Experimental Investigation of Phase Equilibria in the Co-Re-Ta Ternary System
Abstract
:1. Introduction
2. Experimental Procedure
3. Results and Discussion
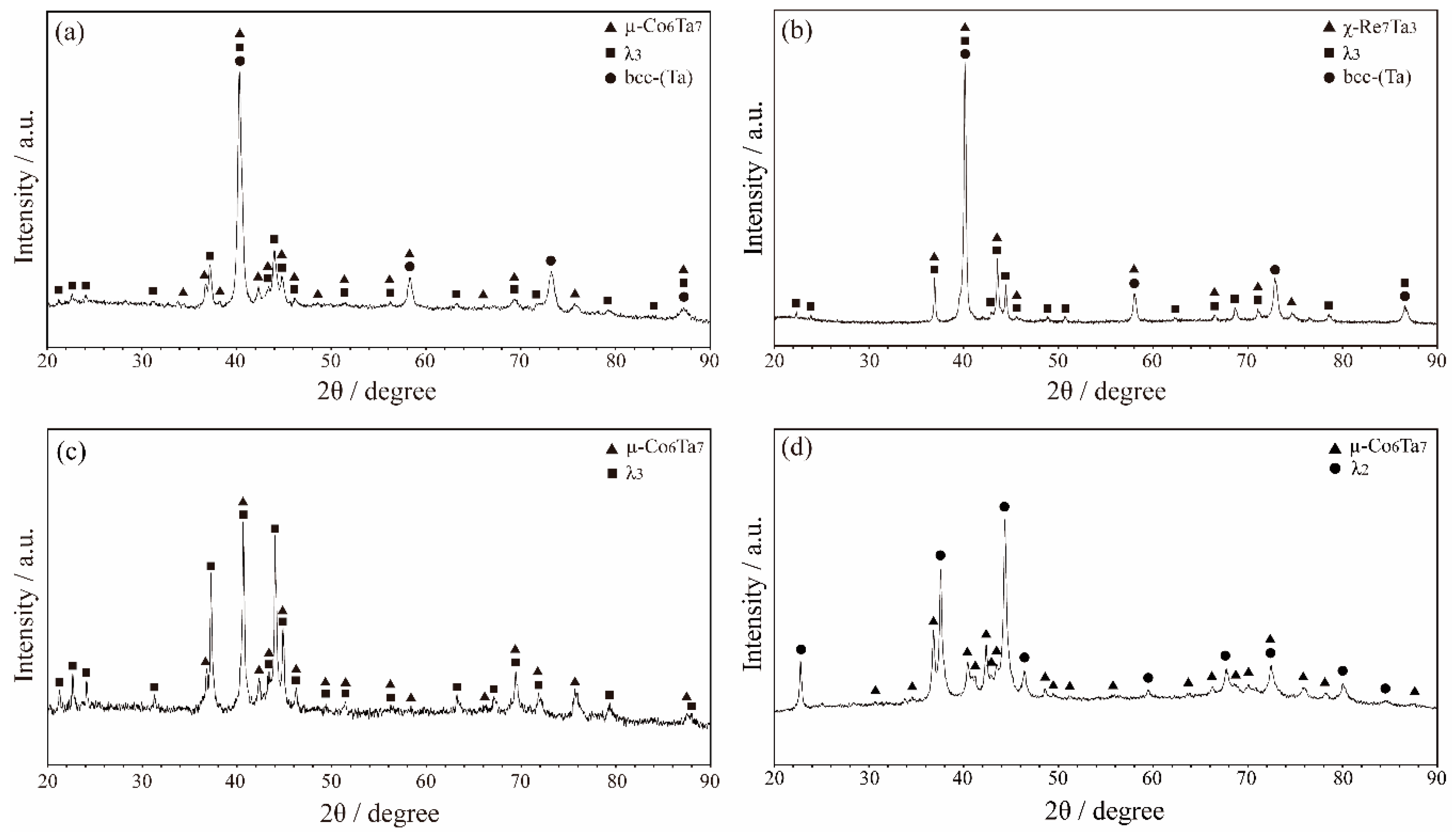
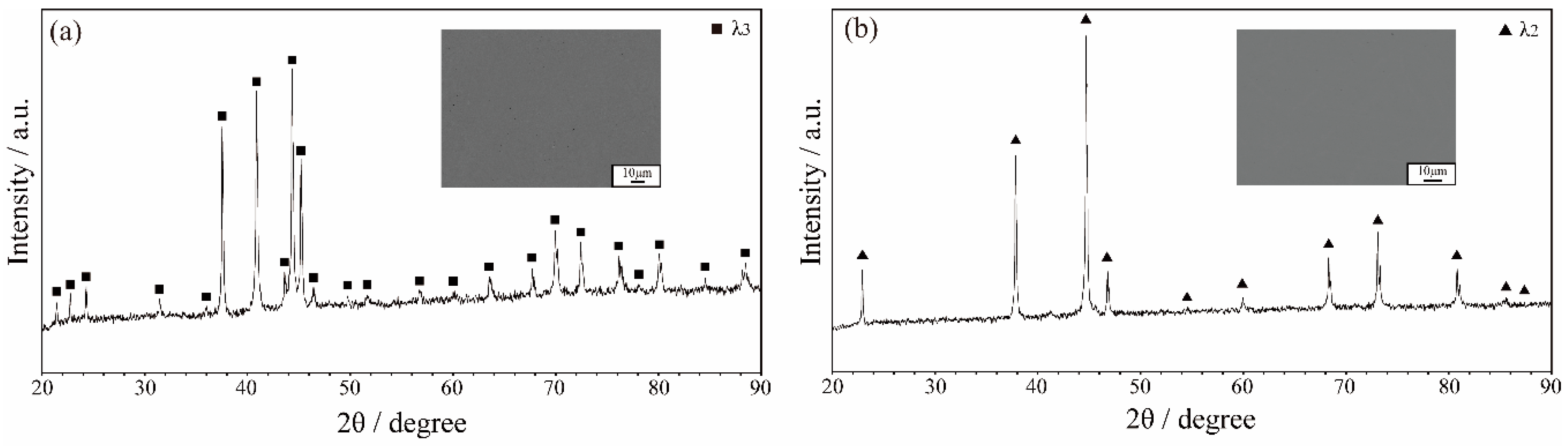
3.1. Microstructure
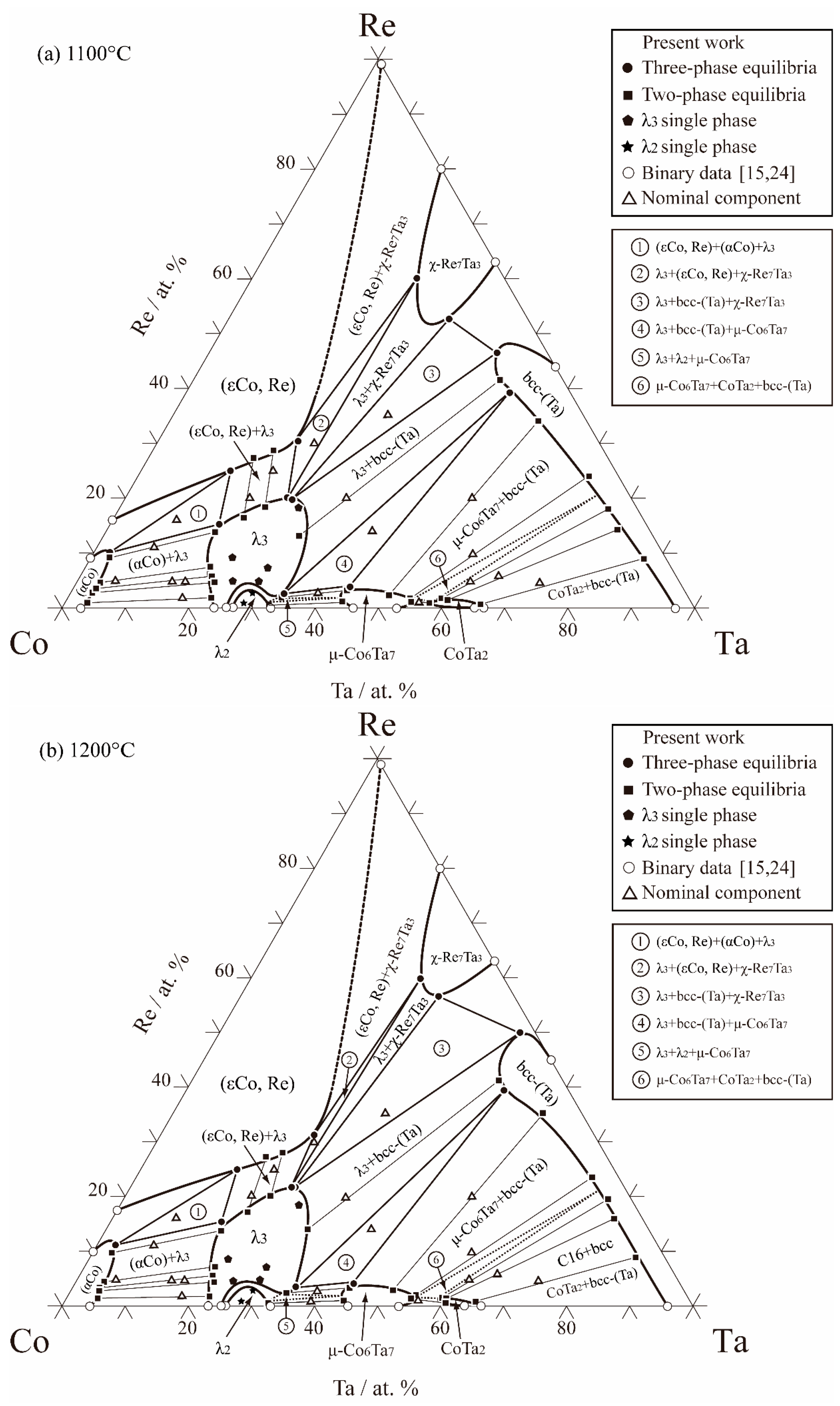
3.2. Isothermal Sections
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sato, J.; Omori, T.; Oikawa, K.; Ohnuma, I.; Kainuma, R.; Ishida, K. Cobalt-base high-temperature alloys. Science 2006, 312, 90–91. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Guan, X.; Zheng, Z. Effect of rhenium on solidification and segregation of nickel-based superalloy. Rare Met. 2011, 30, 320–322. [Google Scholar] [CrossRef]
- Blavette, D.; Caron, P.; Khan, T. An atom probe investigation of the role of rhenium additions in improving creep resistance of Ni-base superalloys. Scr. Metall. 1986, 20, 1395–1400. [Google Scholar] [CrossRef]
- Preußner, J.; Rudnik, Y.; Brehm, H.; Völkl, R.; Glatzel, U. A dislocation density based material model to simulate the anisotropic creep behavior of single-phase and two-phase single crystals. Int. J. Plasticity 2009, 25, 973–994. [Google Scholar] [CrossRef]
- Huang, M.; Zhu, J. An overview of rhenium effect in single-crystal superalloys. Rare Met. 2016, 35, 127–139. [Google Scholar] [CrossRef]
- Shang, L.; Cai, Q.; Liu, C.; Qi, L. Effects of Re doping on properties of Co-based alloy coating treated by laser cladding. Rare Met. 2002, 26, 173–178. [Google Scholar]
- Cardonne, S.M.; Kumar, P.; Michaluk, C.A.; Schwartz, H.D. Tantalum and its alloys. Int. J. Refract. Met. Hard Mater. 1995, 13, 187–194. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, G.; Lee, T.L.; Gorley, M.J.; Wang, Y.; Xiao, C.; Li, Z. The effects of Ta on the stress rupture properties and microstructural stability of a novel Ni-base superalloy for land-based high temperature applications. Mater. Des. 2014, 61, 61–69. [Google Scholar] [CrossRef]
- Seiser, B.; Drautz, R.; Pettifor, D.G. TCP phase predictions in Ni-based superalloys: Structure maps revisited. Acta Mater. 2011, 59, 749–763. [Google Scholar] [CrossRef]
- Rae, C.M.F.; Reed, R.C. The precipitation of topologically close-packed phases in rhenium-containing superalloys. Acta Mater. 2001, 49, 4113–4125. [Google Scholar] [CrossRef]
- Pessah-Simonetti, M.; Donnadieu, P.; Caron, P.T.C.P. Phase particles embedded in a superalloy matrix: Interpretation and prediction of the orientation relationships. Scr. Metall. Mater. 1994, 30, 1553–1558. [Google Scholar] [CrossRef]
- Elliott, R.P. Constitution of binary alloys First supplement. J. Less-Common Met. 1966, 371–372. [Google Scholar]
- Predel, B. Co-Re (Cobalt-Rhenium). In Ca-Cd-Co-Zr; Madelung, O., Ed.; SpringerMaterials; Springer: Berlin, Germany, 1993; Volume 5C, pp. 1–3. [Google Scholar]
- Liu, X.J.; Lin, J.Y.; Lu, Y.; Guo, Y.H.; Wang, C.P. Assessment of the atomic mobility for the fcc phase of Ni–Co–X (X = Re and Ru) system. Calphad 2014, 45, 138–144. [Google Scholar] [CrossRef]
- Guo, C.; Wu, T.; Li, C.; Du, Z. Thermodynamic re-assessment of the Re–X (X = Al, Co, Cr, Ta) binary systems. Calphad 2018, 61, 33–40. [Google Scholar] [CrossRef]
- Greenfield, P.; Beck, P.A. Intermediate phases in binary systems of certain transition elements. JOM 1956, 8, 265–276. [Google Scholar] [CrossRef]
- Cui, Y.; Jin, Z. Assessment of the Re–Ta binary system. J. Alloys Compd. 1999, 285, 150–155. [Google Scholar] [CrossRef]
- Liu, Z.K.; Chang, Y.A. Evaluation of the thermodynamic properties of the Re–Ta and Re–W systems. J. Alloys Compd. 2000, 299, 153–162. [Google Scholar] [CrossRef]
- Itoh, H.; Aoki, Y.; Nakamichi, T.; Yamamoto, M. Crystal structures, homogeneity ranges and magnetic properties of tantalum-cobalt Laves phases. Z. Metallk. 1974, 65, 149. [Google Scholar]
- Okamoto, H. Co-Ta (Cobalt-Tantalum). J. Phase Equilib. Diffus. 2004, 25, 571–572. [Google Scholar] [CrossRef]
- Liu, Z.K.; Chang, Y.A. Thermodynamic assessment of the Co-Ta system. Calphad 1999, 23, 339–356. [Google Scholar] [CrossRef]
- Kaufman, L. Coupled thermochemical and phase diagram data for tantalum based binary alloys. Calphad 1991, 15, 243–259. [Google Scholar] [CrossRef]
- Ponsioen, J.; Van Vucht, J. The Structure of β-TaCo3 and the effect of the substitution of Ta and Co by related elements. Philipp. Res. Rep. 1967, 22, 161–169. [Google Scholar]
- Shinagawa, K.; Chinen, H.; Omori, T.; Oikawa, K.; Ohnuma, I.; Ishida, K.; Kainuma, R. Phase equilibria and thermodynamic calculation of the Co–Ta binary system. Intermetallics 2014, 49, 87–97. [Google Scholar] [CrossRef]


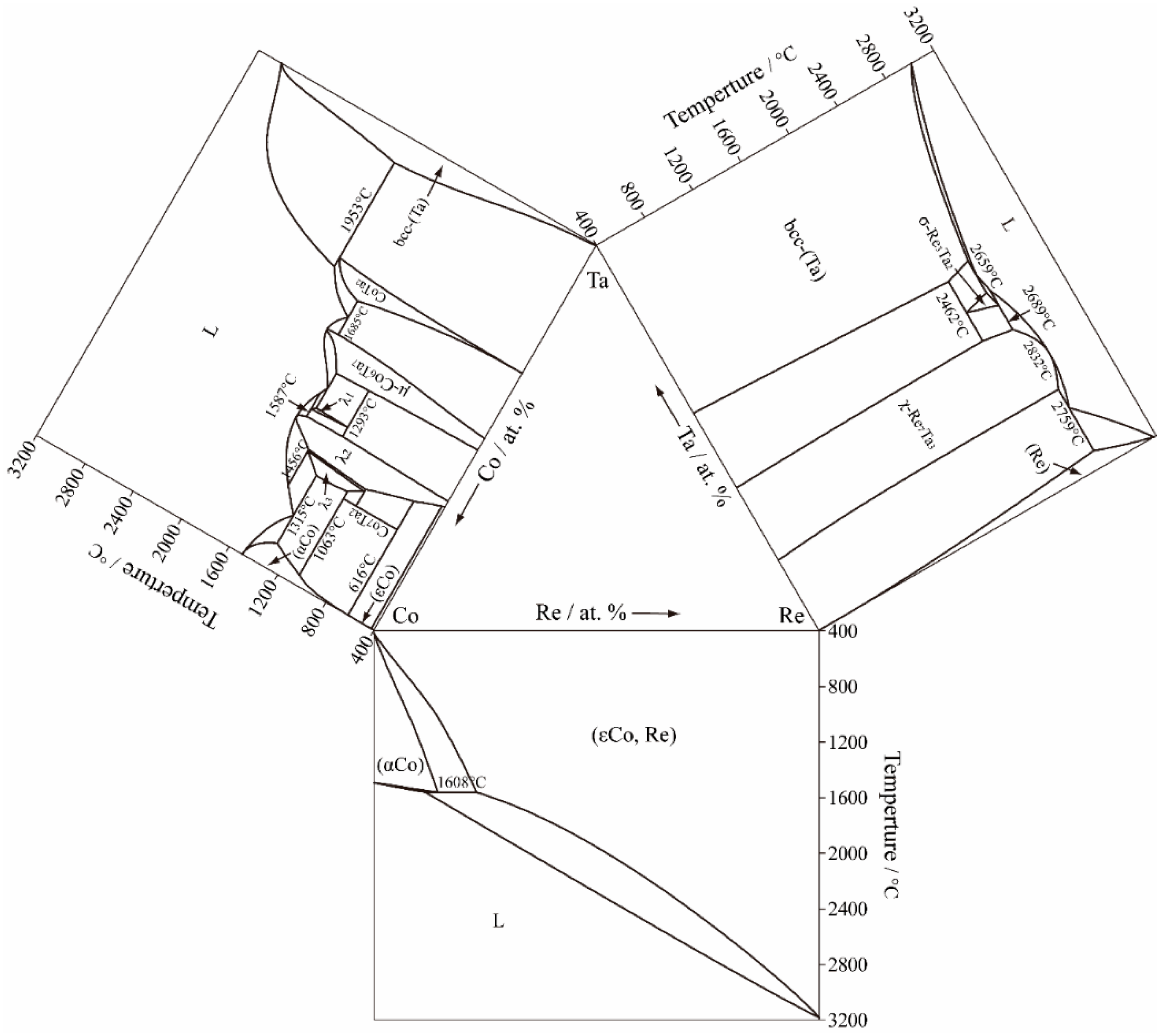
| System | Phase | Pearson Symbol | Space Group | Prototype | Structure Type | References |
|---|---|---|---|---|---|---|
| Re-Ta | (Re) | hP2 | P63/mmc | Mg | A3 | [15] |
| χ-Re7Ta3 | cI58 | I-43m | αMn | A12 | [15] | |
| σ-Re3Ta2 | tP30 | P42/mnm | σCrFe | D8b | [15] | |
| bcc-(Ta) | cI2 | Im-3m | W | A2 | [15] | |
| Co-Re | (αCo) | cF4 | Fm-3m | Cu | A1 | [15] |
| (εCo, Re) | hP2 | P63/mmc | Mg | A3 | [15] | |
| Co-Ta | (αCo) | cF4 | Fm-3m | Cu | A1 | [24] |
| (εCo) | hP2 | P63/mmc | Mg | A3 | [24] | |
| λ3-Co2Ta | hP24 | P63/mmc | Ni2Mg | C36 | [24] | |
| λ2-Co2Ta | cF24 | Fd-3m | Cu2Mg | C15 | [24] | |
| λ1-Co2Ta | hP12 | P63/mmc | Zn2Mg | C14 | [24] | |
| μ-Co6Ta7 | hR13 | R-3m | Fe7W6 | D8b | [24] | |
| CoTa2 | tI12 | I4/mcm | Al2Cu | C16 | [24] | |
| bcc-(Ta) | cI2 | Im-3m | W | A2 | [24] | |
| Co7Ta2 | hR36 | R-3m | BaPb3 [23] | - | [23,24] |
| Nominal Alloys (at. %) | Annealed Time (days) | Phase Equilibrium | Composition (at. %) | |||||
|---|---|---|---|---|---|---|---|---|
| Phase 1/Phase 2/Phase 3 | Phase 1 | Phase 2 | Phase 3 | |||||
| Re | Ta | Re | Ta | Re | Ta | |||
| Co31Re35Ta34 | 65 | λ3/bcc-(Ta)/χ-Re7Ta3 | 19.7 | 26.5 | 46.5 | 45.5 | 52.7 | 34.8 |
| Co45Re30Ta25 | 65 | λ3/(εCo, Re)/χ-Re7Ta3 | 20.1 | 25.5 | 30.4 | 22.0 | 60.1 | 26.0 |
| Co60Re20Ta20 | 65 | (εCo, Re)/λ3 | 27.3 | 16.6 | 16.5 | 20.5 | ||
| Co45Re20Ta35 | 65 | bcc-(Ta)/λ3 | 41.4 | 48.4 | 13.1 | 30.9 | ||
| Co25Re20Ta55 | 65 | μ-Co6Ta7/bcc-(Ta) | 2.3 | 50.6 | 34.1 | 58.3 | ||
| Co54Re25Ta21 | 65 | (εCo, Re)/λ3 | 28.7 | 19.1 | 18.4 | 22.9 | ||
| Co78Re5Ta17 | 50 | (αCo)/λ3 | 2.7 | 3.3 | 4.7 | 21.8 | ||
| Co44Re14Ta42 | 50 | λ3/μ-Co6Ta7/bcc-(Ta) | 2.7 | 33.8 | 3.8 | 43.7 | 39.2 | 51.2 |
| Co22Re5Ta73 | 50 | CoTa2/bcc-(Ta) | 0.7 | 65.8 | 8.9 | 87.5 | ||
| Co58Re3Ta39 | 50 | λ3/μ-Co6Ta7 | 1.9 | 33.6 | 3.2 | 43.4 | ||
| Co80Re2Ta18 | 50 | (αCo)/λ3 | 0.9 | 3.5 | 1.8 | 22.7 | ||
| Co80Re5Ta15 | 50 | (αCo)/λ3 | 3.5 | 3.6 | 5.7 | 20.8 | ||
| Co80Re11Ta9 | 50 | (αCo)/λ3 | 9.2 | 2.7 | 13.8 | 17.2 | ||
| Co74Re16Ta10 | 50 | (αCo)/λ3/(εCo, Re) | 10.1 | 2.4 | 15.2 | 17.3 | 25.0 | 14.2 |
| Co54Re18Ta28 | 50 | λ3 | 18.2 | 28.3 | ||||
| Co89Re5Ta6 | 50 | (αCo)/λ3 | 4.6 | 3.7 | 7.5 | 19.6 | ||
| Co33Re5Ta62 | 50 | CoTa2/bcc-(Ta) | 1.7 | 59.2 | 18.0 | 77.3 | ||
| Co30Re10Ta60 | 50 | μ-Co6Ta7/bcc-(Ta) | 1.7 | 54.2 | 24.0 | 71.3 | ||
| Co69Re9Ta22 | 50 | λ3 | 9.3 | 22.3 | ||||
| Co70Re5Ta25 | 50 | λ3 | 4.5 | 24.9 | ||||
| Co71Re1Ta28 | 50 | λ2 | 0.8 | 28.2 | ||||
| Co68Re3Ta29 | 50 | λ2 | 2.7 | 28.7 | ||||
| Co66Re5Ta29 | 50 | λ3 | 4.7 | 28.5 | ||||
| Co64Re7Ta29 | 50 | λ3 | 7.3 | 28.9 | ||||
| Co60Re1Ta39 | 50 | λ2/μ-Co6Ta7 | 0.7 | 32.3 | 1.2 | 43.7 | ||
| Co28Re6Ta66 | 50 | CoTa2/bcc-(Ta) | 1.4 | 60.2 | 14.2 | 80.7 | ||
| Co43Re1Ta56 | 50 | CoTa2/μ-Co6Ta7 | 0.9 | 57.8 | 1.1 | 54.8 | ||
| Nominal Alloys (at. %) | Annealed Time (days) | Phase Equilibrium | Composition (at. %) | |||||
|---|---|---|---|---|---|---|---|---|
| Phase 1/Phase 2/Phase 3 | Phase 1 | Phase 2 | Phase 3 | |||||
| Re | Ta | Re | Ta | Re | Ta | |||
| Co31Re35Ta34 | 50 | λ3/bcc-(Ta)/χ-Re7Ta3 | 21.7 | 26.1 | 50.0 | 47.6 | 56.6 | 31.4 |
| Co45Re30Ta25 | 50 | λ3/(εCo, Re)/χ-Re7Ta3 | 21.7 | 25.4 | 31.3 | 24.3 | 59.9 | 26.8 |
| Co60Re20Ta20 | 50 | (εCo, Re)/λ3 | 27.2 | 18.7 | 17.2 | 20.9 | ||
| Co45Re20Ta35 | 50 | bcc-(Ta)/λ3 | 41.1 | 48.6 | 14.0 | 31.9 | ||
| Co25Re20Ta55 | 50 | μ-Co6Ta7/bcc-(Ta) | 2.8 | 51.1 | 35.3 | 58.7 | ||
| Co54Re25Ta21 | 50 | (εCo, Re)/λ3 | 28.0 | 20.9 | 20.1 | 22.9 | ||
| Co78Re5Ta17 | 35 | (αCo)/λ3 | 2.7 | 4.4 | 4.3 | 21.9 | ||
| Co44Re14Ta42 | 35 | λ3/μ-Co6Ta7/bcc-(Ta) | 3.5 | 35.3 | 4.0 | 44.2 | 39.4 | 50.4 |
| Co22Re5Ta73 | 35 | CoTa2/bcc-(Ta) | 0.8 | 65.2 | 8.9 | 86.5 | ||
| Co58Re3Ta39 | 35 | λ3/μ-Co6Ta7 | 2.4 | 34.4 | 3.3 | 43.9 | ||
| Co80Re2Ta18 | 35 | (αCo)/λ3 | 1.4 | 4.9 | 1.8 | 22.5 | ||
| Co80Re5Ta15 | 35 | (αCo)/λ3 | 3.2 | 4.2 | 5.5 | 21.1 | ||
| Co80Re11Ta9 | 35 | (αCo)/λ3 | 9.7 | 3.0 | 13.6 | 18.4 | ||
| Co74Re16Ta10 | 35 | (αCo)/λ3/(εCo, Re) | 11.2 | 2.8 | 15.5 | 17.6 | 24.9 | 15.3 |
| Co54Re18Ta28 | 35 | λ3 | 18.3 | 28.2 | ||||
| Co89Re5Ta6 | 35 | (αCo)/λ3 | 4.3 | 4.5 | 7.1 | 20.6 | ||
| Co33Re5Ta62 | 35 | CoTa2/bcc-(Ta) | 1.5 | 59.6 | 19.5 | 76.8 | ||
| Co30Re10Ta60 | 35 | μ-Co6Ta7/bcc-(Ta) | 2.0 | 55.1 | 23.5 | 72.2 | ||
| Co69Re9Ta22 | 35 | λ3 | 8.4 | 22.0 | ||||
| Co70Re5Ta25 | 35 | λ3 | 4.5 | 24.8 | ||||
| Co71Re1Ta28 | 35 | λ2 | 0.8 | 28.0 | ||||
| Co68Re3Ta29 | 35 | λ2 | 2.6 | 28.8 | ||||
| Co66Re5Ta29 | 35 | λ3 | 4.8 | 29.0 | ||||
| Co64Re7Ta29 | 35 | λ3 | 7.0 | 28.8 | ||||
| Co60Re1Ta39 | 35 | λ2/μ-Co6Ta7 | 0.5 | 32.4 | 1.0 | 44.1 | ||
| Co28Re6Ta66 | 35 | CoTa2/bcc-(Ta) | 1.3 | 60.2 | 15.9 | 79.6 | ||
| Co43Re1Ta56 | 35 | CoTa2/μ-Co6Ta7 | 0.6 | 60.5 | 1.3 | 54.8 | ||
| Nominal Alloys (at. %) | Annealed Time (days) | Phase Equilibrium | Composition (at. %) | |||||
|---|---|---|---|---|---|---|---|---|
| Phase 1/Phase 2/Phase 3 | Phase 1 | Phase 2 | Phase 3 | |||||
| Re | Ta | Re | Ta | Re | Ta | |||
| Co31Re35Ta34 | 25 | λ3/bcc-(Ta)/χ-Re7Ta3 | 22.2 | 27.1 | 49.0 | 48.5 | 56.6 | 32.6 |
| Co45Re30Ta25 | 25 | λ3/(εCo, Re)/χ-Re7Ta3 | 22.6 | 26.1 | 32.0 | 23.2 | 58.7 | 25.8 |
| Co60Re20Ta20 | 25 | (εCo, Re)/λ3 | 27.5 | 17.0 | 17.3 | 20.6 | ||
| Co45Re20Ta35 | 25 | bcc-(Ta)/λ3 | 42.8 | 48.9 | 14.2 | 32.8 | ||
| Co25Re20Ta55 | 25 | μ-Co6Ta7/bcc-(Ta) | 3.9 | 51.2 | 35.5 | 58.9 | ||
| Co54Re25Ta21 | 25 | (εCo, Re)/λ3 | 28.8 | 18.9 | 19.7 | 22.1 | ||
| Co78Re5Ta17 | 15 | (αCo)/λ3 | 2.8 | 5.5 | 5.0 | 21.3 | ||
| Co44Re14Ta42 | 15 | λ3/μ-Co6Ta7/bcc-(Ta) | 5.5 | 36.4 | 6.1 | 43.2 | 41.8 | 50.4 |
| Co22Re5Ta73 | 15 | CoTa2/bcc-(Ta) | 0.8 | 65.2 | 8.7 | 87.6 | ||
| Co58Re3Ta39 | 15 | λ3/μ-Co6Ta7 | 2.7 | 35.4 | 3.6 | 44.0 | ||
| Co80Re2Ta18 | 15 | (αCo)/λ3 | 1.3 | 5.6 | 1.9 | 22.3 | ||
| Co80Re5Ta15 | 15 | (αCo)/λ3 | 3.5 | 5.0 | 5.9 | 20.8 | ||
| Co80Re11Ta9 | 15 | (αCo)/λ3 | 10.0 | 3.8 | 13.8 | 17.8 | ||
| Co74Re16Ta10 | 15 | (αCo)/λ3/(εCo, Re) | 13.0 | 2.6 | 16.2 | 17.8 | 26.0 | 14.2 |
| Co54Re18Ta28 | 15 | λ3 | 18.2 | 28.5 | ||||
| Co89Re5Ta6 | 15 | (αCo)/λ3 | 4.8 | 5.0 | 7.2 | 22.4 | ||
| Co33Re5Ta62 | 15 | CoTa2/bcc-(Ta) | 2.1 | 59.2 | 19.2 | 76.2 | ||
| Co30Re10Ta60 | 15 | μ-Co6Ta7/bcc-(Ta) | 2.4 | 54.1 | 24.2 | 71.4 | ||
| Co69Re9Ta22 | 15 | λ3 | 8.9 | 22.0 | ||||
| Co70Re5Ta25 | 15 | λ3 | 4.5 | 24.5 | ||||
| Co71Re1Ta28 | 15 | λ2 | 0.7 | 27.8 | ||||
| Co68Re3Ta29 | 15 | λ2 | 2.7 | 28.6 | ||||
| Co66Re5Ta29 | 15 | λ3 | 4.6 | 28.4 | ||||
| Co64Re7Ta29 | 15 | λ3 | 7.0 | 28.6 | ||||
| Co60Re1Ta39 | 15 | λ2/μ-Co6Ta7 | 0.8 | 32.3 | 1.3 | 44.2 | ||
| Co28Re6Ta66 | 15 | CoTa2/bcc-(Ta) | 1.8 | 58.3 | 14.9 | 81.1 | ||
| Co43Re1Ta56 | 15 | CoTa2/μ-Co6Ta7 | 0.6 | 59.7 | 1.3 | 54.9 | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Wu, D.; Zhang, J.; Yang, M.; Zhu, J.; Li, L.; Chen, Y.; Yang, S.; Han, J.; Lu, Y.; et al. Experimental Investigation of Phase Equilibria in the Co-Re-Ta Ternary System. Metals 2018, 8, 911. https://doi.org/10.3390/met8110911
Liu X, Wu D, Zhang J, Yang M, Zhu J, Li L, Chen Y, Yang S, Han J, Lu Y, et al. Experimental Investigation of Phase Equilibria in the Co-Re-Ta Ternary System. Metals. 2018; 8(11):911. https://doi.org/10.3390/met8110911
Chicago/Turabian StyleLiu, Xingjun, Dan Wu, Jinbin Zhang, Mujin Yang, Jiahua Zhu, Lingling Li, Yuechao Chen, Shuiyuan Yang, Jiajia Han, Yong Lu, and et al. 2018. "Experimental Investigation of Phase Equilibria in the Co-Re-Ta Ternary System" Metals 8, no. 11: 911. https://doi.org/10.3390/met8110911
APA StyleLiu, X., Wu, D., Zhang, J., Yang, M., Zhu, J., Li, L., Chen, Y., Yang, S., Han, J., Lu, Y., & Wang, C. (2018). Experimental Investigation of Phase Equilibria in the Co-Re-Ta Ternary System. Metals, 8(11), 911. https://doi.org/10.3390/met8110911