Electrochemical Surface Treatment of a β-titanium Alloy to Realize an Antibacterial Property and Bioactivity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specimen Preparation
2.2. MAO Treatment
2.3. Surface Characterization and Bioactivity Evaluation
2.4. Bacterial Adhesion Test
3. Results and Discussion
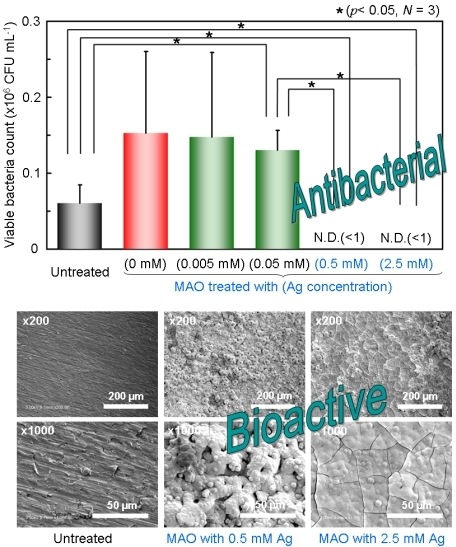
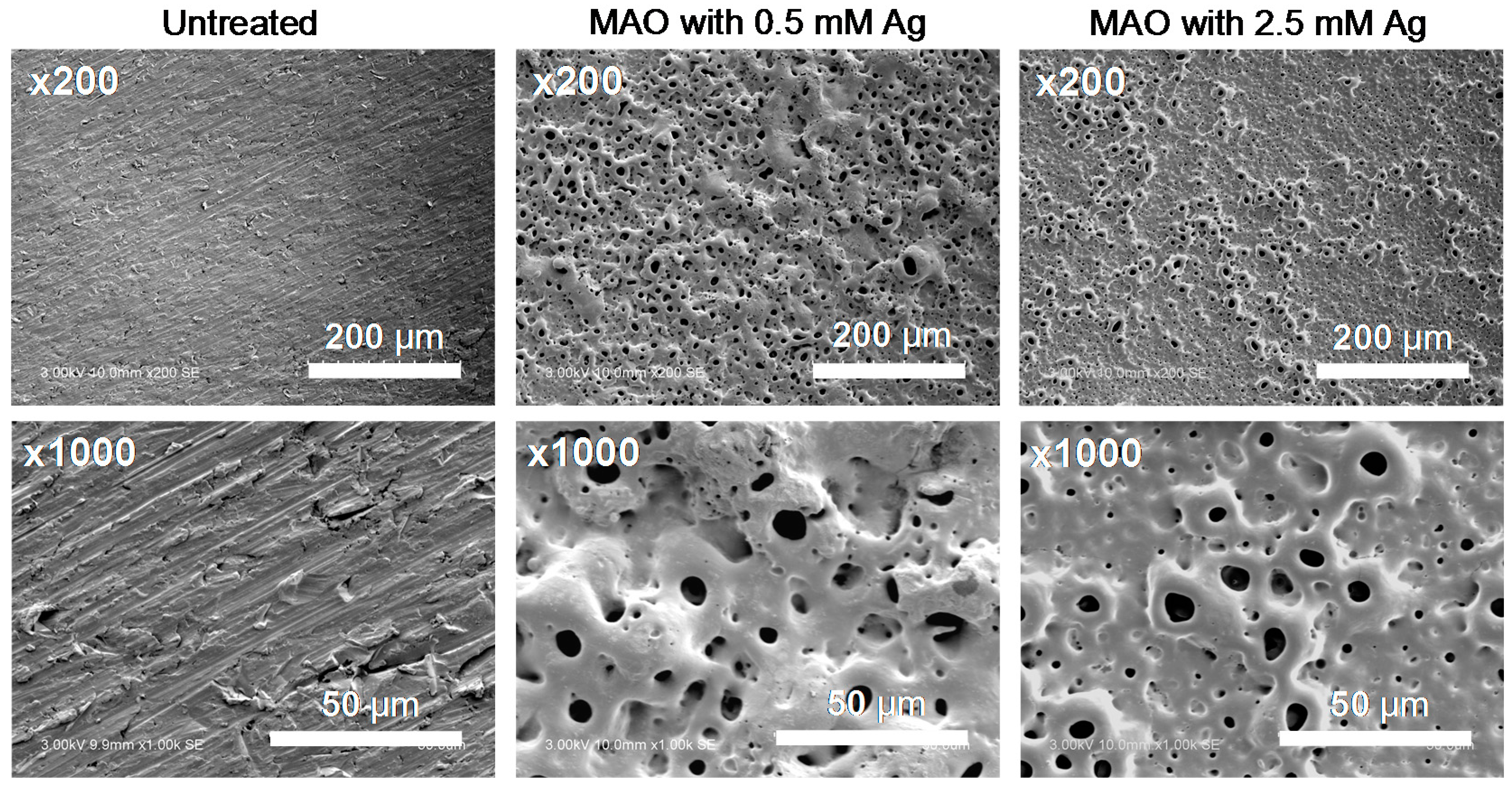
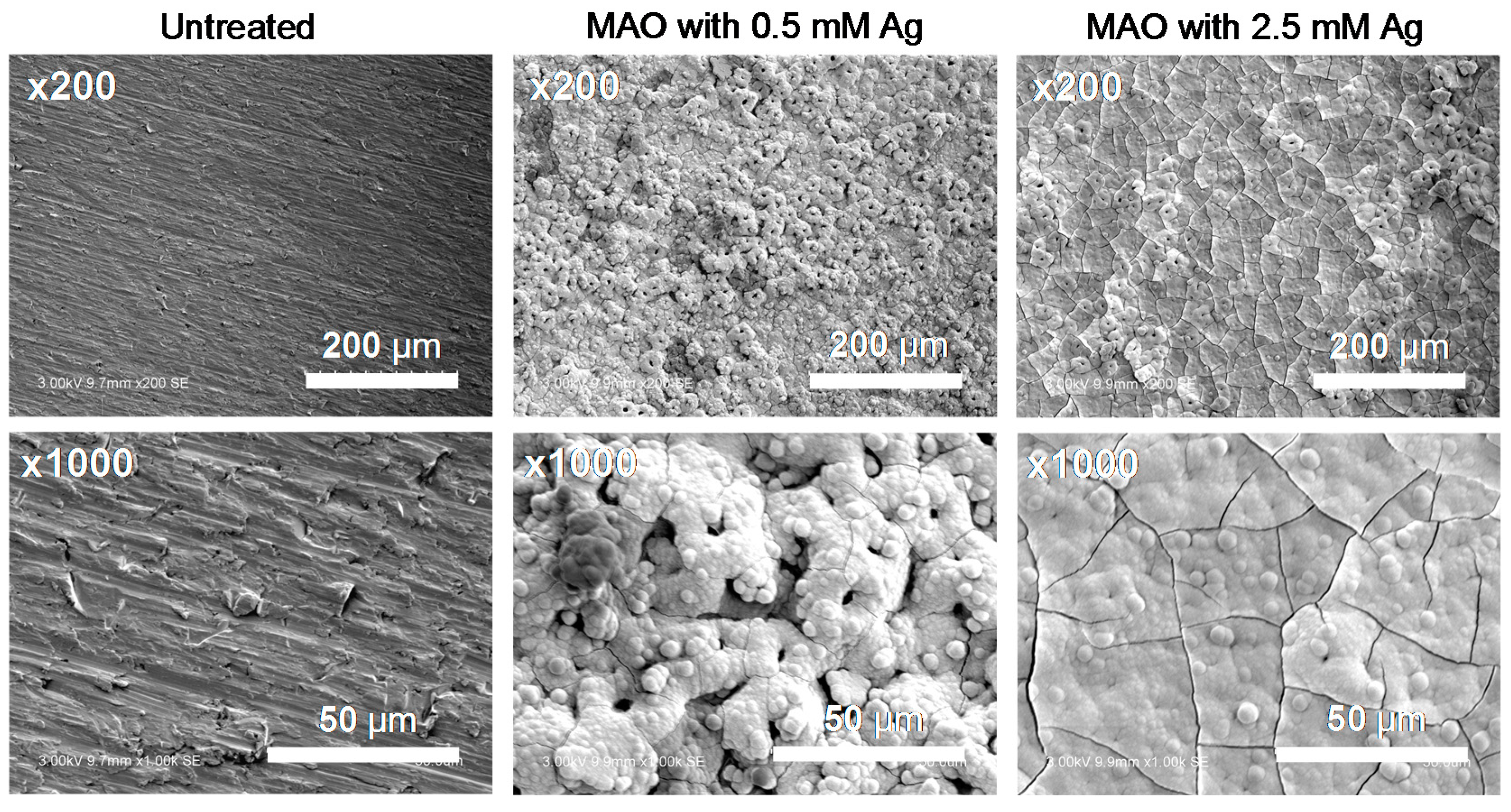
3.1. Surface Properties after MAO Treatment
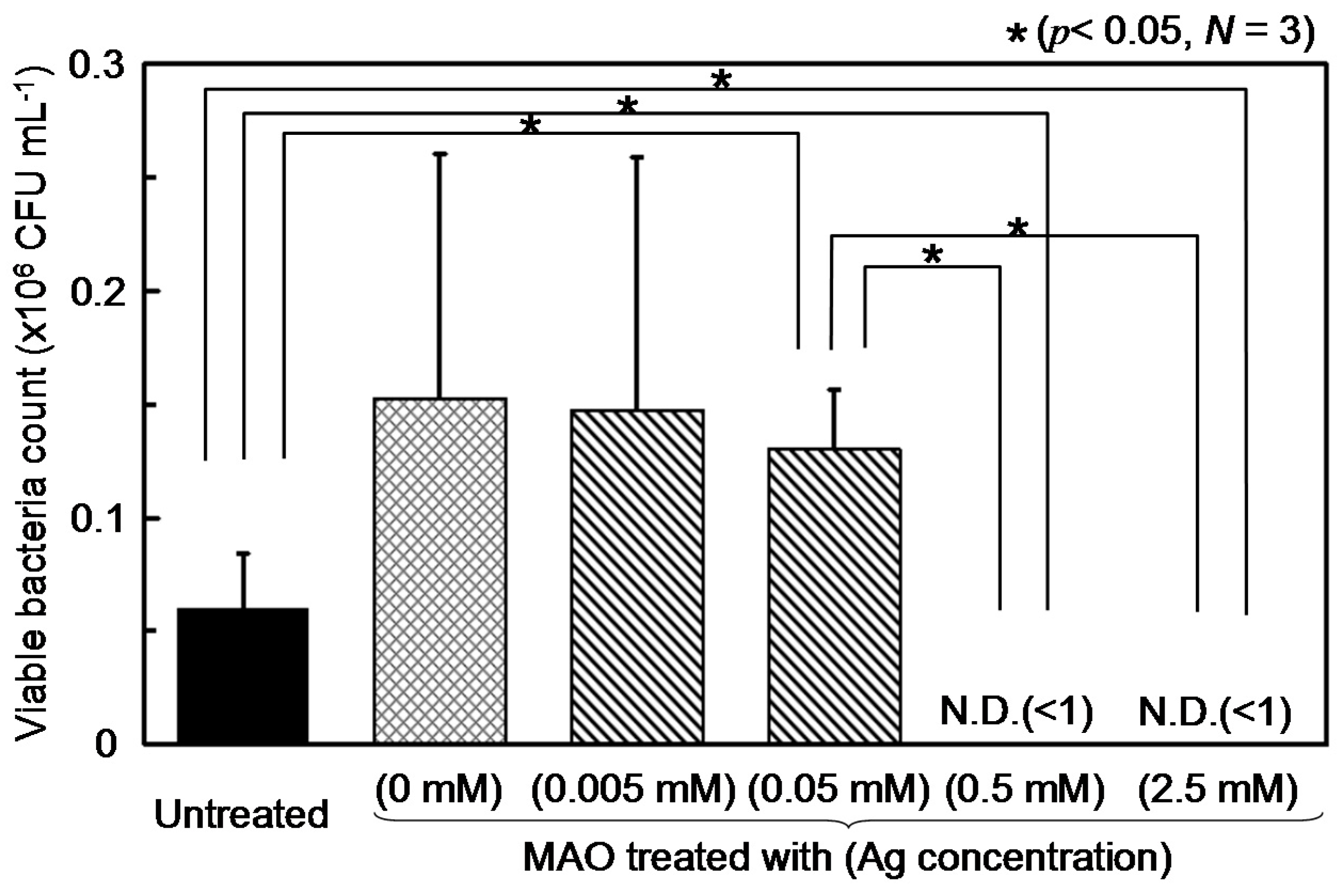
3.2. Antibacterial Property
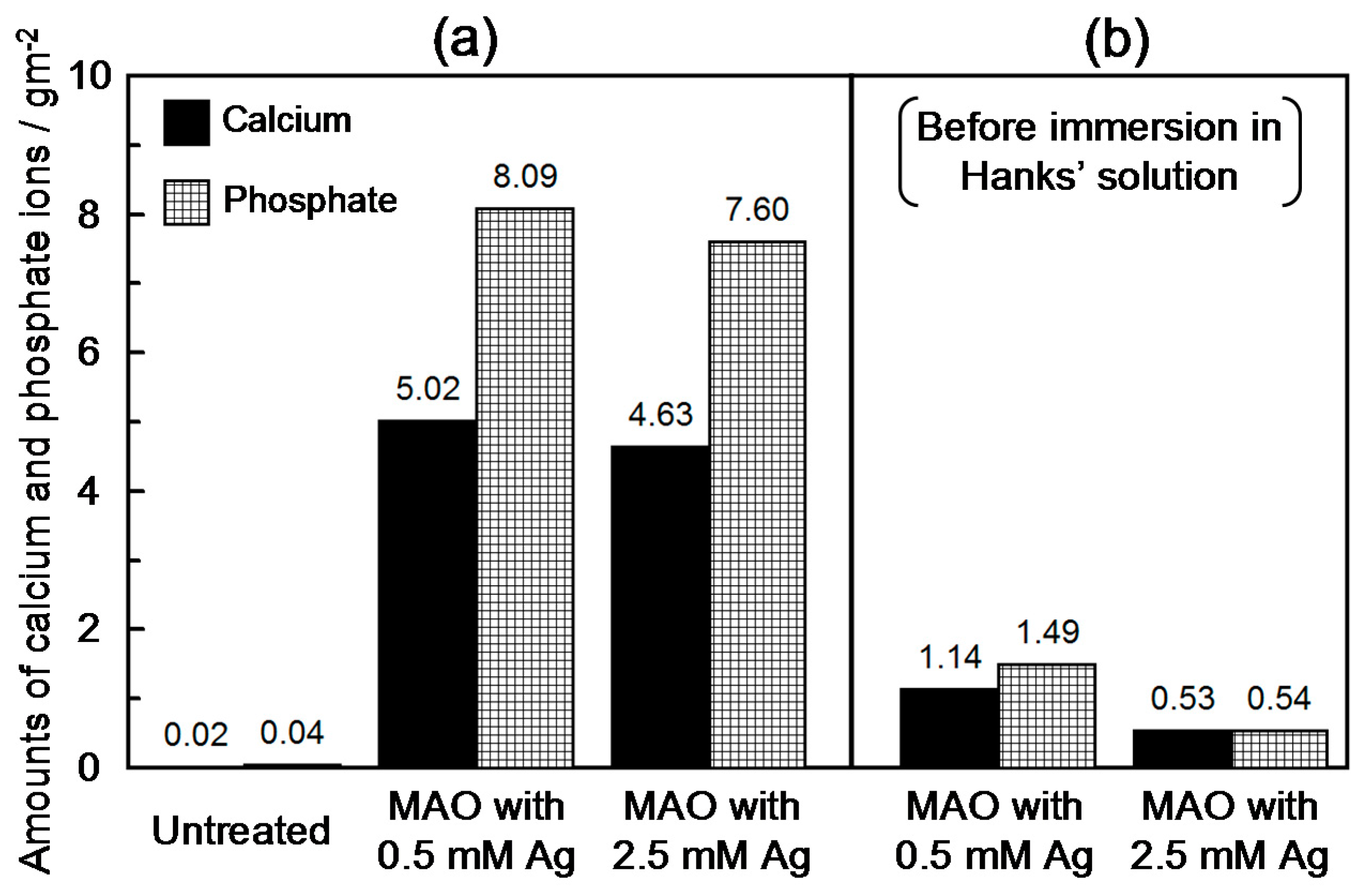
3.3. Calcium Phosphate Formation in Hanks’ Solution
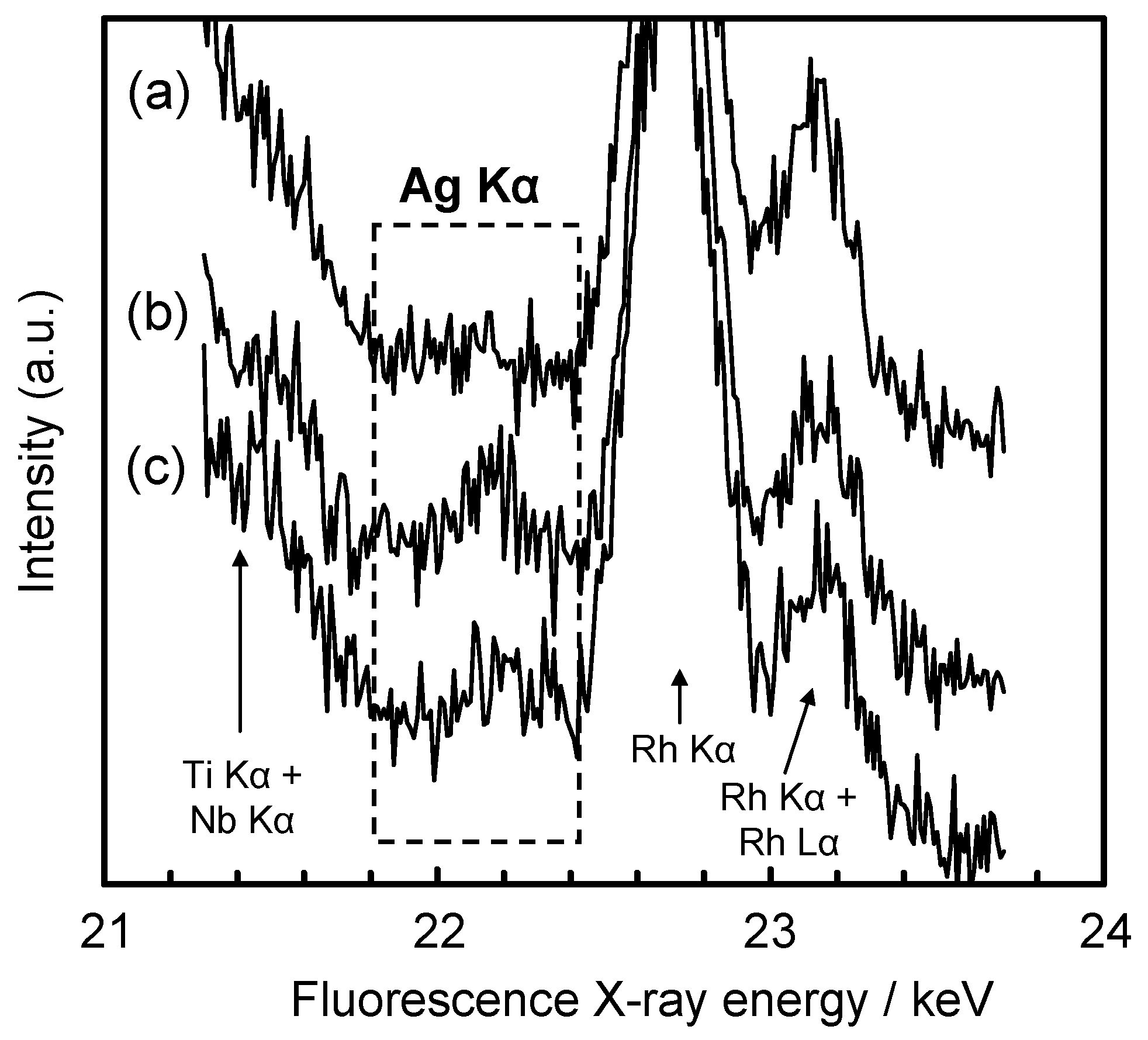
3.4. Efficacy of Ag Incorporated on TNTZ Surface
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| TNTZ | Ti-29Nb-13Ta-4.6Zr alloy |
| MAO | Micro-arc oxidation |
| E. coli | Escherichia coli |
References
- Niinomi, M. Fatigue performance and cyto-toxicity of low rigidity titanium alloy, Ti-29Nb-13Ta-4.6Zr. Biomaterials 2003, 24, 2673–2683. [Google Scholar] [CrossRef]
- Hao, Y.L.; Niinomi, M.; Kuroda, D.; Fukunaga, K.; Yang, R.; Suzuki, A. Aging response of the Young’s modulus and mechanical properties of Ti-29Nb-13Ta-4.6Zr for biomedical applications. Metall. Mater. Trans. A 2003, 34, 1007–1013. [Google Scholar] [CrossRef]
- Niinomi, M.; Hattori, T.; Morikawa, K.; Kasuga, T.; Suzuki, A.; Fukui, H.; Niwa, S. Development of low rigidity β-type titanium alloy for biomedical applications. Mater. Trans. 2002, 43, 2970–2977. [Google Scholar] [CrossRef]
- Suh, J.Y.; Janga, B.C.; Zhu, X.; Ong, J.L.; Kim, K.H. Effect of hydrothermally treated anodic oxide films on osteoblast attachment and proliferation. Biomaterials 2003, 24, 347–355. [Google Scholar] [CrossRef]
- Son, W.W.; Zhu, X.; Shin, H.I.; Ong, J.L.; Kim, K.H. In vivo histological response to anodized and anodized/hydrothermally treated titanium implants. J. Biomed. Mater. Res. Part B Appl. Biomater. 2003, 66B, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Ducheyne, P. Quasi-biological apatite film induced by titanium in a simulated body fluid. J. Biomed. Mater. Res. A 1998, 41, 341–348. [Google Scholar] [CrossRef]
- Hanawa, T. Titanium and Its Oxide Film Substrate for Formation of Apatite. In The Bone-Biomaterial Interface; Davies, J.E., Ed.; University of Toronto Press: Toronto, ON, Canada, 2002; pp. 49–61. [Google Scholar]
- Hanawa, T.; Ota, M. Calcium phosphate naturally formed on titanium in electrolyte solution. Biomaterials 1991, 12, 767–774. [Google Scholar] [CrossRef]
- Wever, D.J.; Veldhuizen, A.G.; de Vries, J.; Busscher, H.J.; Uges, D.R.A.; van Horn, J.R. Electrochemical and surface characterization of a nickel-titanium alloy. Biomaterials 1998, 19, 761–769. [Google Scholar] [CrossRef]
- Hanawa, T.; Okuno, O.; Hamanaka, H. Compositional change in surface of Ti–Zr alloys in artificial bioliquid. J. Jpn. Inst. Met. 1992, 56, 1168–1173. [Google Scholar]
- Ong, J.L.; Lucas, L.C.; Raikar, G.N.; Connatser, R.; Gregory, J.C. Spectroscopic characterization of passivated titanium in a physiologic solution. J. Mater. Sci. Mater. Med. 1995, 6, 113–119. [Google Scholar] [CrossRef]
- Hiromoto, S.; Hanawa, T.; Asami, K. Composition of surface oxide film of titanium with culturing murine fibroblasts L929. Biomaterials 2004, 25, 979–986. [Google Scholar] [CrossRef]
- Tsutsumi, Y.; Niinomi, M.; Nakai, M.; Tsutsumi, H.; Doi, D.; Nomura, N.; Hanawa, T. Micro-arc oxidation treatment to improve the hard-tissue compatibility of Ti-29Nb-13Ta-4.6Zr alloy. Appl. Surf. Sci. 2012, 262, 34–38. [Google Scholar] [CrossRef]
- Ha, J.Y.; Tsutsumi, Y.; Doi, H.; Nomura, N.; Kim, K.H.; Hanawa, T. Enhancement of calcium phosphate formation on zirconium by micro-arc oxidation and chemical treatments. Surf. Coat. Technol. 2011, 205, 4948–4955. [Google Scholar] [CrossRef]
- Dibart, S.; Warbington, M.; Su, M.F.; Skobe, Z. In vitro evaluation of the implant-abutment bacterial seal: The locking taper system. Int. J. Oral Maxillofac Implant. 2005, 20, 732–737. [Google Scholar]
- Glauser, R.; Schupbach, P.; Gottlow, J.; Hammerle, C.H. Periimplant soft tissue barrier at experimental one-piece mini-implants with different surface topography in humans: A light-microscopic overview and histometric analysis. Clin. Implant. Dent. Relat. Res. 2005, 7, S44–S51. [Google Scholar]
- Tesmer, M.; Walle, S.; Koutouzis, T.; Lundgren, T. Bacterial colonization of the dental implant fixture-abutment interface: An in vitro study. J. Periodontol. 2009, 80, 1991–1997. [Google Scholar] [CrossRef] [PubMed]
- MacKintosh, E.E.; Patel, J.D.; Marchant, R.E.; Anderson, J.M. Effects of biomaterial surface chemistry on the adhesion and biofilm formation of Staphylococcus epidermidis in vitro. J. Biomed. Mater. Res. A 2006, 78, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Nikaido, T.; Ikeda, M.; Imai, S.; Hanada, N.; Tagami, J.; Matin, K. Surface properties of resin composite materials relative to biofilm formation. Dent. Mater. J. 2007, 26, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Meredith, D.O.; Eschbach, L.; Wood, M.A.; Riehle, M.O.; Curtis, A.S.; Richards, R.G. Human fibroblast reactions to standard and electropolished titanium and Ti-6Al-7Nb, and electropolished stainless steel. J. Biomed. Mater. Res. A 2005, 75, 541–555. [Google Scholar] [CrossRef] [PubMed]
- Koerner, R.J.; Butterworth, L.A.; Mayer, I.V.; Dasbach, R.; Busscher, H.J. Bacterial adhesion to titanium-oxy-nitride (TiNOX) coatings with different resistivities: A novel approach for the development of biomaterials. Biomaterials 2002, 23, 2835–2840. [Google Scholar] [CrossRef]
- Simonetti, N.N.; Simonetti, G.; Bougnol, F.; Scalzo, M. Electrochemical Ag+ for preservative use. Appl. Environ. Microbiol. 1992, 58, 3834–3836. [Google Scholar] [PubMed]
- Wassall, M.A.; Santin, M.; Isalberti, C.; Cannas, M.; Denyer, S.P. Adhesion of bacteria to stainless steel and silver-coated orthopedic external fixation pins. J. Biomed. Mater. Res. A 1997, 36, 325–330. [Google Scholar] [CrossRef]
- Massè, A.; Bruno, A.; Bosetti, M.; Biasibetti, A.; Cannas, M.; Gallinaro, P. Prevention of pin track infection in external fixation with silver coated pins: Clinical and microbiological results. J. Biomed. Mater. Res. B Appl. Biomater. 2000, 53, 600–604. [Google Scholar]
- Tanaka, Y.; Kobayashi, E.; Hiromoto, S.; Asami, K.; Imai, H.; Hanawa, T. Calcium phosphate formation on titanium by low-voltage electrolytic treatments. J. Mater. Sci. Mater. Med. 2007, 18, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Han, Y.; Lu, C. The effect of chemical treatment on apatite-forming ability of the macroporous zirconia films formed by micro-arc oxidation. Appl. Surf. Sci. 2008, 254, 347–355. [Google Scholar] [CrossRef]
- Pratten, J.; Nazhat, S.N.; Blaker, J.J.; Boccaccini, A.R. In vitro attachment of Staphylococcus epidermidis to surgical sutures with and without Ag-containing bioactive glass coating. J. Biomater. Appl. 2004, 19, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Hardes, J.; Ahrens, H.; Gebert, C.; Streitbuerger, A.; Buerger, H.; Erren, M.; Gunsel, A.; Wedemeyer, C.; Saxler, G.; Winkelmann, W.; et al. Lack of toxicological side-effects in silver-coated megaprostheses in humans. Biomaterials 2007, 28, 2869–2875. [Google Scholar] [CrossRef] [PubMed]
| Na+ | K+ | Mg2+ | Ca2+ | Cl− | HPO42− | SO42− | CO32− |
|---|---|---|---|---|---|---|---|
| 1.42 × 10−1 | 5.81 × 10−3 | 8.11 × 10−4 | 1.26 × 10−3 | 1.45 × 10−1 | 7.78 × 10−4 | 8.11 × 10−4 | 4.17 × 10−3 |
| Specimen | Concentration and triple standard deviation (in brackets), (mass%) | ||||||
|---|---|---|---|---|---|---|---|
| Ti | Nb | Ta | Zr | Ca | P | Ag | |
| Untreated | 52.55 | 29.68 | 13.02 | 4.75 | - | - | - |
| (0.06) | (0.04) | (0.05) | (0.01) | ||||
| MAO 2.5 mM Ag | 35.46 | 28.86 | 11.07 | 4.63 | 9.33 | 10.65 | 0.01 |
| (0.06) | (0.04) | (0.02) | (0.01) | (0.04) | (0.06) | (0.01) | |
| MAO 0.5 mM Ag | 30.95 | 28.94 | 10.52 | 4.52 | 12.62 | 12.44 | 0.01 |
| (0.07) | (0.04) | (0.04) | (0.01) | (0.07) | (0.04) | (0.01) | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsutsumi, Y.; Niinomi, M.; Nakai, M.; Shimabukuro, M.; Ashida, M.; Chen, P.; Doi, H.; Hanawa, T. Electrochemical Surface Treatment of a β-titanium Alloy to Realize an Antibacterial Property and Bioactivity. Metals 2016, 6, 76. https://doi.org/10.3390/met6040076
Tsutsumi Y, Niinomi M, Nakai M, Shimabukuro M, Ashida M, Chen P, Doi H, Hanawa T. Electrochemical Surface Treatment of a β-titanium Alloy to Realize an Antibacterial Property and Bioactivity. Metals. 2016; 6(4):76. https://doi.org/10.3390/met6040076
Chicago/Turabian StyleTsutsumi, Yusuke, Mitsuo Niinomi, Masaaki Nakai, Masaya Shimabukuro, Maki Ashida, Peng Chen, Hisashi Doi, and Takao Hanawa. 2016. "Electrochemical Surface Treatment of a β-titanium Alloy to Realize an Antibacterial Property and Bioactivity" Metals 6, no. 4: 76. https://doi.org/10.3390/met6040076
APA StyleTsutsumi, Y., Niinomi, M., Nakai, M., Shimabukuro, M., Ashida, M., Chen, P., Doi, H., & Hanawa, T. (2016). Electrochemical Surface Treatment of a β-titanium Alloy to Realize an Antibacterial Property and Bioactivity. Metals, 6(4), 76. https://doi.org/10.3390/met6040076