Corrosion Behavior of AlSi10Mg Alloy Produced by Additive Manufacturing (AM) vs. Its Counterpart Gravity Cast Alloy
Abstract
:1. Introduction
2. Materials and Methods
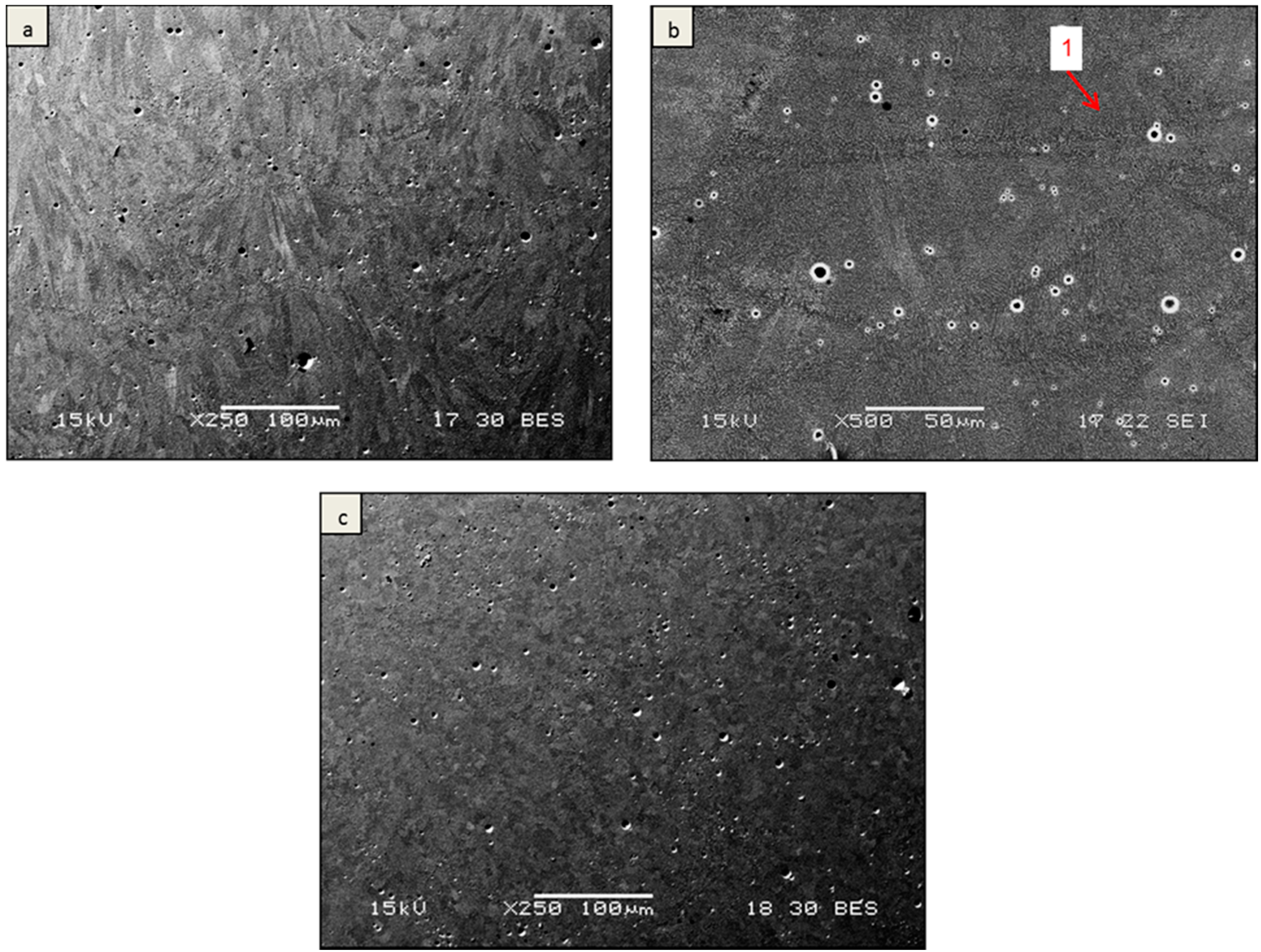
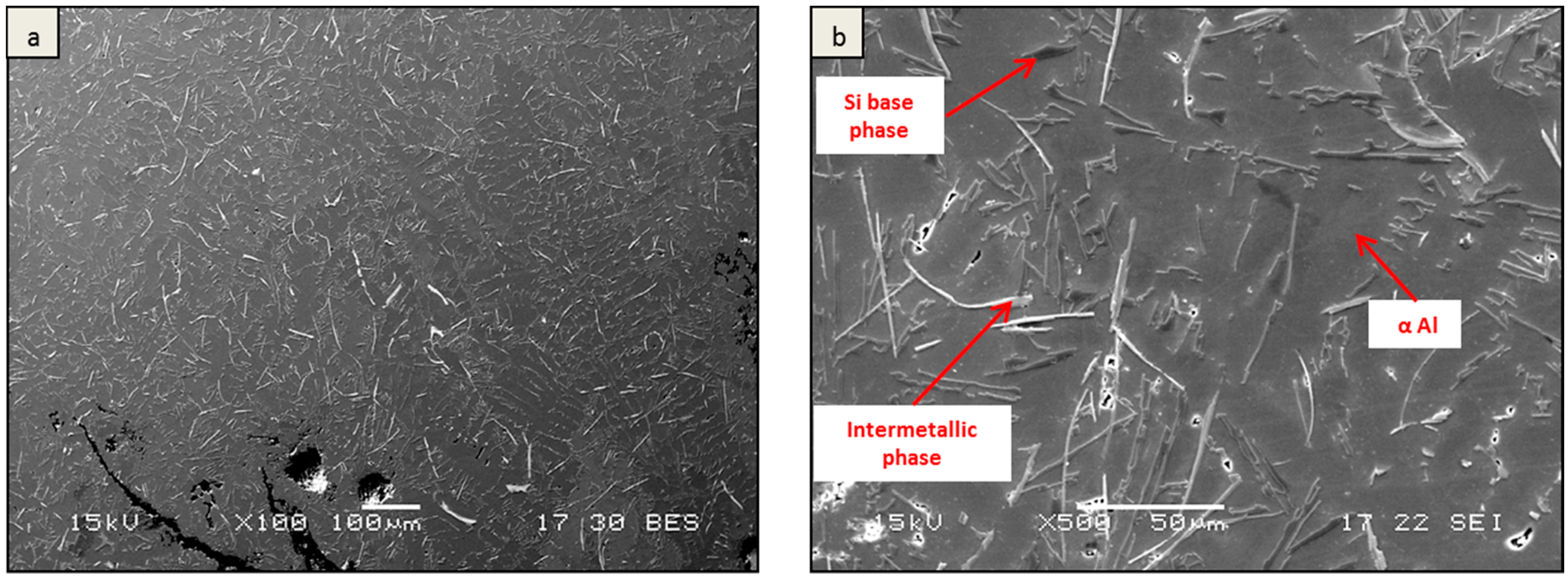
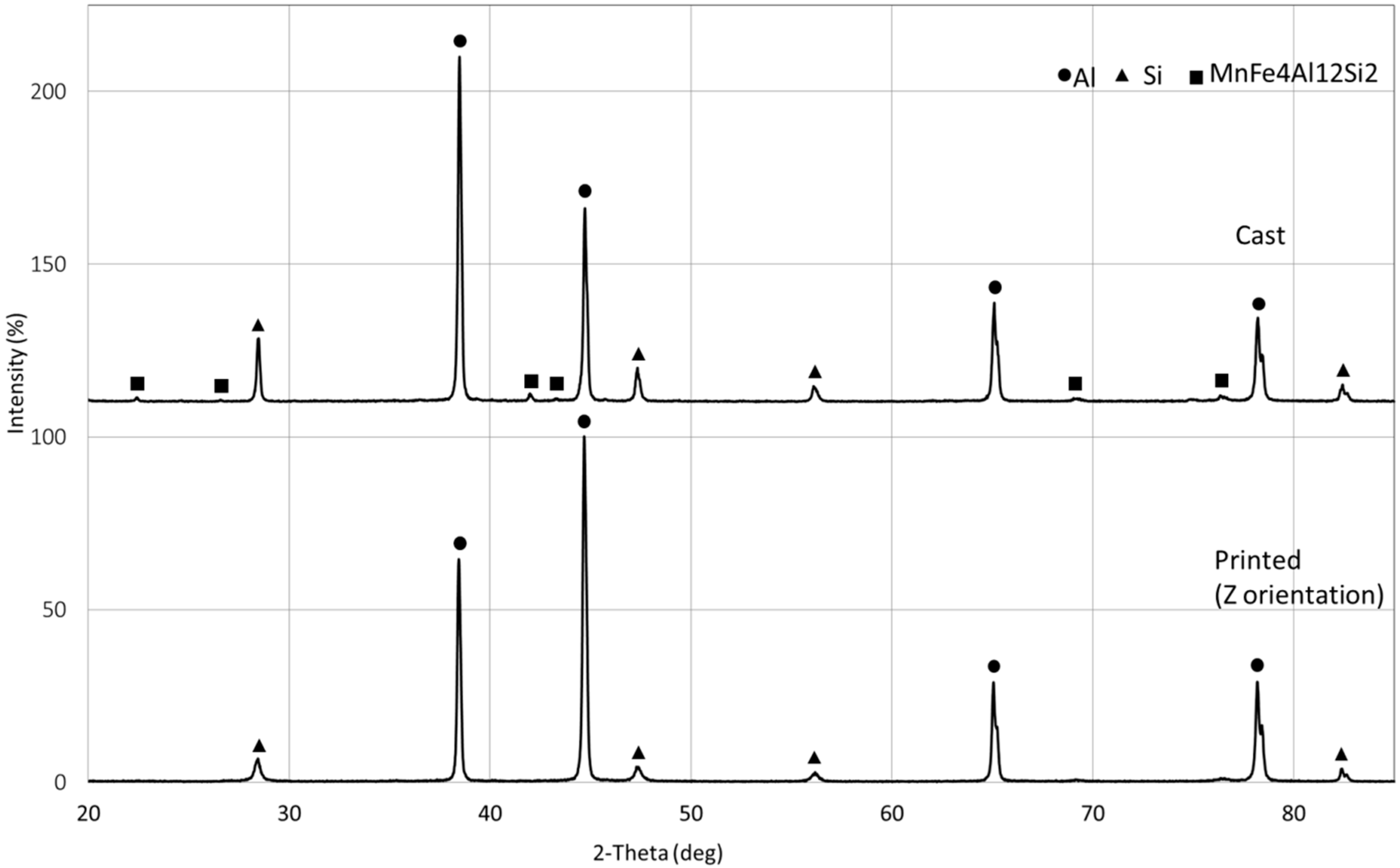
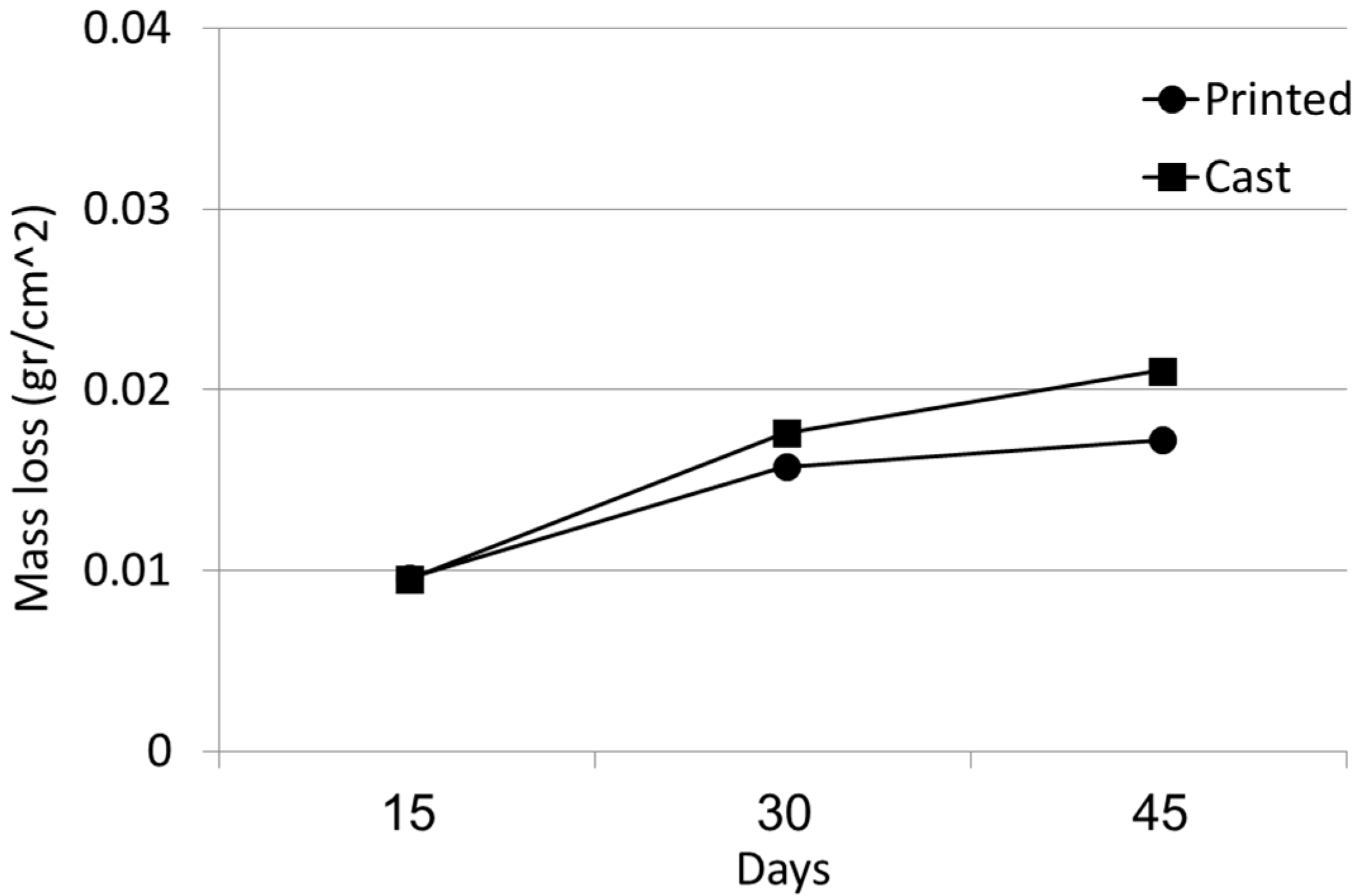
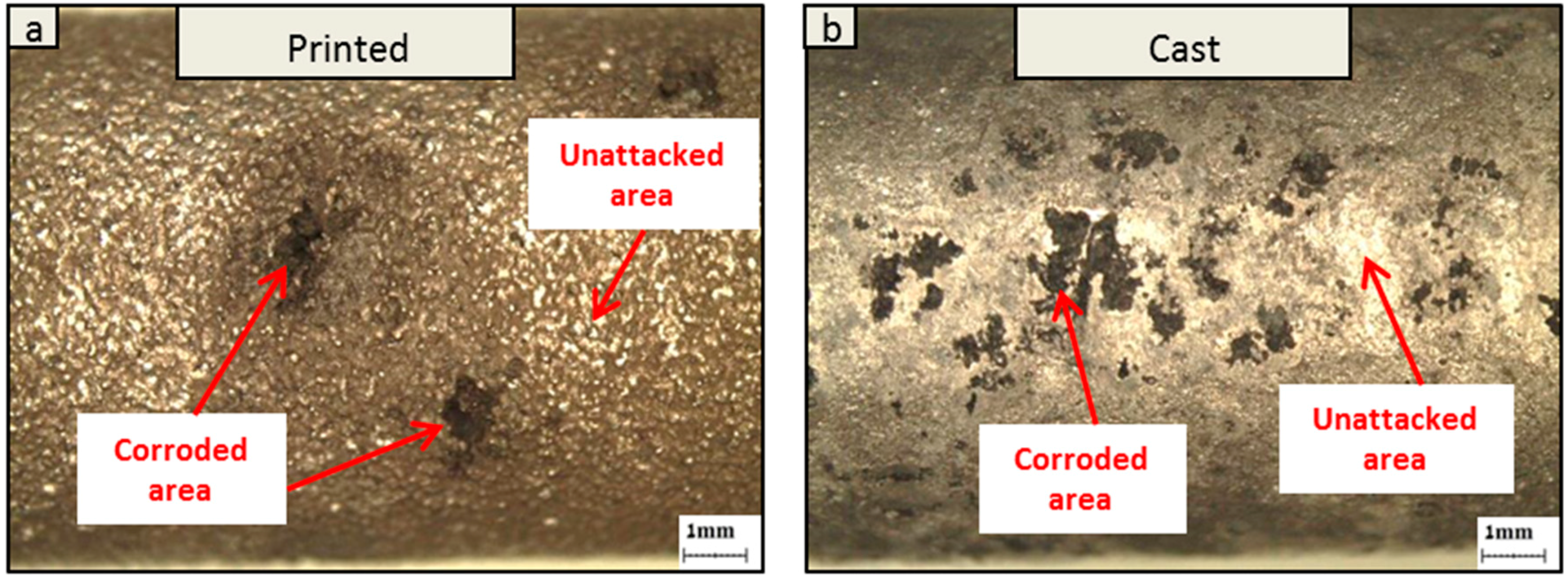
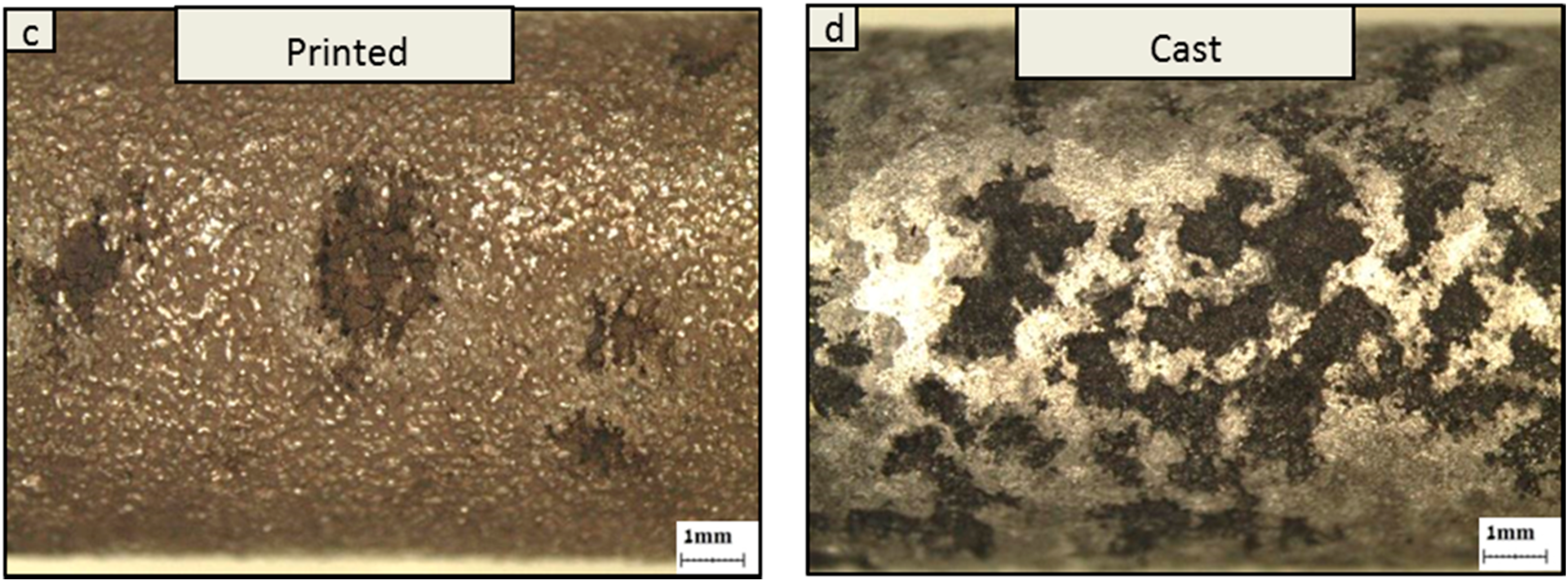
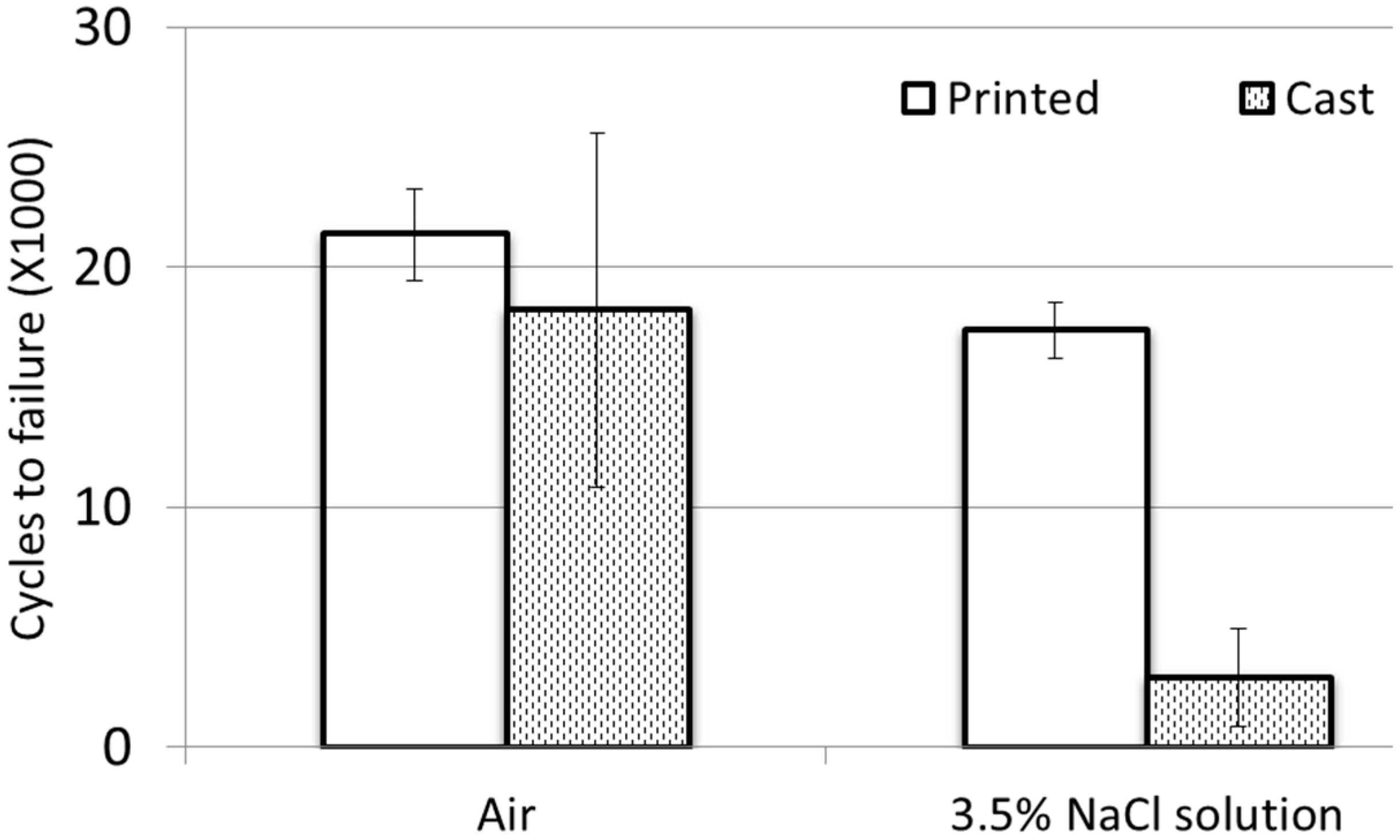
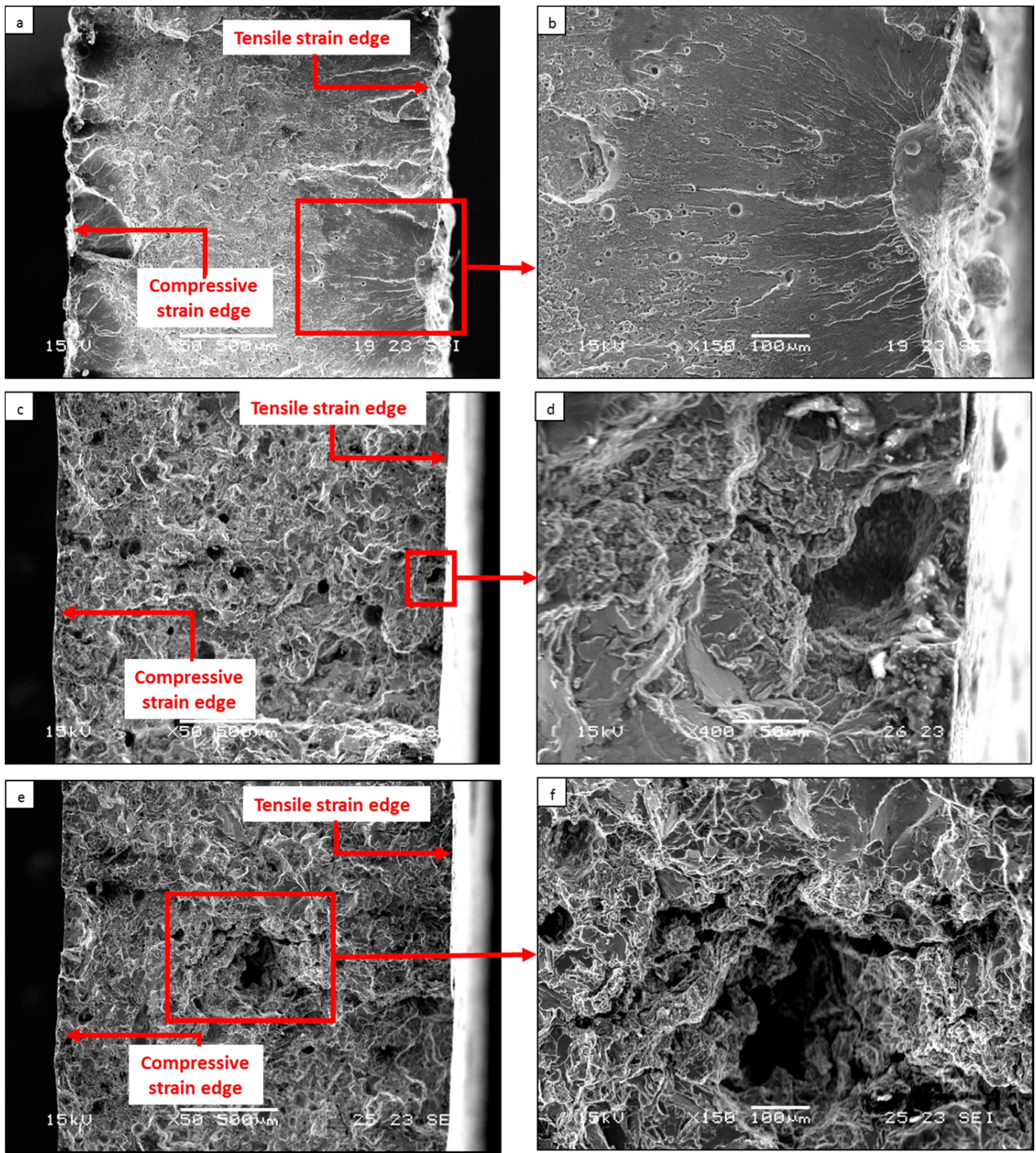
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gao, W.; Zhang, Y.; Ramanujan, D.; Ramani, K.; Chen, Y.; Williams, C.B.; Wang, C.C.L.; Shin, Y.C.; Zhang, S.; Zavattieri, P.D. The status, challenges, and future of additive manufacturing in engineering. Comput. Aided Des. 2015, 69, 65–89. [Google Scholar] [CrossRef]
- Manfredi, D.; Calignano, F.; Krishnan, M.; Canali, R.; Ambrosio, E.P.; Biamino, S.; Ugues, D.; Pavese, M.; Fino, P. Additive Manufacturing of Al Alloys and Aluminium Matrix Composites (AMCs). Light Metal Alloy. Appl. InTech 2014. [Google Scholar] [CrossRef]
- Kempen, L.; Thijs, L.; van Humbeeck, J.; Kruth, J.P. Mechanical properties of AlSi10Mg produced by Selective Laser Melting. Phys. Procedia 2012, 39, 439–446. [Google Scholar] [CrossRef]
- Wang, F. Mechanical property study on rapid additive layer manufacture Hastelloy X alloy by selective laser melting technology. Int. J. Adv. Manuf. Technol. 2012, 58, 545–551. [Google Scholar] [CrossRef]
- Santos, M.C., Jr.; Machado, A.R.; Sales, W.F.; Barrozo, M.A.S.; Ezugwu, M.O. Machining of aluminum alloys: A review. Int. J. Adv. Manuf. Technol. 2016. [Google Scholar] [CrossRef]
- Calignano, F.; Manfredi, D.; Ambrosio, E.P.; Iuliano, L.; Fino, P. Influence of process parameters on surface roughness of aluminum parts produced by DMLS. Int. J. Adv. Manuf. Technol. 2013, 67, 2743–2751. [Google Scholar] [CrossRef]
- Rajamure, R.S.; Vora, H.D.; Srinivasan, S.G.; Dahotre, N.B. Laser alloyed Al-W coatings on aluminum for enhanced corrosion resistance. Appl. Surf. Sci. 2015, 328, 205–214. [Google Scholar] [CrossRef]
- Zaid, B.; Saidi, D.; Benzaid, A.; Hadji, S. Effects of pH and chloride concentration on pitting corrosion of AA6061aluminum alloy. Corros. Sci. 2008, 50, 1841–1847. [Google Scholar] [CrossRef]
- Fulcher, B.A.; Leigh, D.K.; Watt, T.J. Comparison of AlSi10Mg and Al 6061 processed through DMLS. In Proceedings of the Solid Freeform Fabrication (SFF) Symposium, Austin, TX, USA, 4–6 August 2014.
- Levy, G.; Aghion, E. Effect of diffusion coating of Nd on the corrosion resistance of biodegradable Mg implants in simulated physiological electrolyte. Acta Biomater. 2013, 9, 8624–8630. [Google Scholar] [CrossRef] [PubMed]
- Aghion, E.; Levy, G. The effect of Ca on the in vitro corrosion performance of biodegradable Mg-Nd-Y-Zr alloy. J. Mater. Sci. 2010, 45, 3096–3101. [Google Scholar] [CrossRef]
- Unigovski, Y.B.; Lothongkum, G.; Gutman, E.M.; Alush, D.; Cohen, R. Low-cycle fatigue behavior of 316L-type stainless steel in chloride solutions. Corros. Sci. 2009, 51, 3014–3020. [Google Scholar] [CrossRef]
- Itzhak, D.; Aghion, E. An anodic behavior study of an analogical sintered system of austenitic stainless steel in H2SO4 solution. Corros. Sci. 1984, 24, 145–152. [Google Scholar] [CrossRef]
- Itzhak, D.; Aghion, E. Corrosion behavior of hot pressed austenitic stainless steel in H2SO4 solutions at room temperature. Corros. Sci. 1983, 23, 1085–1094. [Google Scholar] [CrossRef]
- Mingo, B.; Arrabal, R.; Pardo, A.; Matykina, E.; Skeldon, P. 3D study of intermetallics and their effect on the corrosion morphology of rheocast aluminium alloy. Mater. Charact. 2016, 112, 122–128. [Google Scholar] [CrossRef]
- Arrabal, R.; Mingo, B.; Pardo, A.; Mohedano, M.; Matykina, E.; Rodríguez, I. Pitting corrosion of rheocast A356 aluminium alloy in 3.5 wt. % NaCl solution. Corros. Sci. 2013, 73, 342–355. [Google Scholar] [CrossRef]
- Szklarska-Smialowska, Z. Pitting corrosion of aluminum. Corros. Sci. 1999, 41, 1743–1767. [Google Scholar] [CrossRef]
- Birbilis, N.; Buchheit, R.G. Electrochemical characteristics of intermetallic phases in aluminum alloys. J. Electrochem. Soc. 2005, 152, 140–151. [Google Scholar] [CrossRef]
- Bronfin, B.; Aghion, E.; von Buch, F.; Schumann, S.; Katsir, M. Die casting magnesium alloys for elevated temperature applications. In Magnesium Technology 2001; Hryn, J.N., Ed.; Wiley & Sons, Inc.: Hoboken, NJ, USA, 2001; pp. 127–130. [Google Scholar] [CrossRef]
- Aghion, E.; Gueta, Y.; Moscovitch, N.; Bronfin, B. Effect of yttrium additions on the properties of grain-refined Mg-3%Nd alloy. J. Mater. Sci. 2008, 43, 4870–4875. [Google Scholar] [CrossRef]
- Hakimi, O.; Aghion, E.; Goldman, J. Improved stress corrosion cracking resistance of a novel biodegradable EW62 Mg alloy by rapid solidification, in simulated electrolytes. Mater. Sci. Eng. 2015, 51, 226–232. [Google Scholar] [CrossRef] [PubMed]


| Wt. % | Si | Mg | Fe | Mn | Ti | Zn | Cu | Ni | Pb | Sn | Al | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alloy | ||||||||||||
| Printed | 10.55 | 0.268 | 0.227 | 0.004 | 0.009 | 0.007 | 0.004 | 0.006 | <0.001 | <0.001 | Bal. | |
| Cast | 10.51 | 0.267 | 0.767 | 0.134 | 0.08 | 0.084 | 0.124 | 0.012 | 0.016 | 0.004 | Bal. | |
| Element (wt. %) | Mn | Fe | Mg | Si | Al | |
|---|---|---|---|---|---|---|
| Tested area | ||||||
| Printed - 1 | 1.55 ± 0.07 | 0.2 ± 0.04 | 0.33 ± 0.01 | 10.56 ± 0.05 | 87.35 ± 0.31 | |
| Cast - 2 | 1.78 ± 0.07 | 0 | 0.1 ± 0.01 | 1.04 ± 0.08 | 96.99 ± 0.35 | |
| Cast - 3 | 1.63 ± 0.06 | 0.07 ± 0.03 | 0.14 ± 0.01 | 37.91 ± 0.13 | 60.25 ± 0.22 | |
| Cast - 4 | 3.81 ± 0.09 | 21.77 ± 0.14 | 0.15 ± 0.02 | 21.29 ± 0.08 | 52.99 ± 0.19 | |
| Parameter | Ecorr (V) | Icorr (µA/cm2) | C.R (mpy) | |
|---|---|---|---|---|
| Specimen | ||||
| Printed | −0.73 | 0.54 | 0.23 | |
| Cast | −0.72 | 0.64 | 0.28 | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leon, A.; Shirizly, A.; Aghion, E. Corrosion Behavior of AlSi10Mg Alloy Produced by Additive Manufacturing (AM) vs. Its Counterpart Gravity Cast Alloy. Metals 2016, 6, 148. https://doi.org/10.3390/met6070148
Leon A, Shirizly A, Aghion E. Corrosion Behavior of AlSi10Mg Alloy Produced by Additive Manufacturing (AM) vs. Its Counterpart Gravity Cast Alloy. Metals. 2016; 6(7):148. https://doi.org/10.3390/met6070148
Chicago/Turabian StyleLeon, Avi, Amnon Shirizly, and Eli Aghion. 2016. "Corrosion Behavior of AlSi10Mg Alloy Produced by Additive Manufacturing (AM) vs. Its Counterpart Gravity Cast Alloy" Metals 6, no. 7: 148. https://doi.org/10.3390/met6070148
APA StyleLeon, A., Shirizly, A., & Aghion, E. (2016). Corrosion Behavior of AlSi10Mg Alloy Produced by Additive Manufacturing (AM) vs. Its Counterpart Gravity Cast Alloy. Metals, 6(7), 148. https://doi.org/10.3390/met6070148








