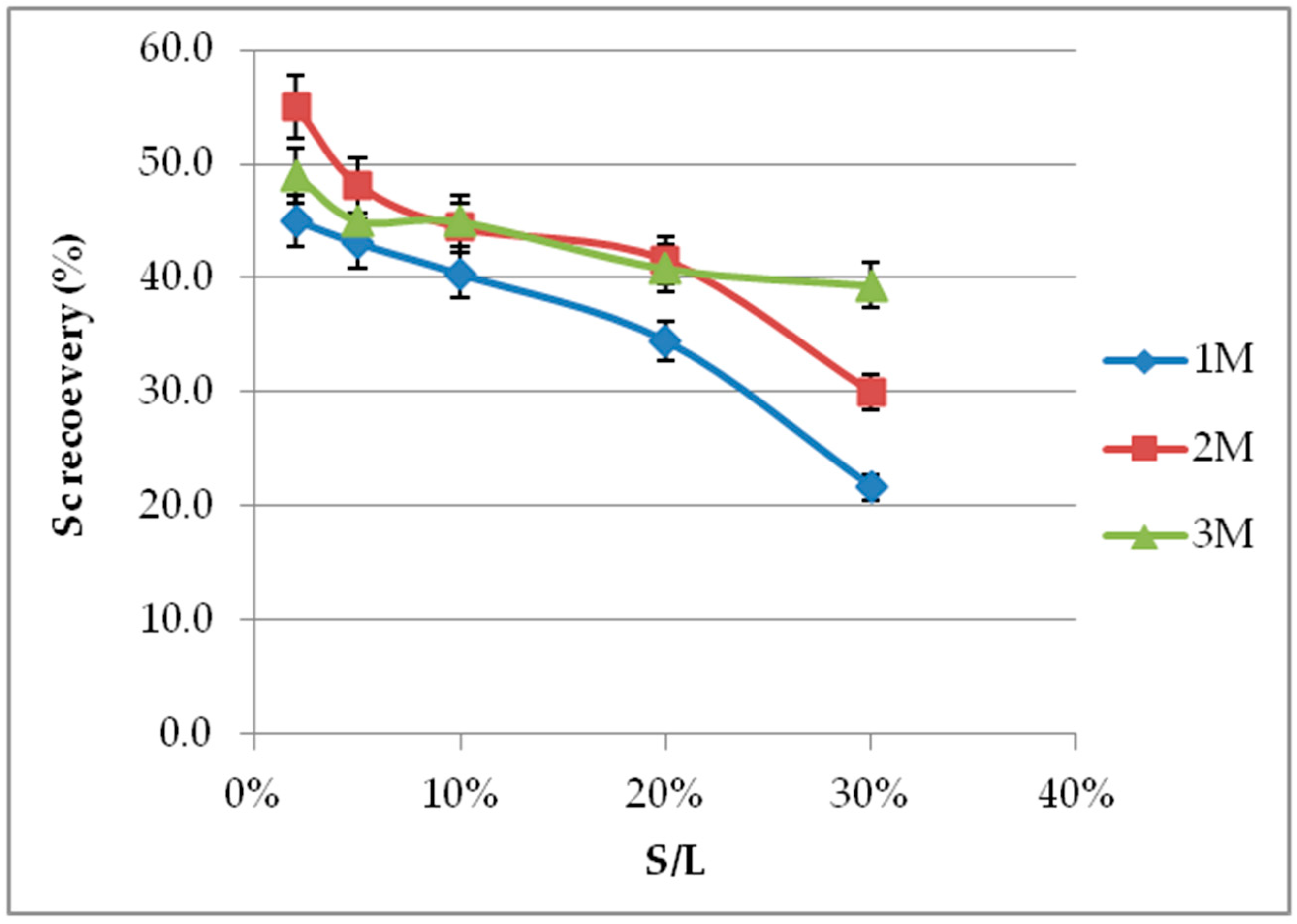
3.4. Effect of S/L
3.5. Effect of pH Adjustment
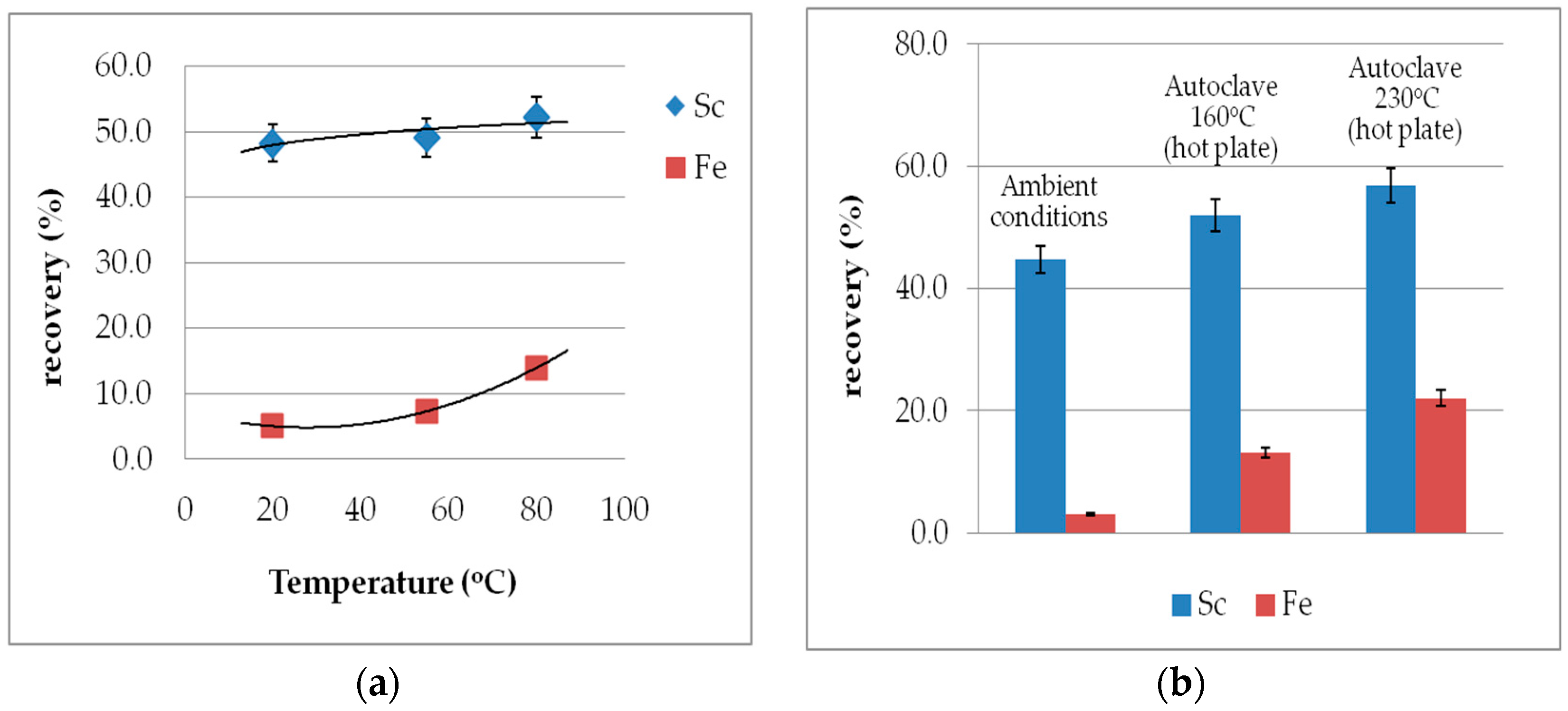
3.6. Temperature Effect
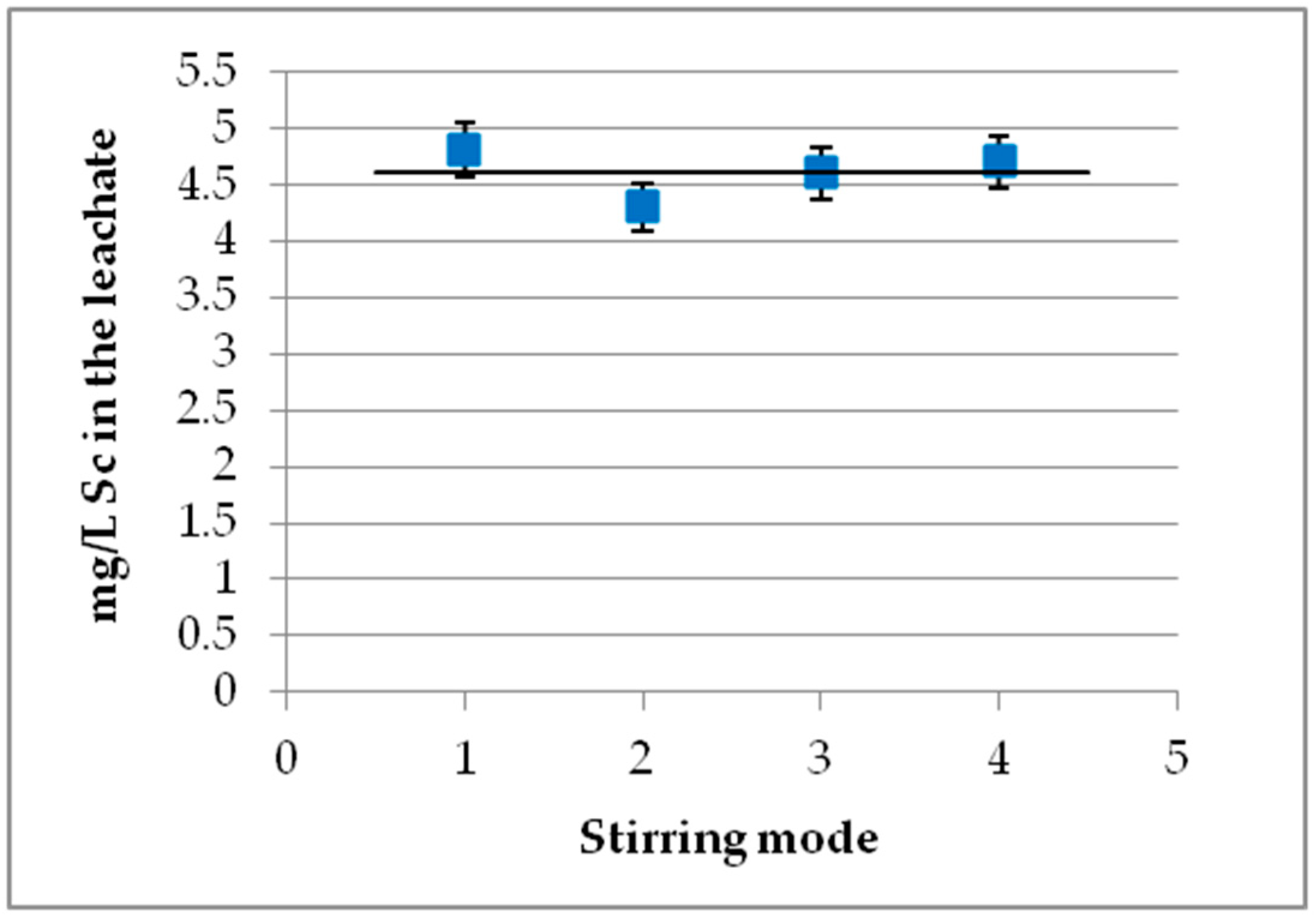
3.7. Effect of Stirring Mode
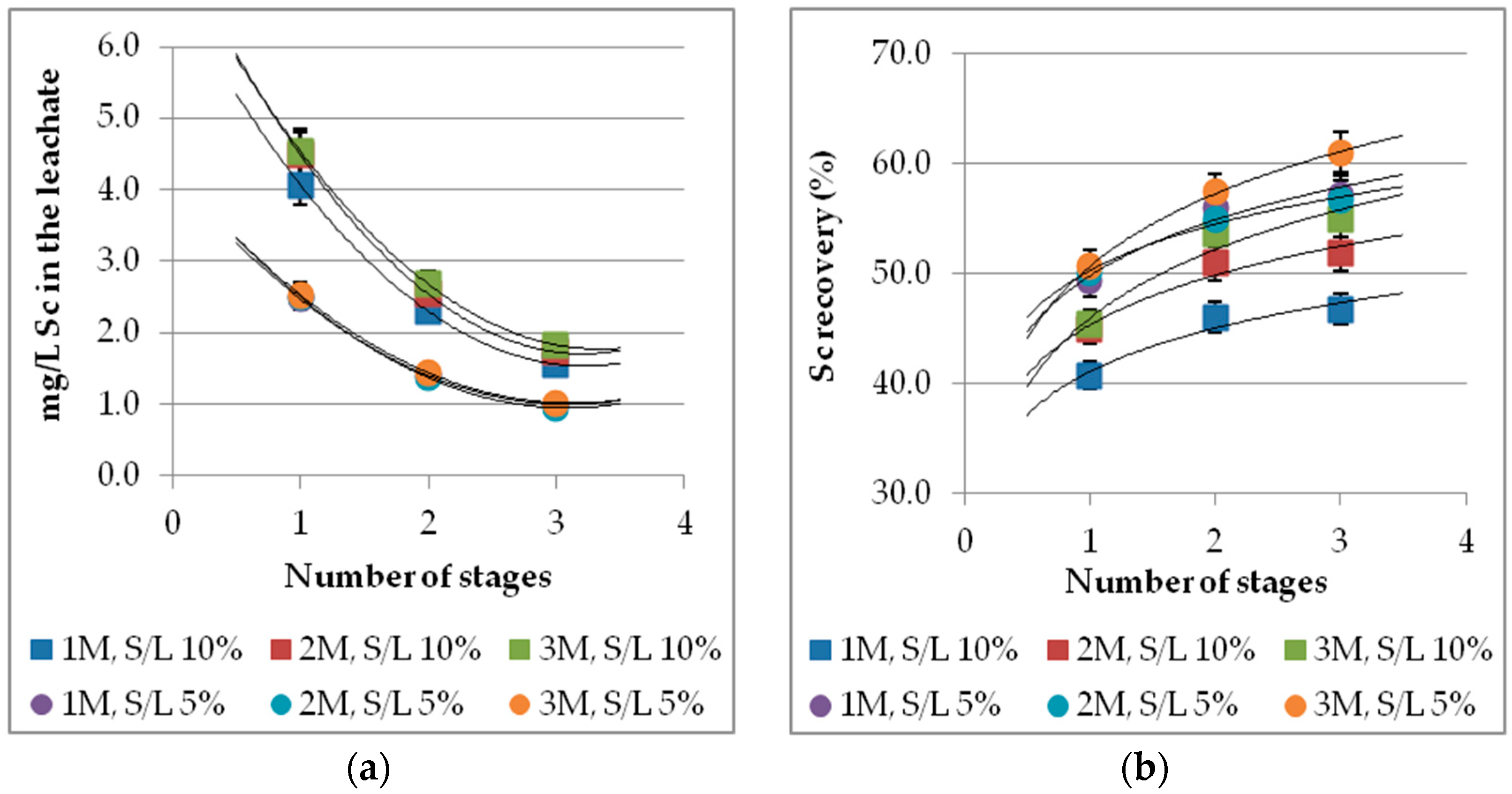
3.8. Effect of Multistage Leaching Process
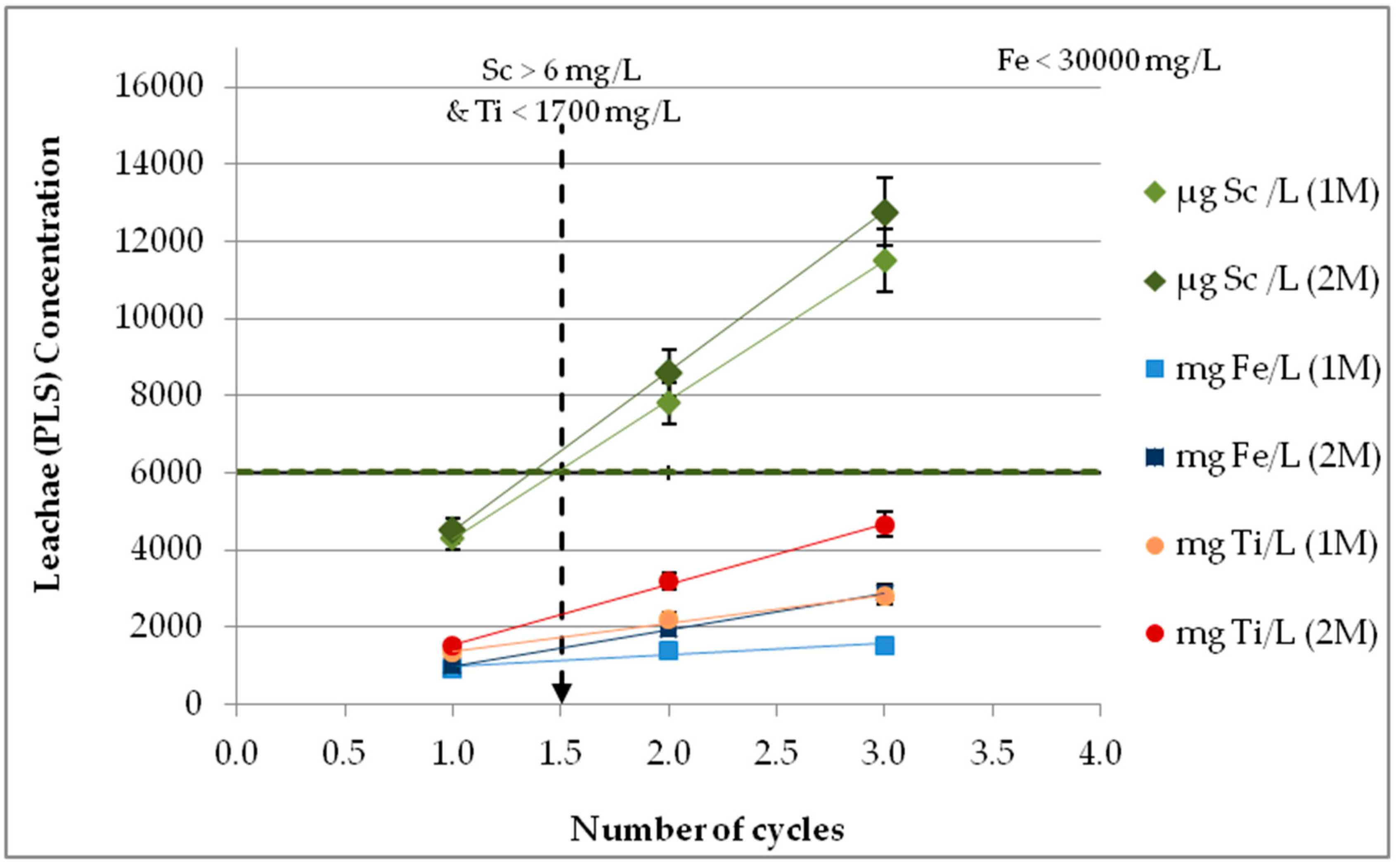
3.9. Effect of Leachate Recycling
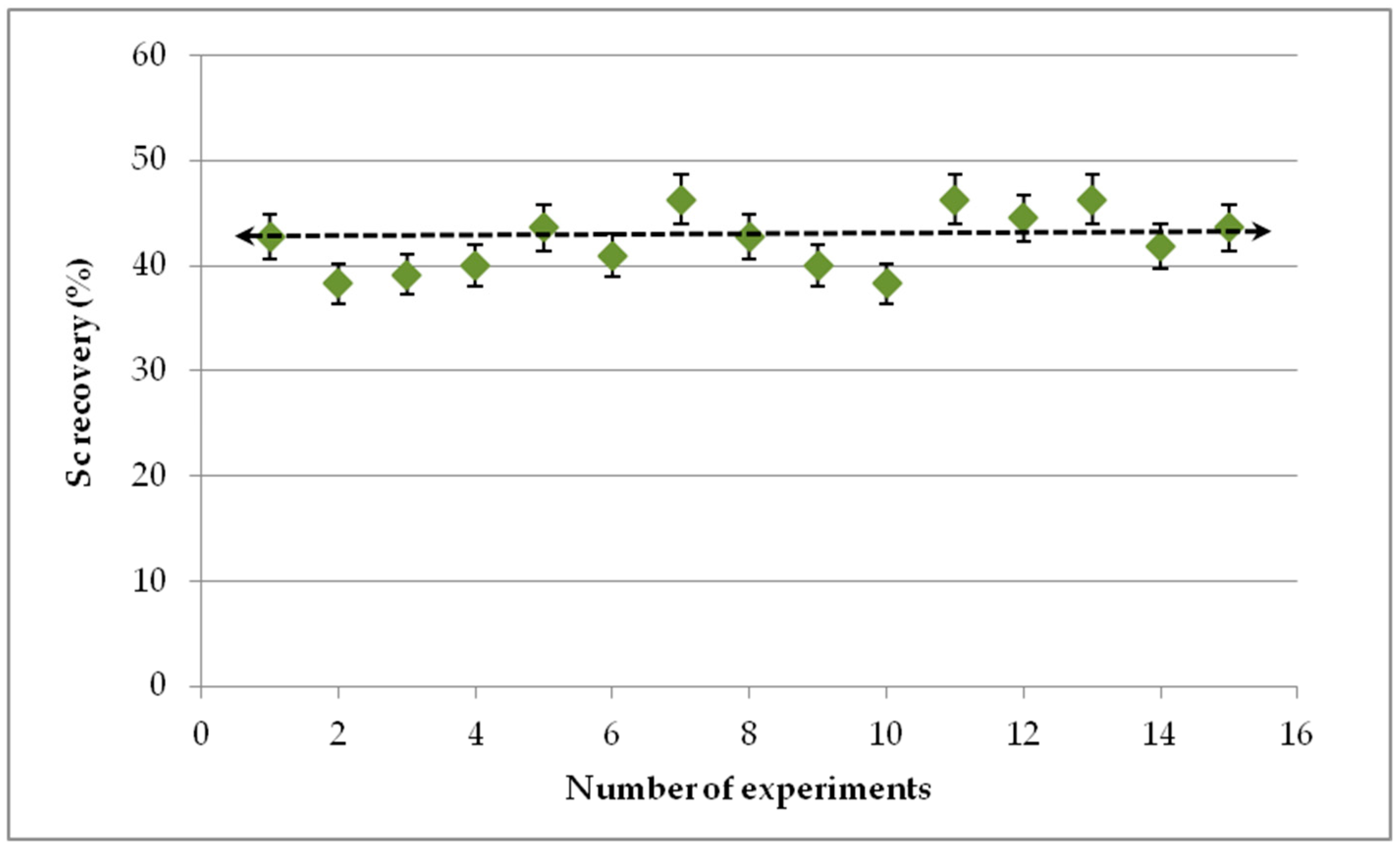
3.10. Statistical Evaluation
4. Assessment and Conclusions
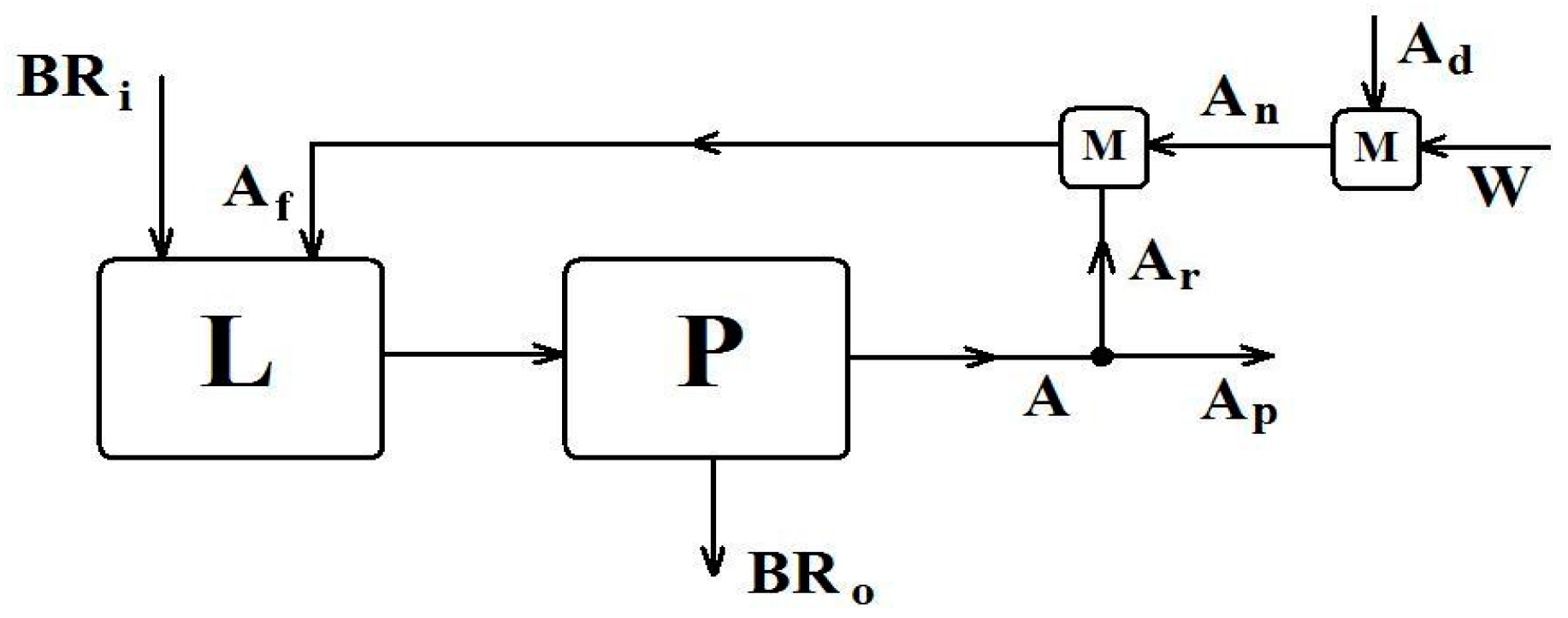
- L = Leaching reactor. Indicative type: CF-CSTR (Continuous Flow-Continuous Stirred Tank Reactor), with a heating/cooling mantle to be an option.
- P = Unit for Purification of leachate from solids. Indicative configuration: Intermediate Tank, Filter Press or Flocculator/Filters, and leachate Tank.
- M = Mixing unit. Indicative configuration: Continuous flow mixing device, with a heat dissipation system.
- BR = Bauxite Residue streams with moisture content.Subscripts: i = input (alkaline solids stream), o = output (acidic solids stream).
- A = Acid streams (sulfuric acid solutions).Subscripts: f = leaching feed, p = product (leachate), n = new (fresh solution), r = recycling leachate, d= dense acid (93–98% w/w). (Reflux ratio) = (Number of cycles in batch processing) − 1.
- W = Water stream; mixture of raw water with used water from Filter Press cleaning.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Power, G.; Grafe, M.; Klauber, C. Bauxite residue issues: I. current management, disposal and storage practices. Hydrometallurgy 2011, 108, 33–45. [Google Scholar] [CrossRef]
- Ochsenkühn-Petropoulou, M.; Lyberopulu, T.; Parissakis, G. Direct determination of lanthanides yttrium and scandium in bauxites and red mud from alumina production. Anal. Chim. Acta 1994, 296, 305–313. [Google Scholar] [CrossRef]
- Lyberopulu, T. Determination and Recovery of Rare Earths from Bauxites and Red Mud. Ph.D. Thesis, National Technical University of Athens, Athens, Greece, 1996. Available online: https://www.didaktorika.gr/eadd/handle/10442/8817 (accessed on 16 September 2018).
- Tsakanika, L.-A. Separation and Recovery of Lanthanides from Red Mud by Use of Selective Extraction and Chromatographic Techniques. Ph.D. Thesis, National Technical University of Athens, Athens, Greece, 2013. [Google Scholar]
- Ochsenkühn-Petropoulou, M.; Tsakanika, L.-A.; Lymperopoulou, T. Process control of an innovative method for the recovery and separation of rare earths from red mud by different analytical techniques. In Proceedings of the 1st European Rare Earth Resources Conference (ERES 2014), Milos Island, Greece, 4–7 September 2014; pp. 28–29. [Google Scholar]
- Scandium Investing. Why Scandium Could Be a Huge Opportunity: Commercially Viable Scandium Deposits Are Rare, but There Is Indeed Opportunity in This Market. Investing News Network. 12 April 2017. Available online: http://investingnews.com/daily/resource-investing/critical-metals-investing/scandiuminvesting/scandium-production-the-problem-and-the-opportunity/ (accessed on 20 September 2018).
- European Commission. Critical Raw Materials. Third List of Critical Raw Materials for the EU of 2017. Available online: https://ec.europa.eu/growth/sectors/raw-materials/specific-interest/critical_en (accessed on 20 September 2018).
- Gambogi, J.; U.S. Geological Survey. Mineral Commodity Summaries, SCANDIUM. January 2017. Available online: https://minerals.usgs.gov/minerals/pubs/commodity/scandium/mcs-2017-scand.pdf (accessed on 20 September 2018).
- Røyset, J.; Ryum, N. Scandium in aluminium alloys. Int. Mater. Rev. 2005, 50, 19–44. [Google Scholar] [CrossRef]
- Hildeman, G.; Koldenhoven, K. Mechanical Properties and Applications of Aluminum Scandium Alloys at Elevated Temperatures. Light Met. 2018, 1581–1587. [Google Scholar] [CrossRef]
- Schmidtke, K.; Palma, F.; Hawkins, A.; Emmelmann, C. Process and mechanical properties: Applicability of a scandium modified al-alloy for laser additive manufacturing. Phys. Procedia 2011, 12, 369–374. [Google Scholar] [CrossRef]
- Pan, F.; Liu, T.; Zhang, X.; Tang, A.; Wang, W. Effects of scandium addition on microstructure and mechanical properties of ZK60 alloy. Mater. Int. 2011, 21, 59–65. [Google Scholar] [CrossRef]
- Duyvesteyn, W.P.C.; Putnam, G.F.; EMC Metals Corporation, White Paper. SCANDIUM: A Review of the Element, Its Characteristics, and Current and Emerging Commercial Applications. May 2014. Available online: http://www.scandiummining.com/i/pdf/Scandium-White-PaperEMC-Website-June-2014-.pdf (accessed on 20 September 2018).
- UC RUSAL Sustainability Report 2016. Available online: https://www.unglobalcompact.org/participation/report/cop/create-and-submit/learner/398571 (accessed on 6 November 2018).
- Project SCALE: Production of Scandium Compounds and Scandium Aluminum Alloys from European Metallurgical by-Products. European Community’s Horizon 2020 Programme. Available online: http://cordis.europa.eu/project/rcn/206331_en.html (accessed on 20 September 2018).
- Awd, M.; Tenkamp, J.; Hirtler, M.; Siddique, S.H.; Bambach, M.; Walther, F. Comparison of microstructure and mechanical properties of scalmalloy® produced by selective laser melting and laser metal deposition. Materials 2018, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- Lymperopoulou, T.; Tsakanika, L.A.; Ochsenkühn, K.M.; Ochsenkühn-Petropoulou, M. Optimization of Mineral Acids Leaching Process for the Recovery of Rare Earth Elements from Greek Red Mud. In Proceedings of the 2nd Conference on European Rare Earth Resources (ERES 2017), Santorini Island, Greece, 28–31 May 2017; pp. 182–184. Available online: https://www.dropbox.com/sh/h1m9r8woixspd3f/AABzcTZ1UXs1Ex9j0kzeleODa/ABSTRACTS/VIA/VIA1.pdf?dl=0 (accessed on 20 September 2018).
- Ochsenkuehn-Petropoulou, M.; Lymperopoulou, T.; Tsakanika, L.A.; Ochsenkuehn, K.M.; Hatzilyberis, K.; Georgiou, P.; Stergiopoulos, C.; Tsopelas, F. Mineral Acid Leaching of Scandium from Bauxite Residue. In Proceedings of the 2nd International Bauxite Residue Valorization and Best Practices Conference (BR2018), Athens, Greece, 7–10 May 2018; pp. 373–379. Available online: http://svs2015-mtm.icts.kuleuven.be/indico/event/39/contribution/30 (accessed on 20 September 2018).
- Hatzilyberis, K.; Lymperopoulou, T.; Tsakanika, L.-A.; Ochsenkühn, K.-M.; Georgiou, P.; Defteraios, N.; Tsopelas, F.; Ochsenkühn-Petropoulou, M. Process Design Aspects for Scandium-Selective Leaching of Bauxite Residue with Sulphuric Acid. Minerals 2018, 8, 79. [Google Scholar] [CrossRef]
- Mineral Prices, Rare Earth Metals. Available online: http://mineralprices.com/default.aspx#rar (accessed on 16 September 2018).
- Liu, Z.; Li, H. Metallurgical process for valuable elements recovery from red mud—A review. Hydrometallurgy 2015, 155, 29–43. [Google Scholar] [CrossRef]
- Wang, W.; Pranolo, Y.; Cheng, C.Y. Metallurgical processes for scandium recovery from various resources: A review. Hydrometallurgy 2011, 108, 100–108. [Google Scholar] [CrossRef]
- Ochsenkühn-Petropulu, M.; Lyberopulu, T.; Ochsenkühn, K.M.; Parissakis, G. Recovery of lanthanides and yttrium from red mud by selective leaching. Anal. Chim. Acta 1996, 319, 249–254. [Google Scholar] [CrossRef]
- Ochsenkühn-Petropoulou, M. Implementation and Demonstration in Pilot Unit of a New Processing Method and Use of Red Sludge, Residue of Metallurgical Process of Greek Industry (EPET II/Research Program); Final Report; Ochsenkühn-Petropoulou, M., Ed.; Laboratory of Inorganic and Analytical Chemistry, School of Chemical Engineering NTUA and Aluminium of Greece S.A.: Athens, Greece, 2001. [Google Scholar]
- Ochsenkühn-Petropoulou, M.; Hatzilyberis, K.; Mendrinos, L.; Salmas, C. Pilot Plant Investigation of the Leaching Process for the Recovery of Scandium from Red Mud. Ind. Eng. Chem. Res. 2002, 41, 5794–5801. [Google Scholar] [CrossRef]
- Jha, M.K.; Kumari, A.; Panda, R.; Kumar, J.R.; Yoo, K.; Lee, J.Y. Review on hydrometallurgical recovery of rare earth metals. Hydrometallurgy 2016, 165, 2–26. [Google Scholar] [CrossRef]
- Yagmurlu, B.; Dittrich, C.; Friedrich, B. Effect of aqueous media on the recovery of scandium by selective precipitation. Metals 2018, 8, 314. [Google Scholar] [CrossRef]
- Borra, C.R.; Pontikes, Y.; Binnemans, K.; Van Gerven, T. Leaching of rare earths from bauxite residue (red mud). Miner. Eng. 2015, 76, 20–27. [Google Scholar] [CrossRef]
- Ujaczki, É.; Zimmermann, Y.S.; Gasser, C.A.; Molnár, M.; Feigl, V.; Lenz, M. Red mud as secondary source for critical raw materials—Extraction study. J. Chem. Technol. Biotechnol. 2017, 92, 2835–2844. [Google Scholar] [CrossRef]
- Petrakova, O.V.; Panov, A.V.; Gorbachev, S.N.; Klimentenok, G.N.; Perestoronin, A.V.; Vishnyakov, S.E.; Anashkin, V.S. Improved efficiency of red mud processing through scandium oxide recovery. In Light Metals; Hyland, M., Ed.; Wiley: New York, NY, USA, 2015; pp. 91–96. [Google Scholar] [CrossRef]
- Alkan, G.; Yagmurlu, B.; Cakmakoglu, S.; Hertel, T.; Kaya, Ş.; Gronen, L.; Stopic, S.; Friedrich, B. Novel Approach for Enhanced Scandium and Titanium Leaching Efficiency from Bauxite Residue with Suppressed Silica Gel Formation. Sci. Rep. 2018, 8, 5676. [Google Scholar] [CrossRef] [PubMed]
- Bonomi, C.; Giannopoulou, I.; Panias, D. Correlation of scandium and titanium during leaching of bauxite residue (red mud) by an imidazolium ionic liquid. In Proceedings of the 2nd Conference on European Rare Earth Resources (ERES2017), Santorini Island, Greece, 28–31 May 2017; Available online: https://www.researchgate.net/publication/321162111_CORRELATION_OF_SCANDIUM_AND_TITANIUM_DURING_LEACHING_OF_BAUXITE_RESIDUE_RED_MUD_BY_AN_IMIDAZOLIUM_IONIC_LIQUID (accessed on 16 September 2018).
- Davris, P.; Balomenos, E.; Panias, D.; Paspaliaris, I. Selective leaching of rare earth elements from bauxite residue (red mud), using a functionalized hydrophobic ionic liquid. Hydrometallurgy 2016, 164, 125–135. [Google Scholar] [CrossRef]
- Qu, Y.; Lian, B. Bioleaching of rare earth and radioactive elements from red mud using Penicillium tricolor RM-10. Bioresour. Technol. 2013, 136, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Borra, C.R.; Mermans, J.; Blanpain, B.; Pontikes, Y.; Binnemans, K.; Van Gerven, T. Selective recovery of rare earths from bauxite residue by combination of sulfation, roasting and leaching. Miner. Eng. 2016, 92, 151–159. [Google Scholar] [CrossRef]
- Borra, C.R.; Blanpain, B.; Pontikes, Y.; Binnemans, K.; Van Gerven, T. Smelting of bauxite residue (red mud) in view of iron and selective rare earths recovery. J. Sustain. Met. 2015, 2, 28–37. [Google Scholar] [CrossRef]
- Tsakanika, L.; Ochsenkühn-Petropoulou, M.; Mendrinos, L. Investigation of the separation of Rare Earth elements from red mud by use of reversed-phase HPLC. Anal. Bioanal. Chem. 2004, 379, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Tsakanika, L.-A.; Ochsenkühn-Petropoulou, M. Separation and recovery of rare earths after red mud leaching by cation-exchange chromatography. In Proceedings of the International Bauxite Residue Valorization and Best Practices Conference (BR2015), Leuven, Belgium, 5–7 October 2015; pp. 309–315. Available online: http://conference2015.redmud.org/wp-content/uploads/2015/10/Lamprini-Areti-TSAKANIKA-secure.pdf (accessed on 16 September 2018).
- Chemical Spot Price. Available online: http://www.sunsirs.com/uk/sectors-14.html (accessed on 23 September 2018).
- China Sulfuric Acid Price. Available online: http://www.sunsirs.com/uk/prodetail-236.html (accessed on 23 September 2018).
- China Nitric Acid Price. Available online: http://www.sunsirs.com/uk/prodetail-723.html (accessed on 23 September 2018).
- Industrial Sulfuric Acid Price H2SO4 98%. Available online: www.alibaba.com/product-detail/industrial-Sulfuric-Acid-Price-H2SO4-98-_2014682840.html?spm=a2700.7724857.normalList.26.466915ebpsz89c (accessed on 23 September 2018).
- Industrial Grade HNO3 Liquid Nitric Acid 68%. Available online: www.alibaba.com/product-detail/Industrial-Grade-HNO3-Liquid-Nitric-Acid_60771810678.html?spm=a2700.7724857.normalList.38.3eca7bb4Ibng1A (accessed on 23 September 2018).
- Sulphuric Acid 98%. Available online: https://www.kemcore.com/catalogsearch/result/?q=sulphuric+acid (accessed on 23 September 2018).
- Nitric Acid 68%. Available online: https://www.kemcore.com/catalogsearch/result/?q=nitric+acid+68%25 (accessed on 23 September 2018).
- Aide, M.; Braden, I.; Mueller, W. Partitioning of Iron and Scandium in Soils Having Water Drainage Limitations. Appl. Environ. Soil Sci. 2009, 2009, 243482. [Google Scholar] [CrossRef]

| Element | BR 1993 | BR 2001 | BR 2007 | BR 2012 | BR 2014 | BR 2016 | Mean |
|---|---|---|---|---|---|---|---|
| Sc | 127.9 | 107.0 | 130.0 | 110.0 | 104.7 | 98.0 | 112.9 ± 13.0 |
| Total REEs | 986.1 | 868.0 | 1010.5 | 1040.3 | 729.7 | 856.0 | 915.1 ± 118.2 |
| S/L | M | Final pH | Recovery % |
|---|---|---|---|
| 30% | 1 | 2.00 | 21.2 |
| 30% | 2 | 1.50 | 28.0 |
| 30% | 3 | 0.32 | 40.0 |
| 20% | 1 | 0.65 | 35.9 |
| 20% | 2 | 0.28 | 42.5 |
| 20% | 3 | 0.20 | 43.2 |
| 20% | pH adjusted * | 0.082 | 49.2 |
| 10% | 1 | 0.32 | 41.8 |
| 10% | 2 | 0.16 | 44.7 |
| 10% | 3 | 0.07 | 46.6 |
| 10% | pH adjusted * | 0.11 | 52.0 |
| 5% | 1 | 0.09 | 44.7 |
| 5% | 2 | 0.01 | 49.0 |
| 5% | 3 | 0.01 | 46.7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ochsenkuehn-Petropoulou, M.; Tsakanika, L.-A.; Lymperopoulou, T.; Ochsenkuehn, K.-M.; Hatzilyberis, K.; Georgiou, P.; Stergiopoulos, C.; Serifi, O.; Tsopelas, F. Efficiency of Sulfuric Acid on Selective Scandium Leachability from Bauxite Residue. Metals 2018, 8, 915. https://doi.org/10.3390/met8110915
Ochsenkuehn-Petropoulou M, Tsakanika L-A, Lymperopoulou T, Ochsenkuehn K-M, Hatzilyberis K, Georgiou P, Stergiopoulos C, Serifi O, Tsopelas F. Efficiency of Sulfuric Acid on Selective Scandium Leachability from Bauxite Residue. Metals. 2018; 8(11):915. https://doi.org/10.3390/met8110915
Chicago/Turabian StyleOchsenkuehn-Petropoulou, Maria, Lamprini-Areti Tsakanika, Theopisti Lymperopoulou, Klaus-Michael Ochsenkuehn, Konstantinos Hatzilyberis, Paraskevas Georgiou, Chrysanthos Stergiopoulos, Olga Serifi, and Fotis Tsopelas. 2018. "Efficiency of Sulfuric Acid on Selective Scandium Leachability from Bauxite Residue" Metals 8, no. 11: 915. https://doi.org/10.3390/met8110915
APA StyleOchsenkuehn-Petropoulou, M., Tsakanika, L.-A., Lymperopoulou, T., Ochsenkuehn, K.-M., Hatzilyberis, K., Georgiou, P., Stergiopoulos, C., Serifi, O., & Tsopelas, F. (2018). Efficiency of Sulfuric Acid on Selective Scandium Leachability from Bauxite Residue. Metals, 8(11), 915. https://doi.org/10.3390/met8110915








