Effects of Charcoal Addition on the Properties of Carbon Anodes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Laboratory Pilot Scale Anode Preparation
2.2. Optimization of the Anode Recipe
2.3. Characterization Techniques
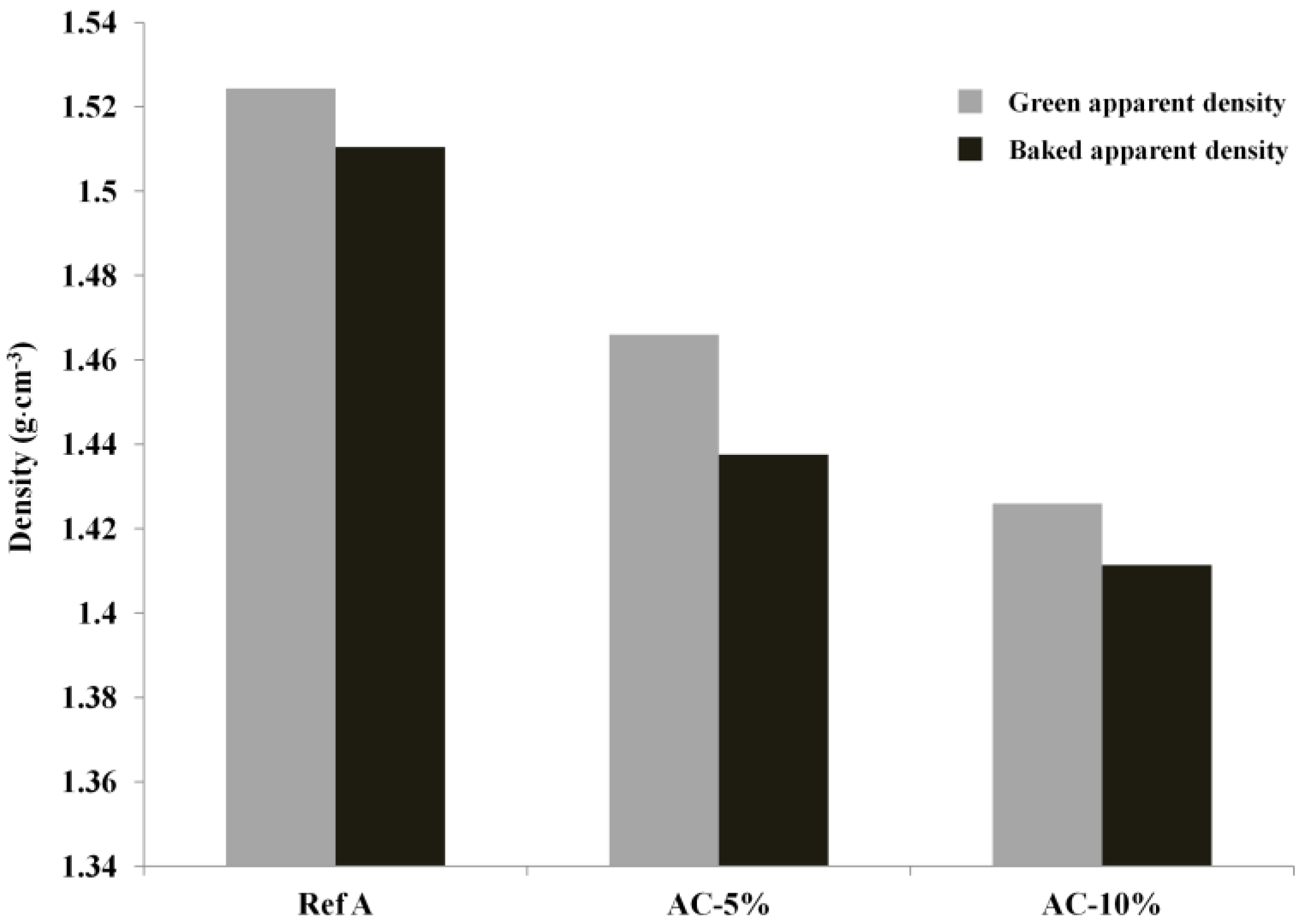
2.3.1. Green and Baked Anode Apparent Density
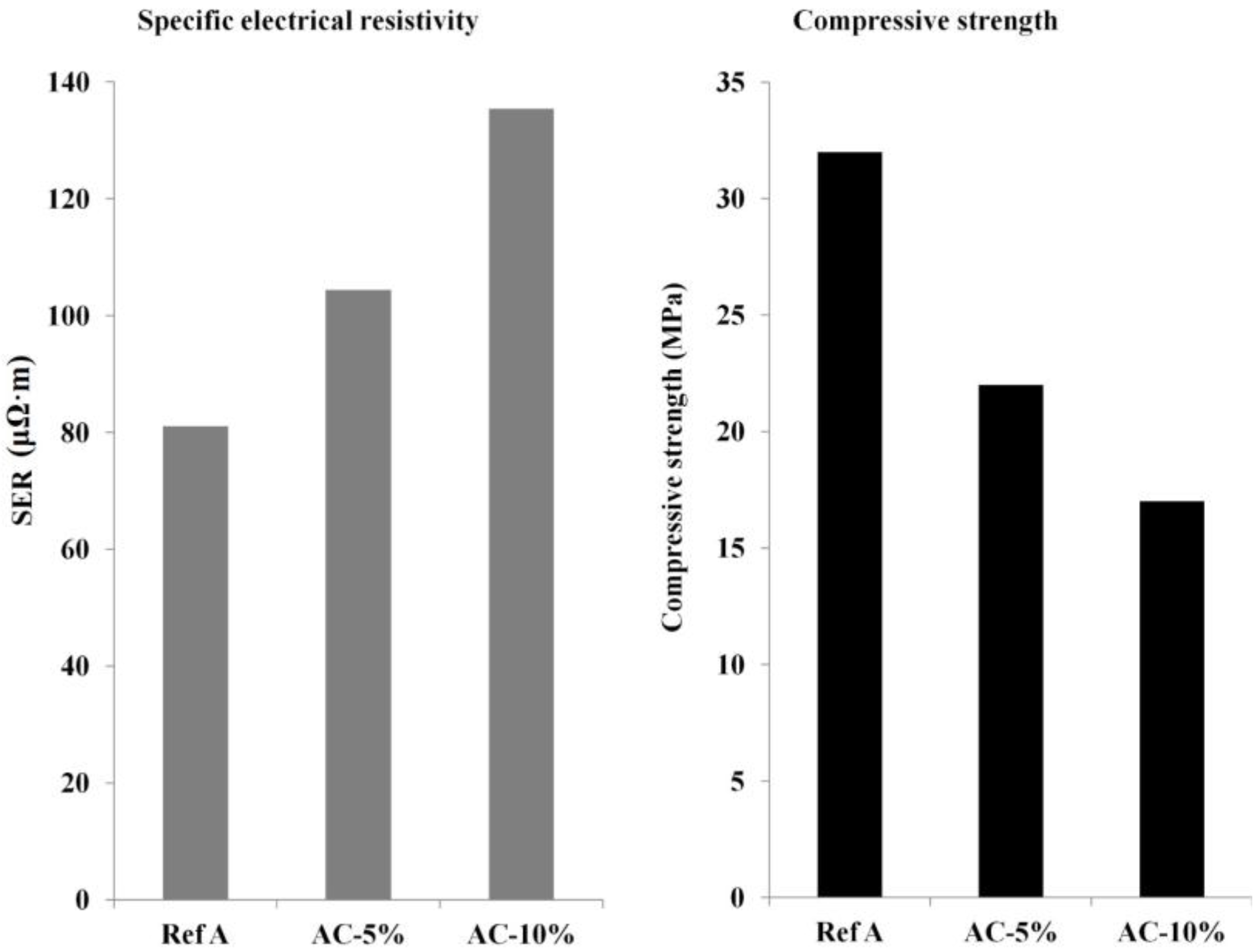
2.3.2. Specific Electrical Resistivity
- ρ = specific electrical resistivity, µΩ·m
- U = voltage drop over the sample, V
- A = cross section of the sample, m2
- L = distance between potential contacts, m
- I = electrical current through the sample, A
2.3.3. Compressive Strength and Young’s Modulus
3. Results and Discussion
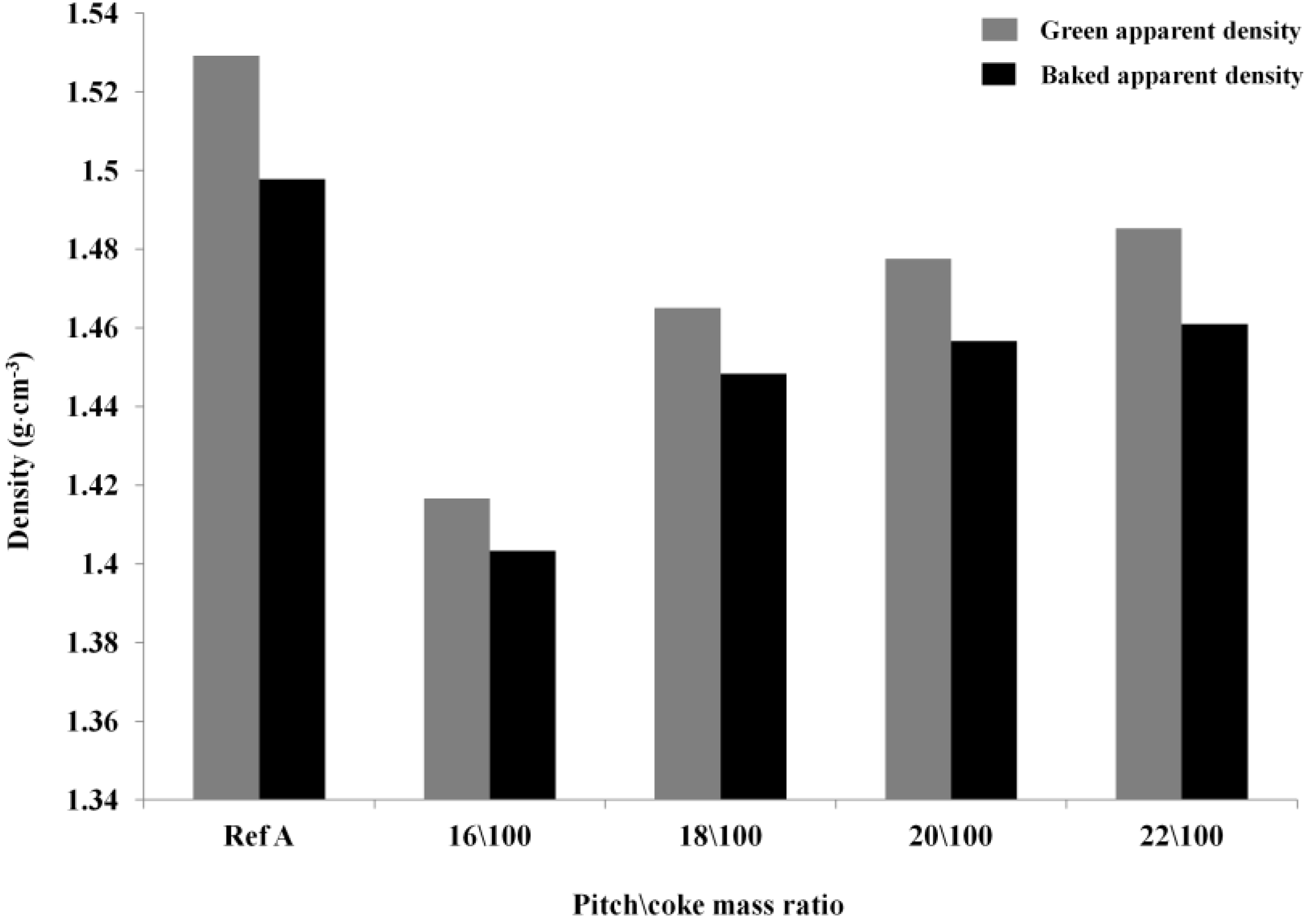
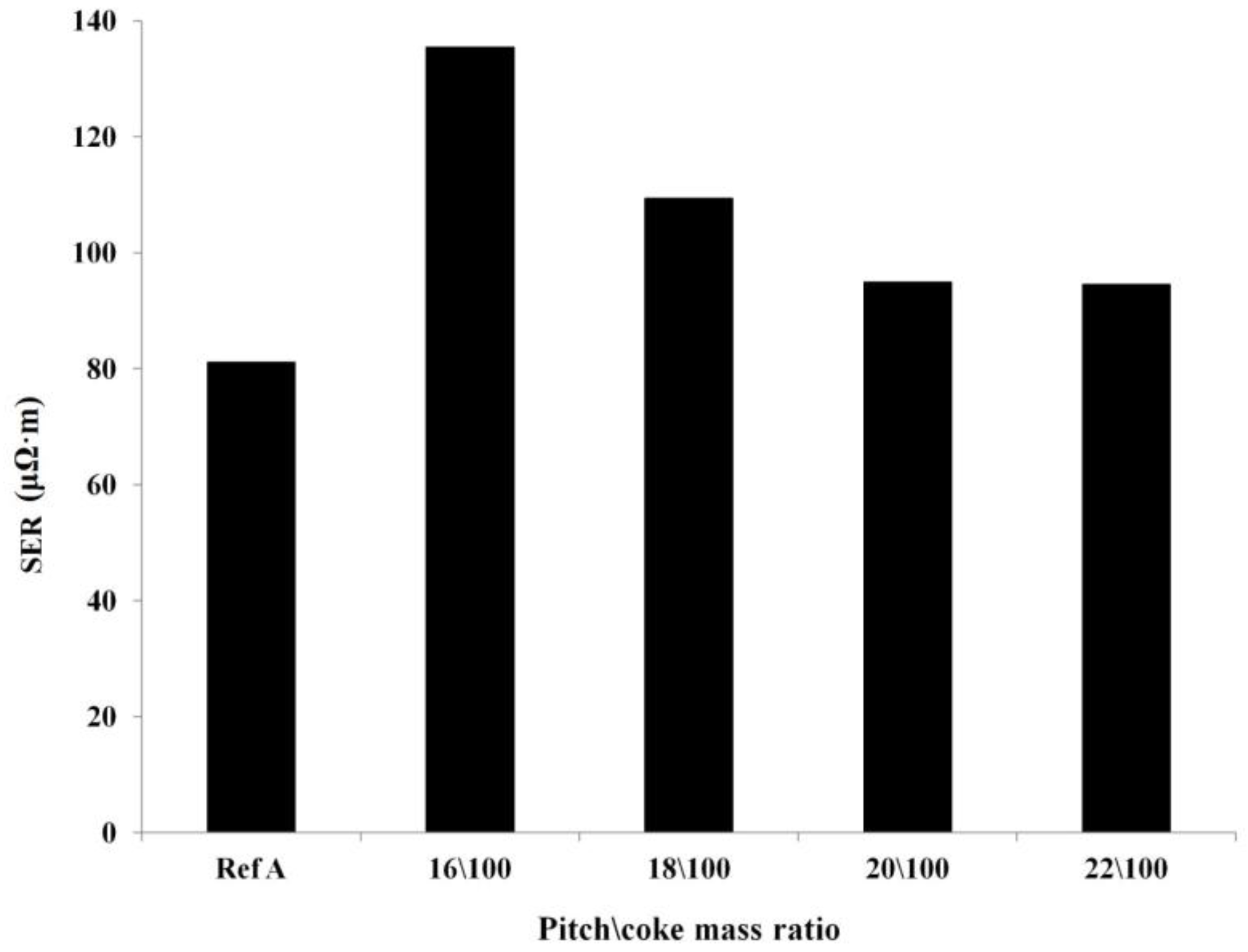
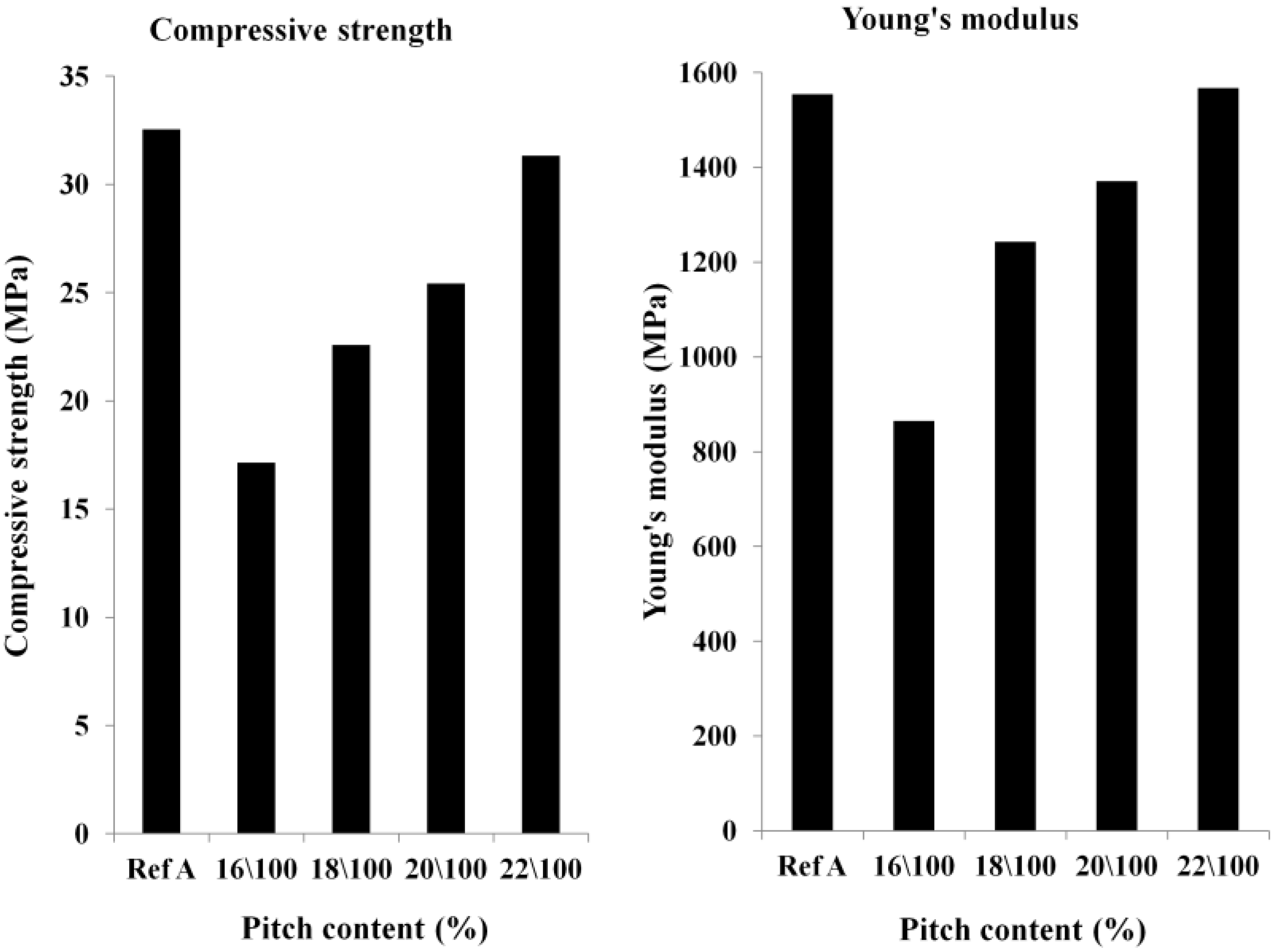
Optimization of the Pitch Content
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Habashi, F. Extractive metallurgy of aluminum. In Handbook of Aluminum; Marcel Dekker: New York, NY, USA, 2003; Volume 2, pp. 1–5. [Google Scholar]
- Meier, M.W. Cracking Behaviour of Anodes. Ph.D. Thesis, Federal Institute of Technology, Zurich, Switzerland, 3 January 1996; pp. 12–40. [Google Scholar]
- Edwards, L. The History and Future Challenges of Calcined Petroleum Coke Production and Use in Aluminum Smelting. JOM 2015, 67, 308–321. [Google Scholar] [CrossRef]
- Jahirul, M.; Rasul, M.; Chowdhury, A.; Ashwath, N. Biofuels Production through Biomass Pyrolysis—A Technological Review. Energies 2012, 5, 4952–5001. [Google Scholar] [CrossRef]
- Monsen, B.; Ratvik, A.P.; Lossius, L.P. Charcoal in anodes for aluminium production. TMS Light Met. 2010, 2010, 929–933. [Google Scholar]
- Elkasabi, Y.; Boateng, A.A.; Jackson, M.A. Upgrading of bio-oil distillation bottoms into biorenewable calcined coke. Biomass Bioenergy 2015, 81, 415–423. [Google Scholar] [CrossRef]
- Coutinho, A.R.; Rocha, J.D.; Luengo, C.A. Preparing and characterizing biocarbon electrodes. Fuel Process. Technol. 2000, 67, 93–102. [Google Scholar] [CrossRef]
- Hussein, A.; Larachi, F.; Zeigler, D.; Alamdari, H. Effects of heat treatment and acid washing on properties and reactivity of charcoal. Biomass Bioenergy 2016, 90, 101–113. [Google Scholar] [CrossRef]
- Azari, K.; Alamdari, H.; Aryanpour, G.; Ziegler, D.; Picard, D.; Fafard, M. Compaction properties of carbon materials used for prebaked anodes in aluminum production plants. Powder Technol. 2013, 246, 650–657. [Google Scholar] [CrossRef]
- Smith, M.A. An Evaluation of the Binder Matrix in Prebaked Carbon Anodes Used for Aluminium Production. Ph.D. Thesis, University of Auckland, Auckland, New Zealand, April 1991. [Google Scholar]
- Hulse, K.L. Anode Manufacture: Raw Materials, Formulation and Processing Parameters, 1st ed.; R & D Carbon Ltd.: Sierre, Switzerland, 2000; pp. 77–158. [Google Scholar]
- Chevarin, F.; Lemieux, L.; Picard, D.; Ziegler, D.; Fafard, M.; Alamdari, H. Characterization of carbon anode constituents under CO2 gasification: A try to understand the dusting phenomenon. Fuel 2015, 156, 198–210. [Google Scholar] [CrossRef]
- ASTM International. ASTM D5502-00(2010), Standard Test Method for Apparent Density by Physical Measurements of Manufactured Anode and Cathode Carbon Used by the Aluminum Industry; ASTM International: West Conshohocken, PA, USA, 2010. [Google Scholar]
- International Organization for Standardization. ISO 11713, Standard Method, Carbonaceous Materials Used in the Production of Aluminium-Cathode Blocks and Baked Anodes-Determination of Electrical Resistivity at Ambient Temperature; International Organization for Standardization: Geneva, Switzerland, 2000. [Google Scholar]
- International Organization for Standardization. O 18515, Carbonaceous Materials for the Production of Aluminium-Cathode Blocks and Baked Anodes-Determination of Compressive Strength; International Organization for Standardization: Geneva, Switzerland, 2014. [Google Scholar]
| Particle size (US Mesh) | −4 | −8 | −14 | −30 | −50 | −100 | −200 | −400 |
| +8 | +14 | +30 | +50 | +100 | +200 | +400 | ||
| Particle size (mm) | −4.75 | −2.36 | −1.40 | −0.600 | −0.300 | −0.150 | −0.075 | −0.038 |
| +2.36 | +1.40 | +0.600 | +0.300 | +0.150 | +0.075 | +0.038 | ||
| Wt. % | 21.8 | 10 | 11.5 | 12.6 | 9 | 10.6 | 14.5 | 10 |
| Material | Na (ppm) | Ca (ppm) | Si (ppm) | V (ppm) | Fe (ppm) | Ni (ppm) | S (%) | Ash (%) |
|---|---|---|---|---|---|---|---|---|
| Pre-treated charcoal | 29 | 1685 | 312 | 0 | 58 | 2 | 0 | 0.55 |
| Pet. Coke | 100 | 130 | 120 | 360 | 460 | 250 | 2.13 | 0.35 |
| Mettler Softening Point (°C) | Quinoline Insoluble (%) | Coking Value (%) | Chemical Compositions (ppm) | |||||
|---|---|---|---|---|---|---|---|---|
| Pb | Fe | Ca | Na | Si | S (%) | |||
| 109.5 | 16.5 | 58.8 | 93 | 209 | 71 | 48 | 254 | 0.55 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussein, A.; Fafard, M.; Ziegler, D.; Alamdari, H. Effects of Charcoal Addition on the Properties of Carbon Anodes. Metals 2017, 7, 98. https://doi.org/10.3390/met7030098
Hussein A, Fafard M, Ziegler D, Alamdari H. Effects of Charcoal Addition on the Properties of Carbon Anodes. Metals. 2017; 7(3):98. https://doi.org/10.3390/met7030098
Chicago/Turabian StyleHussein, Asem, Mario Fafard, Donald Ziegler, and Houshang Alamdari. 2017. "Effects of Charcoal Addition on the Properties of Carbon Anodes" Metals 7, no. 3: 98. https://doi.org/10.3390/met7030098
APA StyleHussein, A., Fafard, M., Ziegler, D., & Alamdari, H. (2017). Effects of Charcoal Addition on the Properties of Carbon Anodes. Metals, 7(3), 98. https://doi.org/10.3390/met7030098





