3.1. Nucleation of Reaction Layer
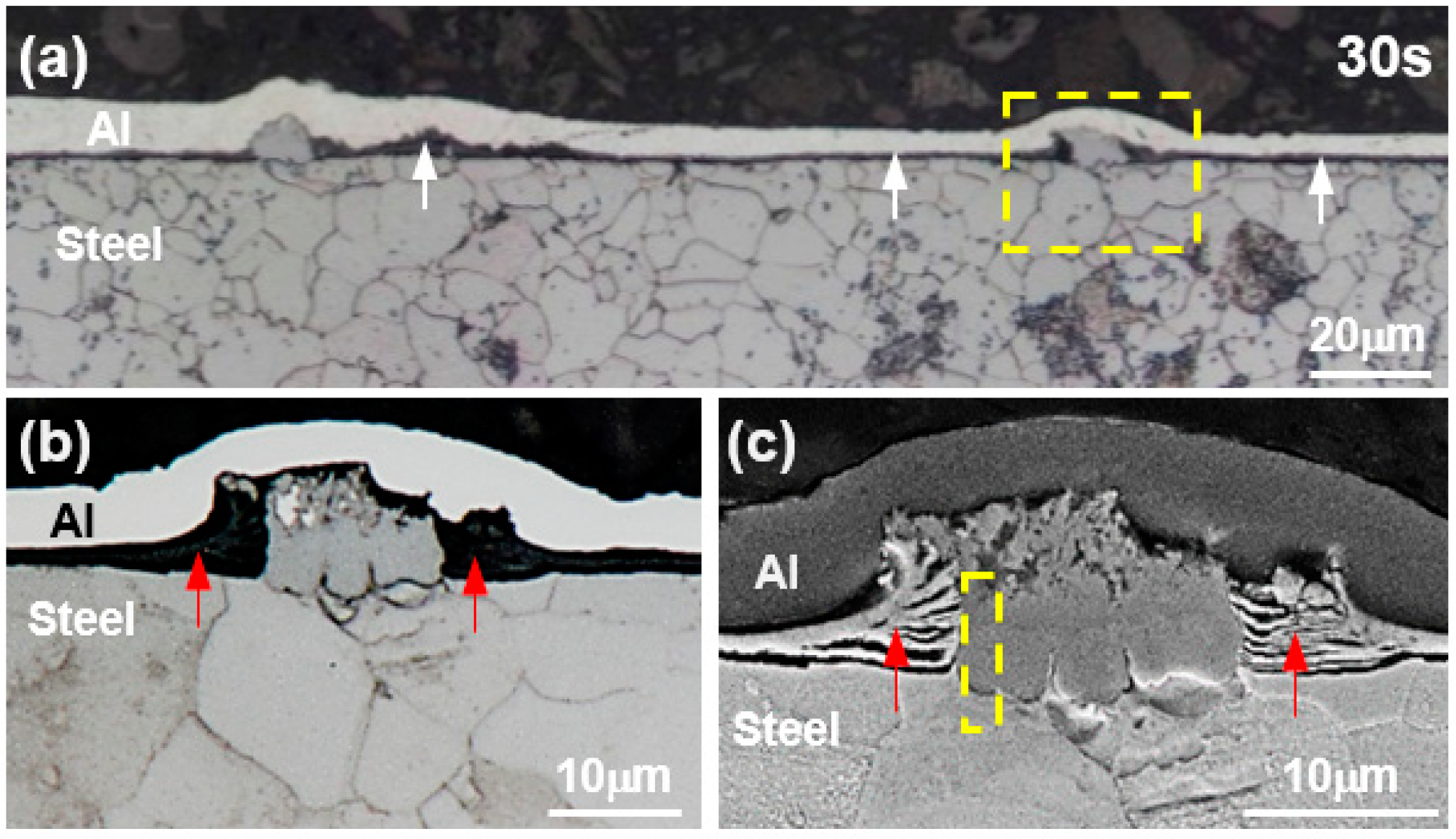
Figure 1a shows a cross-sectional optical microstructure of the specimen hot-dipped for 30 s at 690 °C.
Figure 1b,c shows the optical microstructure and SEM microstructure of the area marked as dotted rectangular area. However, when the hot dipping time was maintained at 30 s or less, no reaction layer was formed.
When hot-dipped for 30 s, as shown in
Figure 1a, a black stripe layer, which is identified with an arrow, formed at the interface between the Al and steel, and a fine reaction layer (width 10–15 μm, height 10 μm) was sporadically formed in this layer. In order to identify the black stripe layer and the reaction phase, we attempted to analyze the reaction layer region marked as dotted rectangular area in
Figure 1a with FE-EPMA.
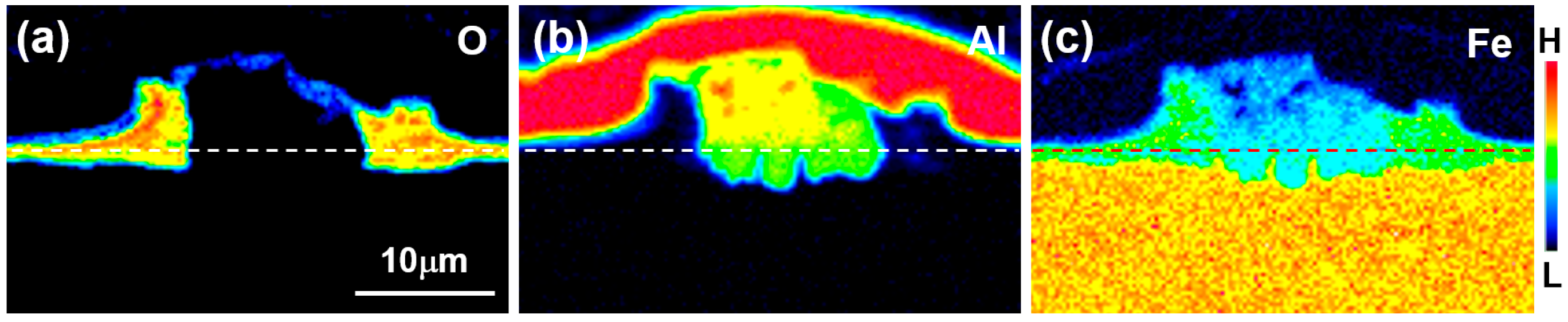
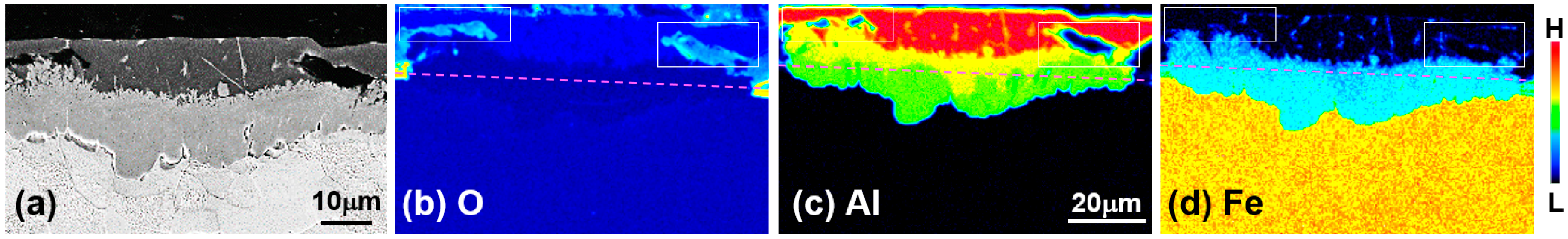
Figure 2 shows the results of the area’s FE-EPMA of O, Al, and Fe as the main component of the steel. The area where O was detected corresponds to the black stripe layer (↑) and the black stripe layer above the reaction layer at the Al/steel interface in
Figure 1b. The black zone adjacent to the reactive layer and the layer at the Al/steel boundary corresponds to the area where Fe was detected. From these results, the black stripe layer is considered to be an Fe-based oxide layer. In the region where no O was detected, a reaction layer formed in which Al, a dipping composition, and Fe, a main component of steel, were mixed. From the shape of the oxide layer around the reaction layer and the EPMA result in
Figure 2c, it can be deduced that when the oxide film reacts with the Al liquid phase and peels off in multiple layers, the liquid phase flows into the oxide film and the reaction layer forms on the surface of the steel.
In the EPMA results of
Figure 2, the dotted line at the interface where Fe and O are present but Al does not represent the original surface of the unoxidized steel. Therefore, it can be concluded that the reaction layer was formed on the liquid phase side and the inner side of the steel with respect to the steel surface and grew longer toward the liquid phase. The Al concentration was also lower in the steel-reactive layer (green) than in the hot-dipped layer (yellow). The fact that the reactive layer was formed on the surface of the steel indicates that the constituent elements of the steel dissolved into the liquid phase to form a new solid phase nucleus.
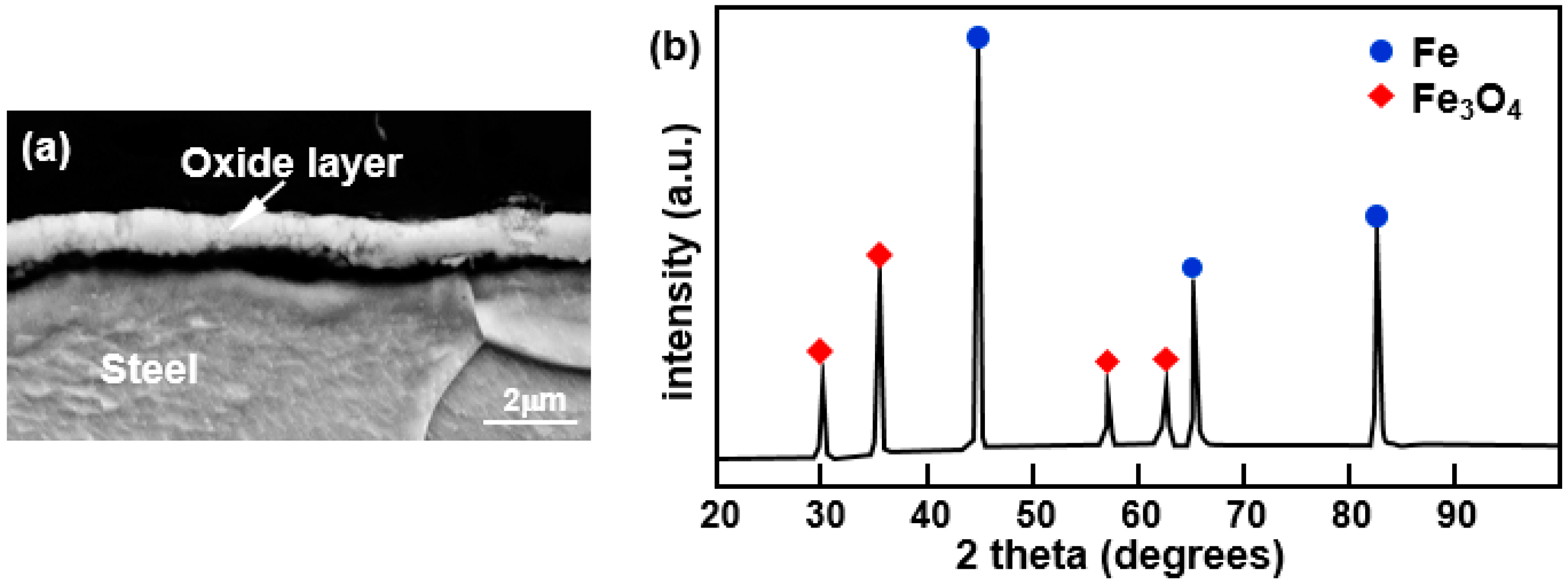
Figure 3a shows the cross-sectional microstructure of the oxide layer observed by SEM by selecting a region of the hot-dipped specimen where there was no reactive layer present on the surface.
Figure 3b is the result from XRD analysis of the surface. From this, it was confirmed that the oxide layer is composed of Fe
3O
4, which is the most thermodynamically stable Fe-based oxide at temperatures below the hot dipping temperature. In contrast, when the boron steel was heated in an electric furnace maintained at 690 °C in the air for 10 s, no oxide layer was observed. From these results, it is presumed that Fe
3O
4 was formed by the reaction of the steel surface with oxygen in the Al melt.
In order to investigate the phase in the nucleated reaction layer in the specimen hot-dipped for 30 s, the TEM was used with an FIB in the area marked as a dotted rectangular area in
Figure 1c and observed by STEM.
Figure 4a shows the TEM high-angle annular dark-field imaged (HAADF) microstructure and
Figure 4b–d shows the enlarged bright-field image X-Z (yellow rectangular) in
Figure 4a.
Figure 4e,f shows dark-field images of
Figure 4b,c, respectively.
Figure 5 shows the diffraction pattern and the result of analysis of the pattern indicated by the labels 1–8 in the figure.
Table 1 shows the result of the EDS point analysis of the phase labelled 1–5 in
Figure 4a.
From the results of the chemical composition analysis of the phases 1–5 (shown in
Table 1) and the diffraction pattern analysis, phases 1 and 5 were composed of Al (main hot dipping component) and α-ferrite (base metal). From the results of the analysis of the diffraction pattern from each phase, phases 2 and 3 were found to be Fe
4Al
13 (
θ phase, monoclinic) and phase 4 was identified as Fe
2Al
5 (
η phase, orthorhombic).
Figure 4b,e shows the bright-field image and dark-field image, respectively, in order to observe in detail the distribution of the phases in the area labelled
X in the HAADF image shown in
Figure 4a.
Figure 5f shows that the phase indicated by diffraction pattern 6 in the dark-field image is composed of Fe
2Al
5 (
η phase). This means that phases such as 6, which appear black in
Figure 4b, are phases that appear white in
Figure 4e. It can, therefore, be seen that the
η phase of size 1 μm or less is dispersed in the Fe
4Al
13 (
θ) phase in the
X region.
Figure 4 c,f shows the bright-field image and dark-field image of the areas labelled
Y in
Figure 2a where the phase of the Al concentration was lower than that of Fe
2Al
5 at the Fe
2Al
5/steel interface.
Figure 5g shows the result of the diffraction pattern analysis of phase 7 in
Figure 4f. This was found to be a FeAl (
α2 or
β, cubic) phase. These features are characterized by a high dislocation density and lateral growth, in contrast to the Fe
2Al
5 (
η) phase.
Figure 4d shows the enlarged bright-field image of the
η phase/steel interface
Z region in
Figure 2a,
Figure 5h shows the diffraction pattern analysis result of the phase labelled 8 in
Figure 4f. From these results, it can be seen that the nanoscale
κ (Fe
3AlC, cubic) phase was formed along the grain boundaries. In addition, α-ferrite grains near the interface exhibit fine dimensionality and were characterized by a high dislocation density.
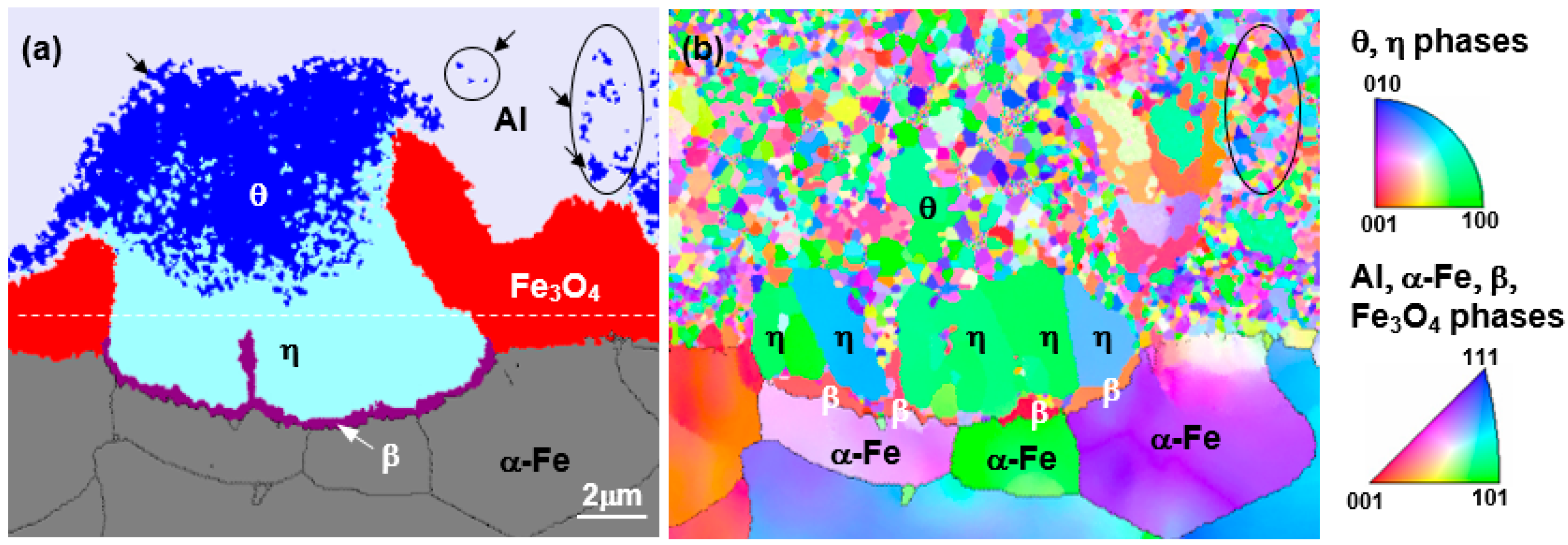
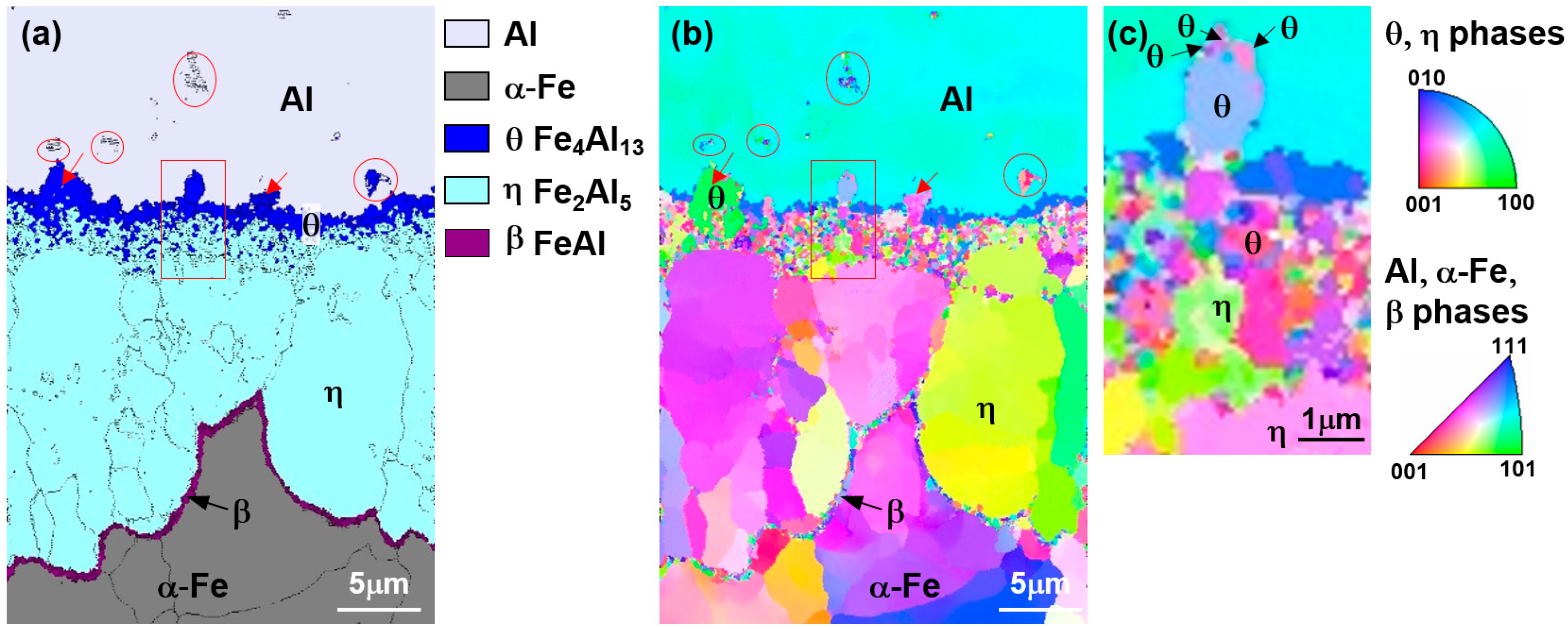
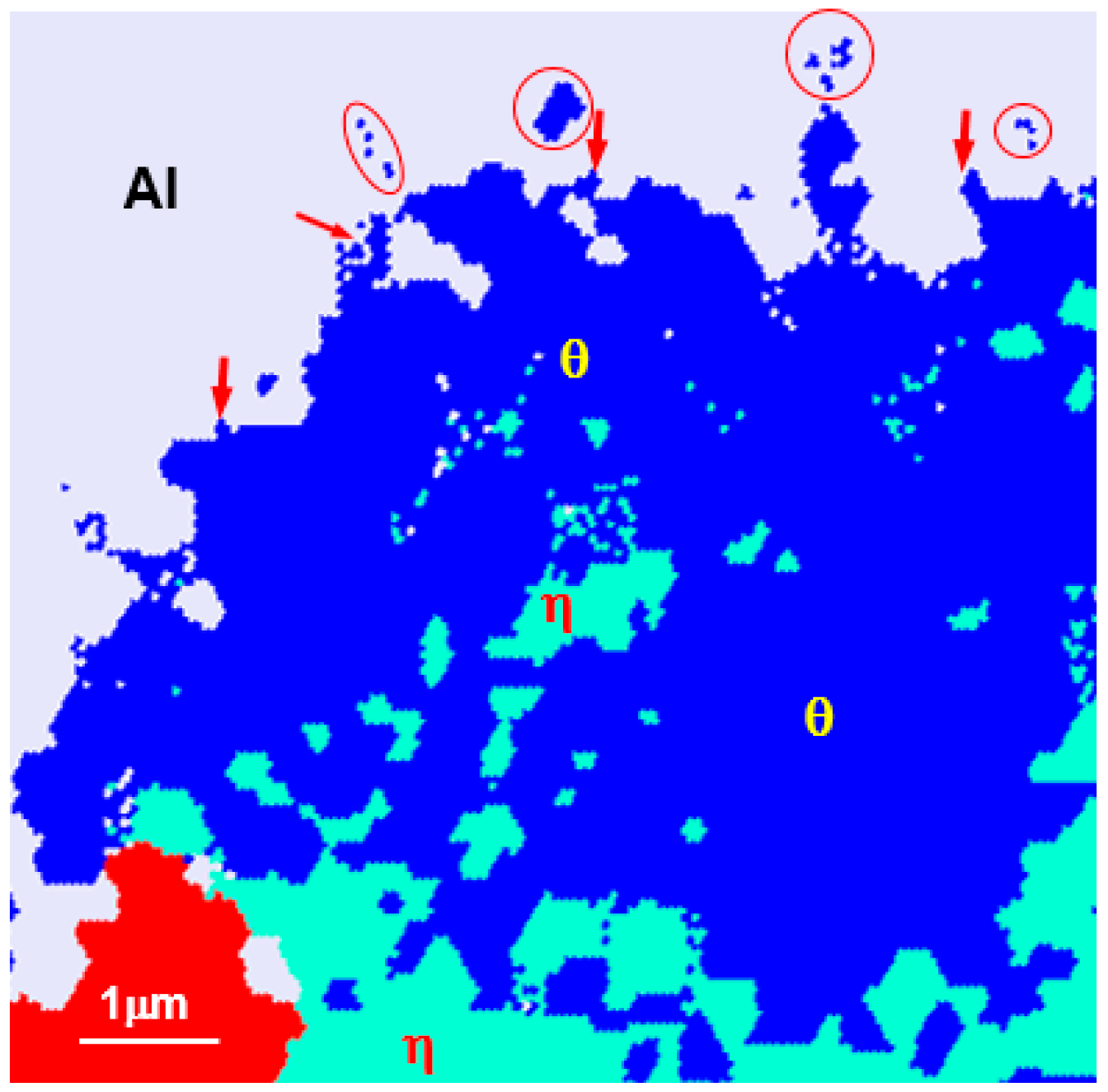
Figure 6 shows the result of electron back-scatter diffraction EBSD analysis of the reaction layer (hot-dipped for 30 s) in
Figure 2, where (a) is the phase map and (b) is the inverse pole figure (IPF).
In the phase map results, the reaction layer features a θ layer on the liquid side and a η layer on the steel side, similar to the TEM observation and a β layer was observed at the η/steel (α) interface. The area where the θ phases exist can be classified into three areas: the area where the θ phase exists in the Al liquid phase (→), the region consisting of the θ phase only, and the region in which θ and η phases are mixed near the θ/η interface. This detail cannot be found in the TEM microstructure that enables observation of only the fine regions.
From the IPF image in
Figure 6b, the
θ grains present in the liquid phase were mostly several hundred nanometers in diameter. Although some grains of the
θ phase-only region were 1–2 μm in diameter, most of the grains were smaller than 500 nm. The
θ grains in the mixed-phase region consisted mostly of those several hundreds of nanometers in diameter. However, it can be seen that the
η phase grows specifically in the <100> crystal orientation to a length of several micrometers in all but the mixed-phase region. At the
η phase/steel interface, band-shaped grains perpendicular to the growth direction of
η were observed. As these crystal grains differ from the
η phase and steel in terms of crystal orientation, they were concluded not to be
η-phase and ferrite. From these observations, it was decided that these grains were
β (FeAl), labelled 7 in
Figure 4c and 7 in
Figure 4f. Meanwhile, the
κ (Fe
3AlC) phase present in the ferrite grain boundary in
Figure 4d is too fine to be described as nanoscale and is not seen in EBSD.
From the above observations and analysis results, in the nucleated reaction layer there appears to be a θ phase region featuring a grain size between several hundred nm to 2 μm near the liquid phase, a mixed region of θ and η phases, a η phase region growing longest toward the steel, a band-type β (FeAl) region of thickness 500 nm at the η phase/steel interface. In addition, κ (Fe3AlC) was formed at the grain boundary of the base material in contact with the reaction layer.
3.3. Formation Mechanism of the Reaction Layer and Phases
As can be seen in
Figure 2,
Figure 6 and
Figure 8, the initially formed nucleated reaction layer was thickest toward the liquid Al region and was thin inside the steel region. The nucleation of the reaction layer was initialized in the liquid phase side, which caused the Fe in the steel to flow into the liquid Al.
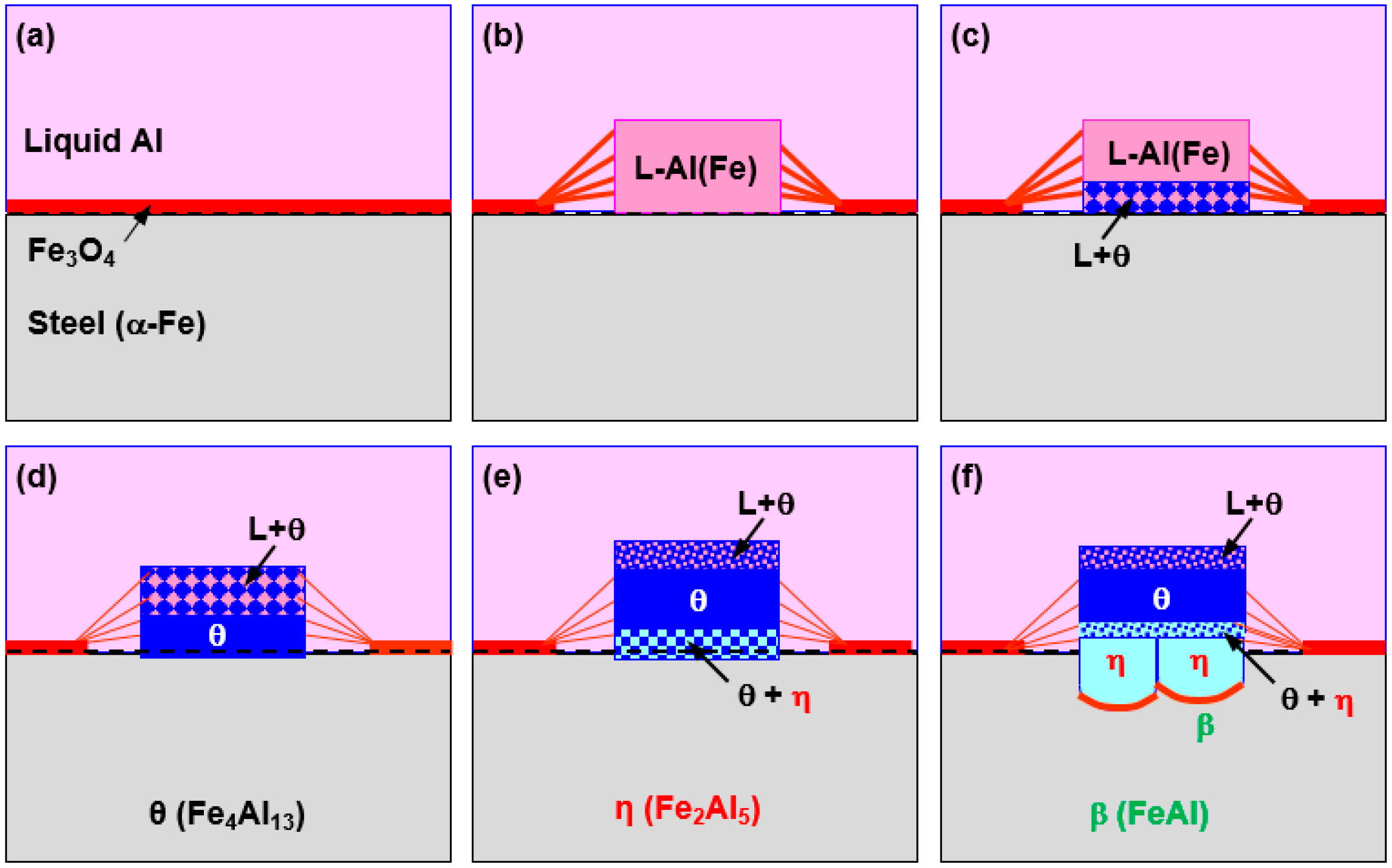
Figure 11 is a schematic diagram displaying the nucleation and formation process of the reaction layer and the reaction phase. Although the reaction layer consists of
θ (Fe
4Al
13 or FeAl
3) and
η (Fe
2Al
5) phases, the
θ phase formed first, later followed by the
η phase.
Figure 11a–d represents the formation process of the
θ phase and
Figure 11e,f depicts that of the
η phase.
An Fe
3O
4 oxide film formed on the surface of the steel while the steel was immersed in the liquid Al. The oxide film was destroyed as it was replaced with Al
2O
3 through the reaction with liquid Al and the reaction of liquid Al with the steel.
Figure 11b The path of Fe into the liquid phase is thought to occur by two mechanisms; one is the dissolution-diffusion of Fe from the surface of the steel, and the other is the generation of Fe by the reduction reaction between the Fe
3O
4 oxide film and the liquid Al.
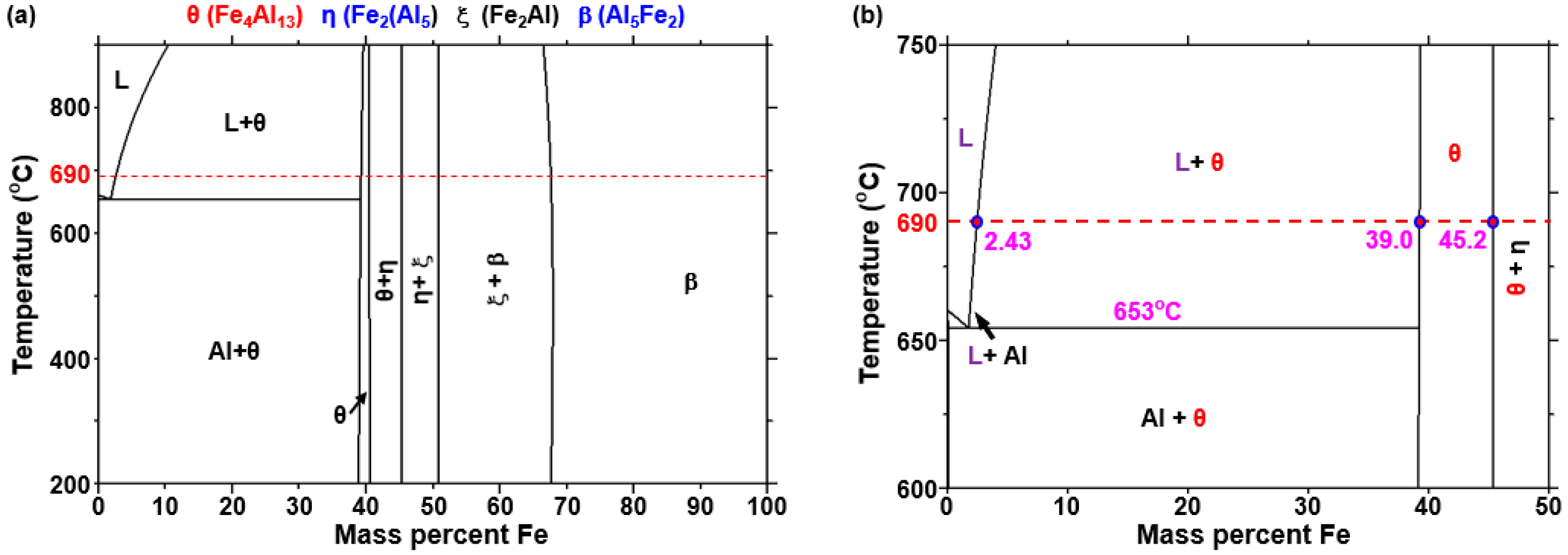
In order to determine the phase transformation of the liquid Al with increasing Fe content at 690 °C, the Al-Fe binary phase diagram was investigated using the software as shown in
Figure 12. There are two processes in which a solid phase can be transformed; when the Fe concentration is increased more than approximately 2.43 wt% at 690 °C, the solid
θ phase (Fe
4Al
13 or FeAl
3) and the liquid Al is generated, so that the
θ phase is solidified in the liquid near the steel interface. In other words, if the concentration of Fe in the liquid is approximately 2.43 wt% or more, the
θ phase can solidify isothermally at 690 °C. Alternatively, when the concentration of Al in the liquid is higher than the eutectic composition (2.0 wt%, 3.97 at%), not only the
θ phase is solidified during the cooling process but also the eutectic reaction of the combined Al and
θ phase can occur.
Figure 13 is a magnified view of
Figure 6a in the vicinity of the liquid Al showing the nucleated reaction layer. The
θ phases (marked with arrows) in the liquid were considered to have formed during isothermal solidification or cooling and to have attached to the already-formed
θ phases. Regarding
Figure 12, if the
η phase was formed during cooling, the Fe concentration should have increased over 40 wt% or more in the liquid. However, it is not considered possible that the Fe concentration in the liquid reached 40 wt% or more in only 30 s. Therefore, the
θ phases in which
η phases coexist are not formed during cooling but are formed by isothermal solidification at 690 °C.
The growth rate of the
θ phase with respect to dipping time was studied differently. Bouche [
8] reported that the growth of FeAl
3 obeyed a parabolic law, whereas Bouayad [
11] reported that growth followed a linear relationship. In this study, it was thought that the growth rate of
θ phases was slow because they were growing toward the liquid phase and because the
θ phases near the steel interface were transformed into
η phases. The complicated formation processes seemed to be the reason the that growth rate could not be accurately measured.
Next, the formation process of the
η phases was considered. When the
θ phase was formed between the liquid Al and the steel, the Al atoms would not diffuse directly into the steel but would instead pass through the solid
θ phase and then diffuse to the steel (liquid Al →
θ → steel). In contrast, Fe atoms diffused in the opposite direction (steel →
θ → liquid Al). In the Fe-Al phase diagram, there are four phases that can be formed by the diffusion of Fe and Al in the solid state:
θ (Fe
4Al
13 or FeAl
3),
η (Fe
2Al
5),
ζ (FeAl
2), and
β or
α2 (FeAl).
Table 2 shows the Al concentration and crystal structure of these phases. It is noted that the
η phase has the least difference in the composition of Al and Fe with the
θ phase. In other words, the
θ phase is 2.4 at% higher than the
η phase, and, in contrast, the Fe concentration is lower by 5.04 at% than the
η phase. If Al diffused from the
θ phase to the steel by 2.5 at% and Fe diffused to the liquid Al along the
θ phase by 2.5 at% in the opposite direction, then the
θ phase will transform into the
η phase. The above phase transformation is considered to be the nucleation mechanism of the
η phase.
From
Figure 6 and
Figure 10, the growth of
η phases is greater than the
θ phase in the two-phase (
θ and
η) region near the steel interface. This is because Al diffuses actively toward the steel and because the
θ phase can transform into the
η phase. The growth of the
η phase is thought to be the nuclei of the
η phase, which itself was transformed from the
θ phase and takes place in the direction of the steel due to the inter-diffusion of Al and Fe. The grain boundary of
η phases is characterized by the longer growth along the
c-axis of the crystal structure, as shown in
Figure 10b. The morphology of
η phases is known as a tongue-like (or sawtooth) grain. The morphology of the
η phases has been studied and explained to be a result of the 30% vacancy rate of the
c-axis of the
η phase, which can be seen as a rapid diffusion tunnel. It causes the
η phase to be orientated by the fixed crystal structure and grows preferentially along the diffusion direction during hot dipping.
The formation mechanism of the
β (FeAl) phase of 500 nm near the
η phase/steel interface was deduced from
Figure 4c,f and
Figure 6. At the interface, Fe diffuses to the
η phase via decreasing Fe concentration, as Al also diffuses into the liquid Al →
θ phase →
η phase via increasing Al concentration, resulting in the formation of new Fe-Al phases. In the Fe-Al phase diagram, there are four representative phases (
Figure 12 and
Table 2). Among them,
β is preferentially formed due to it possessing the lowest Al concentration. However, considering the Al diffusion path, the
η phase near the base material must transform into
β (FeAl) →
ζ (FeAl
2) →
η (Fe
2Al
5) by the mutual diffusion of Al and Fe. However, the
ζ (FeAl
2) phase was not observed in the experimental results.
Another reaction phase formed in the steel near the
η phase was
κ (Fe
3AlC), which was formed at the grain boundary of the ferrite. Springer [
19] recently reported that an 800 nm band consisting of
β and
κ phases was formed between the steel and
η phase by inter-diffusion in the solid reaction of low-carbon steel and pure Al at 600 °C. Although the existence of the phase was revealed, its formation mechanism was not confirmed. The
ζ phase was not observed because it is smaller than the
β phase. According to a previous study on a steel coated with Al alloy through the hot dipping process [
26,
27,
28,
29], the
κ (Fe
3AlC) phase was formed because Al atoms in liquid Al and C atoms emitted from the
η phase, which barely features C, and diffuses preferentially along the grain boundary.
From the experimental results above and subsequent discussion, the formation process of the reaction layer is proposed as follows: Al reacts with the iron oxide layer (Fe3O4) on the surface of the steel that decomposes into Al2O3 and the liquid Al flows into the destroyed oxide layer. When the Fe atoms from the steel dissolve above approximately 2.5 at% (0.6 wt%) into the liquid Al, the θ phase (Fe4Al13) forms individually in a solid state and grows. The η phase (Fe2Al5) is then formed by the inter-diffusion of Al and Fe at the θ phase/steel interface. The η phase grows in a long columnar form towards the steel, and finally, a β phase with a low Al concentration is formed at the steel interface.