Inhibition Effect of Ti on the Formation of Martensite Lath in 14Cr Oxide Dispersion Strengthened Steel
Abstract
1. Introduction
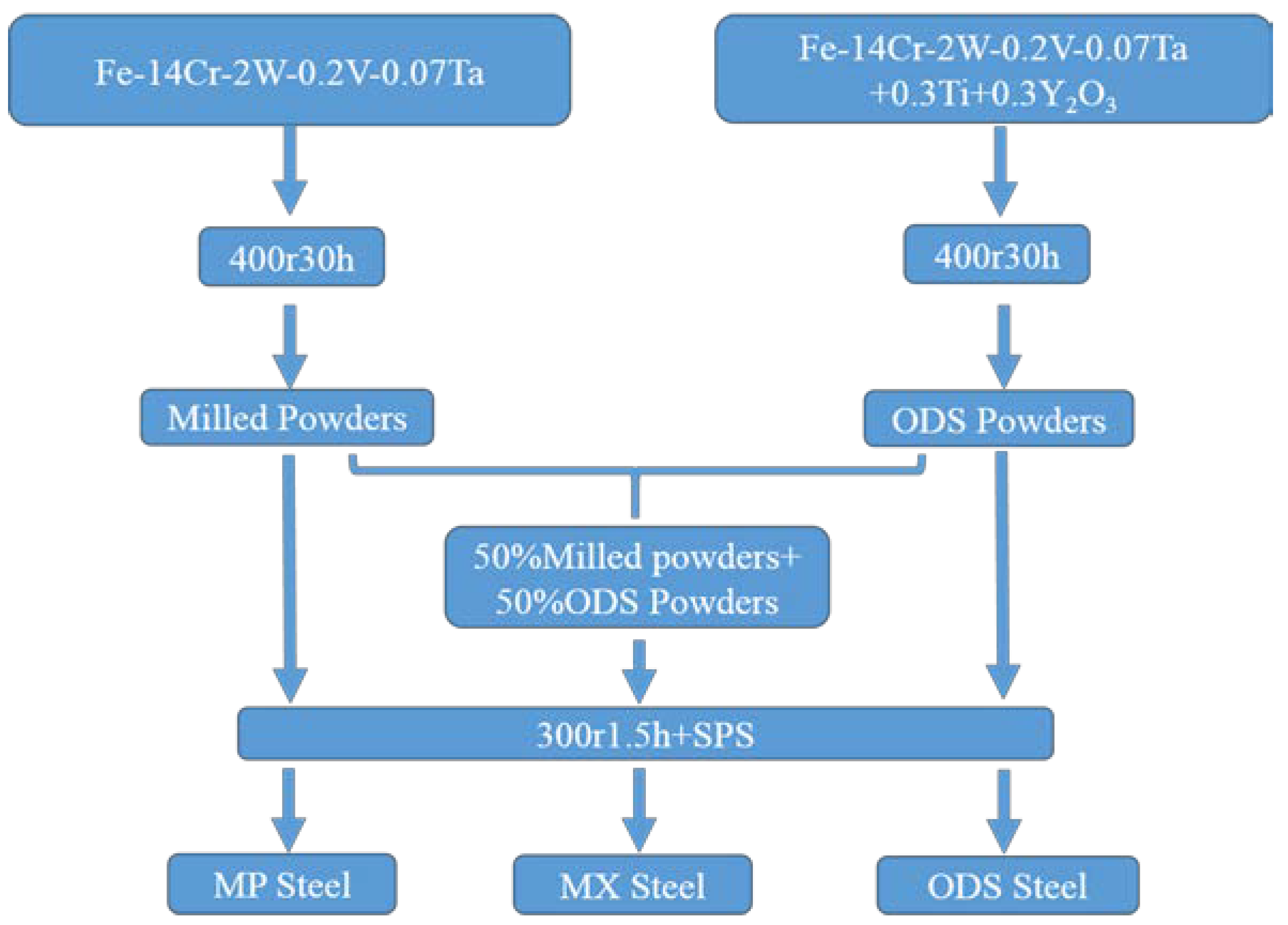
2. Experimental Procedure
3. Results and Discussion
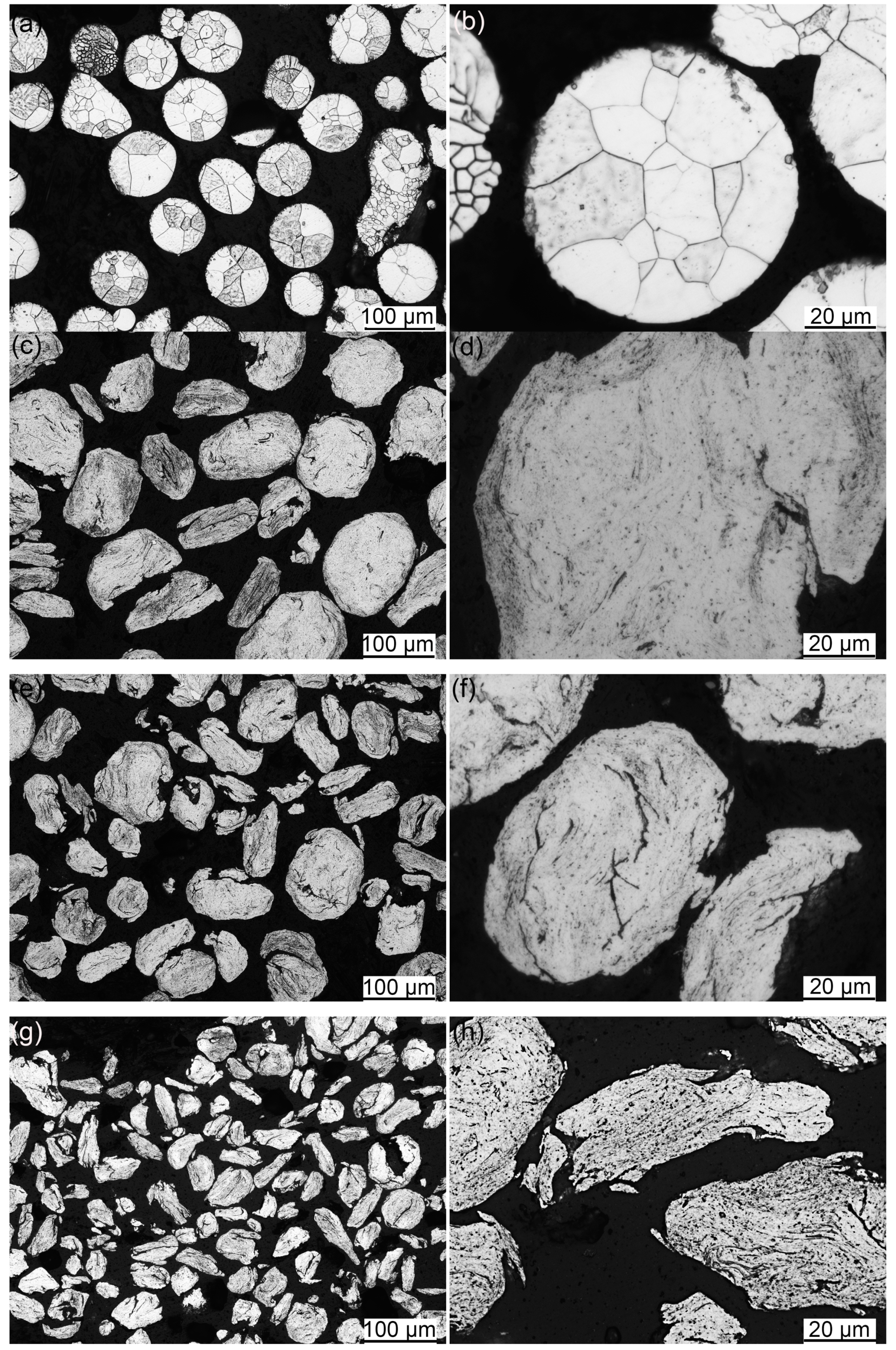
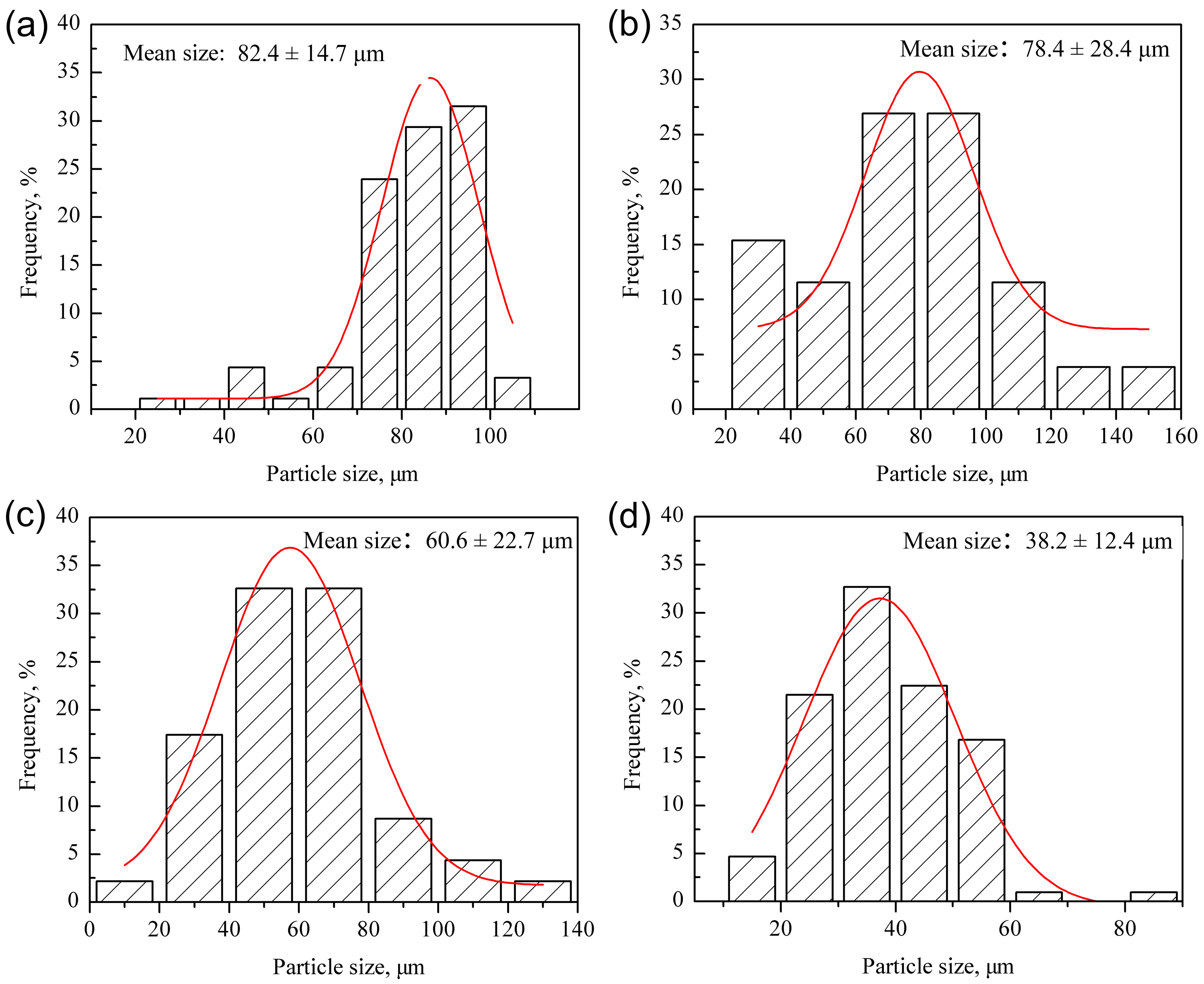
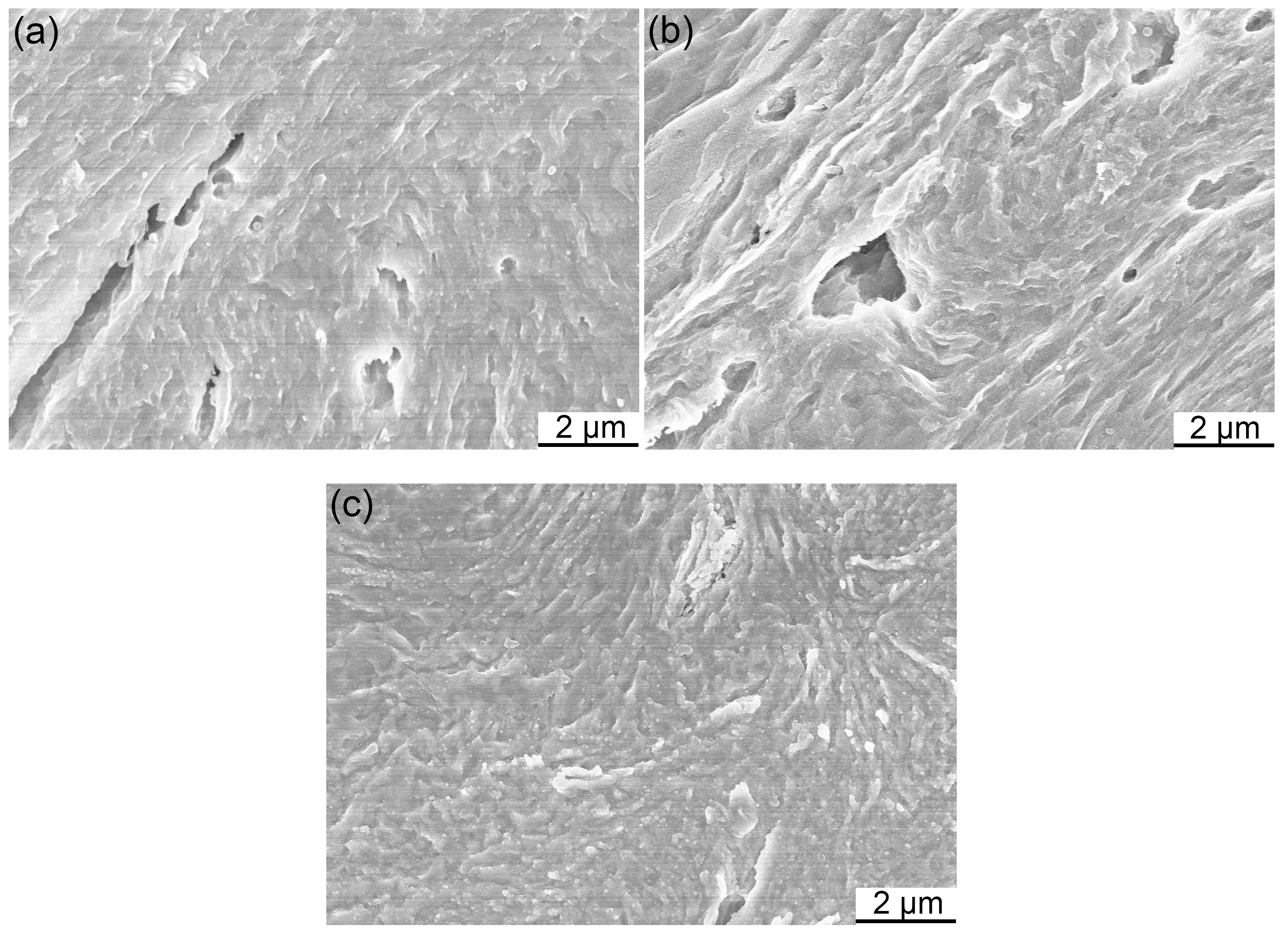
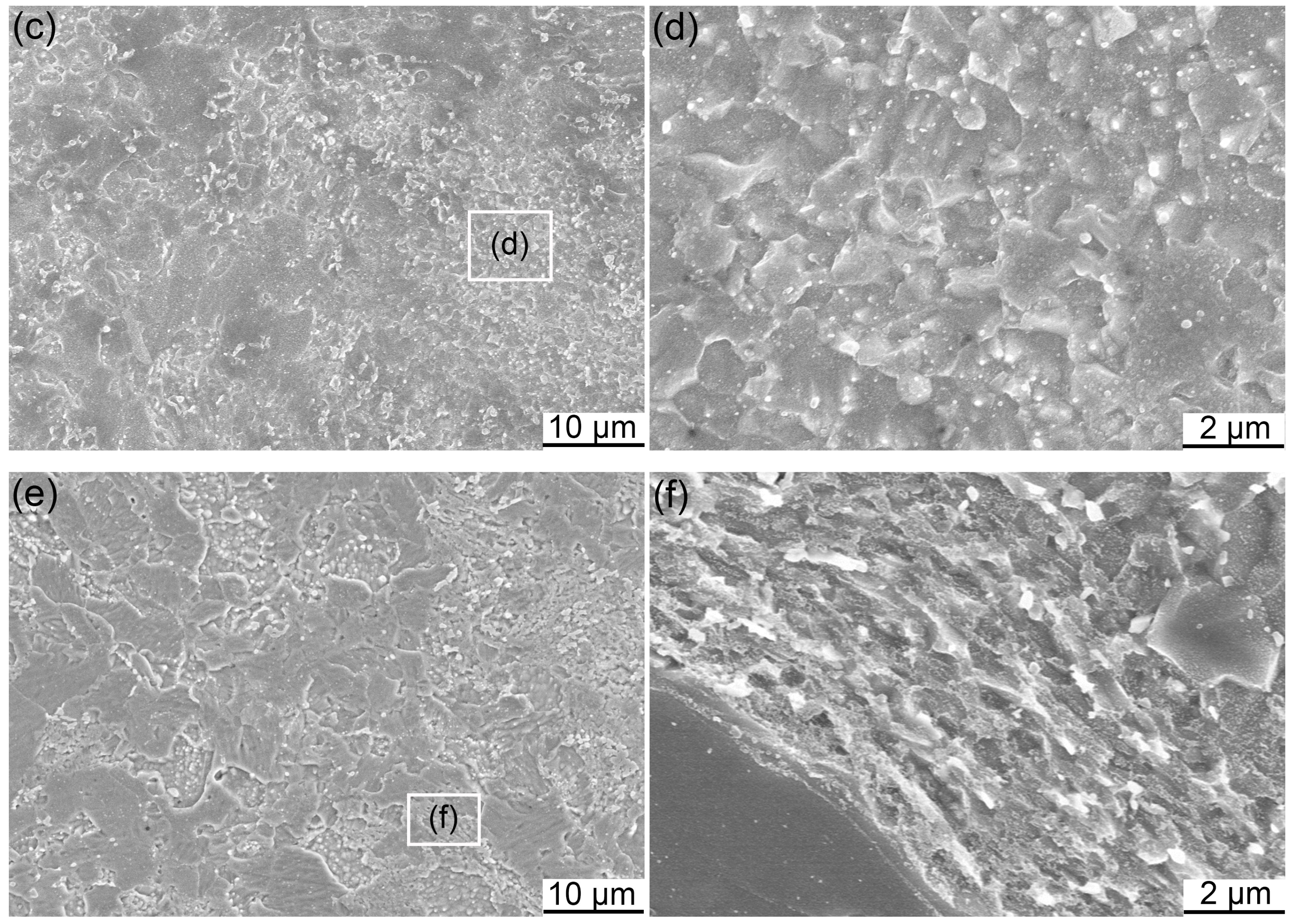
3.1. Microstructure of Powders
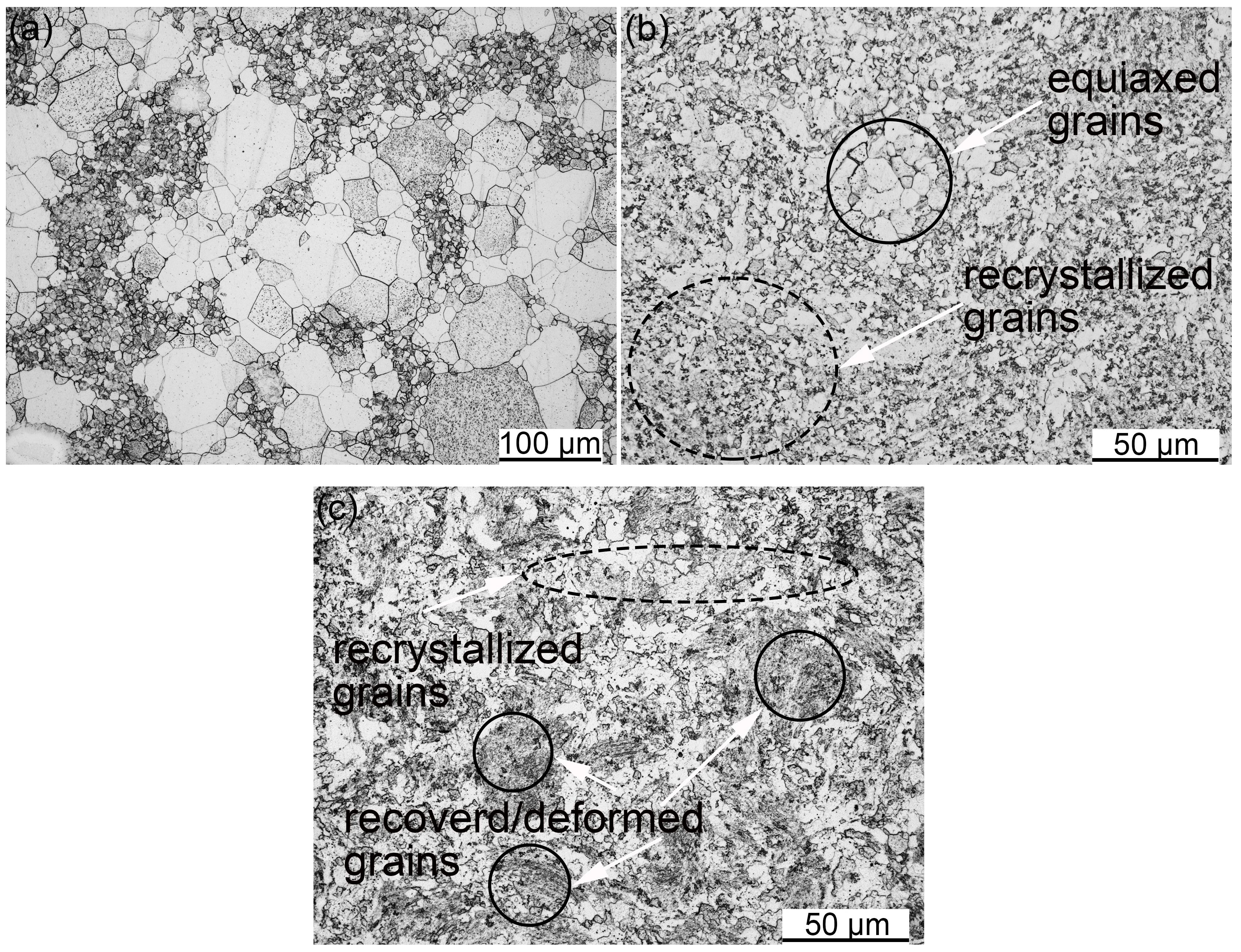
3.2. SPSed Microstructure
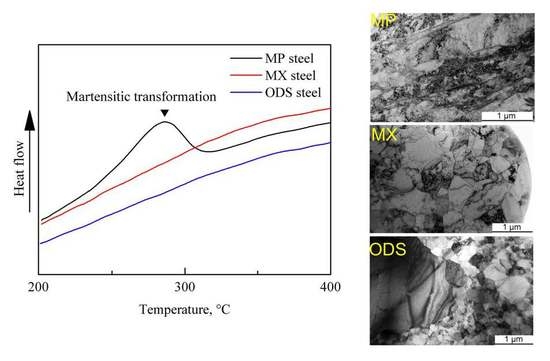
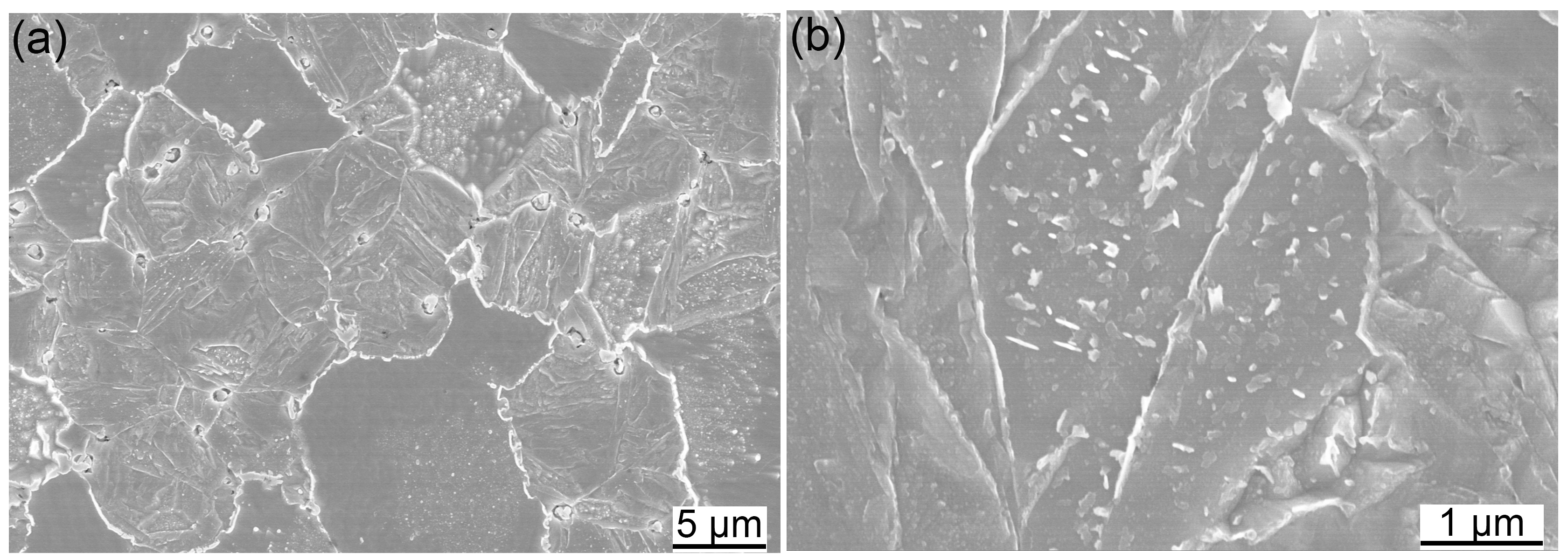
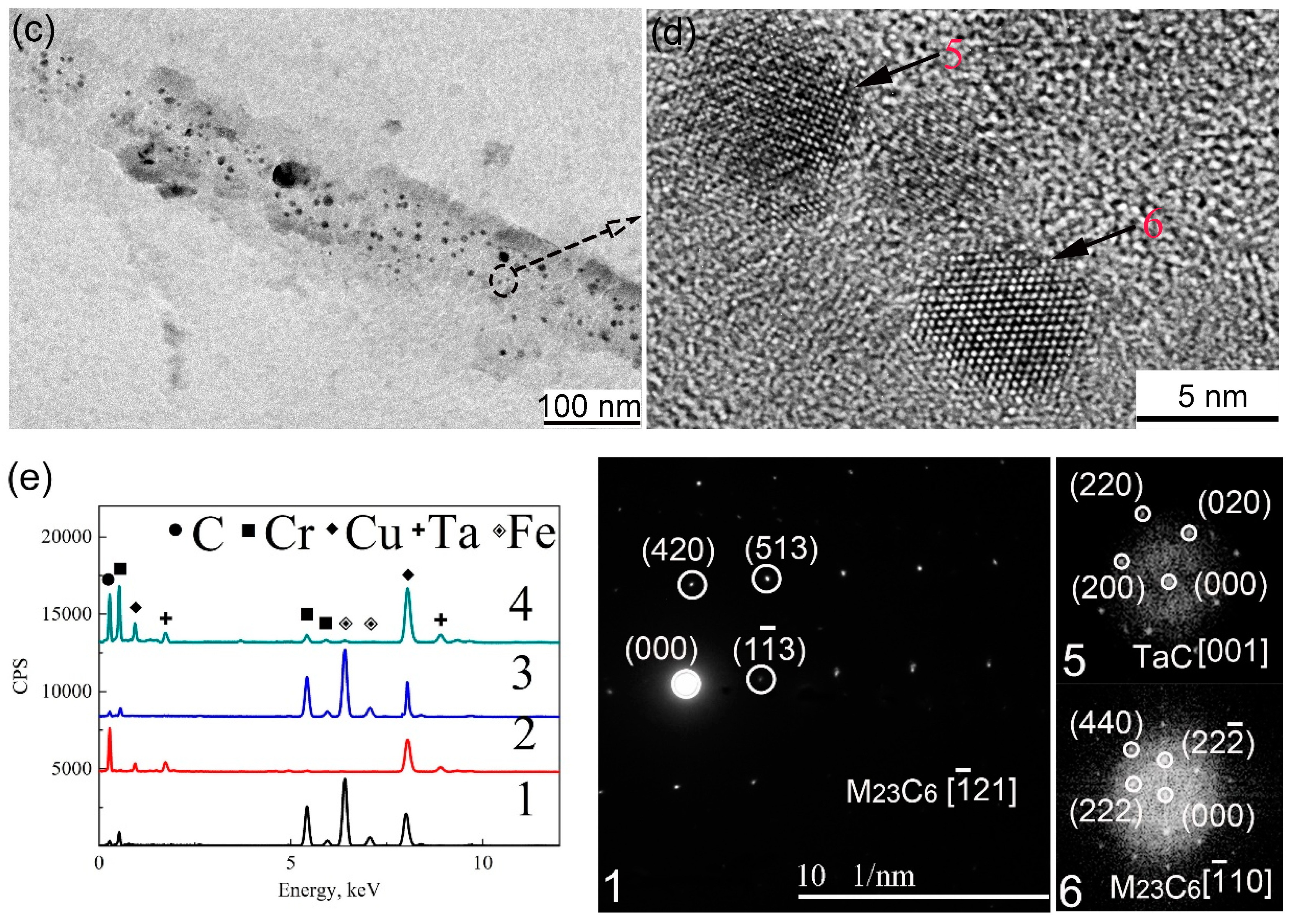
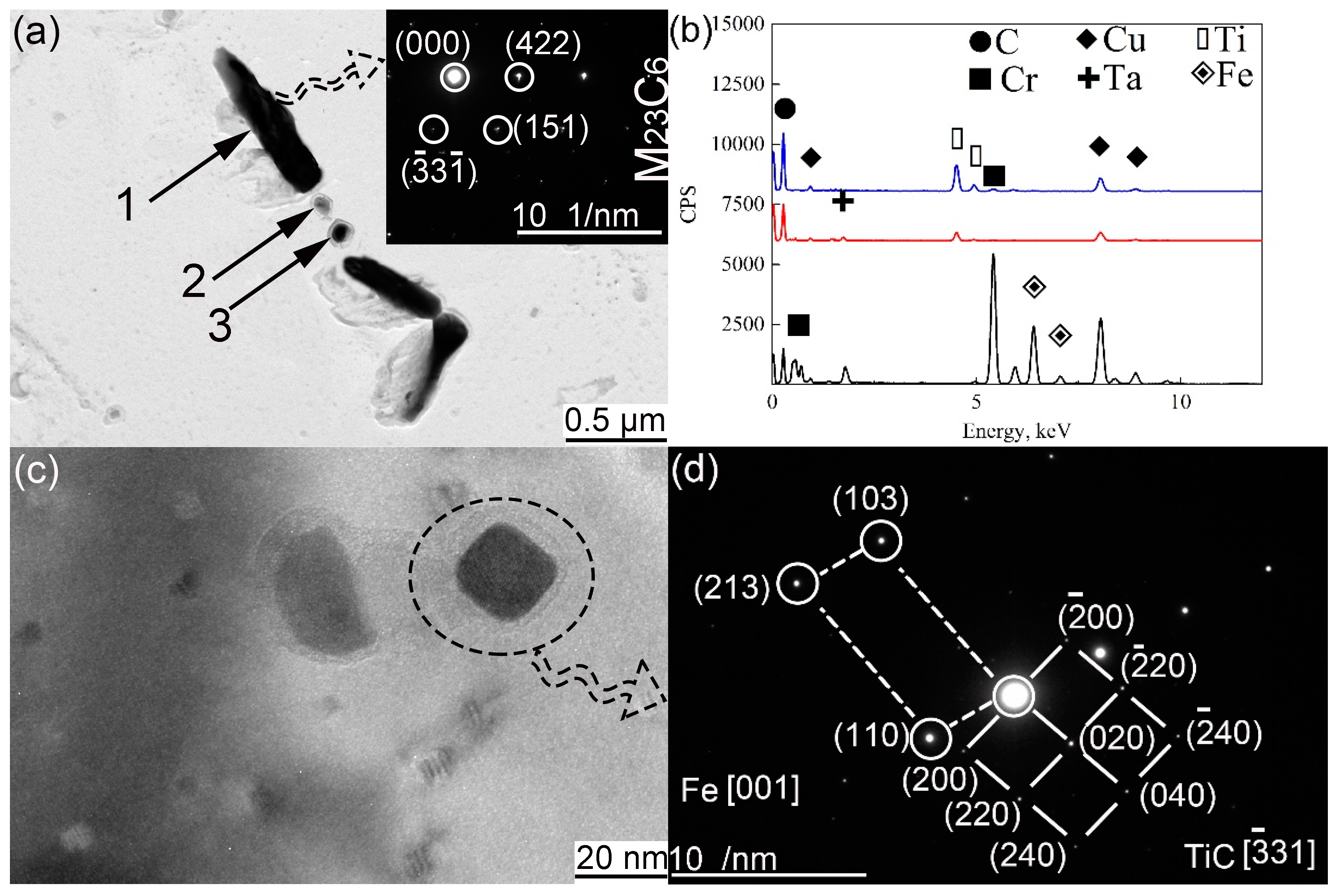
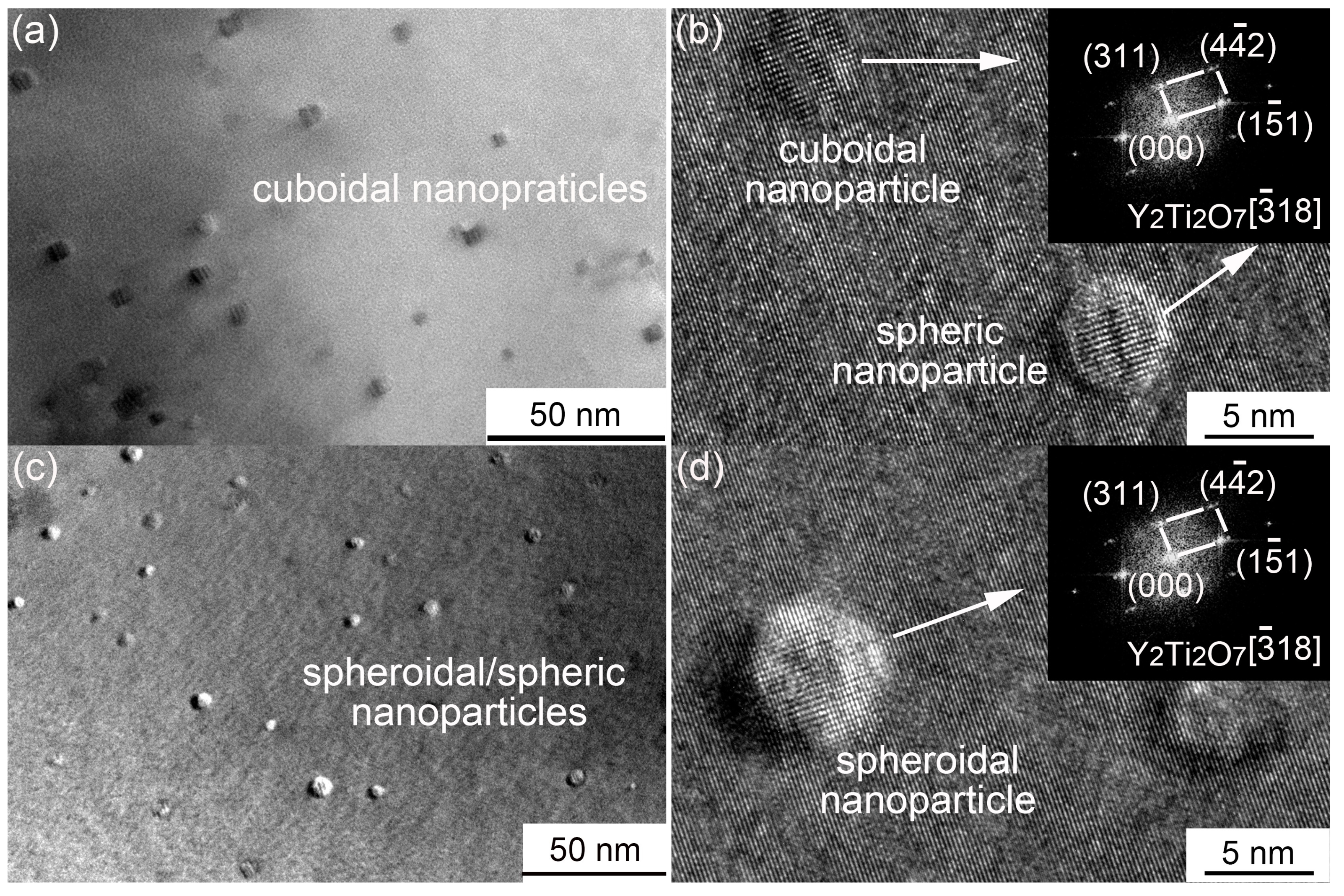
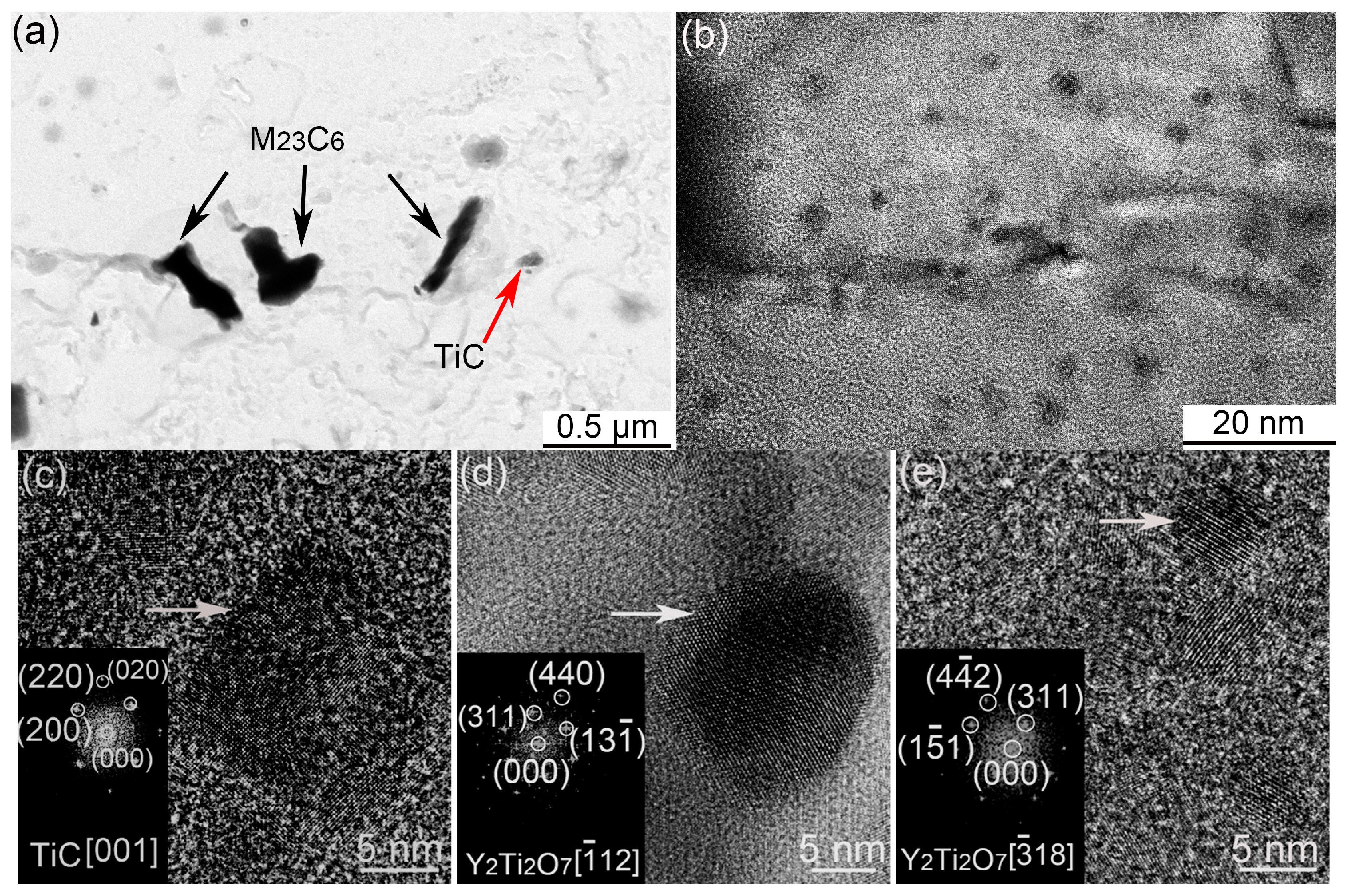
3.3. Secondary Phases
4. Conclusions
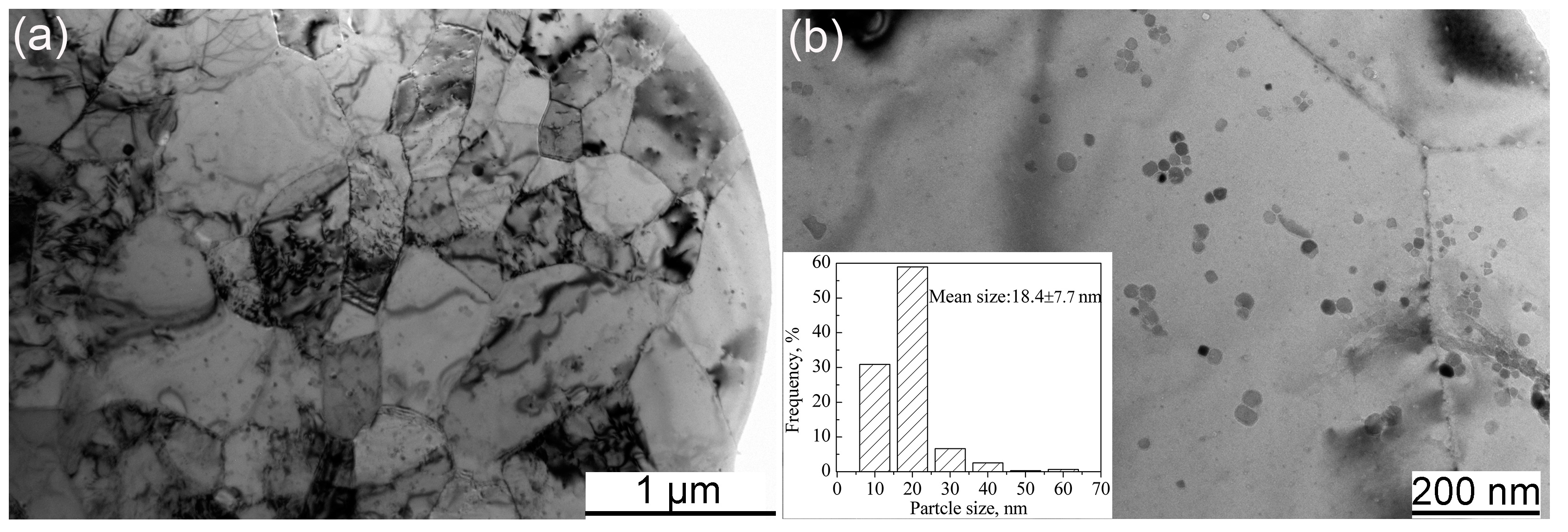
- The particle sizes and morphologies of the MP, MX and ODS powders are different. The excellent refinement effect of ODS powders is attributed to the addition of hard Y2O3 and Ti powders acting as friction material during the mechanical alloying process.
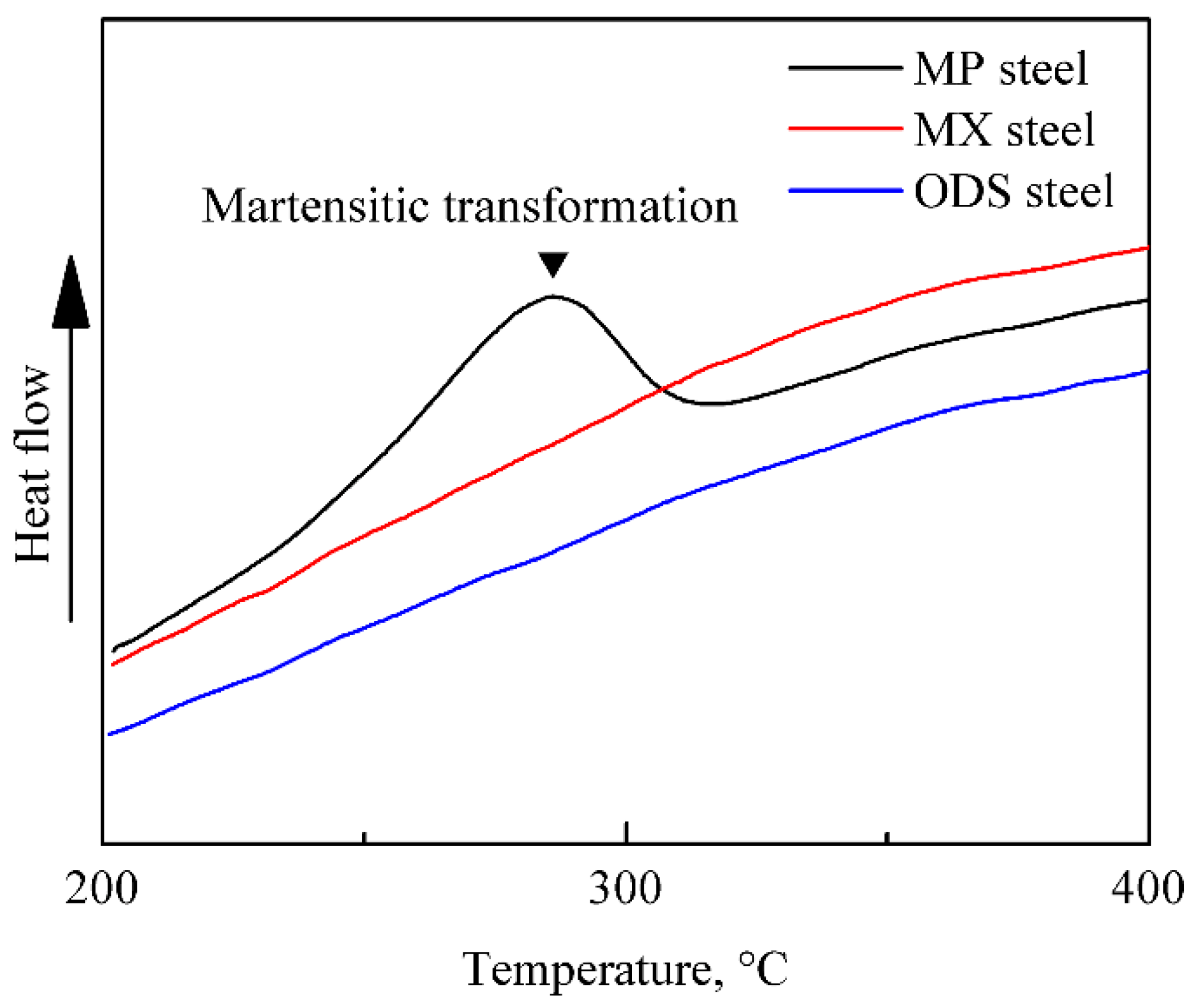
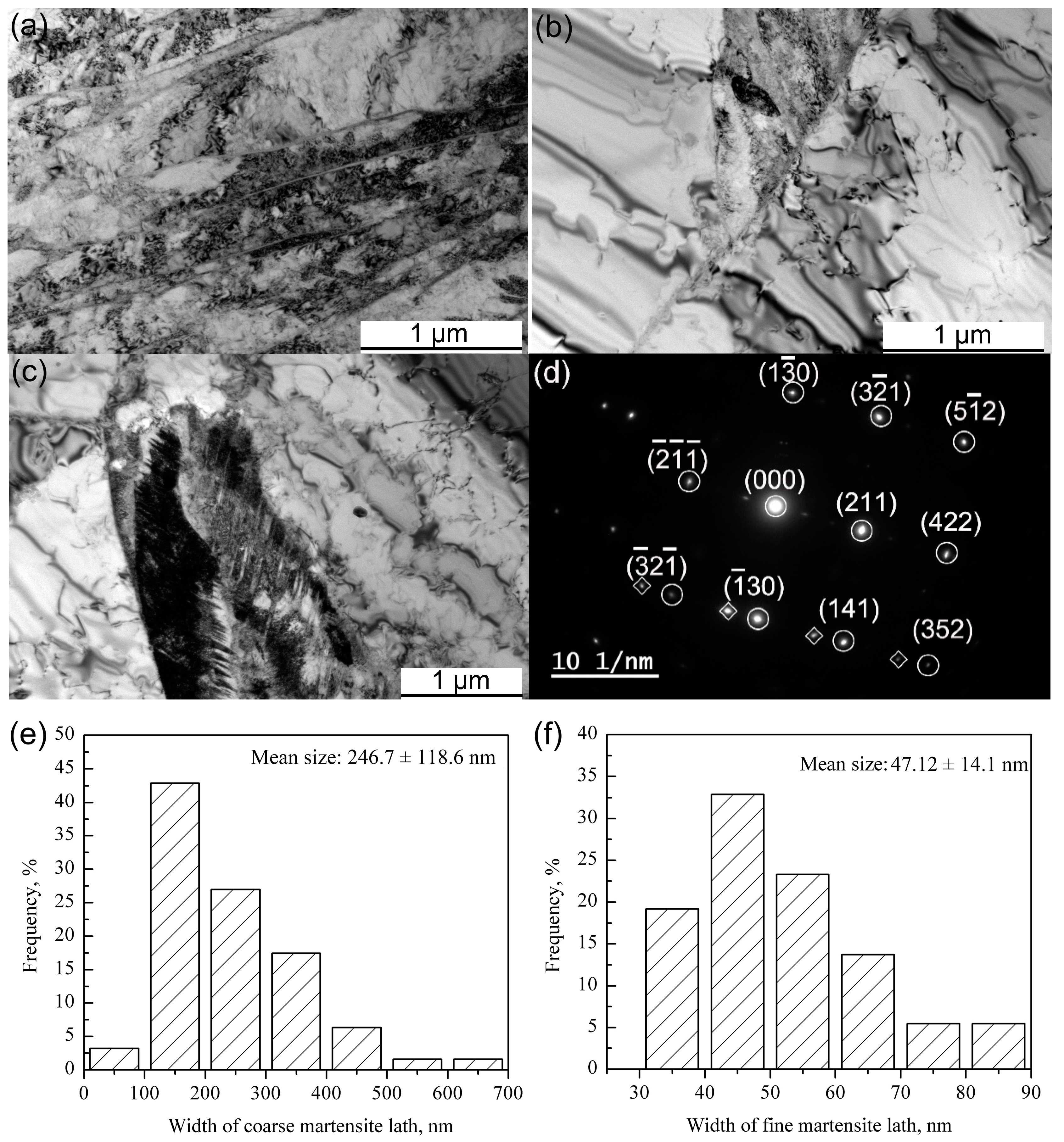
- The generation of martensite lath in the MP steel is due to the large amounts of M23C6, which results in the local Cr depletion.
- In the MX and ODS steel with Ti addition, no martensite transformation occurs during the fabrication process. C prefers to react with Ti since Ti possesses stronger affinity to C than Cr, which effectively hinder the precipitation of M23C6 and inhibit Cr depletion phenomenon. In addition, the long-range diffusion ability and uniform distribution of Ti contributes towards stabilizing the ferrite in the MX steel.
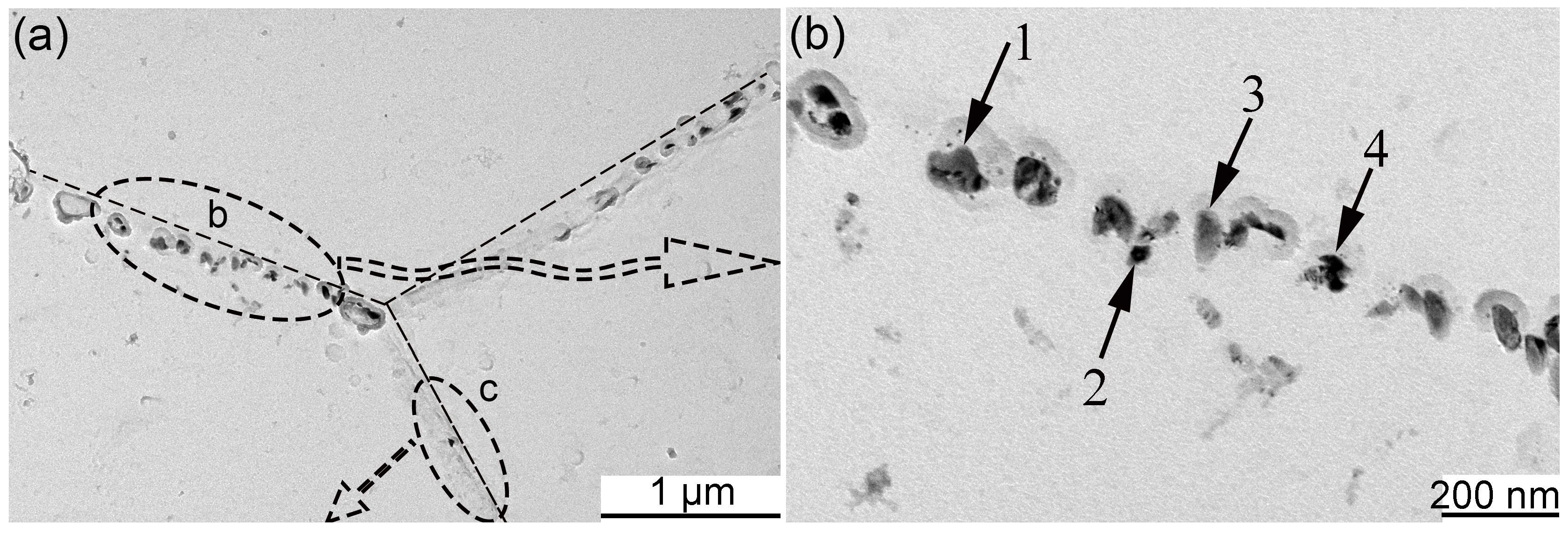
- The diffusion of Y is limited in the matrix, which results in a heterogeneous distribution of Y-Ti-O nanoparticles in the MX steel.
Author Contributions
Funding
Conflicts of Interest
References
- Sornin, D.L.; Karch, A.; Logé, R.E. Competition between intragranular and intergranular deformation mechanisms in ODS ferritic steels during hot deformation at high strain rate. J. Mater. Sci. 2017, 9, 1–11. [Google Scholar] [CrossRef]
- Pimentel, G.; Capdevila, C.; Bartolomé, M.J.; Chao, J.; Serrano, M.; García-Junceda, A.; Campos, M.; Torralba, J.M.; Aldazábal, J. Advanced FeCrAl ODS steels for high-temperature structural applications in energy generation systems. Rev. Metall. 2012, 48, 303–316. [Google Scholar] [CrossRef]
- Sugino, Y.; Ukai, S.; Oono, N.; Hayashi, S.; Kaito, T.; Ohtsuka, S.; Masuda, H.; Taniguchi, S.; Sato, E. High temperature deformation mechanism of 15CrODS ferritic steels at cold-rolled and recrystallized conditions. J. Nucl. Mater. 2015, 466, 653–657. [Google Scholar] [CrossRef]
- Klimenkov, M.; Lindau, R.; Jäntsch, U.; Möslang, A. Effect of irradiation temperature on microstructure of ferritic-martensitic ODS steel. J. Nucl. Mater. 2017, 493, 426–435. [Google Scholar] [CrossRef]
- Robertson, C.; Panigrahi, B.K.; Balaji, S.; Kataria, S.; Serruys, Y.; Mathon, M.H.; Sundar, C.S. Particle stability in model ODS steel irradiated up to 100 dpa at 600 °C: TEM and nano-indentation investigation. J. Nucl. Mater. 2012, 426, 240–246. [Google Scholar] [CrossRef]
- Rogozhkin, S.V.; Aleev, A.A.; Zaluzhnyi, A.G.; Iskanderov, N.A.; Nikitin, A.A.; Vladimirov, P.; Lindau, R.; Möslang, A. Atom probe tomography of nanoscaled features of oxide-dispersion-strengthened ODS Eurofer steel in the initial state and after neutron irradiation. Phys. Met. Metallogr. 2012, 113, 98–105. [Google Scholar] [CrossRef]
- Wharry, J.P.; Swenson, M.J.; Yano, K.H. A review of the irradiation evolution of dispersed oxide nanoparticles in the bcc Fe-Cr system: Current understanding and future directions. J. Nucl. Mater. 2017, 486, 11–20. [Google Scholar] [CrossRef]
- Alinger, M.J.; Odette, G.R.; Hoelzer, D.T. On the role of alloy composition and processing parameters in nanocluster formation and dispersion strengthening in nanostuctured ferritic alloys. Acta Mater. 2009, 57, 392–406. [Google Scholar] [CrossRef]
- Klimiankou, M.; Lindau, R.; Moslang, A. Energy-filtered TEM imaging and EELS study of ODS particles and Argon-filled cavities in ferritic–martensitic steels. Micron 2005, 36, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pasebani, S.; Charit, I. Effect of alloying elements on the microstructure and mechanical properties of nanostructured ferritic steels produced by spark plasma sintering. J. Alloys Compd. 2014, 599, 206–211. [Google Scholar] [CrossRef]
- Ramtani, S.; Dirras, G.; Bui, H.Q. A bimodal bulk ultra-fine-grained nickel: Experimental and micromechanical investigations. Mech. Mater. 2010, 42, 522–536. [Google Scholar] [CrossRef]
- Srinivasarao, B.; Oh-ishi, K.; Ohkubo, T.; Mukai, T.; Hono, K. Synthesis of high-strength bimodally grained iron by mechanical alloying and spark plasma sintering. Scr. Mater. 2008, 58, 759–762. [Google Scholar] [CrossRef]
- Cao, G.H.; Peng, Y.F.; Liu, N.; Li, X.; Lei, Z.S.; Ren, Z.M.; Gerthsen, D.; Russell, A.M. Formation of a bimodal structure in ultrafine Ti–Fe–Nb alloys with high-strength and enhanced ductility. Mater. Sci. Eng. A 2014, 609, 60–64. [Google Scholar] [CrossRef]
- Park, H.W.; Shimojima, K.; Sugiyama, S.; Komine, H.; Yanagimoto, J. Microstructural evolution and formation mechanism of bimodal structure of 0.2% carbon steel subjected to the heavy-reduction controlled rolling process. Mater. Sci. Eng. A 2015, 624, 203–212. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, Z.; Zhou, J. Molecular dynamics simulation of micro-mechanical deformations in polycrystalline copper with bimodal structures. Mater. Lett. 2016, 183, 261–264. [Google Scholar] [CrossRef]
- Zhang, Z.; Orlov, D.; Vajpai, S.K.; Tong, B.; Ameyama, K. Importance of bimodal structure topology in the control of mechanical properties of a stainless steel. Adv. Eng. Mater. 2015, 17, 791–795. [Google Scholar] [CrossRef]
- Zhu, L.; Lu, J. Modelling the plastic deformation of nanostructured metals with bimodal grain size distribution. Int. J. Plast. 2012, 30–31, 166–184. [Google Scholar] [CrossRef]
- Liu, F.; Liu, Y.; Wu, H.; Fang, J.; Zhao, D.; Zhang, L.; Liu, D. Bi-modal microstructure in a powder metallurgical ferritic steel. Trans. Nonferr. Met. Soc. China 2012, 22, 330–334. [Google Scholar] [CrossRef]
- Srinivasarao, B.; Oh-ishi, K.; Ohkubo, T.; Hono, K. Bimodally grained high-strength Fe fabricated by mechanical alloying and spark plasma sintering. Acta Mater. 2009, 57, 3277–3286. [Google Scholar] [CrossRef]
- Auger, M.A.; Castro, V.D.; Leguey, T.; Muñoz, A.; Pareja, R. Microstructure and mechanical behavior of ODS and non-ODS Fe–14Cr model alloys produced by spark plasma sintering. J. Nucl. Mater. 2013, 436, 68–75. [Google Scholar] [CrossRef]
- Boulnat, X.; Perez, M.; Fabregue, D.; Douillard, T.; Mathon, M.H.; Carlan, Y.D. Microstructure evolution in nano-reinforced ferritic steel processed by mechanical alloying and spark plasma sintering. Metall. Mater. Trans. A 2014, 45, 1485–1497. [Google Scholar] [CrossRef]
- Dadé, M.; Malaplate, J.; Garnier, J.; Geuser, F.D.; Barcelo, F.; Wident, P.; Deschamps, A. Influence of microstructural parameters on the mechanical properties of oxide dispersion strengthened Fe-14Cr steels. Acta Mater. 2017, 127, 165–177. [Google Scholar] [CrossRef]
- Mouawad, B.; Boulnat, X.; Fabrègue, D.; Perez, M.; Carlan, Y.D. Tailoring the microstructure and the mechanical properties of ultrafine grained high strength ferritic steels by powder metallurgy. J. Nucl. Mater. 2015, 465, 54–62. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- West, M.K. Processing and Characterization of Oxide Dispersion Strengthened 14YWT Ferritic Alloys. Ph.D. Thesis, University of Tennessee, Knoxville, TN, USA, 2006. [Google Scholar]
- Nagini, M.; Vijay, R.; Ramakrishna, M.; Reddy, A.V.; Sundararajan, G. Influence of the duration of high energy ball milling on the microstructure and mechanical properties of a 9Cr oxide dispersion strengthened ferritic–martensitic steel. Mater. Sci. Eng. A 2015, 620, 490–499. [Google Scholar] [CrossRef]
- Brocq, M.; Radiguet, B.; Breton, J.M.; Cuvilly, F.; Pareige, P.; Legendre, F. Nanoscale characterisation and clustering mechanism in an Fe–Y2O3 model ODS alloy processed by reactive ball milling and annealing. Acta Mater. 2010, 58, 1806–1814. [Google Scholar] [CrossRef]
- Magini, M.; Iasonna, A.; Padella, F. Ball milling: An experimental support to the energy transfer evaluated by the collision model. Scr. Mater. 1996, 34, 13–19. [Google Scholar] [CrossRef]
- Zhang, D.L. Processing of advanced materials using high-energy mechanical milling. Prog. Mater. Sci. 2004, 49, 537–560. [Google Scholar] [CrossRef]
- Ustinovshikov, Y.; Pushkarev, B.; Igumnov, I. Fe-rich portion of the Fe-Cr phase diagram: Electron microscopy study. J. Mater. Sci. 2002, 37, 2031–2042. [Google Scholar] [CrossRef]
- Oksiuta, Z.; Olier, P.; Carlan, Y.D.; Baluc, N. Development and characterisation of a new ODS ferritic steel for fusion reactor application. J. Nucl. Mater. 2009, 393, 114–119. [Google Scholar] [CrossRef]
- Olier, P.; Couvrat, M.; Cayron, C.; Lochet, N.; Chaffron, L. Incidence of mechanical alloying contamination on oxides and carbides formation in ODS ferritic steels. J. Nucl. Mater. 2013, 442, S106–S111. [Google Scholar] [CrossRef]
- Olier, P.; Malaplate, J.; Mathon, M.H.; Nunes, D.; Hamon, D.; Toualbi, L.; Carlan, Y.D.; Chaffron, L. Chemical and microstructural evolution on ODS Fe–14CrWTi steel during manufacturing stages. J. Nucl. Mater. 2012, 428, 40–46. [Google Scholar] [CrossRef]
- London, A.J.; Santra, S.; Amirthapandian, S.; Panigrahi, B.K.; Sarguna, R.M.; Balaji, S.; Vijay, R.; Sundar, C.S.; Lozano-Perez, S.; Grovenor, C.R.M. Effect of Ti and Cr on dispersion, structure and composition of oxide nano-particles in model ODS alloys. Acta Mater. 2015, 97, 223–233. [Google Scholar] [CrossRef]
- Sakasegawa, H.; Chaffron, L.; Legendre, F.; Boulanger, L.; Cozzika, T.; Brocq, M.; Carlan, Y.D. Correlation between chemical composition and size of very small oxide particles in the MA957 ODS ferritic alloy. J. Nucl. Mater. 2009, 384, 115–118. [Google Scholar] [CrossRef]
- Tamura, M.; Sakasegawa, H.; Shiba, K.; Tanigawa, H.; Shinozuka, K.; Esaka, H. Decomposition of Y2Ti2O7 Particles in 8 Pct Cr Oxide-Dispersion-Strengthened Martensitic Steel during Tempering. Metall. Mater. Trans. A 2011, 42, 2176–2188. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, Y.; Ning, H.; Williams, C.A.; London, A.J.; Dawson, K.; Hong, Z.; Gorley, M.J.; Grovenor, C.R.M.; Tatlock, G.J.; et al. Processing and microstructure characterisation of oxide dispersion strengthened Fe–14Cr–0.4Ti–0.25Y2O3 ferritic steels fabricated by spark plasma sintering. J. Nucl. Mater. 2015, 464, 61–68. [Google Scholar] [CrossRef]
- Zhong, S.Y.; Ribis, J.; Lochet, N.; Carlan, Y.D.; Klosek, V.; Ji, V.; Mathon, M.H. The Effect of Y/Ti Ratio on Oxide Precipitate Evolution in ODS Fe-14 Wt Pct Cr Alloys. Metall. Mater. Trans. A 2015, 46, 1413–1418. [Google Scholar] [CrossRef]
- Zhao, Q.; Yu, L.; Liu, Y.; Huang, Y.; Ma, Z.; Li, H.; Wu, J. Microstructure and tensile properties of a 14Cr ODS ferritic steel. Mater. Sci. Eng. A 2017, 680, 347–350. [Google Scholar] [CrossRef]
- Kim, J.K.; Lee, B.J.; Lee, B.H.; Kim, Y.H.; Kim, K.Y. Intergranular segregation of Cr in Ti-stabilized low-Cr ferritic stainless steel. Scr. Mater. 2009, 61, 1133–1136. [Google Scholar] [CrossRef]
- Wang, S.B.; Liu, J.Z.; Qi, Q.; Chang, X.F.; Gao, S.; Zhang, W.; Wang, P. Precipitate formations with self-adaptive elemental diffusion and segregation in T92 steel. J. Alloys Compd. 2017, 693, 264–278. [Google Scholar] [CrossRef]
- Zhu, J.; Yan, X.; Liu, Y. Lath martensite in 1.4% C ultra-high carbon steel and its grain size effect. Mater. Sci. Eng. A 2004, 385, 440–444. [Google Scholar]
- Krauss, G.; Marder, A.R. The morphology of martensite in iron alloys. Metall. Trans. 1971, 2, 2343–2357. [Google Scholar] [CrossRef]
- Inoue, A.; Tomioka, H.; Masumoto, T. Microstructure and mechanical properties of metastable lath martensite wires in the Fe-Ni-Cr-Al-C system produced by melt spinning in rotating water. J. Mater. Sci. 1985, 20, 2603–2613. [Google Scholar] [CrossRef]
- Morito, S.; Saito, H.; Ogawa, T.; Furuhara, T.; Maki, T. Effect of austenite grain size on the morphology and crystallography of lath martensite in low carbon steels. ISIJ Int. 2005, 45, 91–94. [Google Scholar] [CrossRef]
- Fu, C.L.; Krcmar, M.; Painter, G.S.; Chen, X.Q. Vacancy mechanism of high oxygen solubility and nucleation of stable oxygen-enriched clusters in Fe. Phys. Rev. Lett. 2007, 99, 225502. [Google Scholar] [CrossRef] [PubMed]
- Murali, D.; Panigrahi, B.K.; Valsakumar, M.C.; Chandra, S.; Sundar, C.S.; Raj, B. The role of minor alloying elements on the stability and dispersion of yttria nanoclusters in nanostructured ferritic alloys: An ab initio study. J. Nucl. Mater. 2010, 403, 113–116. [Google Scholar] [CrossRef]
- Peng, T.; Zhang, C.; Yang, Z.G.; Hiroyuki, T. Evolution and coarsening of carbides in 2. 25Cr-1Mo steel weld metal during high temperature tempering. J. Iron Steel Res. Int. 2010, 17, 74–78. [Google Scholar]
- Zhang, C.; Cui, L.; Liu, Y.; Liu, C.; Li, H. Microstructures and mechanical properties of friction stir welds on 9% Cr reduced activation ferritic/martensitic steel. J. Mater. Sci. Technol. 2018, 34, 756–766. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Q.; Ma, Z.; Yu, L.; Li, H.; Wang, Z.; Liu, Y. Inhibition Effect of Ti on the Formation of Martensite Lath in 14Cr Oxide Dispersion Strengthened Steel. Metals 2018, 8, 802. https://doi.org/10.3390/met8100802
Zhao Q, Ma Z, Yu L, Li H, Wang Z, Liu Y. Inhibition Effect of Ti on the Formation of Martensite Lath in 14Cr Oxide Dispersion Strengthened Steel. Metals. 2018; 8(10):802. https://doi.org/10.3390/met8100802
Chicago/Turabian StyleZhao, Qian, Zongqing Ma, Liming Yu, Huijun Li, Zumin Wang, and Yongchang Liu. 2018. "Inhibition Effect of Ti on the Formation of Martensite Lath in 14Cr Oxide Dispersion Strengthened Steel" Metals 8, no. 10: 802. https://doi.org/10.3390/met8100802
APA StyleZhao, Q., Ma, Z., Yu, L., Li, H., Wang, Z., & Liu, Y. (2018). Inhibition Effect of Ti on the Formation of Martensite Lath in 14Cr Oxide Dispersion Strengthened Steel. Metals, 8(10), 802. https://doi.org/10.3390/met8100802