Sustainable Steel Carburization by Using Snack Packaging Plastic Waste as Carbon Resources
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
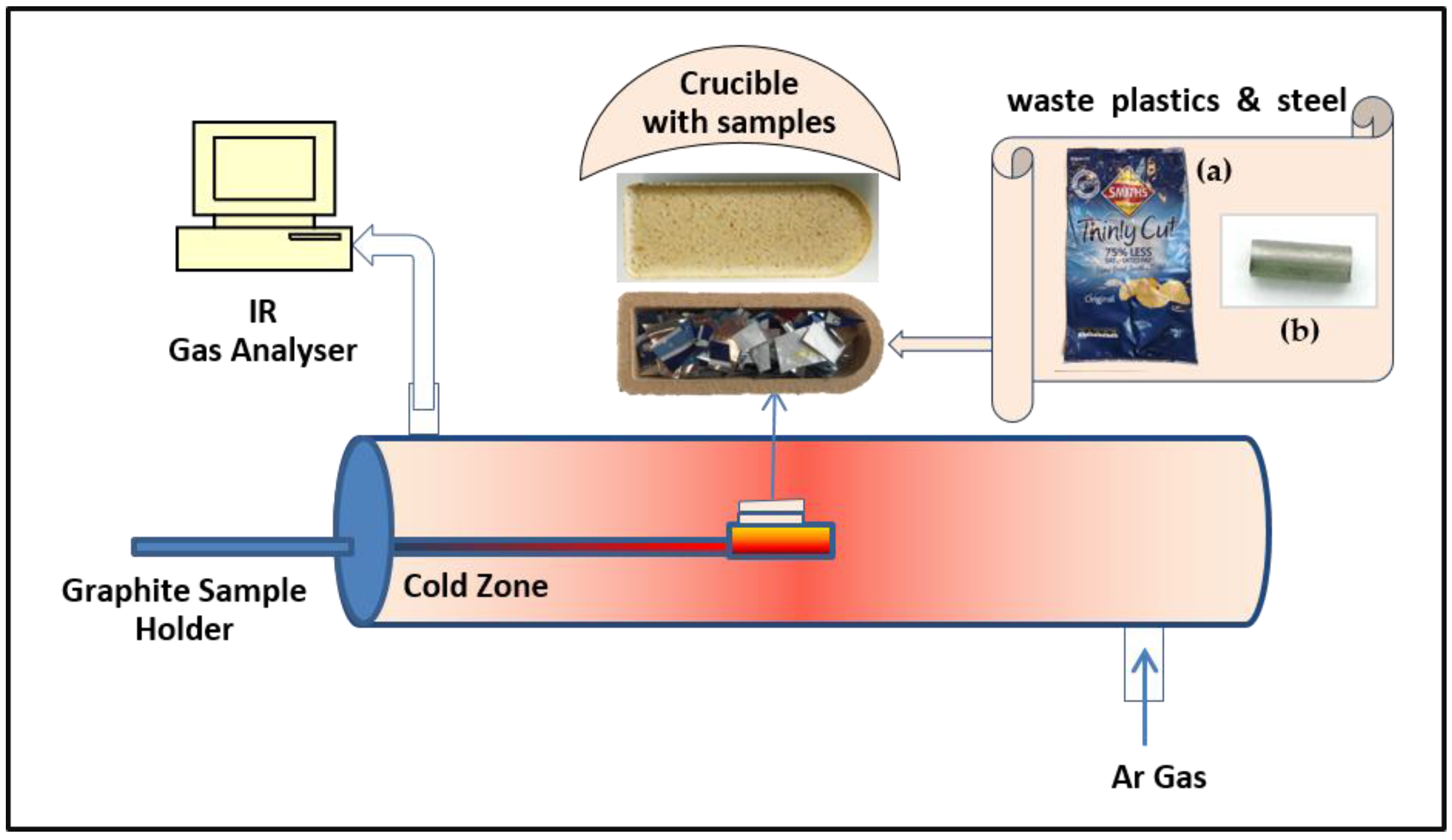
2.2. Experimental Methods
2.3. Characterization of Carburization Effect of Plastic Waste on Steel
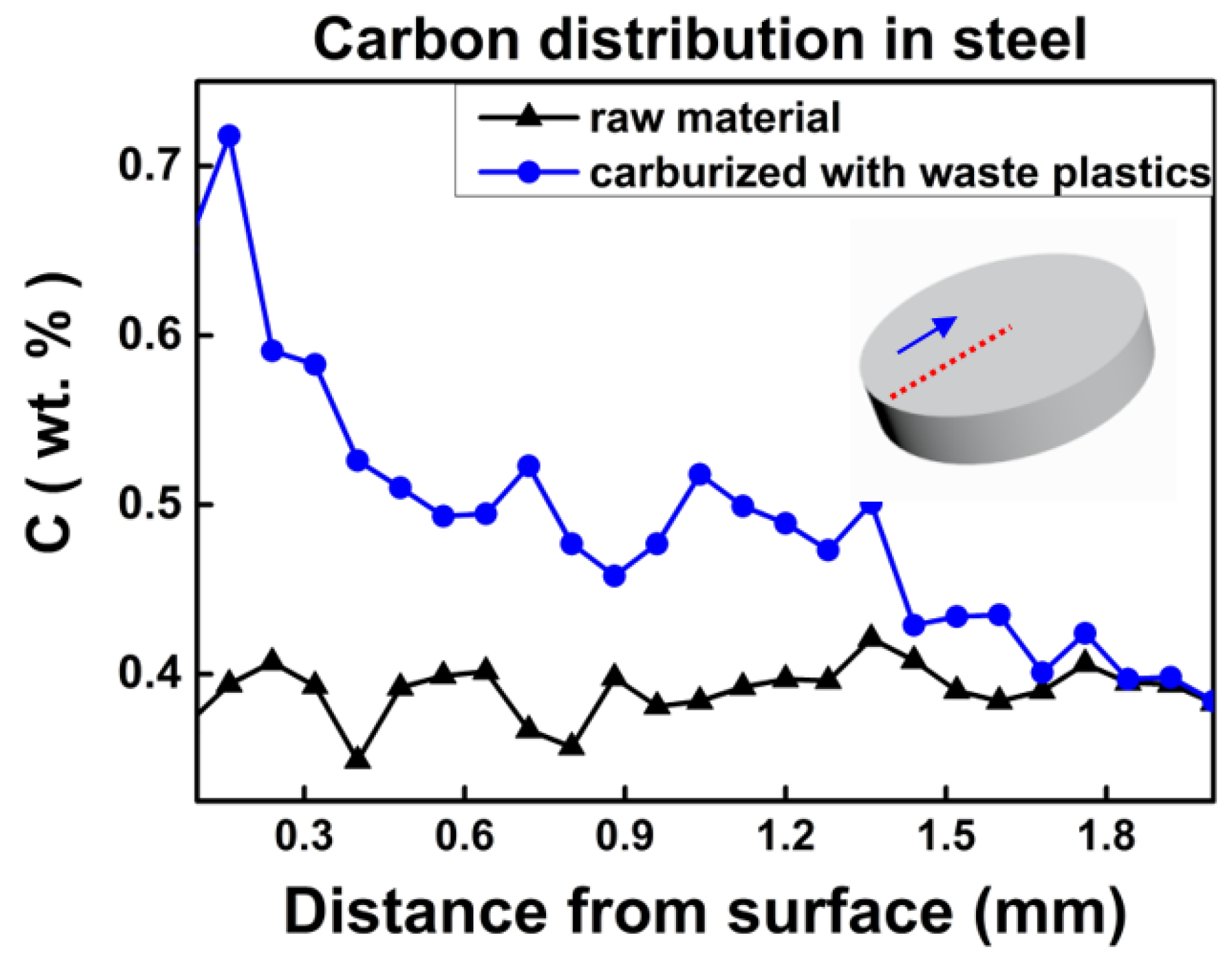
3. Results and Discussion
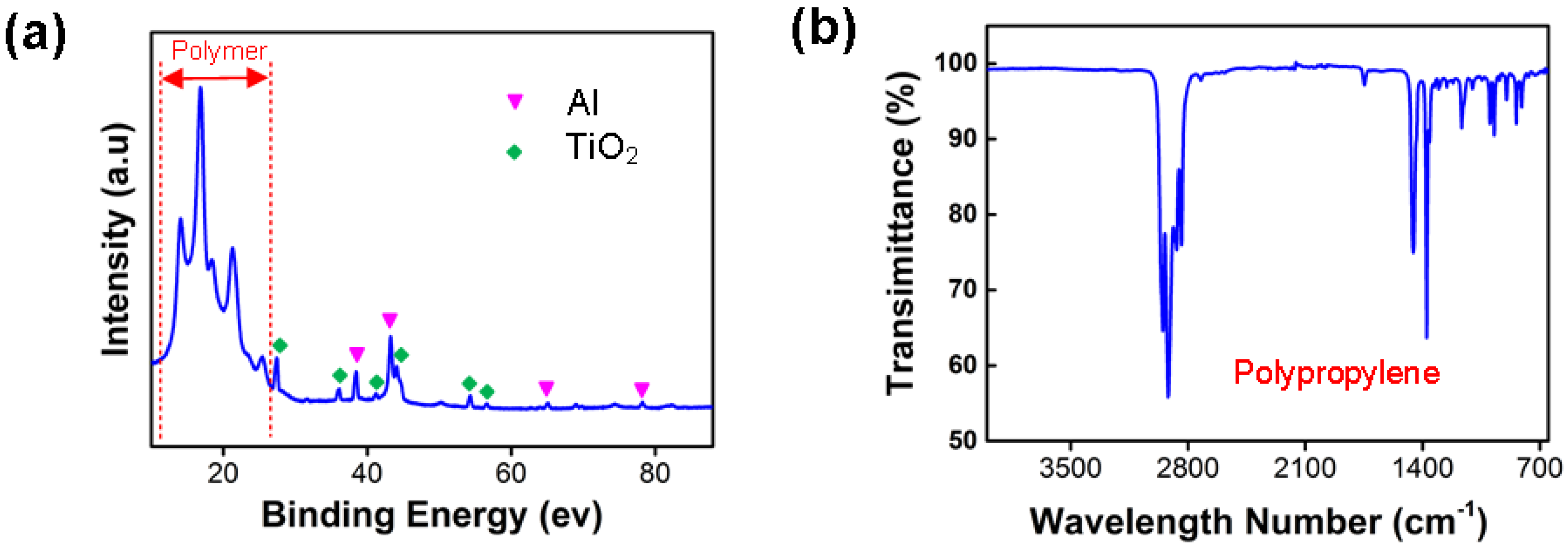
3.1. Characterisation of Snack Packaging Plastic Waste
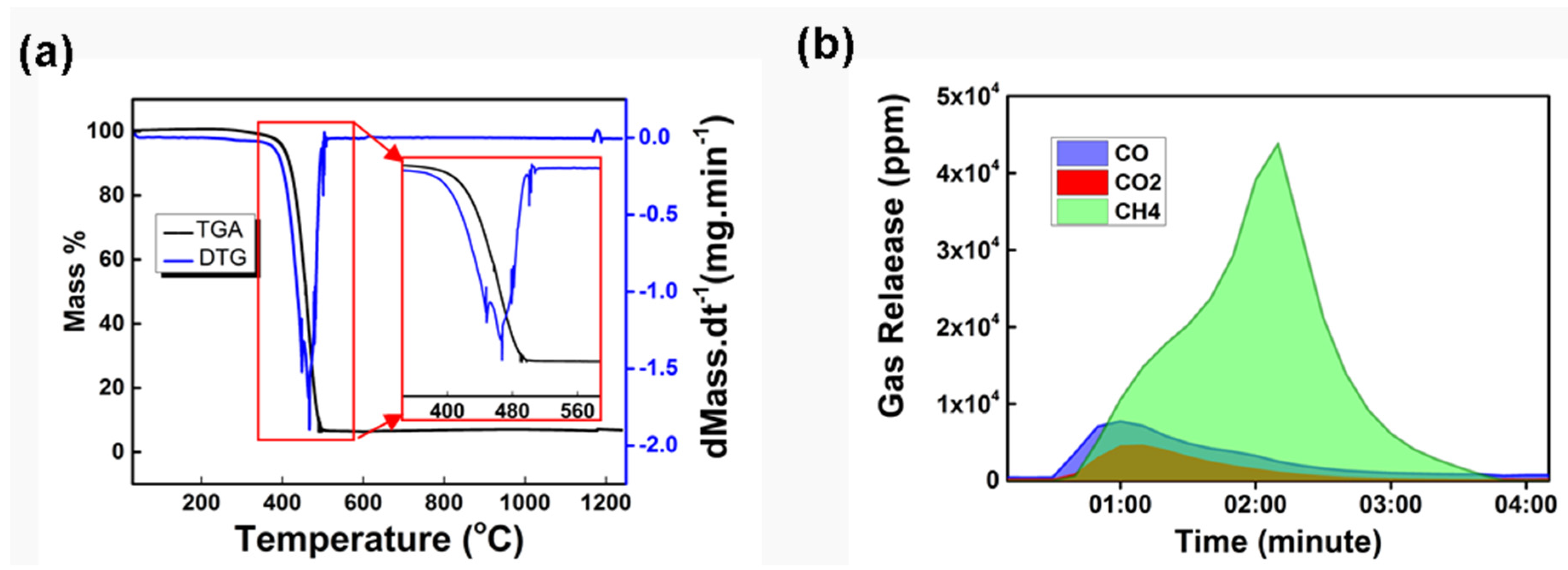
3.2. Mechanism of Steel Carburization by Using Plastic Waste as a Carbon Resource
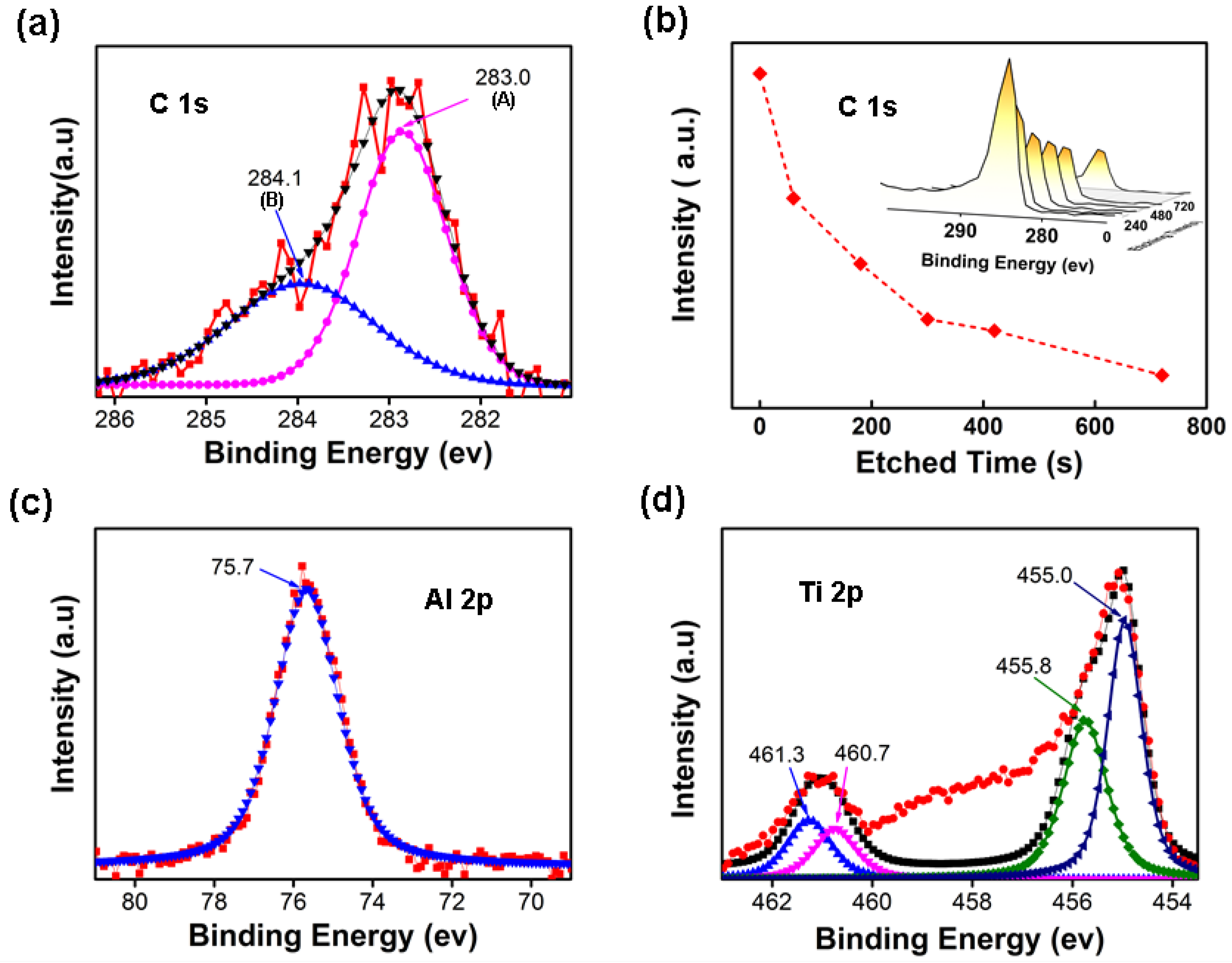
3.3. Characterisation for the Carburization Effect of Metallized Plastics Waste on Steel
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tobie, T.; Hippenstiel, F.; Mohrbacher, H. Optimizing Gear Performance by Alloy Modification of Carburizing Steels. Metals 2017, 7, 415. [Google Scholar] [CrossRef]
- Gray, A. Carburizing and Carbonitriding; ASM: Metals Park, OH, USA, 1977. [Google Scholar]
- Sugimoto, K.-I.; Hojo, T.; Mizuno, Y. Effects of vacuum-carburizing conditions on surface-hardened layer properties of transformation-induced plasticity-aided martensitic steel. Metals 2017, 7, 301. [Google Scholar] [CrossRef]
- Thompson, M.; Davison, M.; Rasmussen, H. Natural gas storage valuation and optimization: A real options application. Nav. Res. Logist. 2009, 56, 226–238. [Google Scholar] [CrossRef]
- Das, P.; Tiwari, P. Valorization of packaging plastic waste by slow pyrolysis. Resour. Conserv. Recycl. 2018, 128, 69–77. [Google Scholar] [CrossRef]
- Yao, D.; Zhang, Y.; Williams, P.T.; Yang, H.; Chen, H. Co-production of hydrogen and carbon nanotubes from real-world waste plastics: Influence of catalyst composition and operational parameters. Appl. Catal. B Environ. 2018, 221, 584–597. [Google Scholar] [CrossRef]
- Bosmans, A.; Vanderreydt, I.; Geysen, D.; Helsen, L. The crucial role of Waste-to-Energy technologies in enhanced landfill mining: A technology review. J. Clean. Prod. 2013, 55, 10–23. [Google Scholar] [CrossRef]
- Iranpour, R.; Stenstrom, M.; Tchobanoglous, G.; Miller, D.; Wright, J.; Vossoughi, M. Environmental engineering: Energy value of replacing waste disposal with resource recovery. Science 1999, 285, 706–711. [Google Scholar] [CrossRef] [PubMed]
- Zaman, A.U. A comprehensive study of the environmental and economic benefits of resource recovery from global waste management systems. J. Clean. Prod. 2016, 124, 41–50. [Google Scholar] [CrossRef]
- Marsh, K.; Bugusu, B. Food packaging—Roles, materials, and environmental issues. J. Food Sci. 2007, 72, R39–R55. [Google Scholar] [CrossRef] [PubMed]
- Barlow, C.; Morgan, D. Polymer film packaging for food: An environmental assessment. Resour. Conserv. Recycl. 2013, 78, 74–80. [Google Scholar] [CrossRef]
- Achilias, D.; Giannoulis, A.; Papageorgiou, G. Recycling of polymers from plastic packaging materials using the dissolution—Reprecipitation technique. Polym. Bull. 2009, 63, 449–465. [Google Scholar] [CrossRef]
- Mo, Y.; Zhao, L.; Wang, Z.; Chen, C.L.; Tan, G.Y.A.; Wang, J.Y. Enhanced styrene recovery from waste polystyrene pyrolysis using response surface methodology coupled with Box-Behnken design. Waste Manag. 2014, 34, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Shen, Y.; Somorjai, G.A. Studies of surface structures and compositions of polyethylene and polypropylene by IR+ visible sum frequency vibrational spectroscopy. Chem. Phys. Lett. 1997, 281, 394–400. [Google Scholar] [CrossRef]
- Morent, R.; De Geyter, N.; Leys, C.; Gengembre, L.; Payen, E. Comparison between XPS- and FTIR-analysis of plasma-treated polypropylene film surfaces. Surf. Interface Anal. 2008, 40, 597–600. [Google Scholar] [CrossRef]
- Busico, V.; Cipullo, R. Microstructure of polypropylene. Prog. Polym. Sci. 2001, 26, 443–533. [Google Scholar] [CrossRef]
- Arkatkar, A.; Arutchelvi, J.; Bhaduri, S.; Uppara, P.V.; Doble, M. Degradation of unpretreated and thermally pretreated polypropylene by soil consortia. Int. Biodeterior. Biodegrad. 2009, 63, 106–111. [Google Scholar] [CrossRef]
- Hedrick, S.A.; Chuang, S.S. Temperature programmed decomposition of polypropylene: In situ FTIR coupled with mass spectroscopy study. Thermochim. Acta 1998, 315, 159–168. [Google Scholar] [CrossRef]
- Park, J.J.; Song, H.-J.; Park, J.-W. The behaviors of H2, CH4, C2H4 and C3H6 from the decomposition of polypropylene by NiO/Si-Al catalyst. J. Mater. Cycles Waste Manag. 2013, 15, 37–41. [Google Scholar] [CrossRef]
- Kwietniewski, C.E.; Tentardini, E.K.; Totten, G.E. Carburizing and Carbonitriding. In Encyclopedia of Tribology; Springer: Berlin, Germany, 2013; pp. 298–306. [Google Scholar]
- Gjerstad, S.; Welch, B. Oxidation of Aluminum by Carbon Dioxide in the Presence of Cryolitic lectrolyte. J. Electrochem. Soc. 1964, 111, 976–980. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, H.; Shu, F.; He, W.; Wang, G. Microstructure and Corrosion Behavior of Simulated Welding HAZ of Q315NS Steel in Sulfuric Acid Solution. Metals 2017, 7, 194. [Google Scholar] [CrossRef]
- Parsons, S.; Edmonds, D. Microstructure and mechanical properties of medium-carbon ferrite-pearlite steel microalloyed with vanadium. Mater. Sci. Technol. 1987, 3, 894–904. [Google Scholar] [CrossRef]
- Smith, W.F.; Hashemi, J. Foundations of Materials Science and Engineering; McGraw-Hill: New York, NY, USA, 2011. [Google Scholar]
- Gladshtein, L.; Larionova, N.; Belyaev, B. Effect of ferrite-pearlite microstructure on structural steel properties. Metallurgist 2012, 56, 579–590. [Google Scholar] [CrossRef]
- Toribio, J.; González, B.; Matos, J.C.; Ayaso, F.J. Influence of Microstructure on Strength and Ductility in Fully Pearlitic Steels. Metals 2016, 6, 318. [Google Scholar] [CrossRef]
- Zhou, D.; Shiflet, G. Ferrite: Cementite crystallography in pearlite. Metall. Mater. Trans. A 1992, 23, 1259–1269. [Google Scholar] [CrossRef]
- Samuels, L.E. Optical Microscopy of Carbon Steels; American Society for Metals: Metals Park, OH, USA, 1980. [Google Scholar]
- Chastain, J.; King, R.C.; Moulder, J. Handbook of X-Ray Photoelectron Spectroscopy: A Reference Book of Standard Spectra for Identification and Interpretation of XPS Data; Physical Electronics Division, Perkin-Elmer Corporation: Eden Prairie, MN, USA, 1992. [Google Scholar]
- Lopez, D.; Schreiner, W.H.; De Sánchez, S.R.; Simison, S.N. The influence of carbon steel microstructure on corrosion layers: An XPS and SEM characterization. Appl. Surf. Sci. 2003, 207, 69–85. [Google Scholar] [CrossRef]
- Whangbo, S.; Choi, Y.K.; Jang, H.K.; Chung, Y.D.; Lyo, I.W.; Whang, C.N. Effect of oxidized Al prelayer for the growth of polycrystalline Al2O3 films on Si using ionized beam deposition. Thin Solid Films 2001, 388, 290–294. [Google Scholar] [CrossRef]
- Jeurgens, L.; Sloof, W.G.; Tichelaar, F.D.; Mittemeijer, E.J. Structure and morphology of aluminium-oxide films formed by thermal oxidation of aluminium. Thin solid films 2002, 418, 89–101. [Google Scholar] [CrossRef]
- Braic, M.; Braic, V.; Balaceanu, M.; Kiss, A.; Cotrut, C.; Drobe, P.; Vladescu, A.; Vasilescu, C. On Some Characteristics of Ti Oxynitrides Obtained by Pulsed Magnetron Sputtering. Plasma Process. Polym. 2007, 4, S171–S174. [Google Scholar] [CrossRef]
- Yin, S.; Rajarao, R.; Kong, C.; Wang, Y.; Gong, B.; Sahajwalla, V. Sustainable Fabrication of Protective Nanoscale TiN Thin Film on a Metal Substrate by Using Automotive Waste Plastics. Sustain. Chem. Eng. 2016, 5, 1549–1556. [Google Scholar] [CrossRef]
- Bhadeshia, H.; Honeycombe, R. Steels: Microstructure and Properties; Butterworth-Heinemann: Oxford, UK, 2017. [Google Scholar]

| Element | wt. % |
|---|---|
| C | 0.39 |
| Fe | 98.95 |
| Mn | 0.564 |
| Cu | 0.108 |
| Cr | 0.085 |
| Ni | 0.06 |
| Mo | 0.019 |
| Ca | 0.015 |
| Zn | 0.005 |
| Al | 0.004 |
| Ba | 0.004 |
| Co | 0.004 |
| As | 0.003 |
| Ultimate Analysis | ||
|---|---|---|
| C | 90.4 | wt. % |
| N | 0.4 | |
| S | 0.04 | |
| Al | 4153 | mg/kg |
| Ca | 419 | |
| Cu | 309 | |
| P | 203 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, S.; Rajarao, R.; Pahlevani, F.; Sahajwalla, V. Sustainable Steel Carburization by Using Snack Packaging Plastic Waste as Carbon Resources. Metals 2018, 8, 78. https://doi.org/10.3390/met8010078
Yin S, Rajarao R, Pahlevani F, Sahajwalla V. Sustainable Steel Carburization by Using Snack Packaging Plastic Waste as Carbon Resources. Metals. 2018; 8(1):78. https://doi.org/10.3390/met8010078
Chicago/Turabian StyleYin, Songyan, Ravindra Rajarao, Farshid Pahlevani, and Veena Sahajwalla. 2018. "Sustainable Steel Carburization by Using Snack Packaging Plastic Waste as Carbon Resources" Metals 8, no. 1: 78. https://doi.org/10.3390/met8010078
APA StyleYin, S., Rajarao, R., Pahlevani, F., & Sahajwalla, V. (2018). Sustainable Steel Carburization by Using Snack Packaging Plastic Waste as Carbon Resources. Metals, 8(1), 78. https://doi.org/10.3390/met8010078







