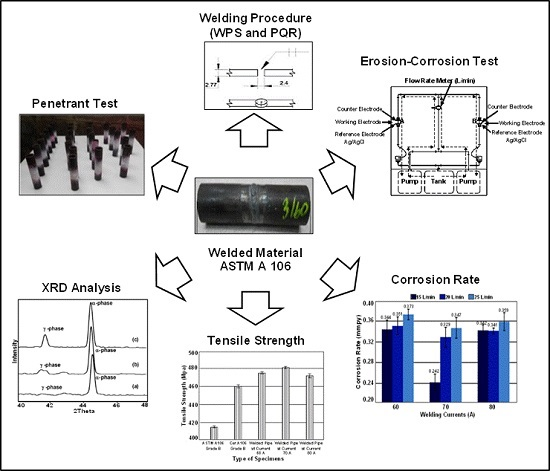
The Electrochemical Investigation of the Corrosion Rates of Welded Pipe ASTM A106 Grade B
Abstract
:1. Introduction
2. Experimental Procedure
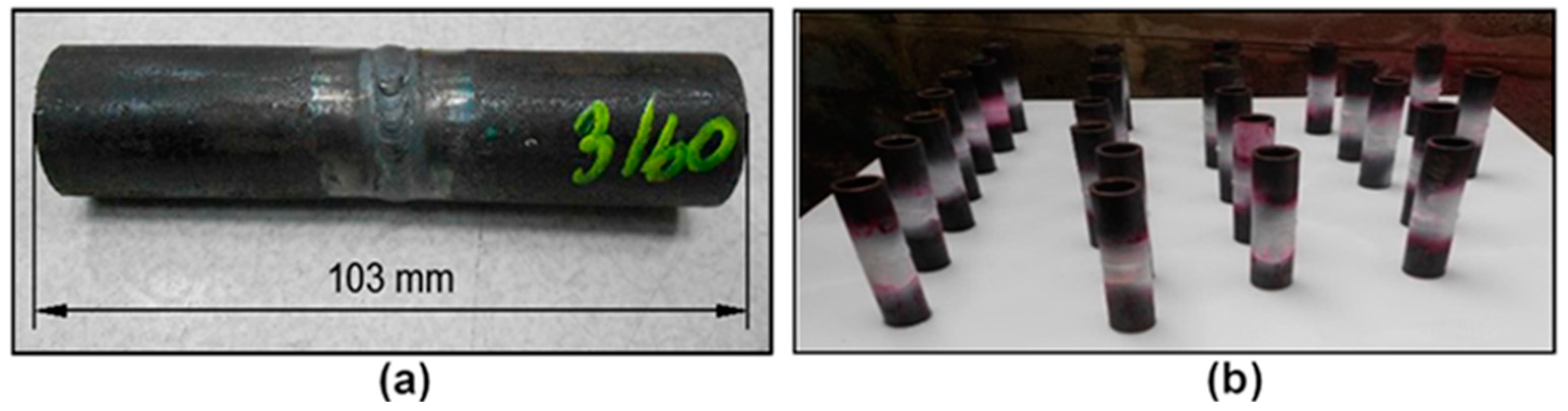
2.1. Materials Preparation and Welding
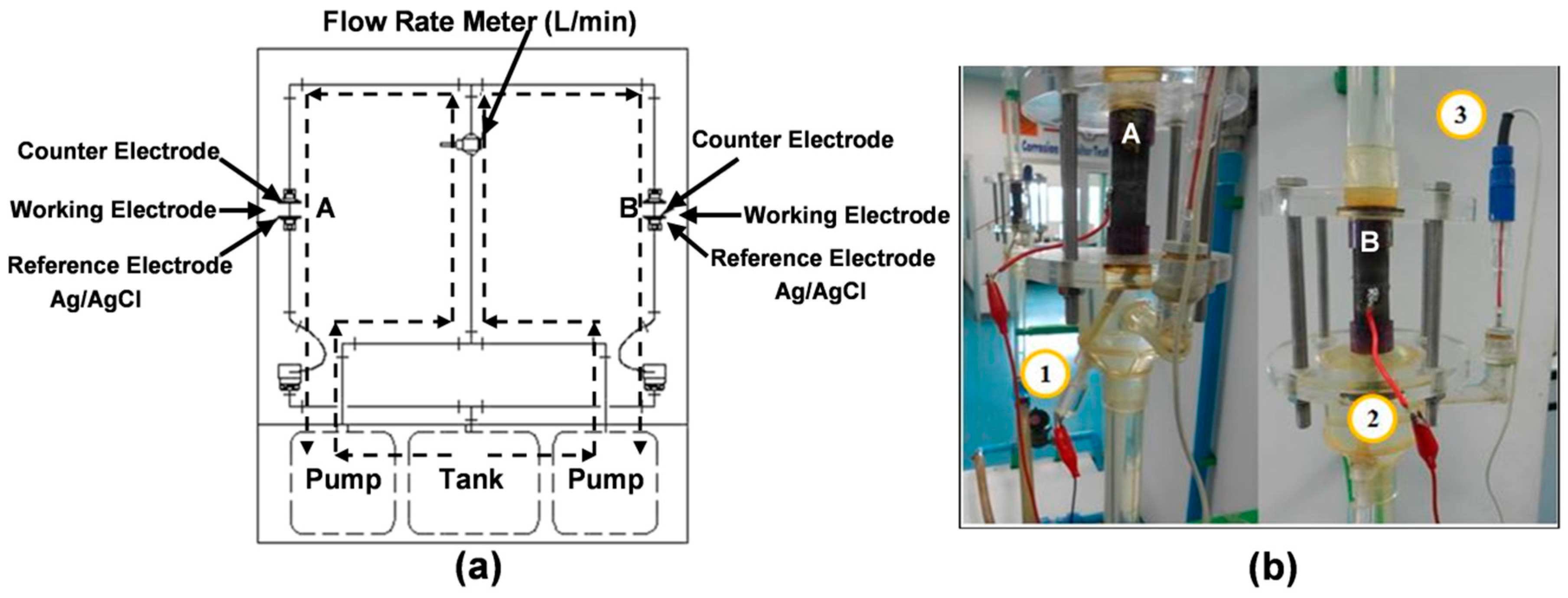
2.2. Corrosion Test
2.3. Characterization and Equipment
3. Results and Discussion
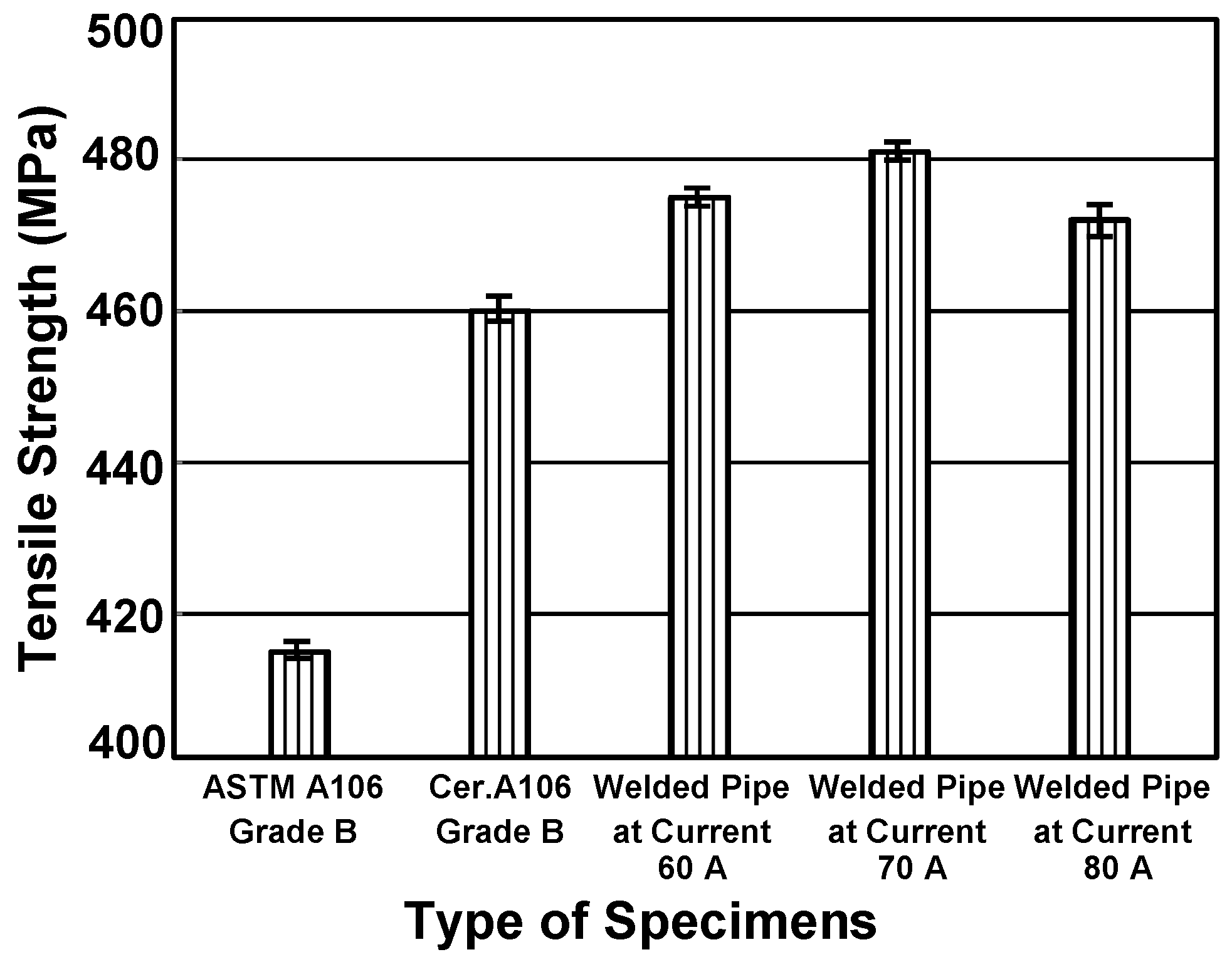
3.1. Material Verification
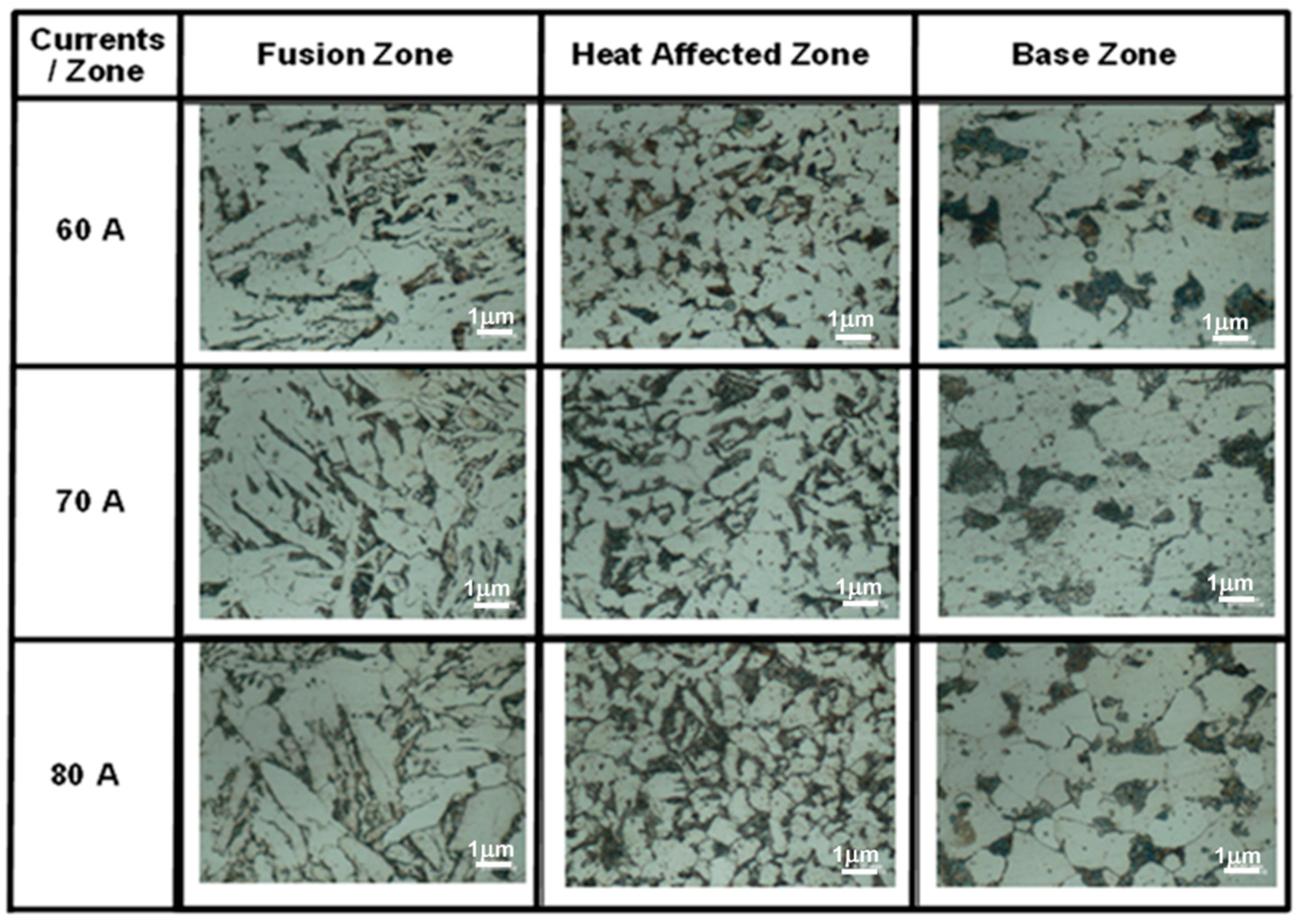
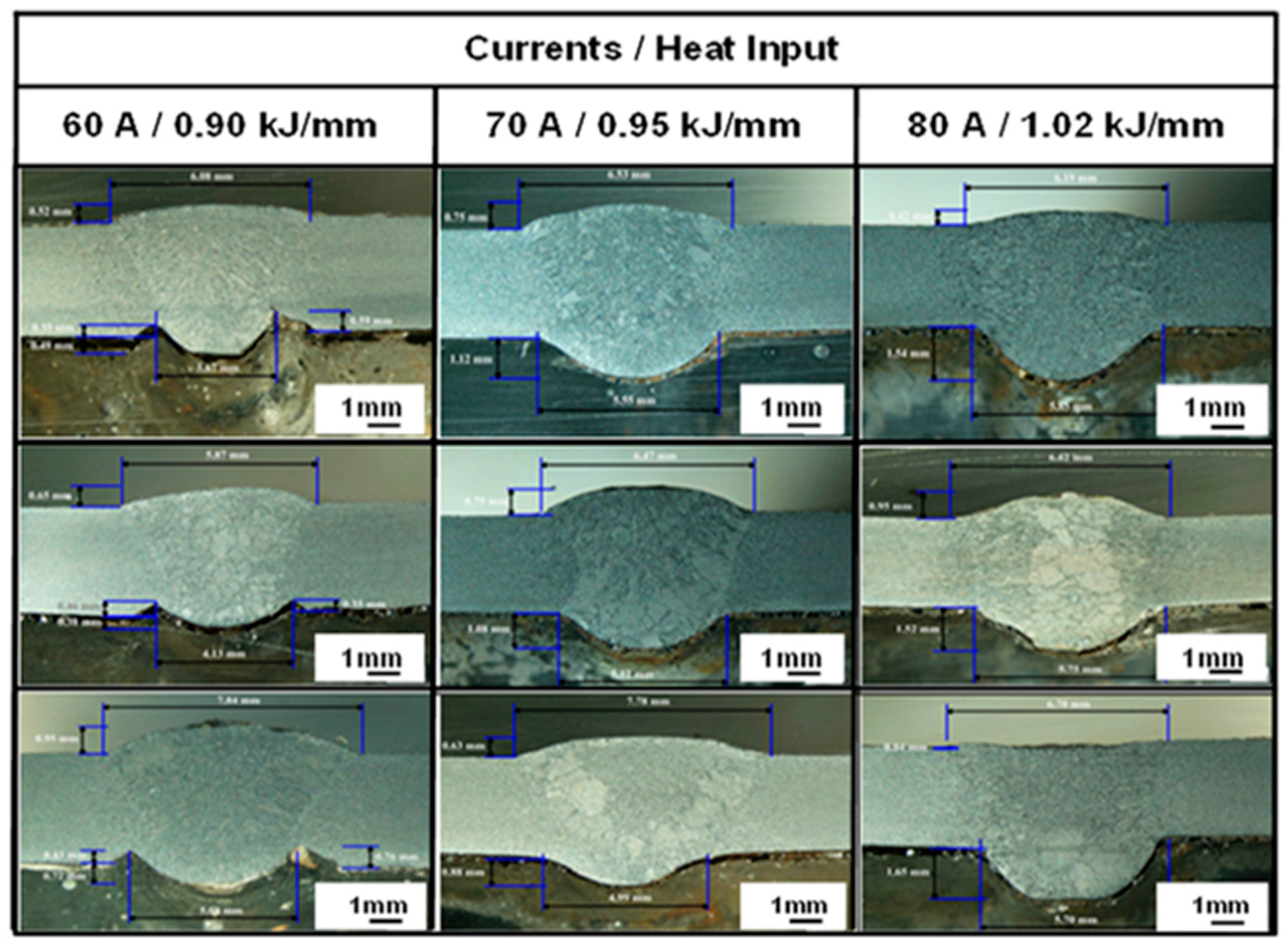
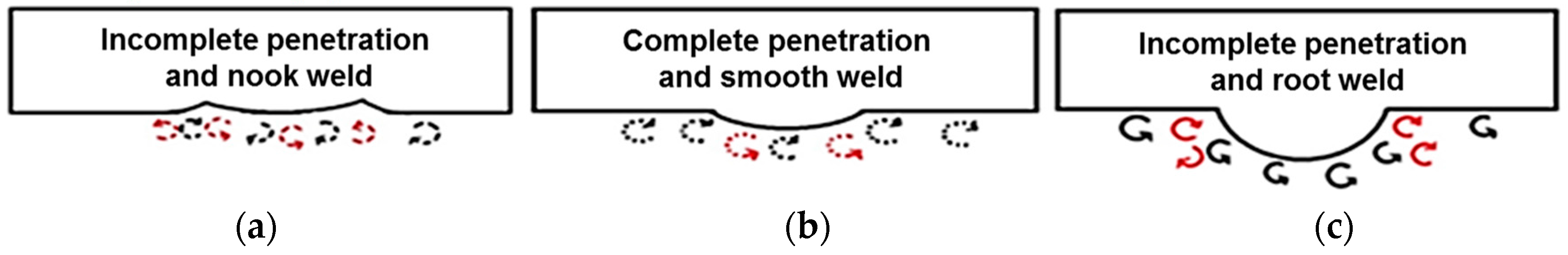
3.2. Microstructure Observation
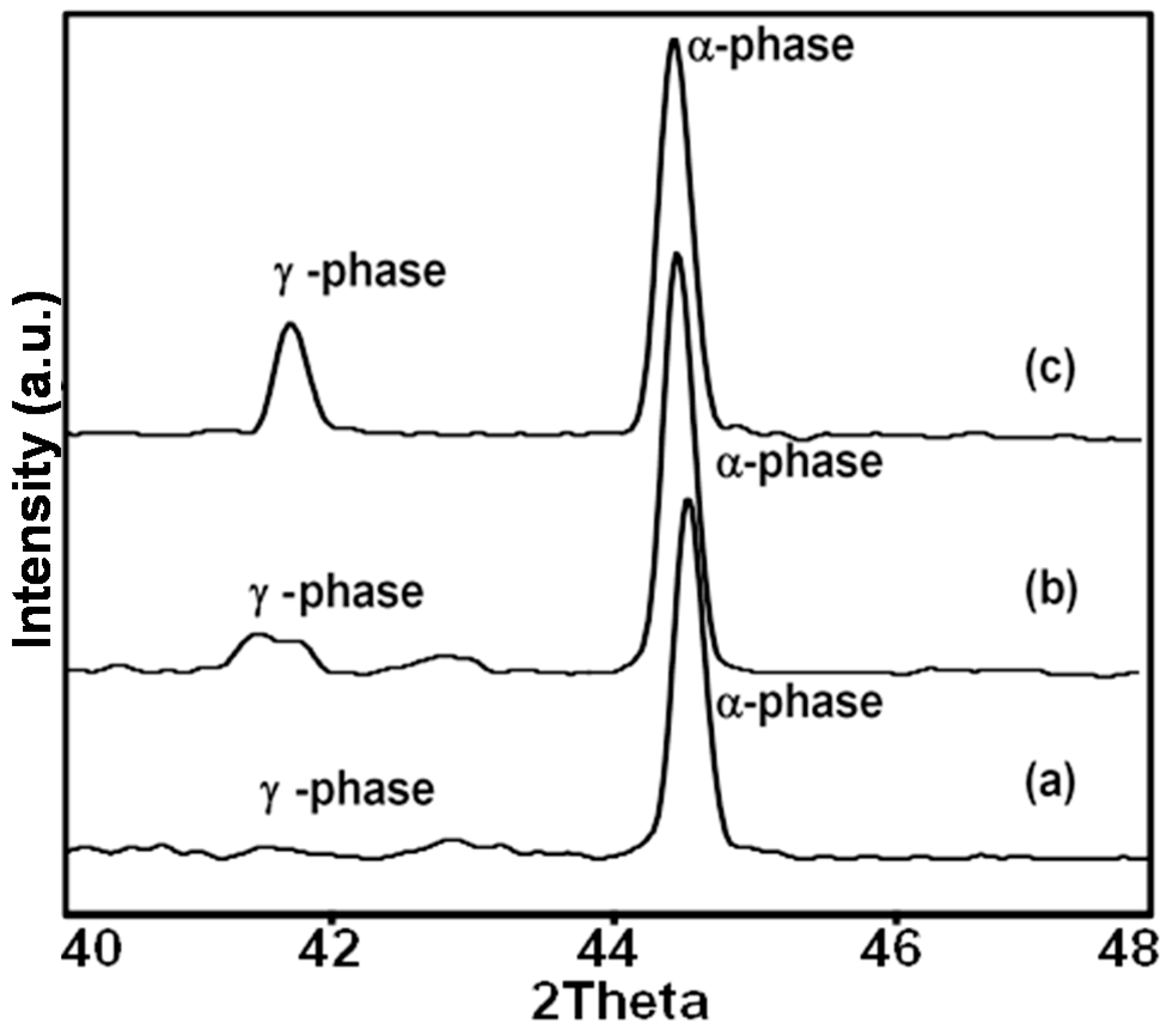
3.3. Phase Transformation Information Using XRD
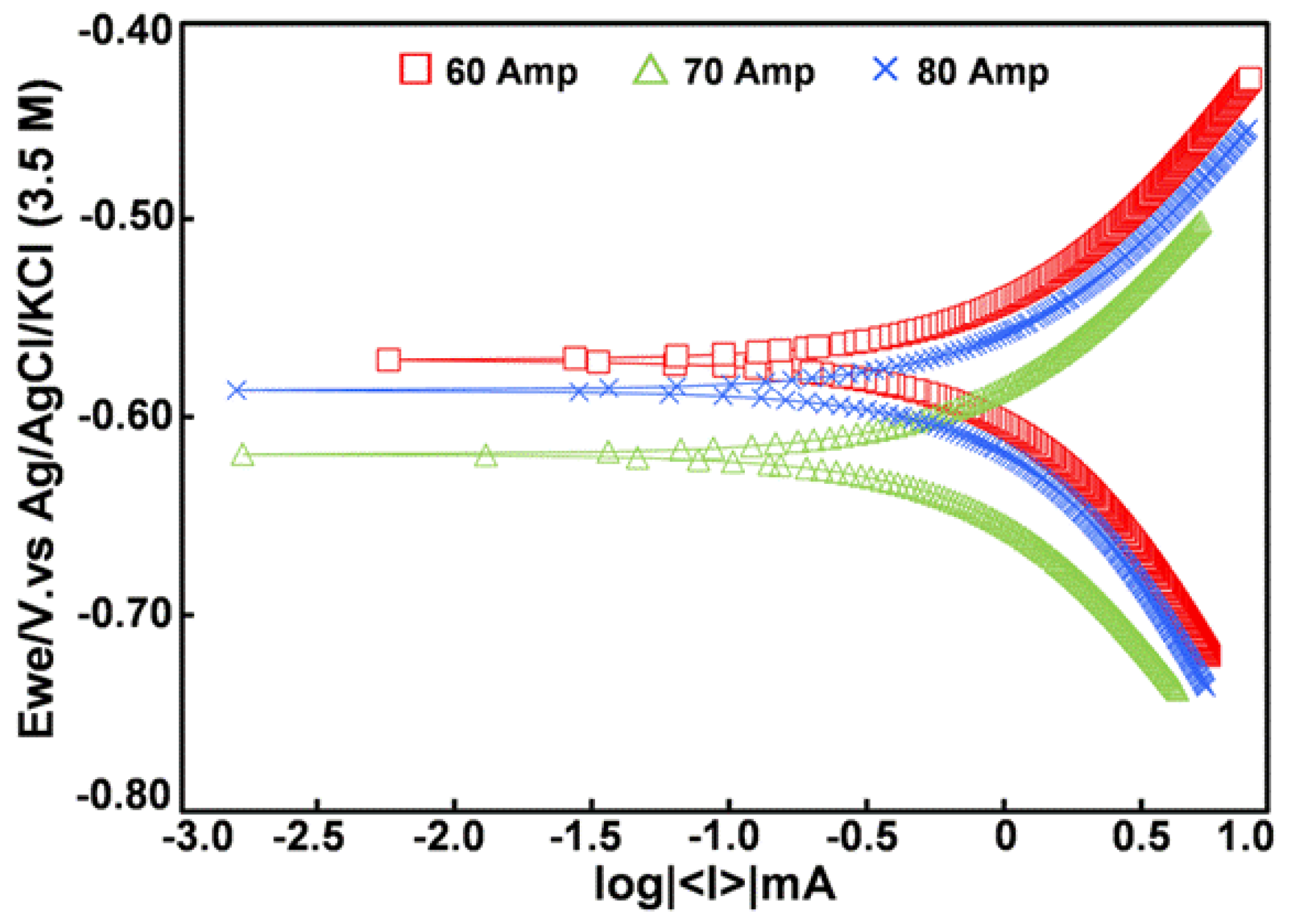
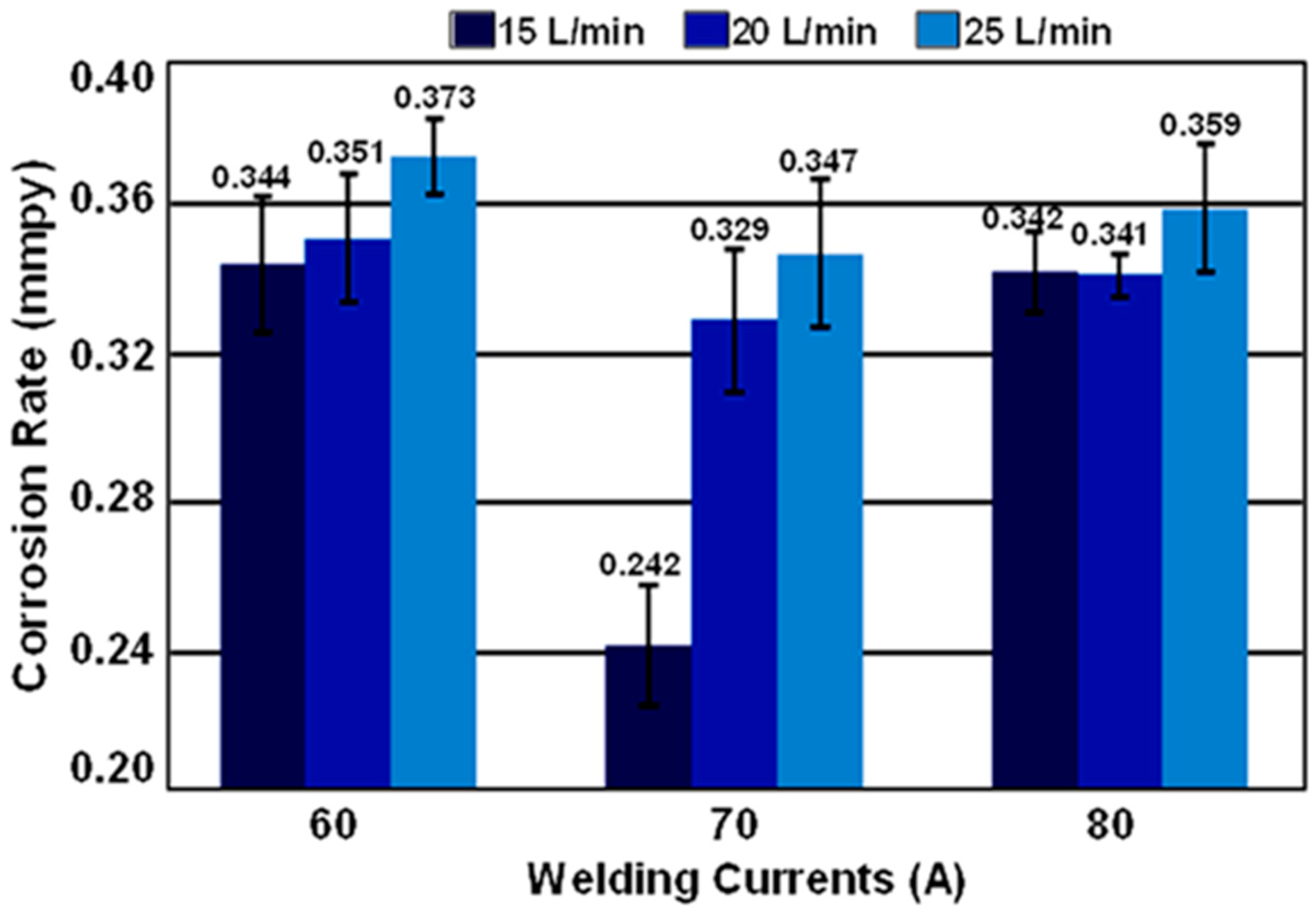
3.4. Corrosion Rate Determination by Tafel Slope Polarization
- Icorr = the density electricity/metal surface area samples (A/cm2)
- E.W. = the mass of metal inherent (27.9)
- A = metal surface area sample (150 cm2)
- ρ = the density metal samples (7.87/cm3)
- K = constants of the calculated mmpy unit, 3.27 × 10−3
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- McKetta, J.J. Piping Design Handbook; Marcel Dekker Inc: New York, NY, USA, 1992. [Google Scholar]
- Bae, D.; Kim, C.H. Characteristics in the weld of multi-pass welded A106 Gr B steel pipe. KSME Int. J. 2004, 18, 114–121. [Google Scholar]
- Bakour, S.; Guenbour, A.; Bellaouchou, A.; Escrivà-Cerdán, C.; Sánchez-Tovar, R.; Leiva-García, R.; García-Antón, J. Effect of welding on the corrosion behaviour of a highly alloyed austenitic stainless steel UNS N06027 in polluted phosphoric acid media. Int. J. Electrochem. Sci. 2012, 7, 10530–10543. [Google Scholar]
- Guo, H.X.; Lu, B.T.; Luo, J.L. Interaction of mechanical and electrochemical factors in erosion-corrosion of carbon steel. Electrochim. Acta 2005, 51, 315–323. [Google Scholar] [CrossRef]
- Shrestha, S.; Hodgkiess, T.; Neville, A. Erosion-corrosion behaviour of high-velocity oxy-fuel Ni-Cr-Mo-Si-B coatings under high-velocity seawater jet impingement. Wear 2005, 259, 208–218. [Google Scholar] [CrossRef]
- Zheng, Y.G.; Yao, Z.M.; Wei, X.Y.; Ke, W. The synergistic effect between erosion and corrosion in acidic slurry medium. Wear 1995, 186–187, 555–561. [Google Scholar] [CrossRef]
- Flow-Associated Corrosion in Power Plants. Available online: http://www.epri.com/abstracts/Pages/ProductAbstract.aspx?ProductId=TR-106611-R1 (accessed on 15 August 2016).
- Bermudez, M.D.; Jimenez, A.Z.; Martinez, N.G. Study of surface interactions of ionic liquids with aluminium alloys in corrosion and erosion-corrosion processes. Appl. Surf. Sci. 2007, 253, 7295–7302. [Google Scholar] [CrossRef]
- Shrestha, S.; Hodgkiess, T.; Neville, A. The effect of post-treatment of a high-velocity oxy-fuel Ni–Cr–Mo–Si–B coating part 2: Erosion-corrosion behavior. J. Therm. Spray Technol. 2001, 10, 656–665. [Google Scholar] [CrossRef]
- Zhang, J.S.; Li, N. Analysis on liquid metal corrosion-oxidation interactions. Corros. Sci. 2007, 49, 4154–4184. [Google Scholar] [CrossRef]
- Scuratia, A.; Fekeb, D.L.; Manas, Z.I. Analysis of the kinetics of agglomerate erosion in simple shear flows. Chem. Eng. Sci. 2005, 60, 6564–6573. [Google Scholar] [CrossRef]
- Stack, M.M.; Abd El Badia, T.M. On the construction of erosion-corrosion maps for WC/Co–Cr-based coatings in aqueous conditions. Wear 2006, 261, 1181–1190. [Google Scholar] [CrossRef]
- Meng, H.; Hu, X.; Neville, A. A systematic erosion-corrosion study of two stainless. Wear 2007, 263, 355–362. [Google Scholar] [CrossRef]
- Liu, G.J.; Zhu, S. Shaft corrosion problem for the circulating water pum pand treatment. Electr. Power 2006, 39, 52–55. [Google Scholar]
- ASTM G5-14: Standard Reference Test Method for Making Potentiodynamic Anodic Polarization Measurements. Available online: http://www.astm.org/Standards/G5 (accessed on 15 August 2016).
- Stack, M.M.; Abd El Badia, T.M. Mapping erosion-corrosion of WC/Co-Cr based composite coatings: Particle velocity and applied potential effects. Surf. Coat. Technol. 2006, 201, 1335–1347. [Google Scholar] [CrossRef]
- Houle, M.J.; McGuire, R.D. CASTI Guidebook to ASME Section IX—Welding Qualifications; CASTI Publishing Inc.: Edmonton, AB, Canada, 2011. [Google Scholar]
- Essentials–Section V: Nondestructive Examination. Available online: https://www.asme.org/products/courses/essentials-bpv-code-section-v-nondestructive (accessed on 15 August 2016).
- Specification for Carbon Steel Electrodes and Rods for Gas Shielded Arc Welding. Available online: https://pubs.aws.org/p/21/a518a518m2005-specification-for-carbon-steel-electrodes-and-rods-for-gas-sheilded-arc-welding (accessed on 15 August 2016).
- Specification for Tungsten and Oxide Dispersed Tungsten Electrodes for Arc Welding and Cutting. Available online: http://sif.library.illinois.edu/bibframe/html/7654275.html (accessed on 15 August 2016).
- Douglas, J.F.; Gasiorek, J.M.; Swaffield, J.A. Fluid Mechanics, 3rd ed.; Longman Scientific & Technical: London, UK, 1995; p. 300. [Google Scholar]
- Kaewvilai, A.; Wattanathana, W.; Jongrungruangchok, S.; Veranitisagul, C.; Koonsaeng, N.; Laobuthee, A. 3,4-Dihydro-1,3-2H-benzoxazines: Novel reducing agents through one electron donation mechanism and their application as the formation of nano-metallic silver coating. Mater. Chem. Phys. 2015, 167, 9–13. [Google Scholar] [CrossRef]
- Zhang, J.; Guyot, F. Experimental study of the bcc-fcc phase transformations in the Fe-Si at high pressures. Phys. Chem. Miner. 1999, 26, 419–424. [Google Scholar] [CrossRef]
- Srisuwan, N.; Eidhed, K.; Kreatsereekul, N.; Yingsamphanchareon, T.; Kaewvilai, A. The study of heat treatment effects on chromium carbide precipitation of 35Cr-45Ni-Nb alloy for repairing furnace tubes. Metals 2016, 6, 26. [Google Scholar] [CrossRef]


| Atomic Percentage (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| C | Mn | P | S | Si | Cr | Cu | Mo | Ni | Va |
| 0.30 max | 0.29–1.06 | 0.035 max | 0.035 max | 0.10 min | 0.40 max | 0.40 max | 0.15 max | 0.40 max | 0.08 max |
| AWS Classification A5.18 | Atomic Percentage (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | Mn | Si | P | S | Ni | Cr | Mo | V | Cu | |
| ER70S-6 | 0.06–0.15 | 1.40–1.85 | 0.80–1.15 | 0.025 | 0.035 | 0.15 | 0.15 | 0.15 | 0.03 | 0.50 |
| Welding Currents for Welded Pipe Preparation | Crystallite Sizes (nm) | |
|---|---|---|
| α-phase | γ-phase | |
| 60 A | 60.54 | - |
| 70 A | 60.19 | 23.75 |
| 80 A | 59.34 | 48.31 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yingsamphancharoen, T.; Srisuwan, N.; Rodchanarowan, A. The Electrochemical Investigation of the Corrosion Rates of Welded Pipe ASTM A106 Grade B. Metals 2016, 6, 207. https://doi.org/10.3390/met6090207
Yingsamphancharoen T, Srisuwan N, Rodchanarowan A. The Electrochemical Investigation of the Corrosion Rates of Welded Pipe ASTM A106 Grade B. Metals. 2016; 6(9):207. https://doi.org/10.3390/met6090207
Chicago/Turabian StyleYingsamphancharoen, Trinet, Nakarin Srisuwan, and Aphichart Rodchanarowan. 2016. "The Electrochemical Investigation of the Corrosion Rates of Welded Pipe ASTM A106 Grade B" Metals 6, no. 9: 207. https://doi.org/10.3390/met6090207
APA StyleYingsamphancharoen, T., Srisuwan, N., & Rodchanarowan, A. (2016). The Electrochemical Investigation of the Corrosion Rates of Welded Pipe ASTM A106 Grade B. Metals, 6(9), 207. https://doi.org/10.3390/met6090207