Study on Interface Structure of Cu/Al Clad Plates by Roll Casting
Abstract
1. Introduction
2. Experimental Materials and Method
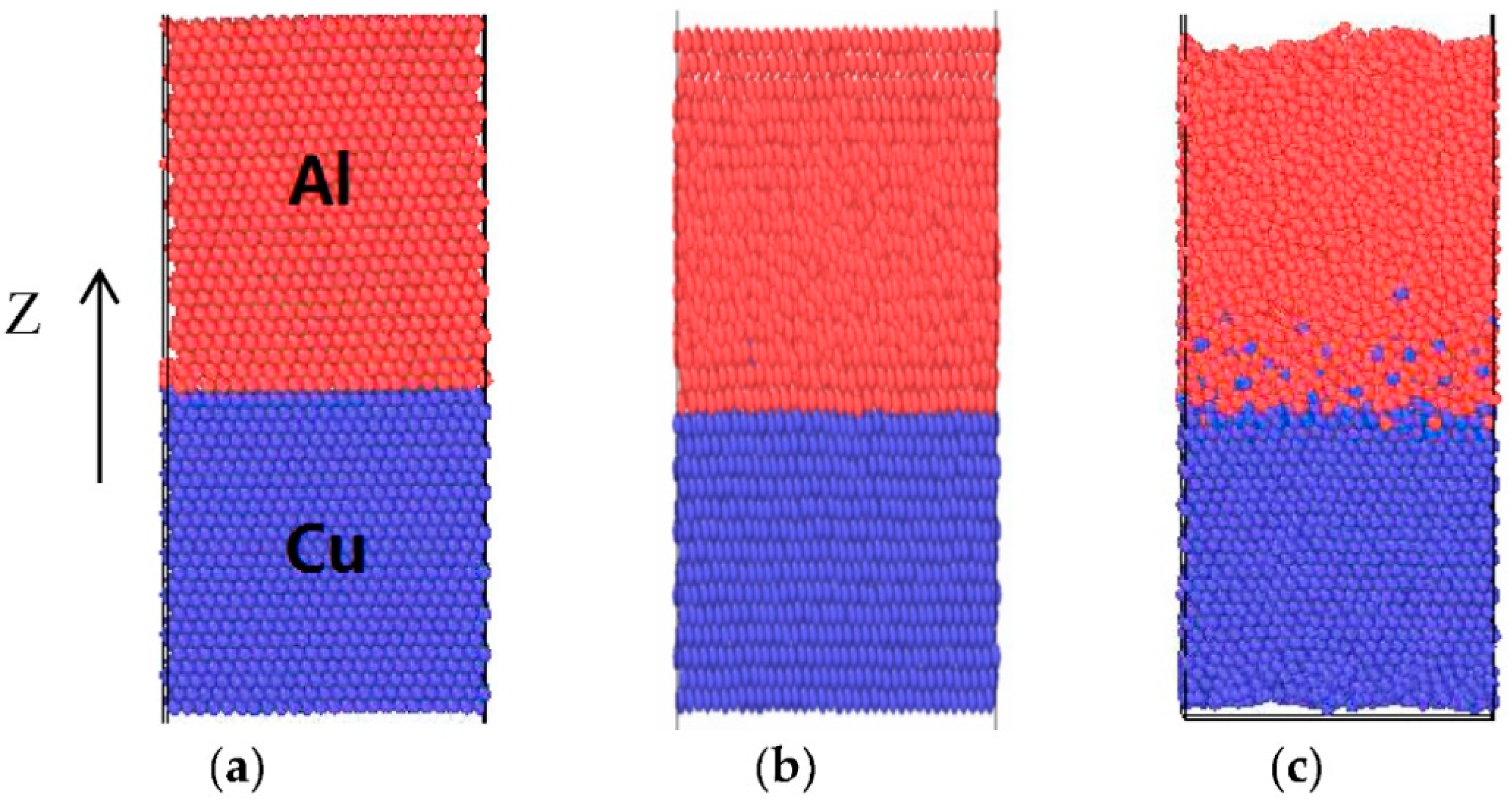
2.1. Molecular Dynamics Simulation of the Structure Evolution in the Semi-Molten Roll Casting Interface of Cu/Al Composite Panels
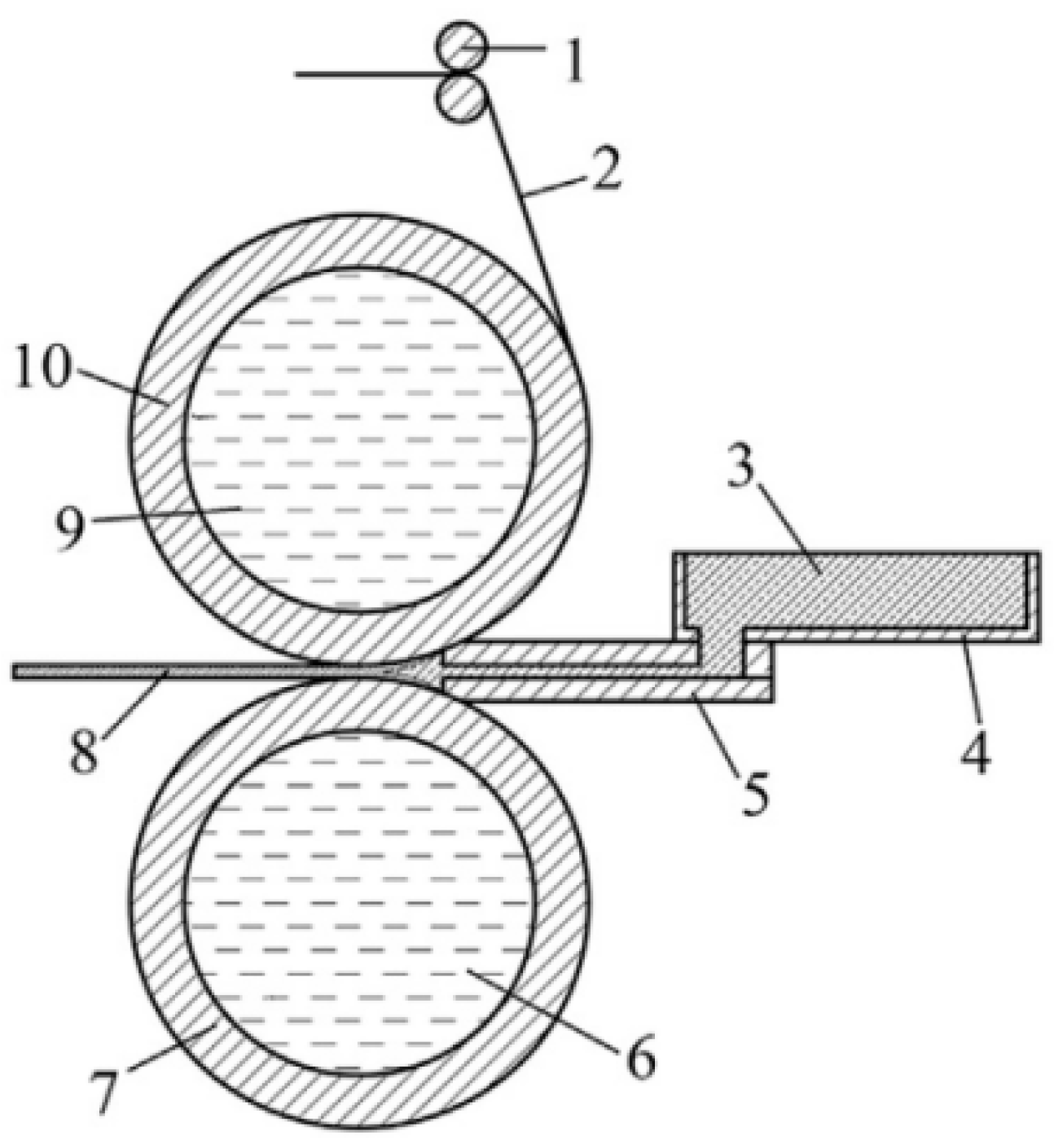
2.2. Overview of the Solid-Liquid Roll Casting Composite Process
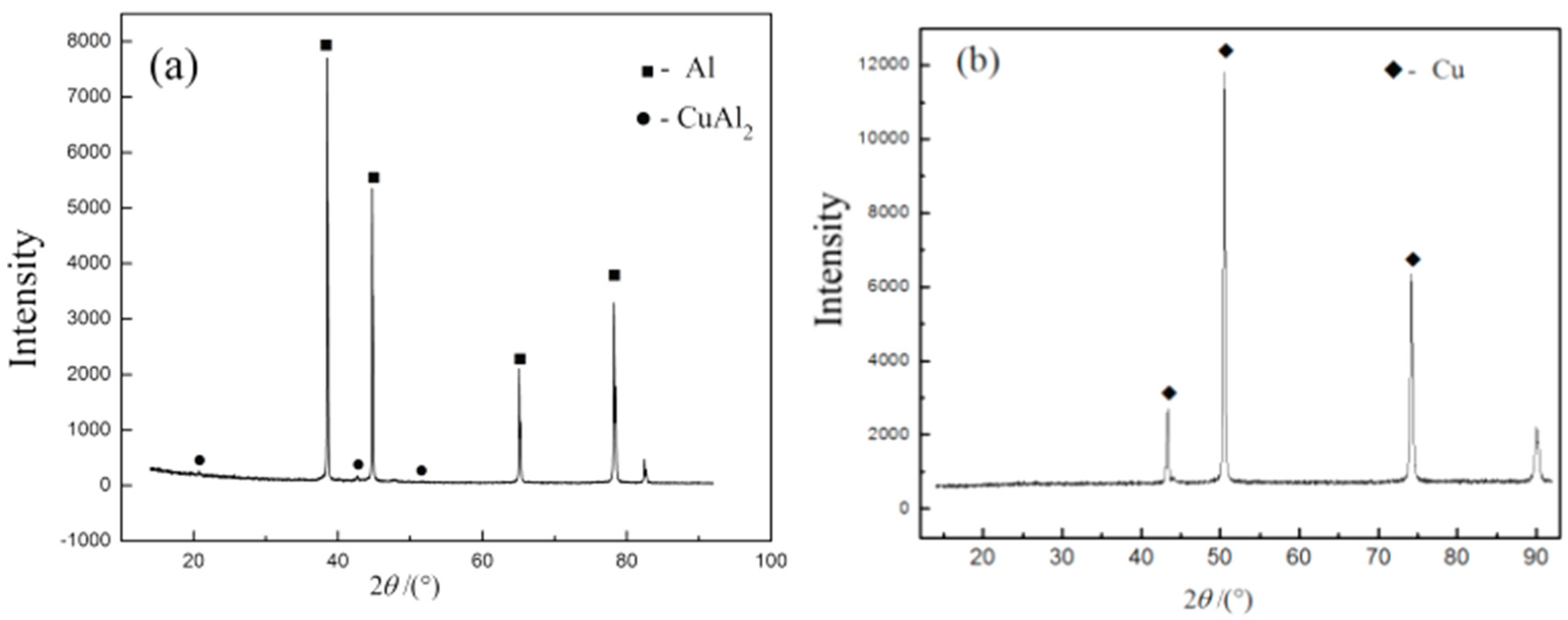
2.2.1. Analysis of Interface Structure and Chemical Composition
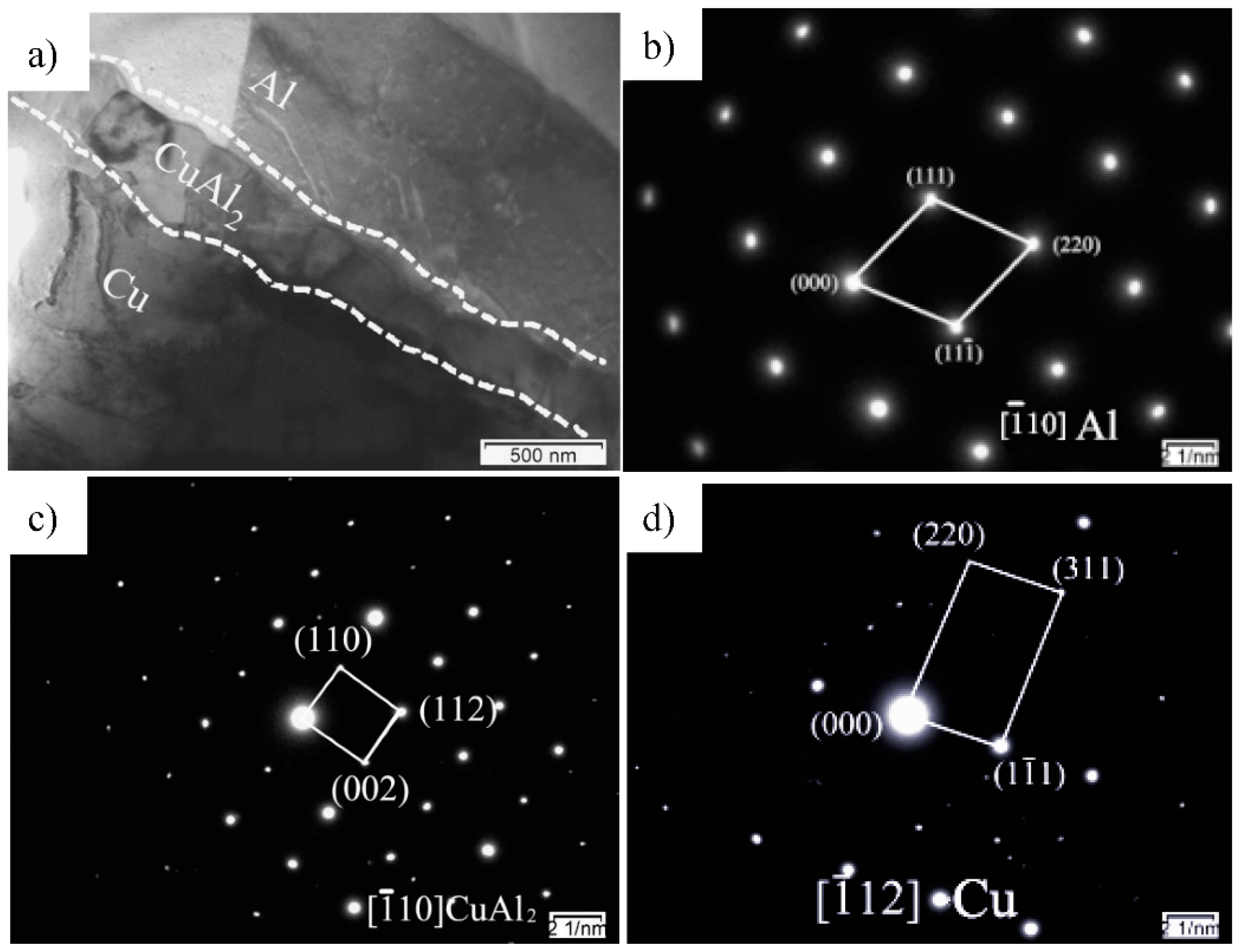
2.2.2. Analysis of TEM Images
3. Conclusions
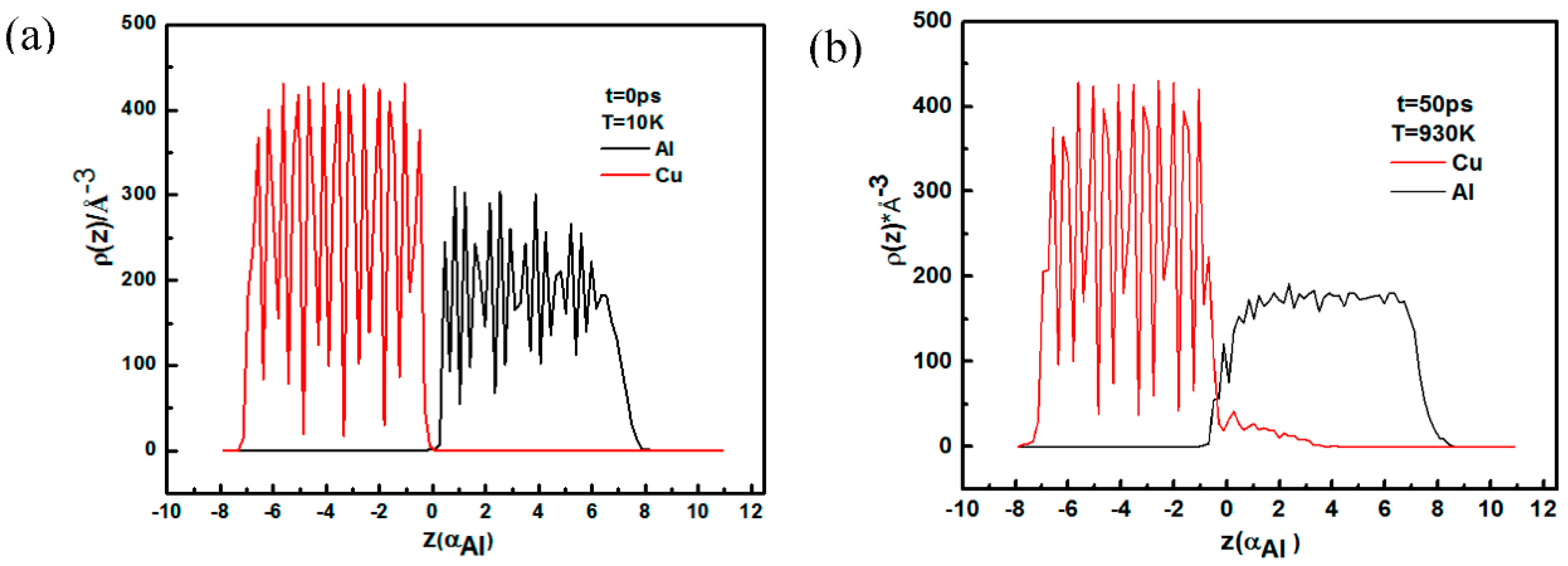
- The results of LAMMPS molecular dynamics simulation show that Cu atoms are arranged orderly, while Al lattice structures are destroyed at high temperature. Additionally, the diffusion rate of Cu atoms is higher than that of Al atoms. Cu atoms at the interface transfer into the Al matrix.
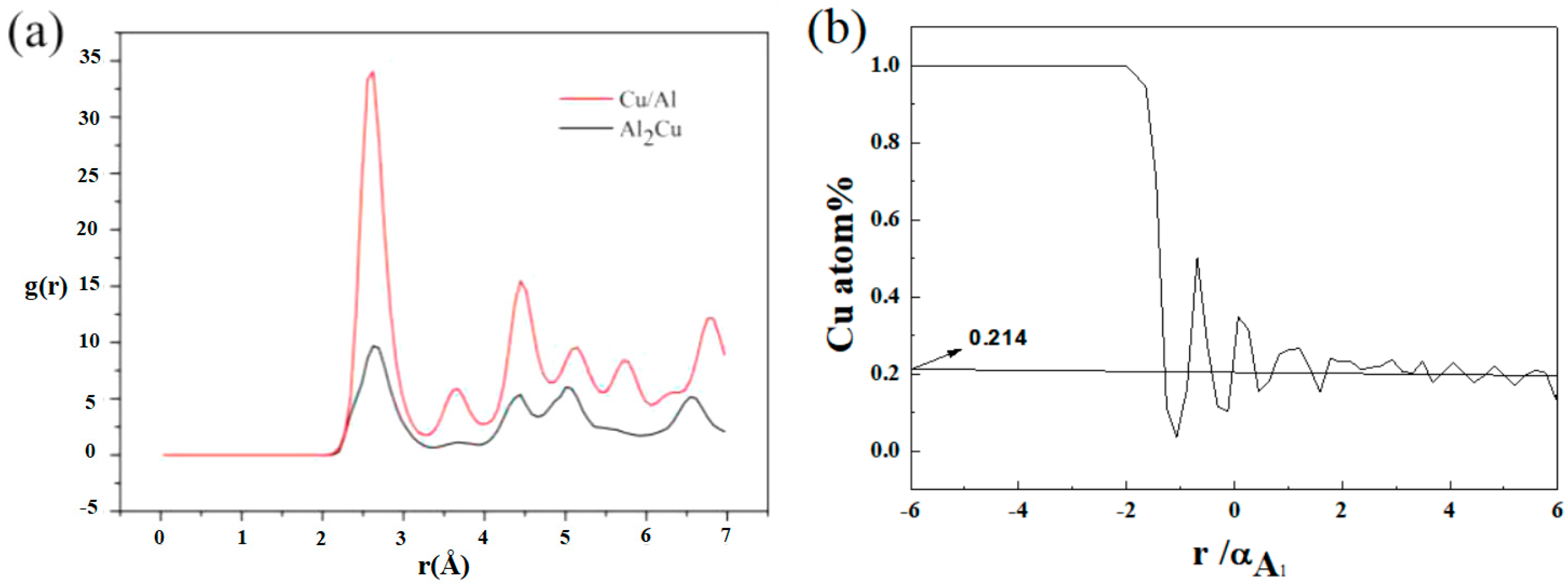
- When T = 930K, t = 50 ps, the diffusion distance of Cu atoms in the Al layer is nearly four times the Al lattice constants, while for Al atoms, the distance is just half a lattice constant. The radial distribution function shows that CuAl2 compound will be formed at the interface when the temperature is 300 K.
- The TEM results show that the thickness of compound at the interface is about 0.35 μm, and there is only one compound phase tetragonal CuAl2 formed, according to the electron diffraction patterns of experimental interface. The results of experiments are same as LAMMPS simulation. That is to say, the parameters of simulation can be used in industrialization.
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Wadsworth, J.; Lesuer, D.R. Ancient and modern laminated composites—From the Great Pyramid of Gizeh to Y2K. Mater. Charact. 2000, 45, 289–313. [Google Scholar] [CrossRef]
- Lacaze, J.; Tierce, S.; Lafont, M.C.; Thebault, Y.; Pébère, N.; Mankowski, G.; Blanc, C.; Robidou, H.; Vanumousse, D.; Daloz, D. Study of the microstructure resulting from brazed aluminium materials used in heat exchangers. Mater. Sci. Eng. A 2005, 413, 317–321. [Google Scholar] [CrossRef]
- Foley, J.; Clauberg, H.; Chylak, B. Enabling high volume fine pitch copper wire bonding: Enhancements to process and equipment capability. In Proceedings of the 2010 3rd Electronic System-Integration Technology Conference (ESTC), Berlin, Germany, 13–16 September 2010; pp. 1–4. [Google Scholar] [CrossRef]
- Lee, T.H.; Lee, Y.J.; Park, K.T.; Jeong, H.G.; Lee, J.H. Mechanical and asymmetrical thermal properties of Al/Cu composite fabricated by repeated hydrostatic extrusion process. Met. Mater. Int. 2015, 21, 402–407. [Google Scholar] [CrossRef]
- Mahdavian, M.M.; Khatami-Hamedani, H.; Abedi, H.R. Macrostructure evolution and mechanical properties of accumulative roll bonded Al/Cu/Sn multilayer composite. J. Alloys Compd. 2017, 703, 605–613. [Google Scholar] [CrossRef]
- Watanabe, T.; Yoneda, A.; Yanagisawa, A.; Konuma, S.; Ohashi, O. Ultrasonic Welding of Al-Cu and Al-SUS304. Q. J. Jpn. Weld. Soc. 2009, 17, 223–233. [Google Scholar] [CrossRef]
- Sun, X.J.; Tao, J.; Guo, X.Z. Bonding properties of interface in Fe/Al clad tube prepared by explosive welding. Trans. Nonferrous Met. Soc. China 2011, 21, 2175–2180. [Google Scholar] [CrossRef]
- Rhee, K.Y.; Han, W.Y.; Park, H.J.; Kim, S.S. Fabrication of aluminum/copper clad composite using hot hydrostatic extrusion process and its material characteristics. Mater. Sci. Eng. A 2004, 384, 70–76. [Google Scholar] [CrossRef]
- Xu, H.; Liu, C.; Silberschmidt, V.V.; Pramana, S.S.; White, T.J.; Chen, Z.; Acoff, V.L. Behavior of aluminum oxide, intermetallics and voids in Cu–Al wire bonds. Acta Mater. 2011, 59, 5661–5673. [Google Scholar] [CrossRef]
- Lee, K.S.; Ha, T.K.; Ahn, S.; Chang, Y.W. High temperature deformation behavior of the Zr 41.2 Ti 13.8 Cu 12.5 Ni 10 Be 22.5, bulk metallic glass. J Non-Cryst. Solids 2003, 317, 193–199. [Google Scholar] [CrossRef]
- Li, X.B.; Zu, G.Y.; Wang, P. Microstructural development and its effects on mechanical properties of Al/Cu laminated composite. Trans. Nonferrous Met. Soc. China 2015, 25, 36–45. [Google Scholar] [CrossRef]
- Chen, G.; Li, J.T.; Yu, H.L.; Su, L.H.; Xu, G.M.; Pan, J.S.; You, T.; Zhang, G.; Sun, K.M.; He, L.Z. Investigation on bonding strength of steel/aluminum clad sheet processed by horizontal twin-roll casting, annealing and cold rolling. Mater. Des. 2016, 112, 263–274. [Google Scholar] [CrossRef]
- Majure, D.L.; Haskins, R.W.; Lee, N.J.; Ebeling, R.M.; Maier, R.S.; Marsh, C.P.; Bednar, A.J.; Kirgan, R.A.; Welch, C.R.; Cornwell Charles, F. Large-Scale Atomic/Molecular Massively Parallel Simulator (LAMMPS) Simulations of the Effects of Chirality and Diameter on the Pullout Force in a Carbon Nanotube Bundle. In Proceedings of the DoD HPCMP Users Group Conference, Seattle, WA, USA, 14–17 July 2008; IEEE: Piscataway, NJ, USA, 2009; pp. 201–207. [Google Scholar] [CrossRef]
- Ramakrishnan, R.; Phanikumar, G.; Sankarasubramanian, R. Crystal–Melt Interface Growth Velocity of Ni–Zr Alloys Through Molecular Dynamics Simulations. Trans. Indian Inst. Met. 2015, 68, 1113–1117. [Google Scholar] [CrossRef]
- Geysermans, P.; Pontikis, V. The structure of the solid-liquid interface: Atomic size effect. J. Phys. IV 2002, 12, 239–248. [Google Scholar] [CrossRef]
- Kim, S.P.; Chung, Y.C.; Lee, S.C.; Lee, K.R.; Lee, K.H. Surface alloy formation of Co on Al surface: Molecular dynamics simulation. J. Appl. Phys. 2003, 93, 8564–8566. [Google Scholar] [CrossRef]
- Chen, C.Y.; Chen, H.L.; Hwang, W.S. Influence of Interfacial Structure Development on the Fracture Mechanism and Bond Strength of Aluminum/Copper Bimetal Plate. Mater Trans. 2006, 47, 1232–1239. [Google Scholar] [CrossRef]
- Lim, A.B.; Long, X.; Shen, L.; Chen, X.; Ramanujan, R.V.; Gan, C.L.; Chen, Z. Effect of palladium on the mechanical properties of Cu–Al intermetallic compounds. J. Alloys Compd. 2015, 628, 107–112. [Google Scholar] [CrossRef]
- Zhu, D.; Li, C.; Guo, C.; Du, Z. Thermodynamic assessment of the Ni–Sc binary system. Calphad 2015, 48, 106–112. [Google Scholar] [CrossRef]
- Kawakami, H.; Suzuki, J.; Nakajima, J. Bonding process of Al/Cu dissimilar bonding with liquefaction in air. Weld. Int. 2007, 21, 836–843. [Google Scholar] [CrossRef]
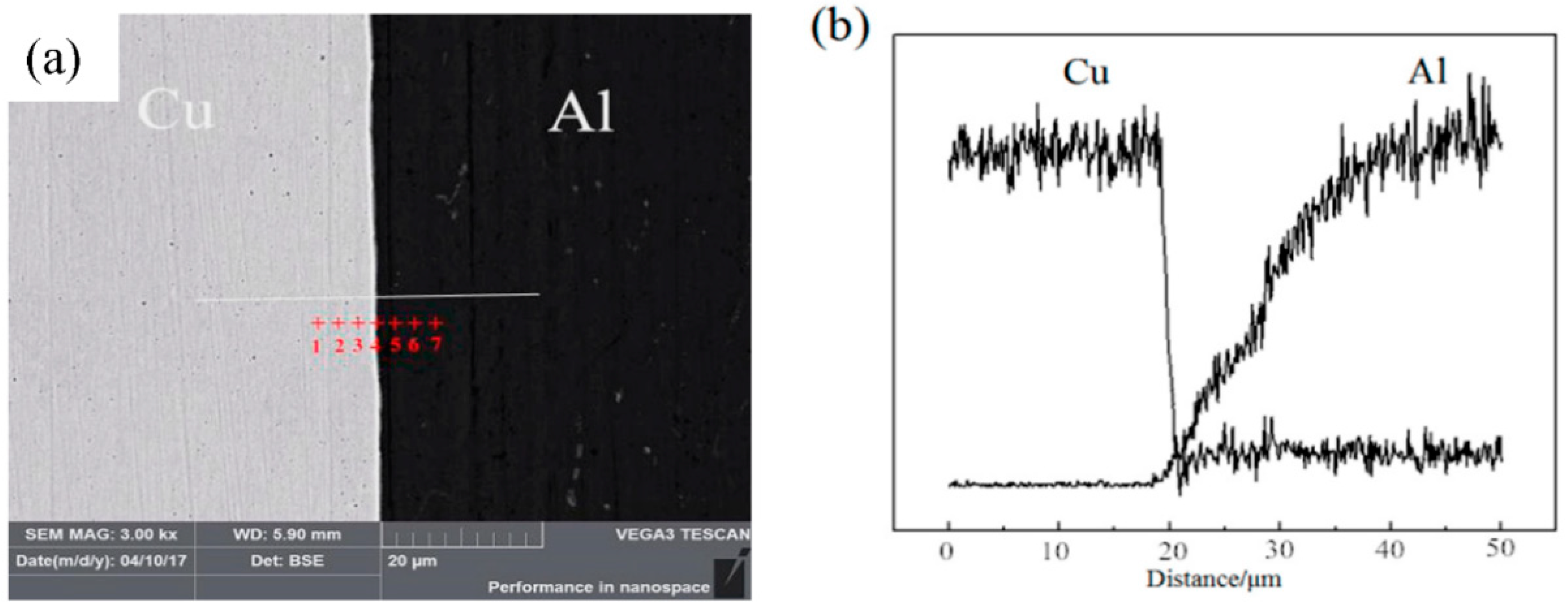
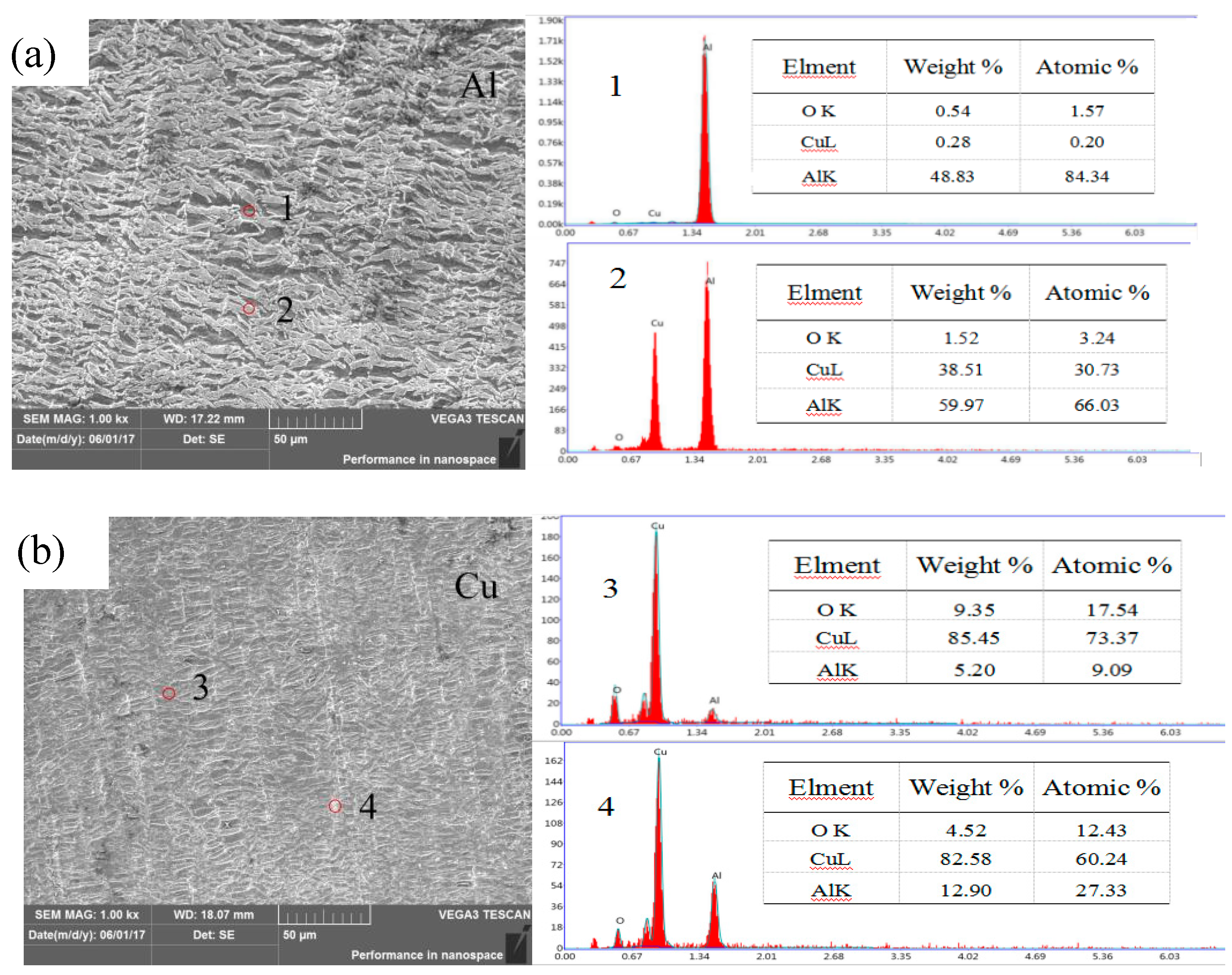
| Points | Cu (At%) | Al (At%) | Al (At%) | Phase |
|---|---|---|---|---|
| 1 | 98.81 | 1.19 | Cu Matrix | |
| 2 | 97.38 | 2.42 | Cu Matrix | |
| 3 | 68.76 | 32.14 | Cu and CuAl2 | |
| 4 | 43.57 | 56.43 | CuAl2 | |
| 5 | 11.13 | 88.87 | CuAl2 and Al | |
| 6 | 4.36 | 95.64 | Al Matrix | |
| 7 | 2.37 | 97.63 | Al Matrix |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Q.; Xie, J.; Mao, A.; Wang, W. Study on Interface Structure of Cu/Al Clad Plates by Roll Casting. Metals 2018, 8, 770. https://doi.org/10.3390/met8100770
Chang Q, Xie J, Mao A, Wang W. Study on Interface Structure of Cu/Al Clad Plates by Roll Casting. Metals. 2018; 8(10):770. https://doi.org/10.3390/met8100770
Chicago/Turabian StyleChang, Qinghua, Jingpei Xie, Aixia Mao, and Wenyan Wang. 2018. "Study on Interface Structure of Cu/Al Clad Plates by Roll Casting" Metals 8, no. 10: 770. https://doi.org/10.3390/met8100770
APA StyleChang, Q., Xie, J., Mao, A., & Wang, W. (2018). Study on Interface Structure of Cu/Al Clad Plates by Roll Casting. Metals, 8(10), 770. https://doi.org/10.3390/met8100770




