Metal Injection Molding (MIM) of Magnesium and Its Alloys
Abstract
:1. Introduction
- PM processing of Mg facilitates homogeneous distribution of elements.
- PM allows us to obtain a fine-grained microstructure. The low grain size of the magnesium alloy metal powder can be kept or even reduced to the sub-micrometer scale using PM metal forming techniques, e.g., extrusion or equal channel angular pressing (ECAP) [1,2,3]. However, these techniques are suitable for the production of bar stock and semi-finished products only.
- Difficulties occurring during metal forming, which are due to the hexagonal lattice structure of magnesium, can be avoided by using PM-techniques. In particular, near net shape techniques like powder pressing and sintering of Mg [4], powder forging or metal injection molding (MIM) of Mg [5,6] can be applied.
2. Materials and Methods
2.1. Powder and Feedstock (or Feedstockpreparation)
2.2. Metal Injection Moulding (MIM)
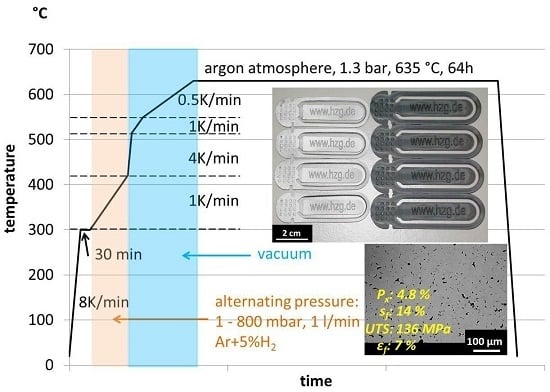
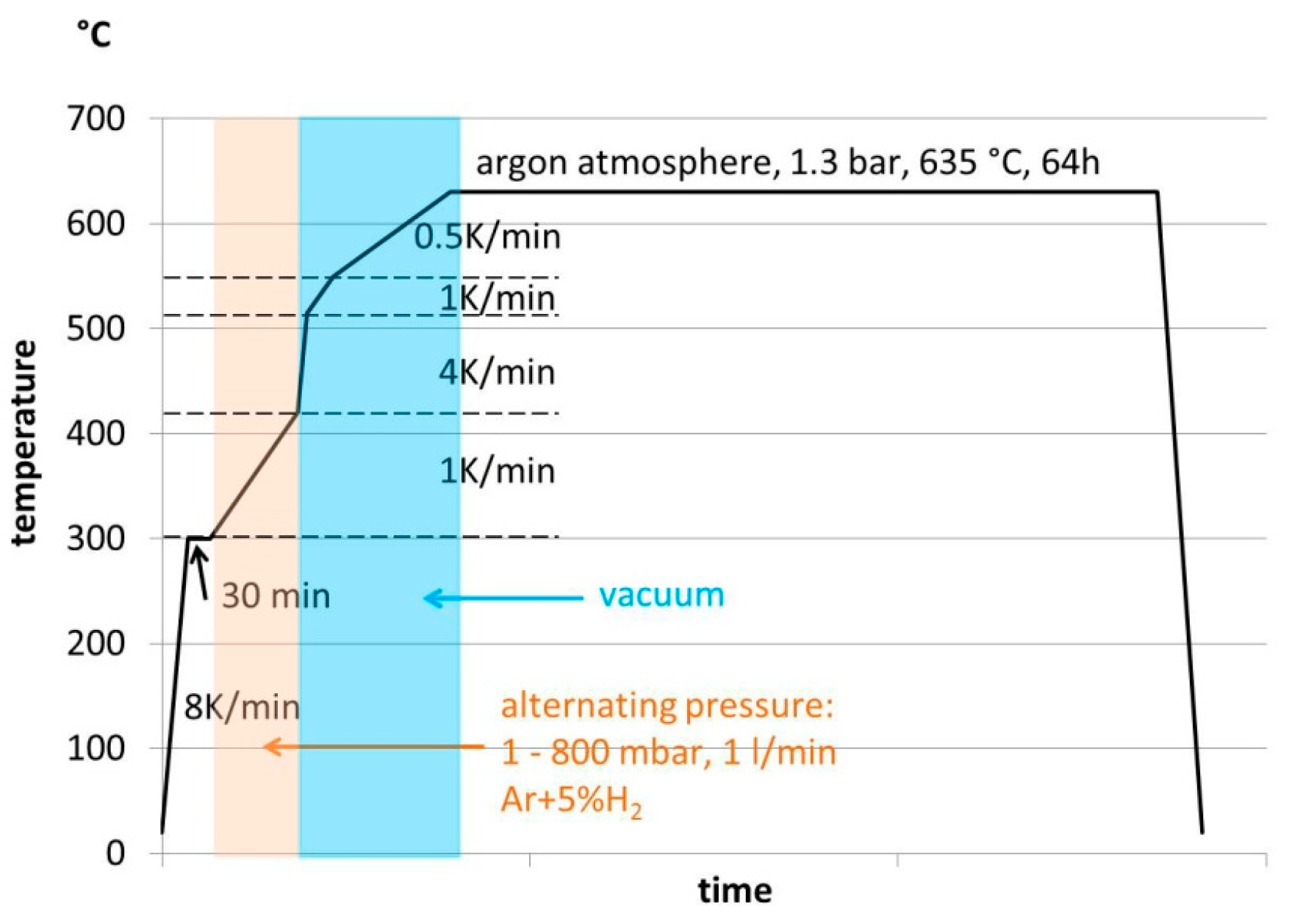
2.3. Debinding and Sintering
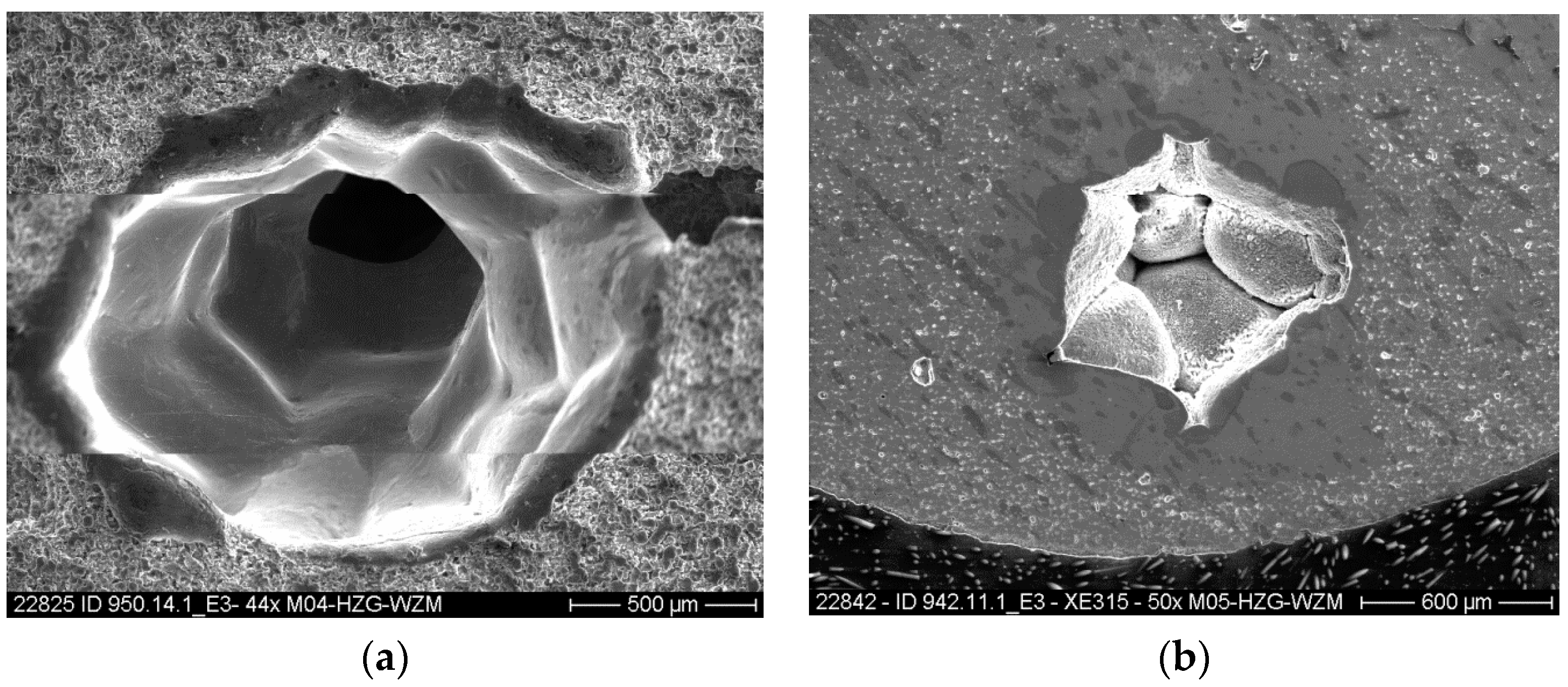
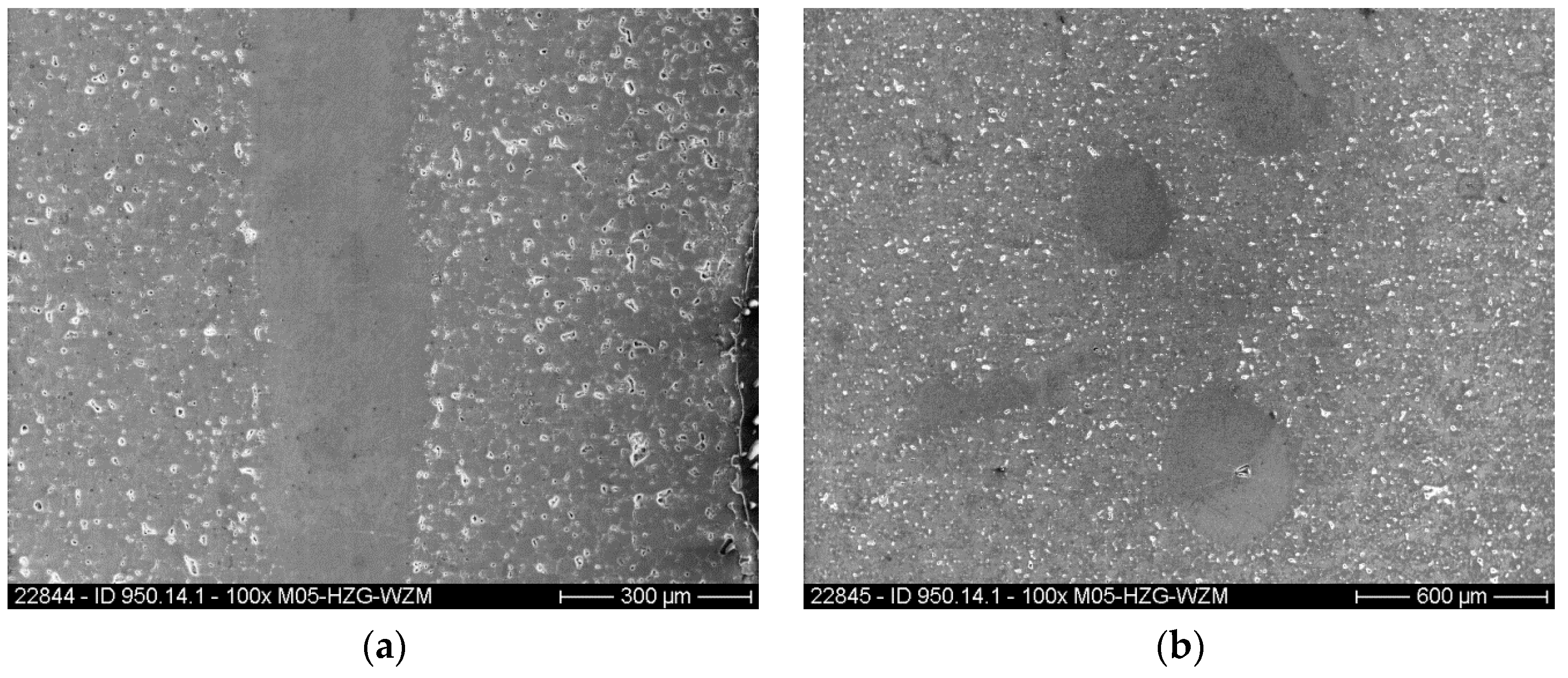
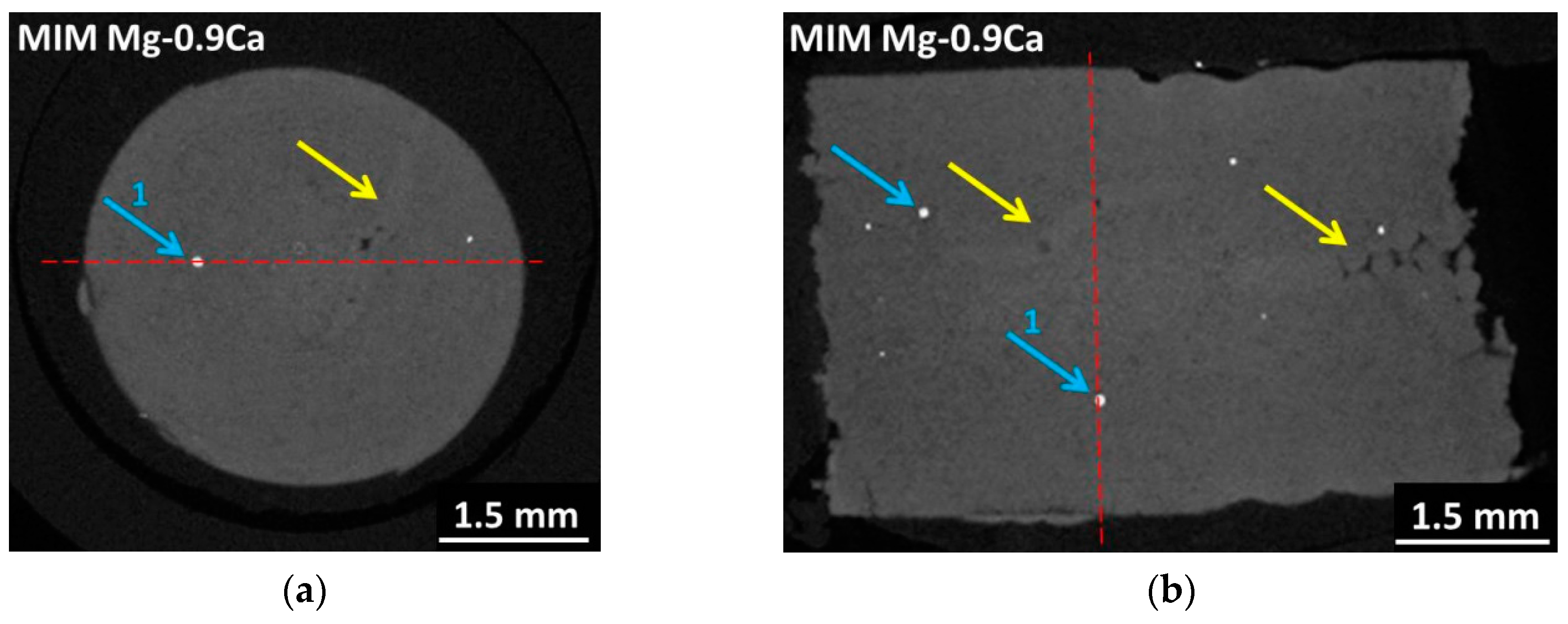
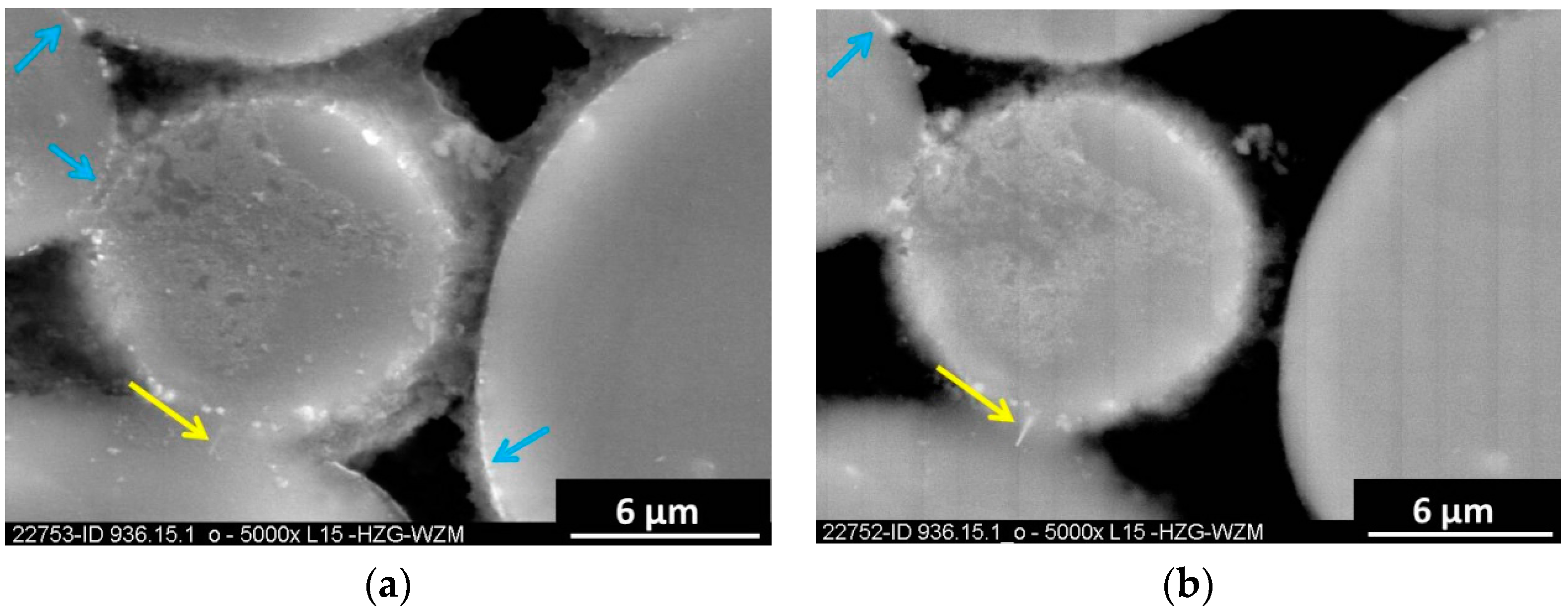
2.4. Characterisation Methods
3. Results and Discussion
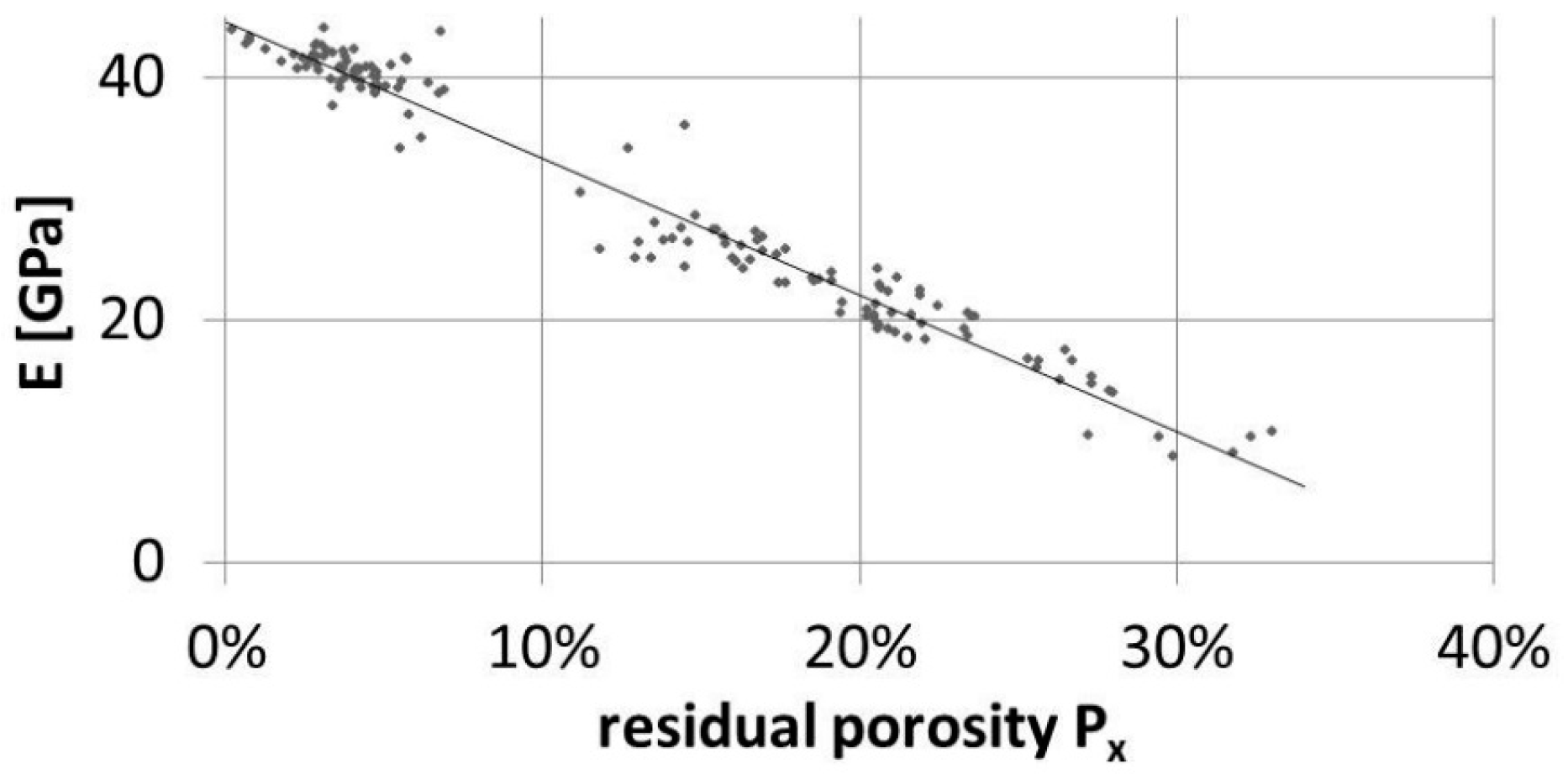
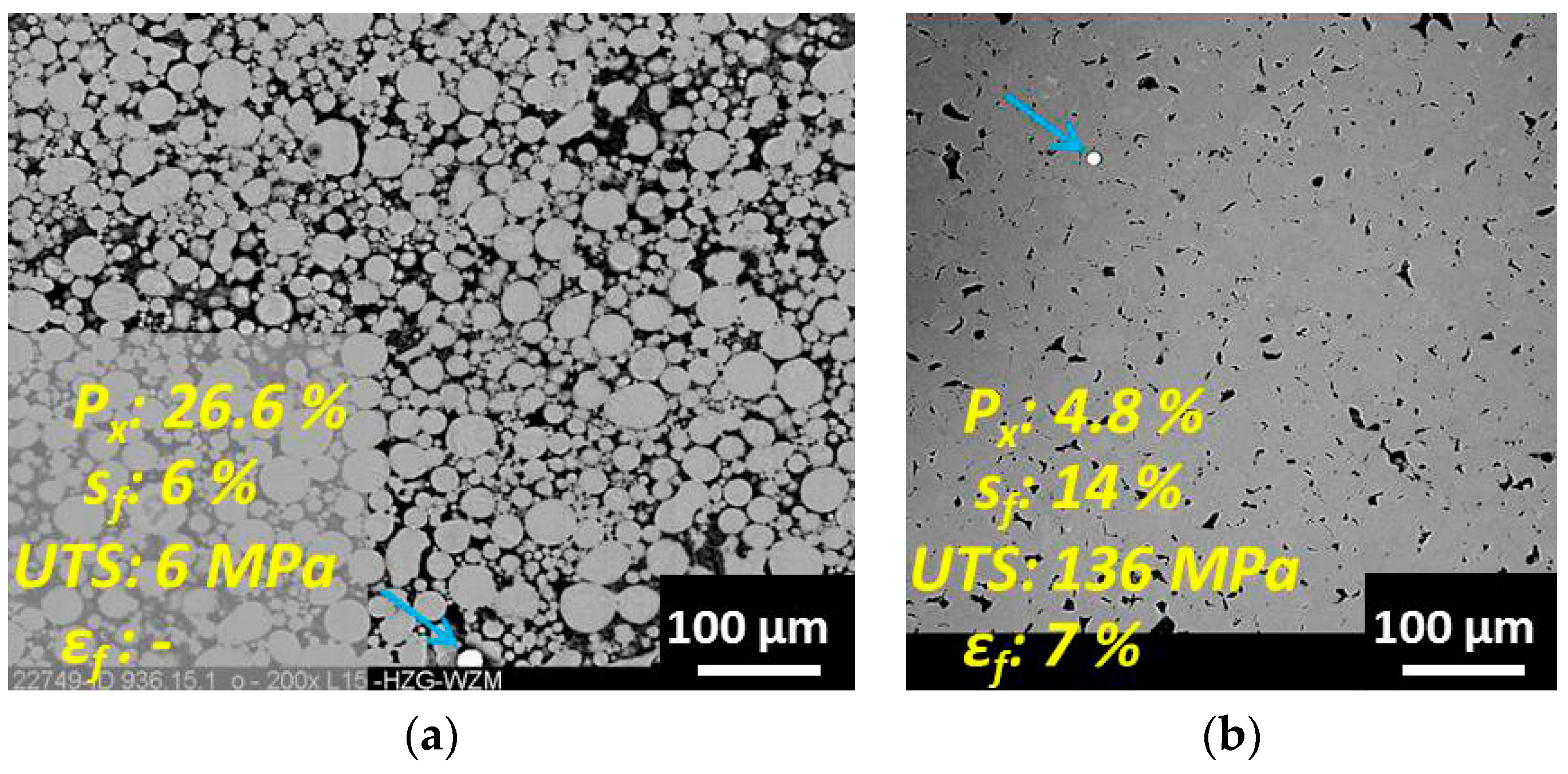
3.1. Young’s Modulus vs. Residual Porosity Relationship
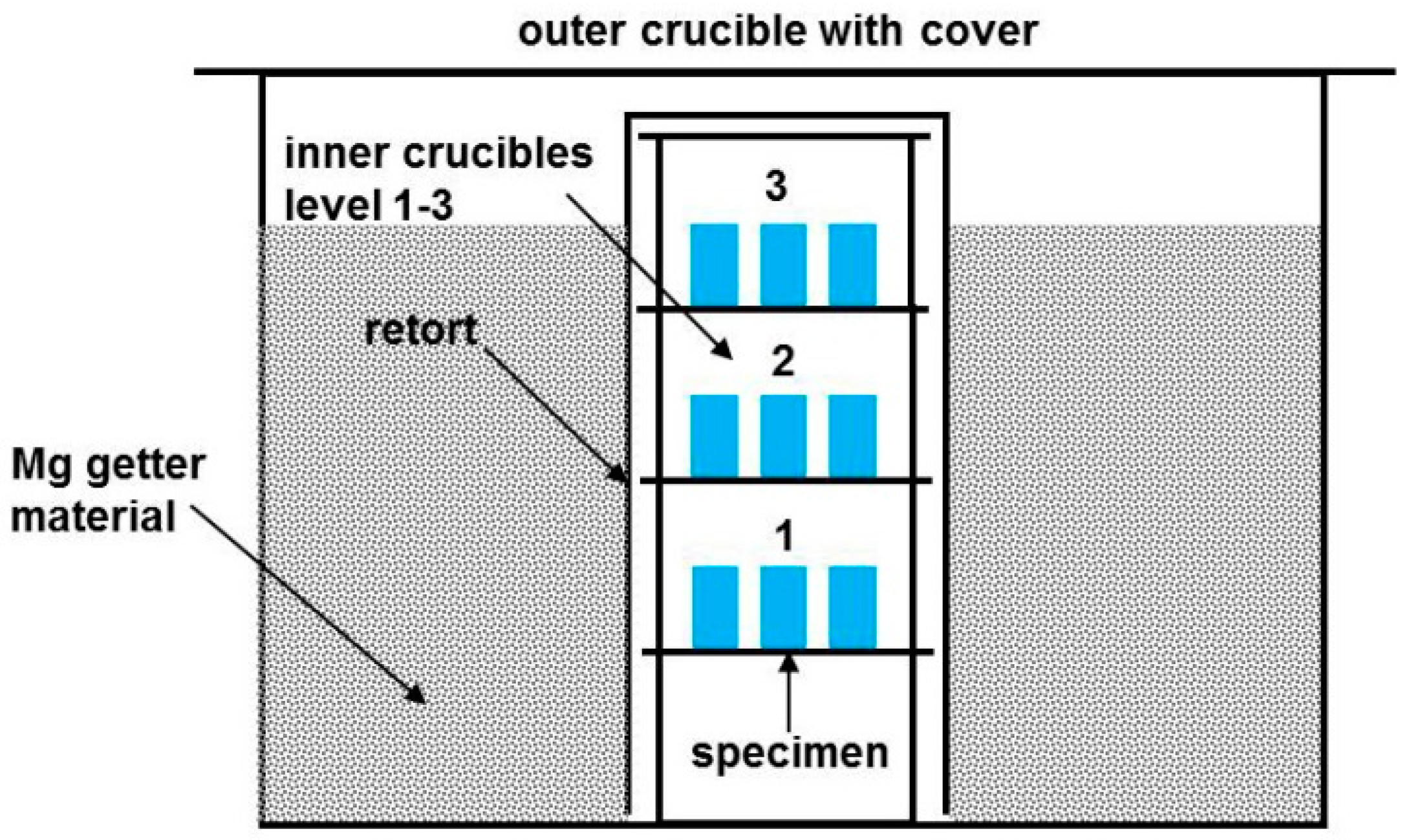
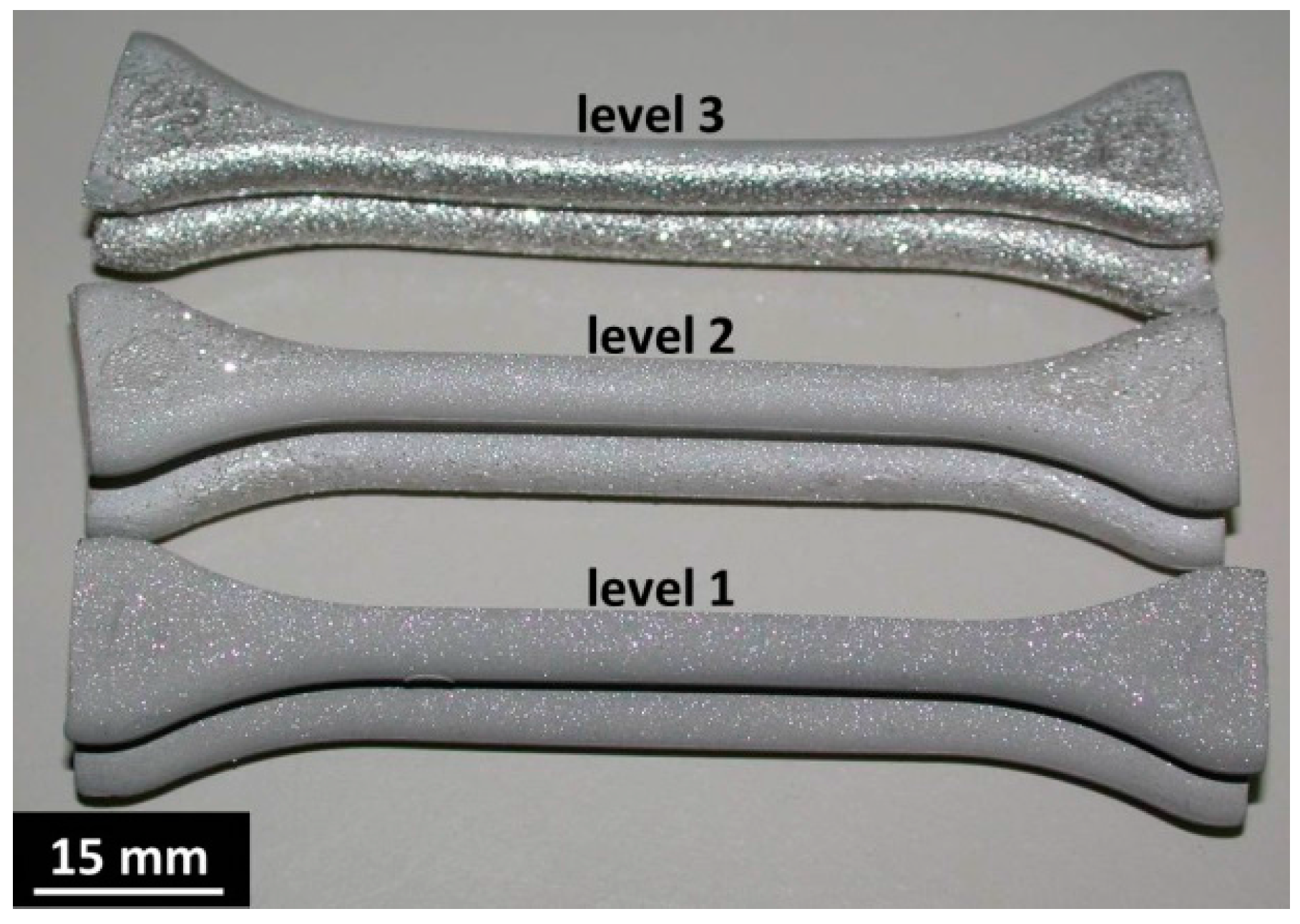
3.2. Furnace and Crucible Requirements
3.3. Feedstock and Recycling Requirements
3.4. Organic Polymer Binder Requirements
- The binder system has to possess a perfect rheological behavior to enable failure-free injection molding of the feedstock. Hence, no jetting blistering or cavity formation in the green compact is acceptable.
- The binder or its thermal debinding products shall not harm the sintering performance of the magnesium powder compact.
- PE contains quite different and more Mg-affine production residue than PP or PB.
- PE degrades in more Mg-affine olefins than PP or PB do.
4. Conclusions
Author Contributions
Conflicts of Interest
Abbreviations
| MIM | metal injection molding |
| PM | powder metallurgy |
| HZG | Helmholtz-Zentrum Geesthacht, Centre for Materials and Coastal Research |
| UTS | ultimate tensile strength |
| TYS | tensile yield strength |
| εf | elongation at fracture |
| E | Young’s modulus |
| Px | residual porosity |
| sf | shrinkage |
| PLDLA | poly L-lactide/DL-lactide copolymer |
| PW 65 | paraffin wax, melting point 65 °C |
| PW 55 | paraffin wax, melting point 55 °C |
| StA | stearic acid |
| PP | polypropylene |
| PB | polybuthene |
| PPcoPB | polypropylene copolymer polybutene |
| PPcoPE | polypropylene copolymer polyethylene |
| PE-VA | polyethylene copolymer vinylacetat |
| Ar + 5% H2 | argon with 5% hydrogen, Arcal 15 |
| Ar 6.0 | argon high purity 99.9999% |
Appendix
References
- Vrátná, J.; Janecek, M. Thermal stability of ultra-fine grained Magnesium Alloy processed by Extrusion and ECAP. In Magnesium Technology 2013; Hort, N., Mathaudhu, S.N., Neelameggham, N.R., Alderman, M., Eds.; Wiley: New York, NY, USA; p. 175ff.
- Dieringa, H.; Hort, N.; Müller, S.; Kainer, K.U. Compression creep at 240 °C of extruded Magnesium Alloy containing Gadolinium. In Proceedings of the Fifth International Light Metals Technology Conference 2011, Lüneburg, Germany, 19–22 July 2011; Dieringa, H., Hort, N., Kainer, K.U., Eds.; Trans Tech Publications Ltd.: Durnten-Zurich, Switzerland; Enfield, NH, USA, 2011; p. 270ff. [Google Scholar]
- Lapovok, R.; Estrin, Y. Superplasticity in Magnesium Alloys by severe plastic deformation. In Advances in Wrought Magnesium Alloys: Fundamentals of Processing, Properties and Applications; Bettles, C., Barnett, M., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2012; Chapter 4; p. 148ff. [Google Scholar]
- Wolff, M.; Dahms, M.; Ebel, T. Sintering of Magnesium. Adv. Eng. Mater. 2010, 12, 829–836. [Google Scholar] [CrossRef]
- Wolff, M.; Schaper, J.G.; Dahms, M.; Ebel, T.; Kainer, K.U.; Klassen, T. Magnesium Powder Injection Moulding for biomedical application. Powder Metall. 2014, 57, 331–340. [Google Scholar] [CrossRef]
- Wolff, M.; Schaper, J.G.; Suckert, M.R.; Dahms, M.; Ebel, T.; Willumeit-Römer, R.; Klassen, T. Magnesium Powder Injection Moulding (MIM) of Orthopedic Implants for Biomedical Applications. JOM 2016, 68, 1191–1197. [Google Scholar] [CrossRef]
- Li, Z.; Gu, X.; Lou, S.; Zheng, Y. The development of binary Mg-Ca alloys for use as biodegradable materials within bone. Biomaterials 2008, 29, 1329–1344. [Google Scholar] [CrossRef] [PubMed]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Witte, F.; Kaese, V.; Haferkamp, H.; Switzer, E.; Meyer-Lindenberg, A.; Wirth, C.J.; Windhagen, H. In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials 2005, 26, 3557–3563. [Google Scholar] [CrossRef] [PubMed]
- Witte, F.; Reifenrath, J.; Müller, P.P.; Crostack, H.A.; Nellesen, J.; Bach, F.W.; Bormann, D.; Rudert, M. Cartilage repair on magnesium scaffolds used as a subchondral bone replacement. Mater. Werkst. 2006, 37, 504–508. [Google Scholar] [CrossRef]
- Witte, F.; Feyerabend, F.; Maier, P.; Fischer, J.; Störmer, M.; Blawert, C.; Dietzel, W.; Hort, N. Biodegradable magnesium-hydroxyapatite metal matrix composites. Biomaterials 2007, 28, 2163–2174. [Google Scholar] [CrossRef] [PubMed]
- Witte, F.; Ulrich, H.; Rudert, M.; Willbold, E. Biodegradable magnesium scaffolds: Part I: Appropriate inflammatory response. J. Biomed. Mater. Res. 2007, 81A, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Witte, F.; Fischer, J.; Nellesen, J.; Crostack, H.A.; Kraese, V.; Pisch, A.; Beckmann, F.; Windhagen, H. In vitro and in vivo corrosion measurements of magnesium alloys. Biomaterials 2006, 27, 1013–1018. [Google Scholar] [CrossRef] [PubMed]
- Witte, F.; Ulrich, H.; Palm, C.; Willbold, E. Biodegradable magnesium scaffolds: Part II: Peri-implant bone remodeling. J. Biomed. Mater. Res. 2007, 81A, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Poumarat, G.; Squire, P. Comparison of mechanical properties of human, bovine bone and a new processed bone xenograft. Biomaterials 1993, 14, 337–340. [Google Scholar] [CrossRef]
- Cunha, A.R.; Umbelino, B.; Correia, M.L.; Neves, M.F. Magnesium and vascular changes in hypertension. Int. J. Hypertens. 2012, 2012, 754250. [Google Scholar] [CrossRef] [PubMed]
- Janning, C.; Willbold, E.; Vogt, C.; Nellesen, J.; Meyer-Lindenberg, A.; Windbergen, H.; Thorey, F.; Witte, F. Magnesium hydroxide temporarily enhancing osteoblast activity and decreasing the osteoclast number in peri-implant bone remodeling. Acta Biomater. 2010, 6, 1861–1868. [Google Scholar] [CrossRef] [PubMed]
- Hort, N.; Dieringa, H.; Kumar, S.T.; Kainer, K.U. Magnesium matrix Composites. In Magnesium Technology, Metallurgy, Design Data, Applications; Friedrich, H.E., Mordike, B.L., Eds.; Springer Berlin Heidelberg: Berlin, Germany, 2006; Chapter 6.3; p. 327ff. [Google Scholar]
- Wolff, M.; Guelck, T.; Ebel, T. Sintering of Mg and MgCa alloys for biomedical applications. In Proceedings of the Euro PM 2009 Powder Metallurgy Congress & Exhibition—PM Biomaterials, Copenhagen, Denmark, 12–14 October 2009; pp. 417–422.
- Wolff, M.; Deussing, J.; Dahms, M.; Ebel, T.; Kainer, K.U.; Klassen, T. Advances in the Metal Injection Moulding of Mg-Ca alloys for biomedical applications. Powder Inject. Mould. Int. 2012, 6, 59–63. [Google Scholar]
- Wolff, M.; Bischof, C.; Dahms, M.; Ebel, T.; Klassen, T. Production of biodegradable Mg-0.9Ca implants by Powder Injection Moulding (PIM). In Proceedings of the 9th International Conference on Magnesium and Their Applications 2012, Vancouver, BC, Canada, 8–12 July 2012; Poole, W.J., Kainer, K.U., Eds.; p. 102.
- Kammer, C. Eigenschaften von reinem Magnesium. In Magnesium Taschenbuch; Aluminium-Verlag: Düsseldorf, Germany, 2000; Chapter 4; p. 86. [Google Scholar]
- Lacroix, D.; Planell, J.A.; Prendergast, P.J. Computer-aided design and finite-element modelling of biomaterial scaffolds for bone tissue engineering. Philos. Trans. R. Soc. A 2009, 367, 1993–2009. [Google Scholar] [CrossRef] [PubMed]
- Bram, M.; Ebel, T.; Wolff, M.; Barbosa, A.P.C.; Tuncer, N. Applications of powder metallurgy in biomaterials. In Advances in Powder Metallurgy; Chang, I., Zhao, Y., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2013; Chapter 18; p. 537ff. [Google Scholar]
- Kashef, S.; Asgari, A.; Hilditch, T.B.; Yan, W.; Goel, V.K.; Quadbeck, P.; Hodgson, P.D. Fracture mechanics of stainless steel foams. Mater. Sci. Eng. A 2013, 578, 115–124. [Google Scholar] [CrossRef]
- Ebel, T.; Ferri, O.M.; Limberg, W.; Oehring, M.; Pyczak, F.; Schimansky, F.-P. Metal Injection Moulding of Titanium and Titanium-Aluminides. Key Eng. Mater. 2012, 520, 153–160. [Google Scholar] [CrossRef]
- Wolff, M.; Wiese, B.; Dahms, M.; Ebel, T. Binder development for Magnesium Powder Injection Moulding. In Proceedings of the Euro PM 2011 Powder Metallurgy Congress & Exhibition—PM Lightweight Materials, Barcelona, Spain, 9–12 October 2011; Volume 2, pp. 271–276.
- Wiese, B. Binder für die MIM-Verarbeitung von Magnesium. Bachelor’s Thesis, University of Applied Sciences FH-Flensburg, Flensburg, Germany, 9 February 2010. [Google Scholar]

| Binder | Abbreviation | Manufacturer |
|---|---|---|
| paraffin wax | PW 65 | Fisher Scientific |
| paraffin wax | PW 55 | Merck |
| stearic acid | StA | Merck |
| polypropylene | PP | - |
| polybuthene | PB | - |
| polypropylene copolymer polybutene | PPcoPB | Sigma-Aldrich |
| polypropylene copolymer polyethylene | PPcoPE | * |
| polyethylene copolymer venylacetate | PE-VA | Bassell |
| Polymer | UTS (MPa) | YTS (MPa) | εf |
|---|---|---|---|
| 35% PE-VA | 4 ± 1 | - | - |
| 35% PPcoPE | 136 ± 6 | 68 ± 2 | 7 ± 2 |
| 25% PPcoPE | 142 ± 5 | 67 ± 1 | 8 ± 1 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolff, M.; Schaper, J.G.; Suckert, M.R.; Dahms, M.; Feyerabend, F.; Ebel, T.; Willumeit-Römer, R.; Klassen, T. Metal Injection Molding (MIM) of Magnesium and Its Alloys. Metals 2016, 6, 118. https://doi.org/10.3390/met6050118
Wolff M, Schaper JG, Suckert MR, Dahms M, Feyerabend F, Ebel T, Willumeit-Römer R, Klassen T. Metal Injection Molding (MIM) of Magnesium and Its Alloys. Metals. 2016; 6(5):118. https://doi.org/10.3390/met6050118
Chicago/Turabian StyleWolff, Martin, Johannes G. Schaper, Marc René Suckert, Michael Dahms, Frank Feyerabend, Thomas Ebel, Regine Willumeit-Römer, and Thomas Klassen. 2016. "Metal Injection Molding (MIM) of Magnesium and Its Alloys" Metals 6, no. 5: 118. https://doi.org/10.3390/met6050118
APA StyleWolff, M., Schaper, J. G., Suckert, M. R., Dahms, M., Feyerabend, F., Ebel, T., Willumeit-Römer, R., & Klassen, T. (2016). Metal Injection Molding (MIM) of Magnesium and Its Alloys. Metals, 6(5), 118. https://doi.org/10.3390/met6050118