Abstract
The recovery of vanadium from sulfuric and hydrofluoric mixed acid solutions generated by the direct leaching of black shale was investigated using solvent extraction and precipitation methods. The process consisted of reduction, solvent extraction, and stripping, followed by precipitation and calcination to yield vanadium pentoxide. The influence of various operating parameters on the extraction and recovery of vanadium was studied. Vanadium (IV) was selectively extracted using a mixture of 10% (v/v) di(2-ethylhexyl)phosphoric acid and 5% (v/v) tri-n-butylphosphate in sulfonated kerosene. Using six extraction and five stripping stages, the extraction efficiency for vanadium was 96.7% and the stripping efficiency was 99.7%. V2O5 with a purity of 99.52% was obtained by oxidation of the loaded strip solution and precipitation of ammonium polyvanadate at pH 1.8 to 2.2, followed by calcination of the dried precipitate at 550 °C for 2 h. It was concluded that the combination of solvent extraction and precipitation is an efficient method for the recovery of vanadium from a multi-element leach solution generated from black shale.
1. Introduction
Black shale (also called stone coal) is an important vanadium resource in China, where it is found extensively in the southern provinces and autonomous regions of the country. The gross reserves of vanadium in black shale in Hunan, Hubei, Guangxi, Jiangxi, Zhejiang, Anhui, Guizhou, Henan, and Shanxi provinces are 18 million tons in terms of V2O5, which accounts for more than 87% of the domestic reserves of vanadium [1]. The vanadium grade in black shale generally ranges from 0.13% to 1.2%, while grades higher than a cut-off of 0.5% account for about 40% of the total vanadium reserves [2]. Besides vanadium, some 60 different metallic and nonmetallic elements have been identified in these sources, including molybdenum, nickel, uranium, copper, selenium, gallium, and precious metals [3,4,5]. Black shale is therefore regarded as a complicated low-grade multi-element ore. Because of its relatively high vanadium grade and abundant deposits, studies of the recovery of vanadium from black shale have received considerable attention.
Vanadium exists as the (II), (III), (IV), and (V) species in nature [6]. A previous study showed that vanadium in black shale is found mainly as vanadium (III), then as vanadium (IV) and vanadium (V) [7]. Vanadium (III), which usually exists in the crystal lattices of aluminosilicate clay minerals, comprises more than 90% of the total vanadium content because black shale originates from reducing environments [8,9,10]. The principle of recovering vanadium from black shale is first to decompose the crystal lattice of the aluminosilicate clay minerals and oxidize insoluble vanadium (III) to acid-soluble vanadium (IV) and/or water-soluble vanadium (V) compounds, then to dissolve these species by acid, water, or alkaline leaching. Finally, pure vanadium products are produced from the leach solution by separation and purification, using methods including solvent extraction, ion exchange, and precipitation [11,12]. The traditional technique for the extraction of vanadium from black shale was by roasting with sodium salts and water leaching [13,14,15]. Using this technique, the roasting efficiency is about 45%–55%, the total vanadium recovery is less than 45%, and the released exhaust gas containing Cl2, HCl, and SO2 pollutes the environment. These serious disadvantages have limited further development of this technology and the sodium salt roasting process has been prohibited by the local government since 2006. A recently developed technique that does not involve roasting is direct acid leaching [16,17,18,19,20,21,22]. Investigations have found that vanadium in aluminosilicate and mica clay minerals can be leached by sulfuric acid with the addition of HF, NaF, or CaF2 to the leaching processing [23,24,25]. The resultant hydrofluoric acid decomposes the vanadium-bearing aluminosilicate clay minerals, allowing the exposed vanadium (III) to be oxidized to vanadium (IV) by Fe (III) and converted to soluble VOSO4 in the leach solution. The extent of vanadium leaching can exceed 85%, which is much higher than that of traditional roasting processes. Furthermore, this process completely avoids the discharge of roasting exhaust gas and lowers energy consumption. However, the various impurities elements in black shale, for instance, Ni, Al, Fe, Mg, Mn, Si, and P, leach into the solution with vanadium in the acid-leaching process. Selective extraction of vanadium is key to the recovery of pure vanadium products from the multi-element leach solutions of vanadium-bearing black shale [26,27].
Solvent extraction offers an attractive alternative for the extraction and recovery of pure metals from multi-element leach solutions [28,29,30]. Although the extraction of vanadium from various acidic solutions has been reported, there has been no systematic study of the recovery of vanadium from multi-element H2SO4-HF leach solutions from black shale by combined solvent extraction and precipitation, which is important for the practical application of this technology [31,32,33]. The aim of the present work was to extract and recover vanadium from such solutions using solvent extraction and precipitation. The effects of the aqueous-phase pH, extractant concentration, and organic-to-aqueous phase ratio (O/A) on the extraction of vanadium, and the effects of pH, temperature, and vanadium concentration on the precipitation of vanadium, are reported. This study provides data to develop a flow sheet for the recovery of a high purity vanadium product from the solutions generated by the direct leaching of black shale.
2. Experimental
2.1. Materials and Reagents
In our previous tests, a direct acid leaching process using sulfuric acid and hydrofluoric acid solutions was proposed to recover vanadium from black shale and a high leaching ratio of 86% was obtained. In the present study, the starting solution was generated by the same conditions as our previous studies, using a liquid/solid ratio of 4 mL/g, a sulfuric acid concentration of 87.5 g/L, a hydrofluoric acid concentration of 15 g/L, a sodium hypochlorite concentration of 1 g/L, a temperature of 95 °C, and a reaction time of 6 h [24]. The composition of the leach solution generated from black shale from Hunan Province, China, is listed in Table 1.

Table 1.
Composition of the liquor generated from the leaching of black shale in the H2SO4-HF mixture.
D2EHPA (di(2-ethylhexyl)phosphoric acid) and TBP (tri-n-butylphosphate), supplied by Shanghai Rare-Earth Chemical Co., Ltd., Shanghai, China, were used without further purification. Commercial kerosene was contacted with 98% concentrated sulfuric acid to produce sulfonated kerosene for use as the diluent. All other reagents and chemicals were of analytical reagent grade.
2.2. Experimental Procedure
2.2.1. Solvent Extraction
The metal equilibrium distributions between the organic and the aqueous phase were obtained by contacting a given volume ratio of the two liquids in a 250 mL mechanically agitated extractor. After the extraction, the two phases were separated in a separating funnel. The batch simulation counter-current experiments were performed in a box-style mixer-settler unit which contained six mixer-settler stages, while it was just used in the former five stages in the stripping. Each stage consisted of square-box type mixing and settling compartments. The active mixer volume was 0.5 L and the settler volume was 2.0 L. The agitation speed was 800 rpm. The vanadium (VO2+ and total vanadium concentrations) in the aqueous solutions was determined by the ferrous ammonium sulfate titration using 2-(phenylamino) benzoic acid as an indicator [34]. The Fe (II) concentration was determined by potassium dichromate titration using sodium diphenylamine sulfonate as an indicator. Fluoride ion selective electrode (Crison 9655, HACH, Loveland, CO, USA) was used to measure fluoride concentration and the phosphorus concentration was measured by the ammonium molybdate spectrometric method using UV-vis spectrophotometer (UV-vis, UV-2550, Shimadzu, Kyoto, Japan). The compositions of other elements in the aqueous phases were analyzed by inductively coupled, plasma atomic emission spectroscopy (ICP-AES) using an OPTIMA 5300DV (Perkin-Elmer, Waltham, MA, USA). Metal concentrations in the organic solutions were calculated by mass balance. The analysis method used for the vanadium pentoxide product is in accordance with the relevant industry standard of YB/T5304-2011 in China [35].
The solution pH was adjusted using 3 mol/L H2SO4 or 20% ammonia water, and the pH was measured using a digital pH meter (INESA instrucment, Shanghai, China). All extraction and stripping experiments were carried out at 25 ± 1 °C.
2.2.2. Oxidation and Precipitation
The oxidation and precipitation processes were conducted at atmospheric pressure in a 1000 mL stirred glass reactor that was placed in a temperature-controlled water bath. Hydrogen peroxide was used to oxidize vanadium (IV) to vanadium (V). Aqueous ammonia was used to control the pH and precipitate ammonium polyvanadate. The solid ammonium polyvanadate derived from the precipitation process was separated by vacuum filtration (0.7 atm) and washed twice with distilled water.
2.2.3. Calcination
The ammonium polyvanadate was dried at 60 °C in a constant-temperature laboratory oven. The V2O5 product was obtained by calcining the dried ammonium polyvanadate in a muffle furnace at a determined temperature and time.
3. Results and Discussion
3.1. Ferric Ion Reduction
Because the extraction order of D2EHPA is Fe3+ > VO2+ > VO2+ > Fe2+ [36], this means that Fe3+ in solution is more strongly extracted by D2EHPA than VO2+. The presence of Fe3+ not only reduces the maximum loading of vanadium on the extractant, but also compromises the purity of the final vanadium product. To selectively extract vanadium and avoid the co-extraction of Fe3+, it is necessary to reduce Fe (III) to Fe (II) before the extraction of vanadium. Sodium sulfite was employed as the reductant. The reaction can be expressed as:
VO2+ in the leach solution is also reduced to VO2+ in the reduction process:
To determine the amount of reductant necessary for Fe (III) reduction, different masses of Na2SO3 were added into 300 mL of leach solution for 2 h at 30 °C at different nR/nFe(III) coefficients, where nR/nFe(III) is the molar ratio of Na2SO3 to Fe3+. The reduction results are shown in Table 2. With a nR/nFe(III) coefficient of 0.8, more than 99.5% of the Fe (III) present was reduced to Fe (II). The further addition of Na2SO3 gave no further reduction of Fe (III). In subsequent experiments, the leach solution was reduced by Na2SO3 using a nR/nFe(III) coefficient of 0.8. The reduced leach solution was used as the feed solution for the solvent extraction test work.

Table 2.
Effect of Na2SO3/Fe (III) molar ratio on Fe (III) and V (V) reduction efficiency.
3.2. Solvent Extraction and Stripping of Vanadium (IV)
3.2.1. Effect of pH and Extractant Concentration
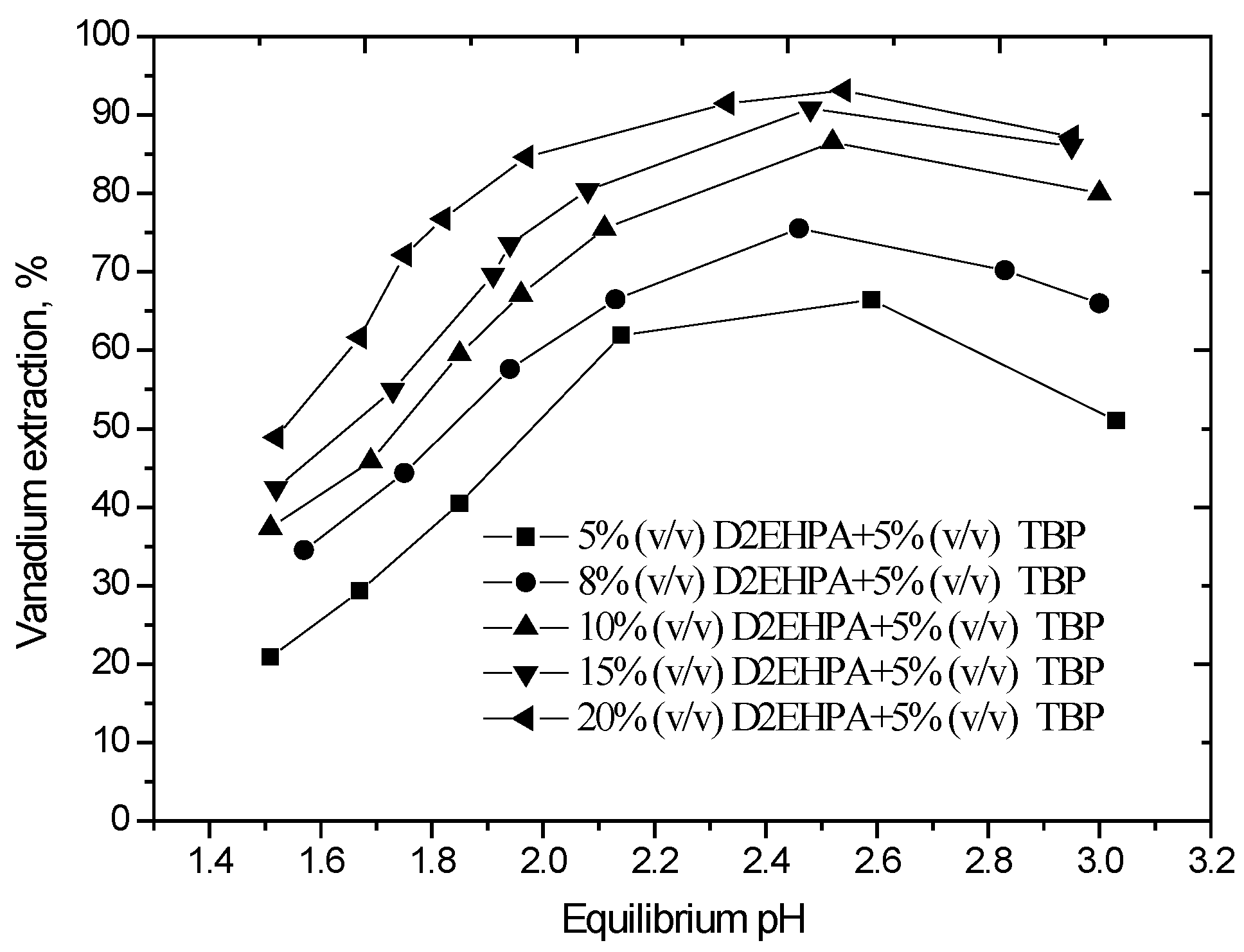
The effect of equilibrium pH in the range of pH 1.5 to 3.0 on the extraction of vanadium, carried out using different concentrations of D2EHPA with 5% (v/v) TBP in sulfonated kerosene, is shown in Figure 1.
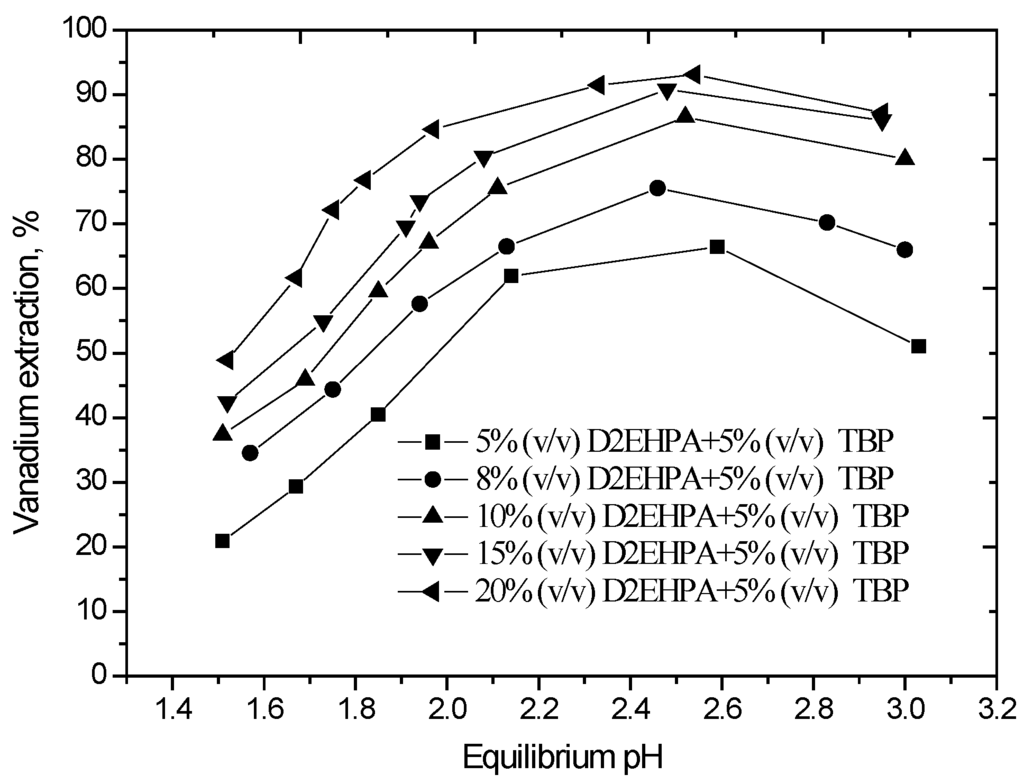
Figure 1.
Effect of equilibrium pH on vanadium extraction. (O/A phase ratio = 1/1, agitation speed 800 rpm, extraction time 10 min, 25 °C).
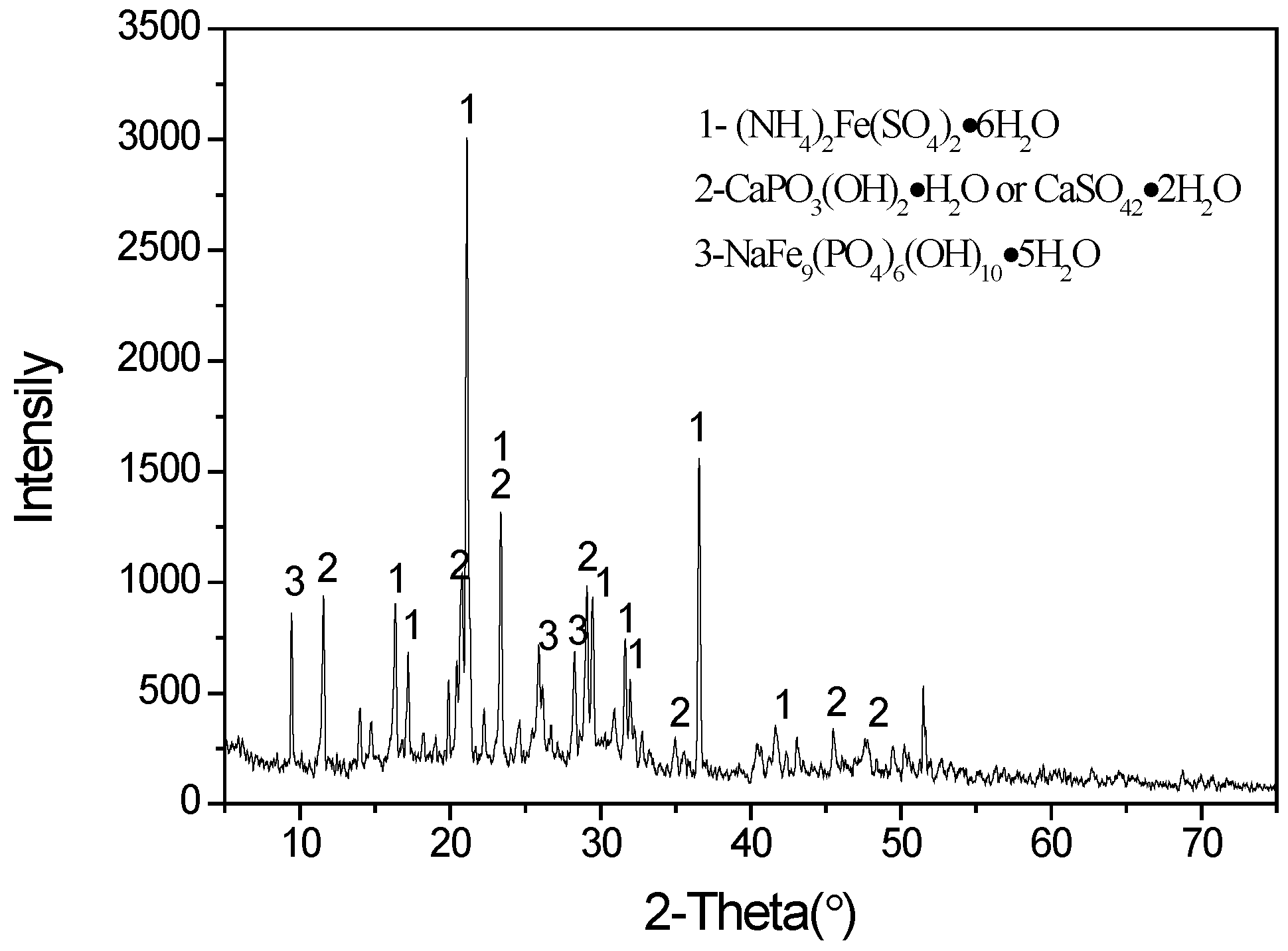
The results show that vanadium extraction efficiency initially increases with increasing pH because this is a cation exchange process. The maximum extraction was achieved at an equilibrium pH of 2.5 for the various D2EHPA concentrations employed. Vanadium extraction reached 85.6% at an equilibrium pH of 2.5 using 10% (v/v) D2EHPA and 5% (v/v) TBP in a single-stage extraction. Furthermore, when the aqueous pH exceeded 2.5, the solution became turbid and there was a formation of a third phase during the solvent extraction process. An X-ray diffraction (XRD) of the dried third phase (Figure 2) indicates that the major phases precipitating were (NH4)2Fe(SO4)2·6H2O, CaPO3(OH)2·H2O or CaSO4·2H2O, and NaFe9(PO4)6(OH)10·5H2O. The optimum aqueous pH for vanadium extraction should therefore be determined by considering both the vanadium extraction efficiency and the need to avoid third phase formation. An equilibrium pH of 2.5 was considered optimal and chosen for subsequent extraction experiments.
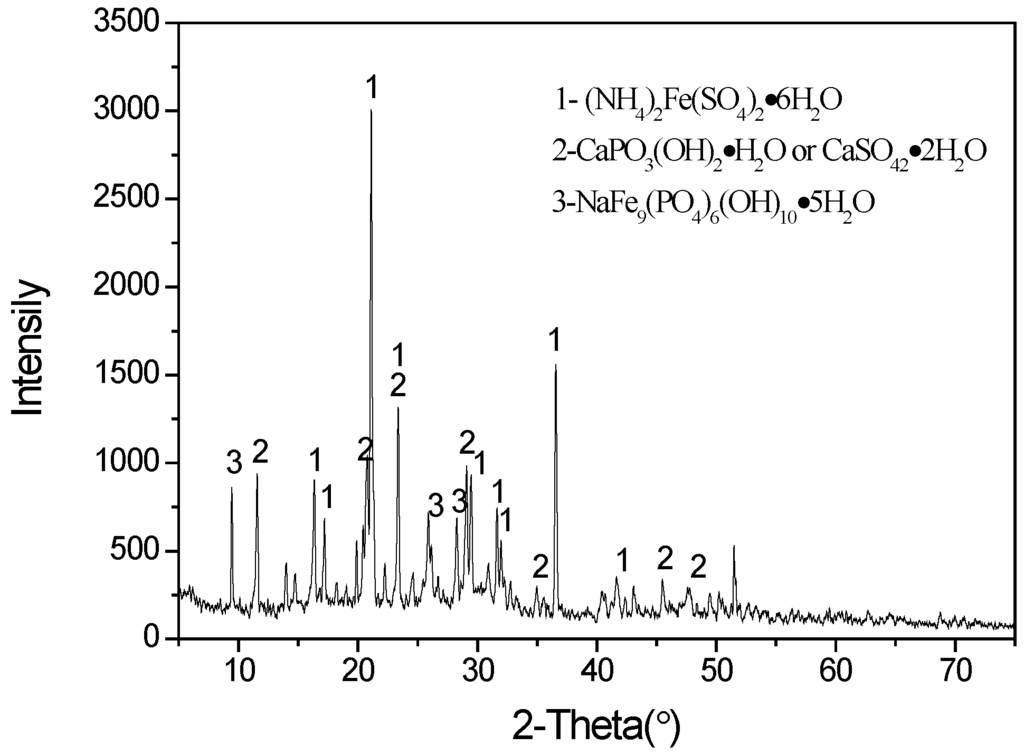
Figure 2.
XRD pattern of dried third phase produced during extraction process.
From the results of Figure 1, it also can be observed that the extraction of vanadium increased from 65.1% to 85.6% with the increase in D2EHPA concentration from 5% (v/v) to 10% (v/v), and upon the further increase in the D2EHPA concentration to 20% (v/v), the vanadium extraction increase slowly. Based on the experimental results, 10% (v/v) D2EHPA was sufficient to extract vanadium from the leach solution.
3.2.2. Effect of Fluoride Ion Concentration
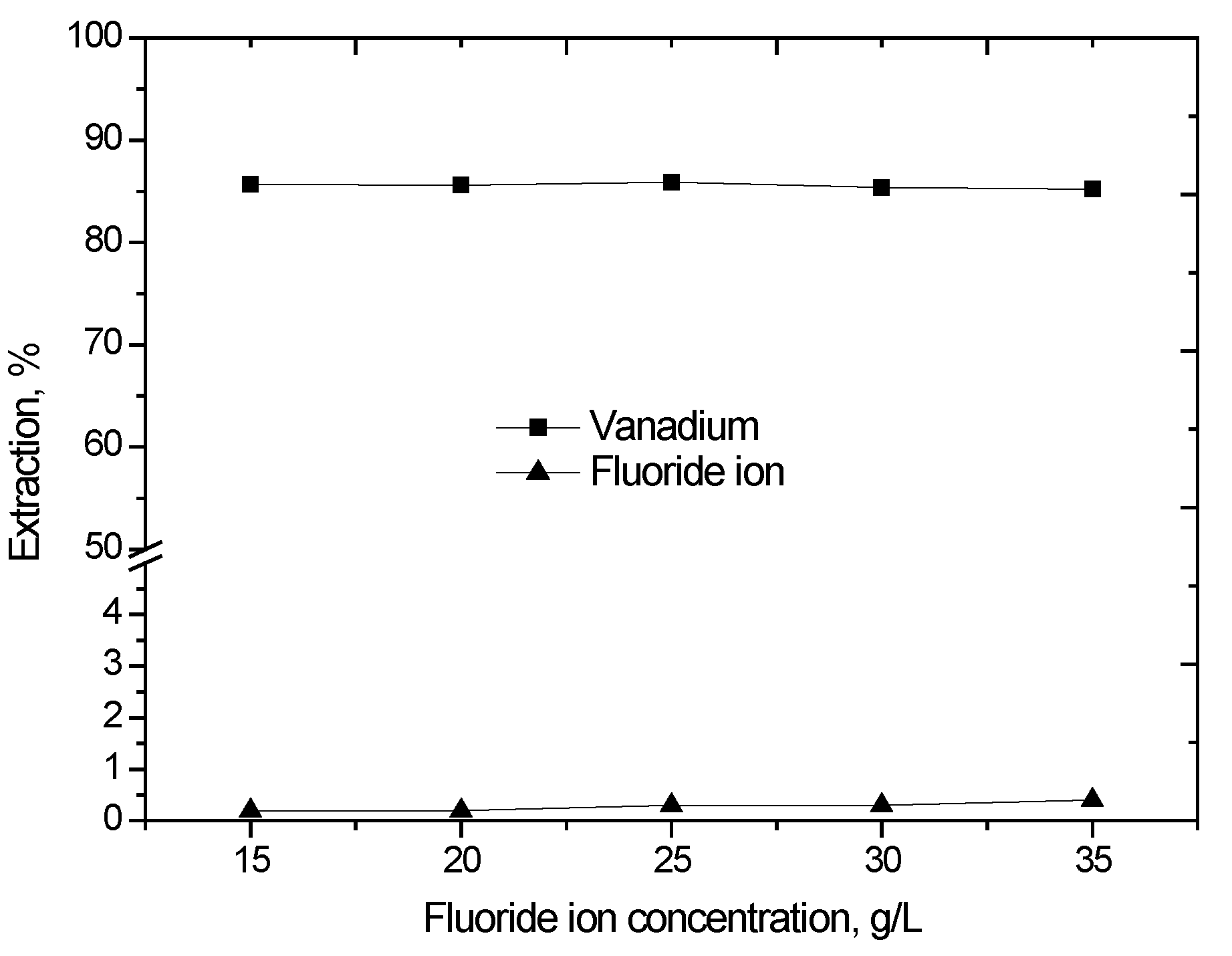
The effect of fluoride ion concentration on the extraction of vanadium (IV) was studied. The fluoride ion concentration varied in step with different concentrations of sodium fluoride from 15 g/L to 35 g/L, the results of which are shown in Figure 3. It was observed that the change in fluoride ion concentration in the investigated range had almost no effect on the vanadium (IV) extraction, and the extraction of fluoride ion is very low, which indicates that the fluoride ion does not participate in the extracted complexes.
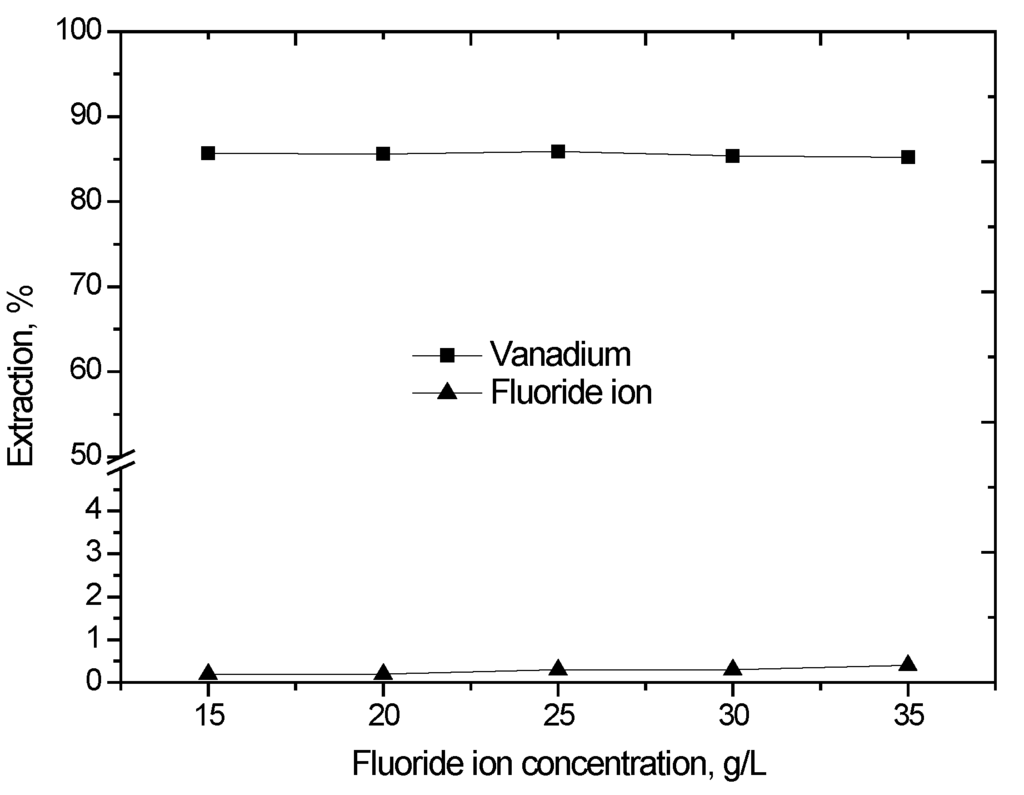
Figure 3.
Effect of fluoride ion concentration on the extraction of vanadium (IV) with D2EHPA and TBP (D2EHPA = 10% (v/v), TBP = 5% (v/v), O/A phase ratio = 1/1, agitation speed 800 rpm, extraction time 10 min, 25 °C).
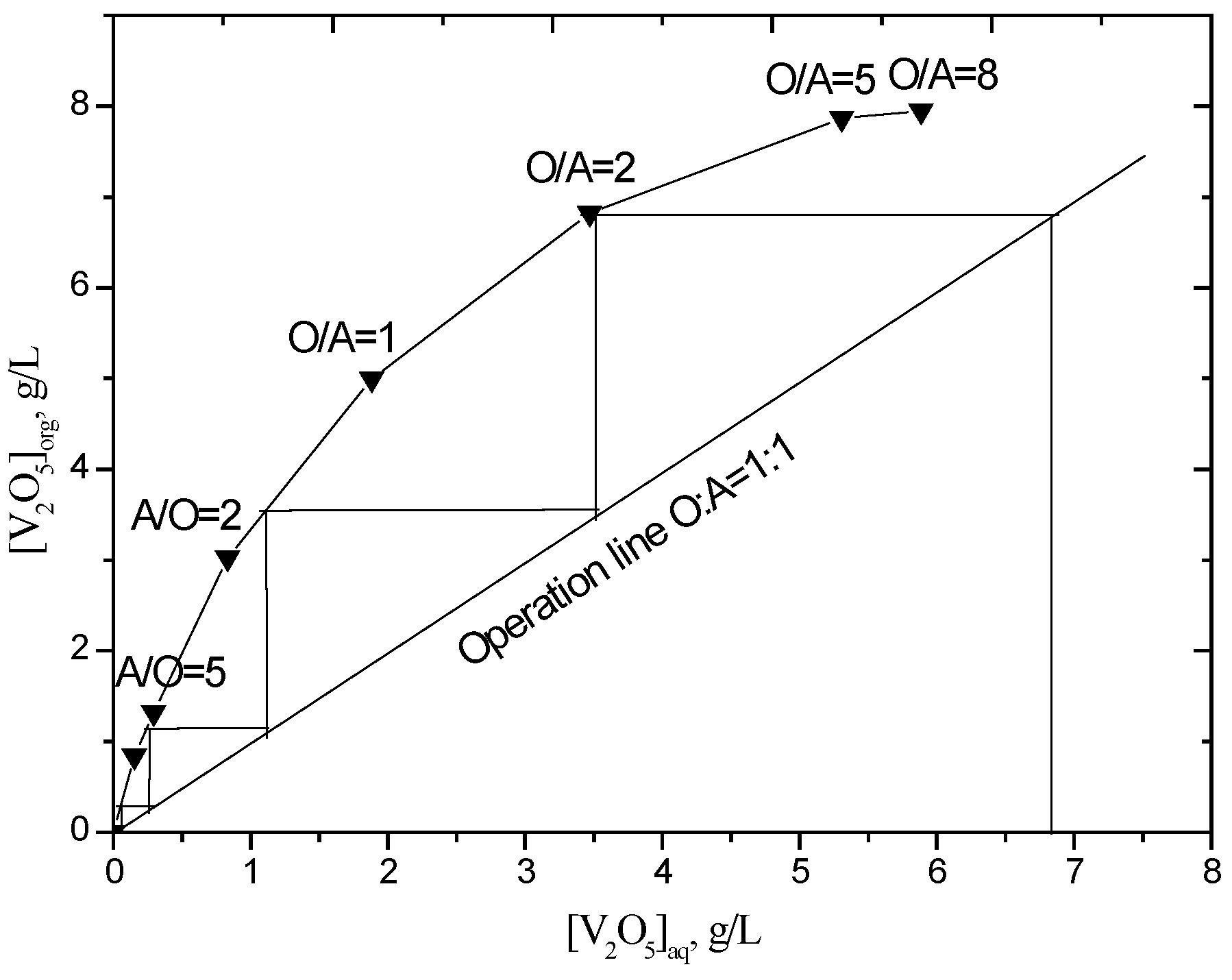
3.2.3. McCabe-Thiele Plot for Vanadium (IV) Extraction
To determine the theoretical number of stages required for the maximum extraction of vanadium at a chosen phase ratio and D2EHPA concentration, an extraction isotherm was drawn from the results obtained by contacting the leach solution with organic phase (10% (v/v) D2EHPA and 5% (v/v) TBP in sulfonated kerosene at different O/A phase ratios (O/A = 1/8, 1/5, 1/2, 1/1, 2/1, 5/1). The McCabe-Thiele plot (Figure 4) shows that quantitative extraction of vanadium can be theoretically achieved in five stages of counter-current extraction at an O/A phase ratio of 1.0. Because the pH will decrease as the number of extraction stages increases in a continuous counter-current extraction process, which influences the extraction efficiency, one more stage was added. A batch simulation of six stages of counter-current extraction was carried out and the raffinates were analyzed for vanadium and other metals concentration (Table 3). The raffinate contained 222 mg/L V2O5, which corresponds to 96.7% extraction efficiency. The initial and final pH of the solutions are 2.5 and 1.52, respectively. The loaded organic, containing 6.45 g/L V2O5, was then used to carry out stripping studies.
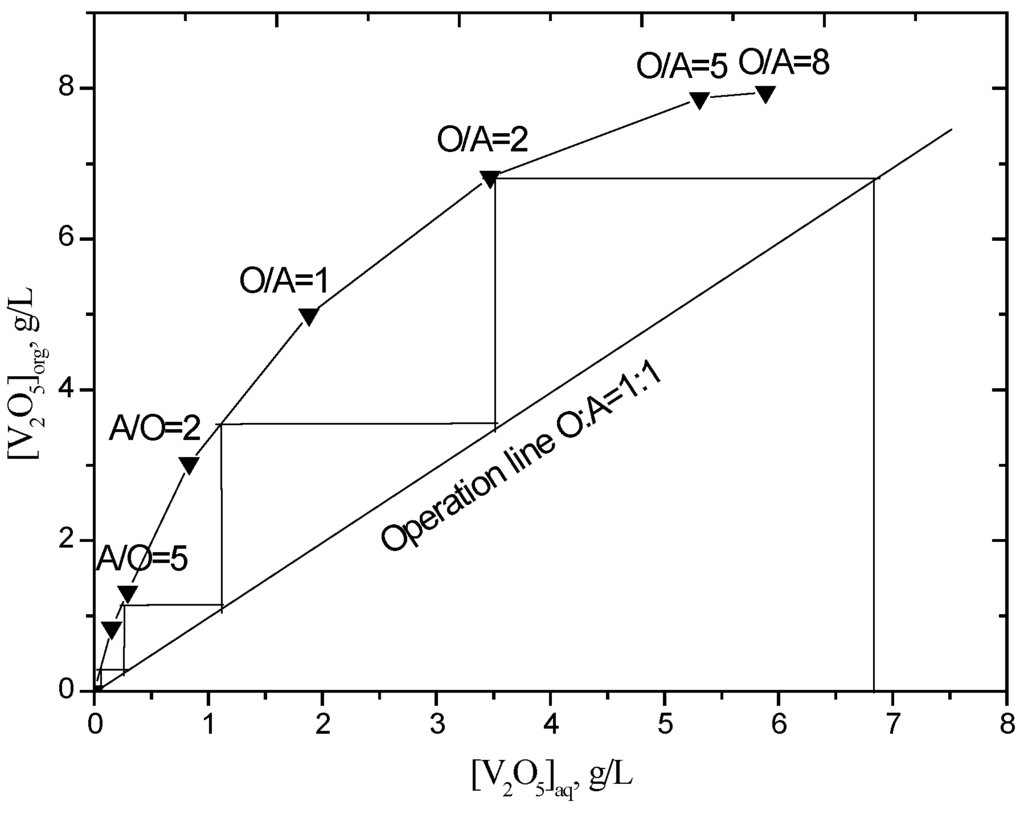
Figure 4.
Extraction isotherm for vanadium (D2EHPA = 10% (v/v), TBP = 5% (v/v), equilibrium pH = 2.5, 25 °C).

Table 3.
Chemical analysis of the raffinate.
3.2.4. Stripping of Vanadium (IV)
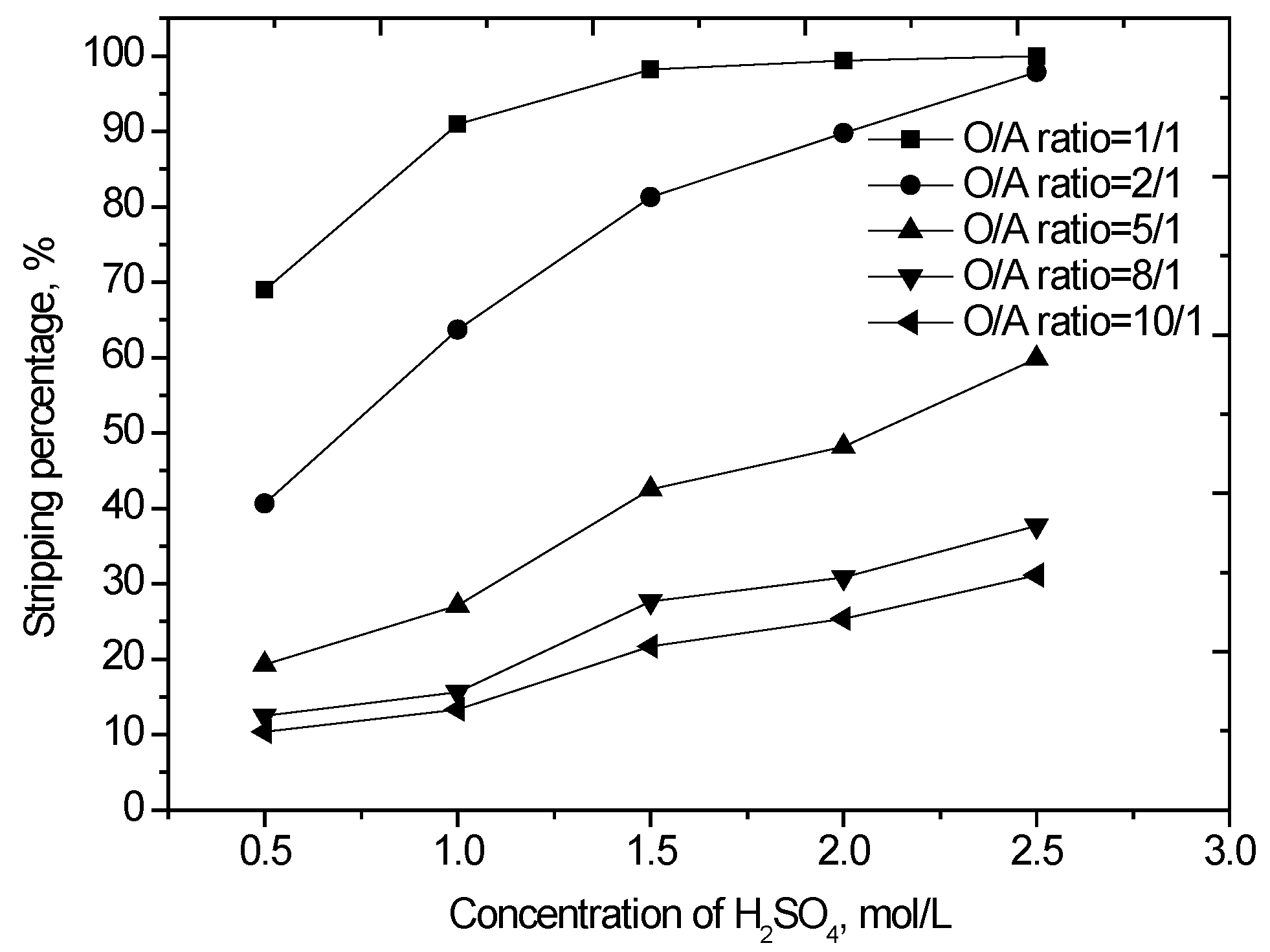
The loaded organic was scrubbed using 0.15 mol/L sulfuric acid solution at an O/A phase ratio of 2.0 to remove the entrainment impurities. The scrubbed loaded organic phase was stripped with sulfuric acid. Different concentrations of sulfuric acid were contacted with the loaded organic phase at various O/A phase ratios. The results (Figure 5) show that the vanadium stripping increases from 40.6% to 97.9% as the sulfuric acid concentration increased from 0.5 to 2.5 mol/L at an O/A phase ratio of 2/1. Additionally, the residual sulfuric acid in the spent strip solution also increased with the increasing acid concentration. As the initial concentration of H2SO4 increased from 0.5 to 2.5 mol/L, the residual H2SO4 in the strip solution increased from 0.26 to 2.1 mol/L. Greater residual sulfuric acid in the strip solution means that more aqueous ammonia will be consumed during the subsequent vanadium precipitation process. The optimum amount of H2SO4 was determined by considering both the vanadium stripping efficiency and the residual sulfuric acid concentration in the strip solution. For the present study, 1.5 mol/L H2SO4 was chosen as optimum.
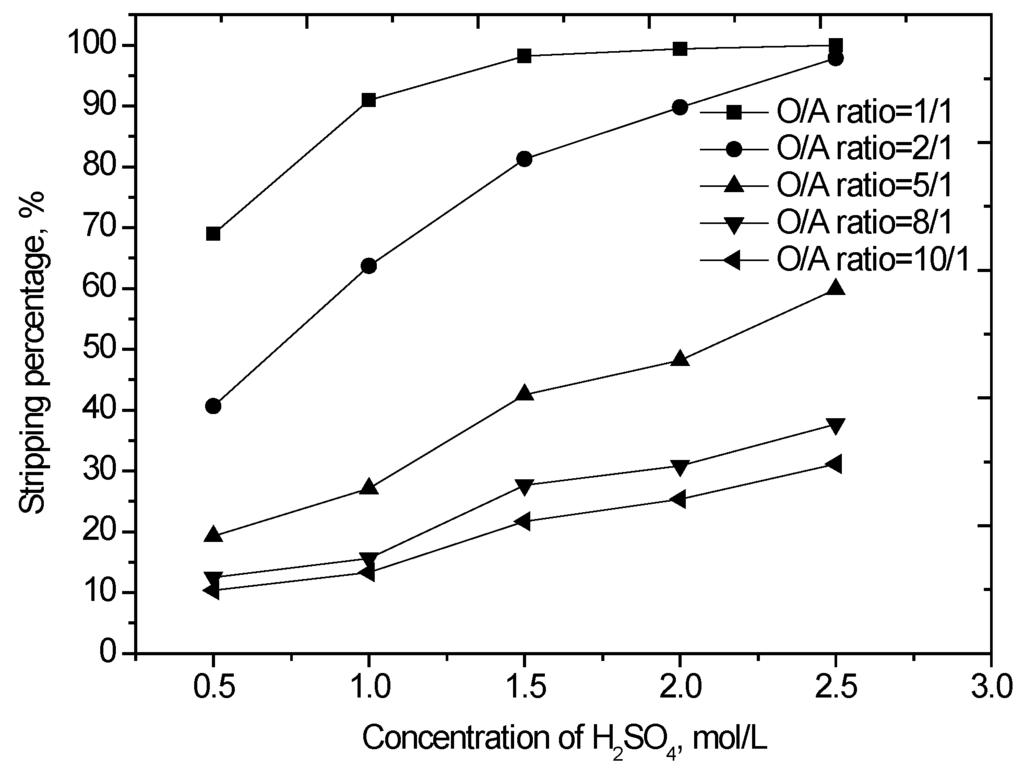
Figure 5.
Effect of H2SO4 concentration and organic-to-aqueous (O/A) phase ratio on vanadium stripping (agitation speed 1000 rpm, stripping time 10 min, 25 °C).
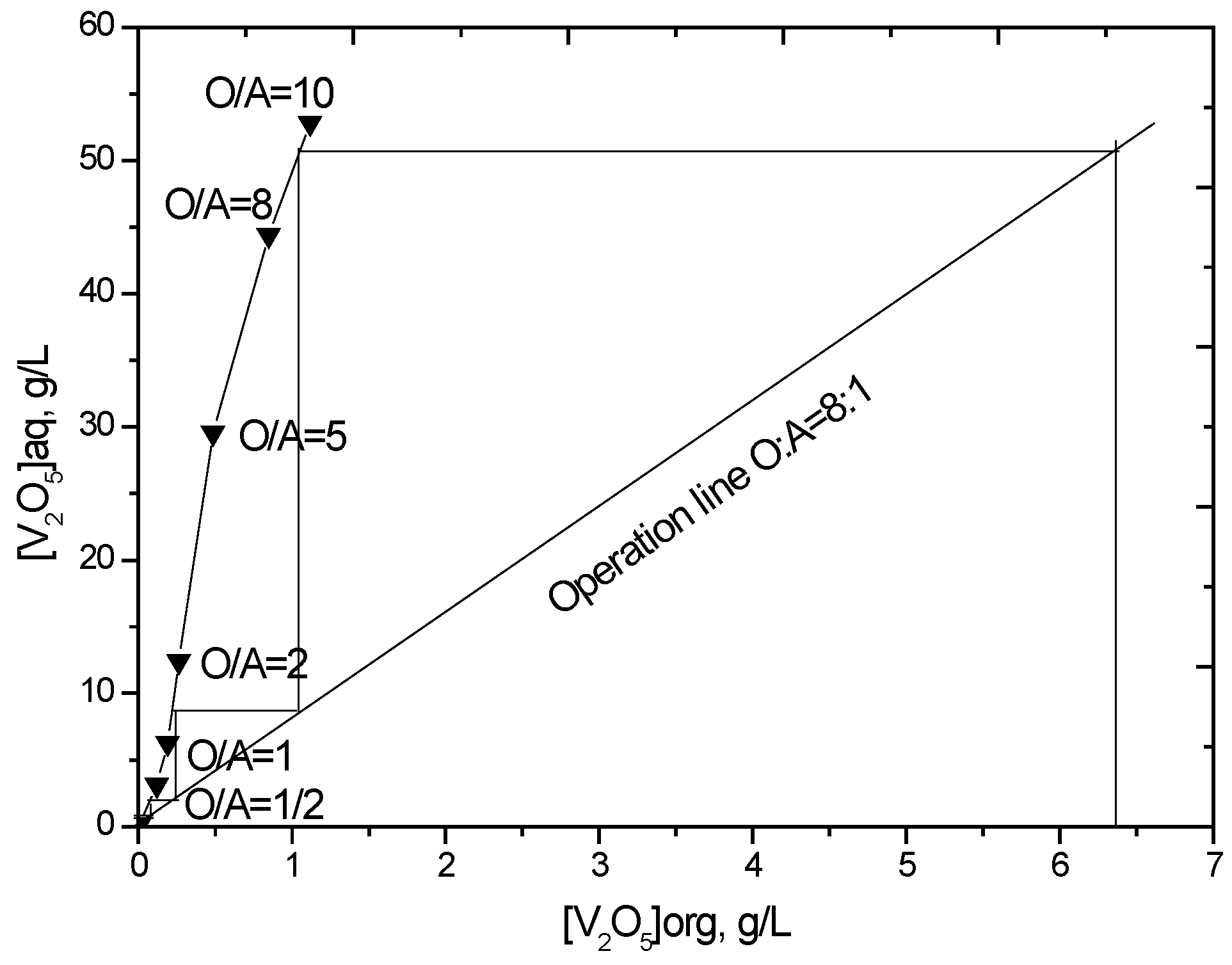
3.2.5. Stripping Isotherm for Vanadium (IV)
The stripping isotherm for vanadium was constructed to determine the number of stages required for stripping at a chosen phase ratio. The loaded organic phase and 1.5 mol/L H2SO4 were contacted at different O/A phase ratios from 10:1 to 1:2, while keeping the total volume constant. The McCabe-Thiele plot (Figure 6) illustrates that greater than 99% of the loaded vanadium can be theoretically stripped using four counter-current stages at an O/A flow ratio of 8. Considering that the sulfuric acid concentration will decrease as the number of stripping stages increases, which influences the stripping efficiency, one more stage was added. A batch simulation of five stages of counter-current stripping was carried out at an O/A ratio of 8 using 1.5 mol/L H2SO4. The stripped organic phase contained only 20 mg/L V2O5, which indicates that greater than 99.7% vanadium stripping efficiency was achieved.
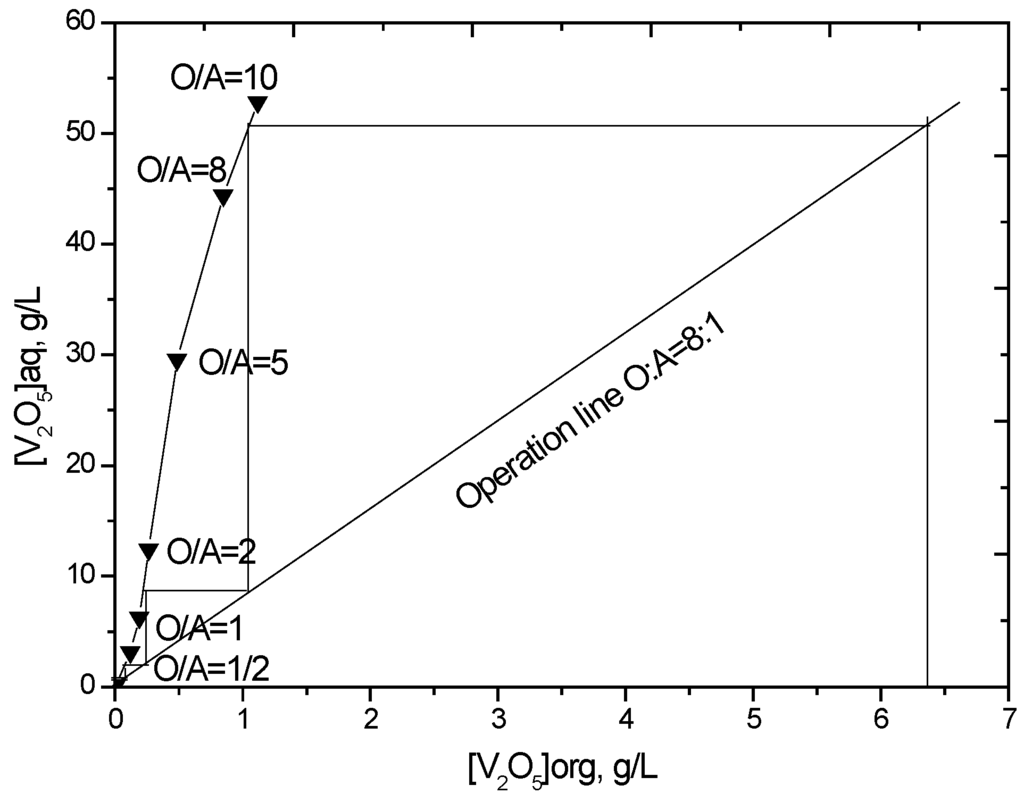
Figure 6.
McCabe-Thiele plot for vanadium stripping (V2O5 in loaded organic phase = 6.45 g/L, strip solution = 1.5 mol/L H2SO4, 25 °C).
3.3. Oxidation and Precipitation
3.3.1. Oxidation
The vanadium (VO2+) in the strip solution should be oxidized to VO2+ before precipitation. KMnO4, NaClO3, H2O2, and H2SO5 are common oxidants that can be used. Among these, H2O2 was chosen for its strong oxidizing ability, low cost, and easy operation:
In the present study, oxidation was carried out by adding H2O2 at six times the stoichiometric amount at 50 °C for 1 h. The color of the strip solution changed from blue to yellow at the end of the reaction. Sample testing showed that the vanadium (IV) concentration in the oxidized solution was below 0.1 g/L, which meant that almost all of the vanadium (IV) was oxidized to vanadium (V). The vanadium (V) solution was used as the feed for the precipitation experiments.
3.3.2. Precipitation
After the oxidation of the strip solution, vanadium can be precipitated from the vanadium (V)-containing solution by adding an ammonium salt, such as (NH4)2SO4, (NH4)2CO3, or NH4Cl. Because aqueous ammonia was added to the feed solution to neutralize the residual acid in the present study, a large amount of (NH4)2SO4 was produced during the neutralization reaction and additional ammonium salt was not required.
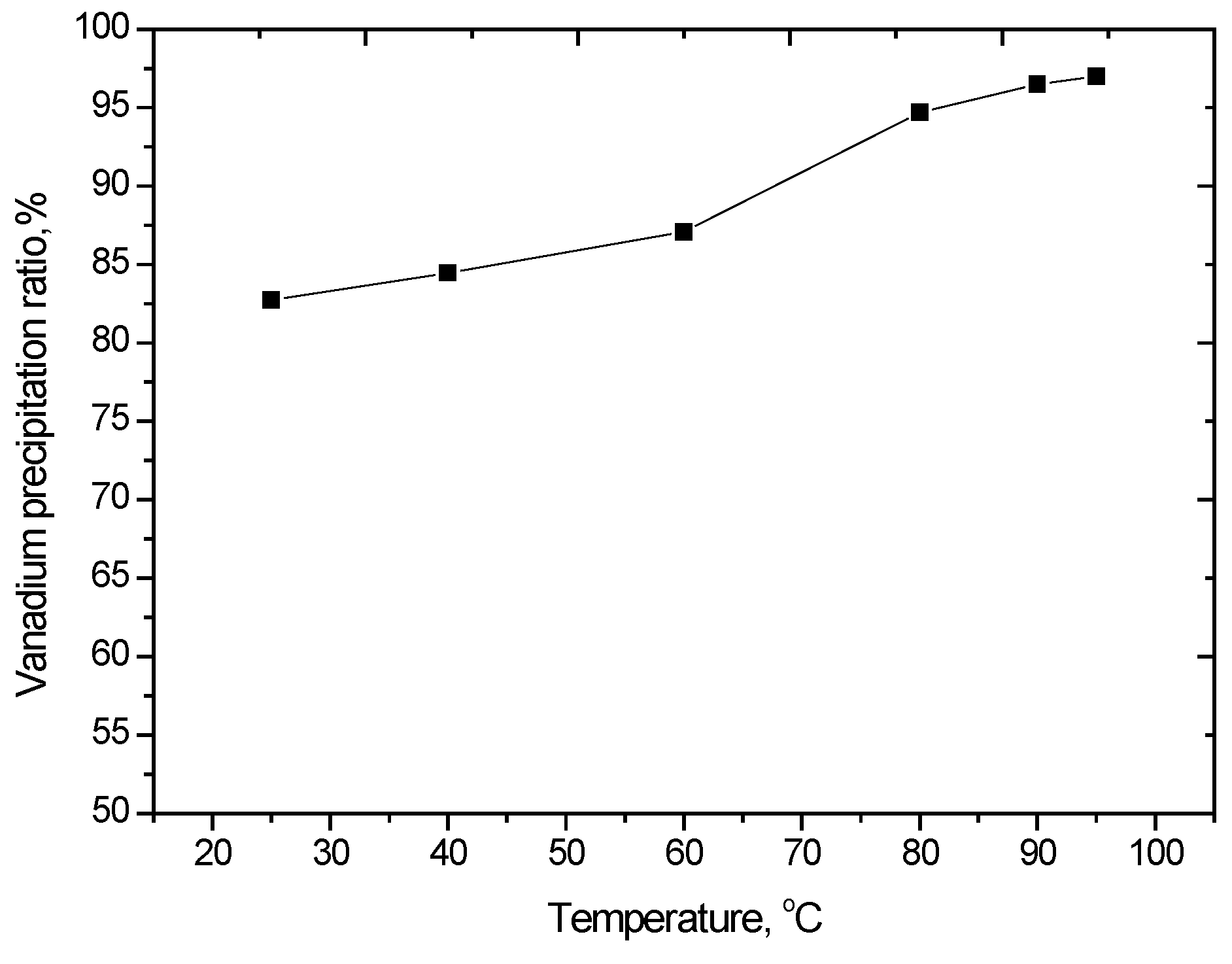
The effect of temperature on vanadium precipitation was studied in the range of 25 to 95 °C. The results shown in Figure 7 indicate that precipitation efficiency increases with increasing reaction temperature. Increasing the solution temperature from 60 to 90 °C caused an increase in the extent of vanadium precipitation; however, a further increase in temperature had little effect. According to these data, vanadium precipitation should be carried out between 90 and 95 °C.
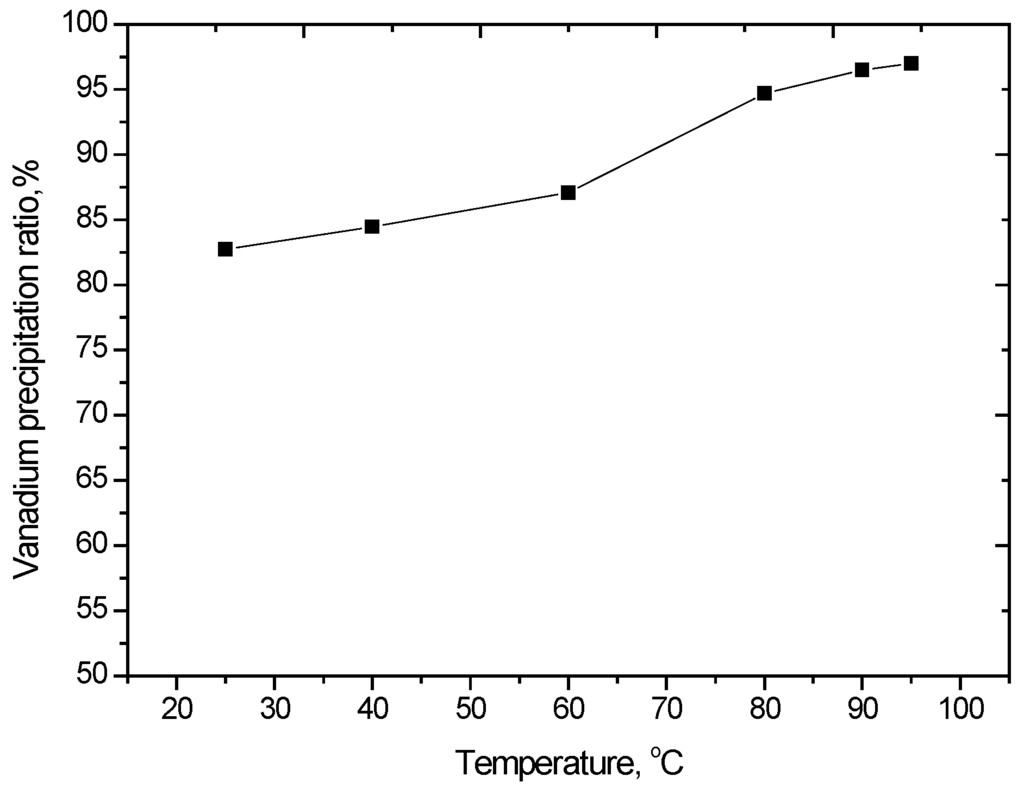
Figure 7.
Effect of temperature on vanadium precipitation (V2O5 concentration 52 g/L, equilibrium pH of 1.8–2.2, reaction time of 2 h).
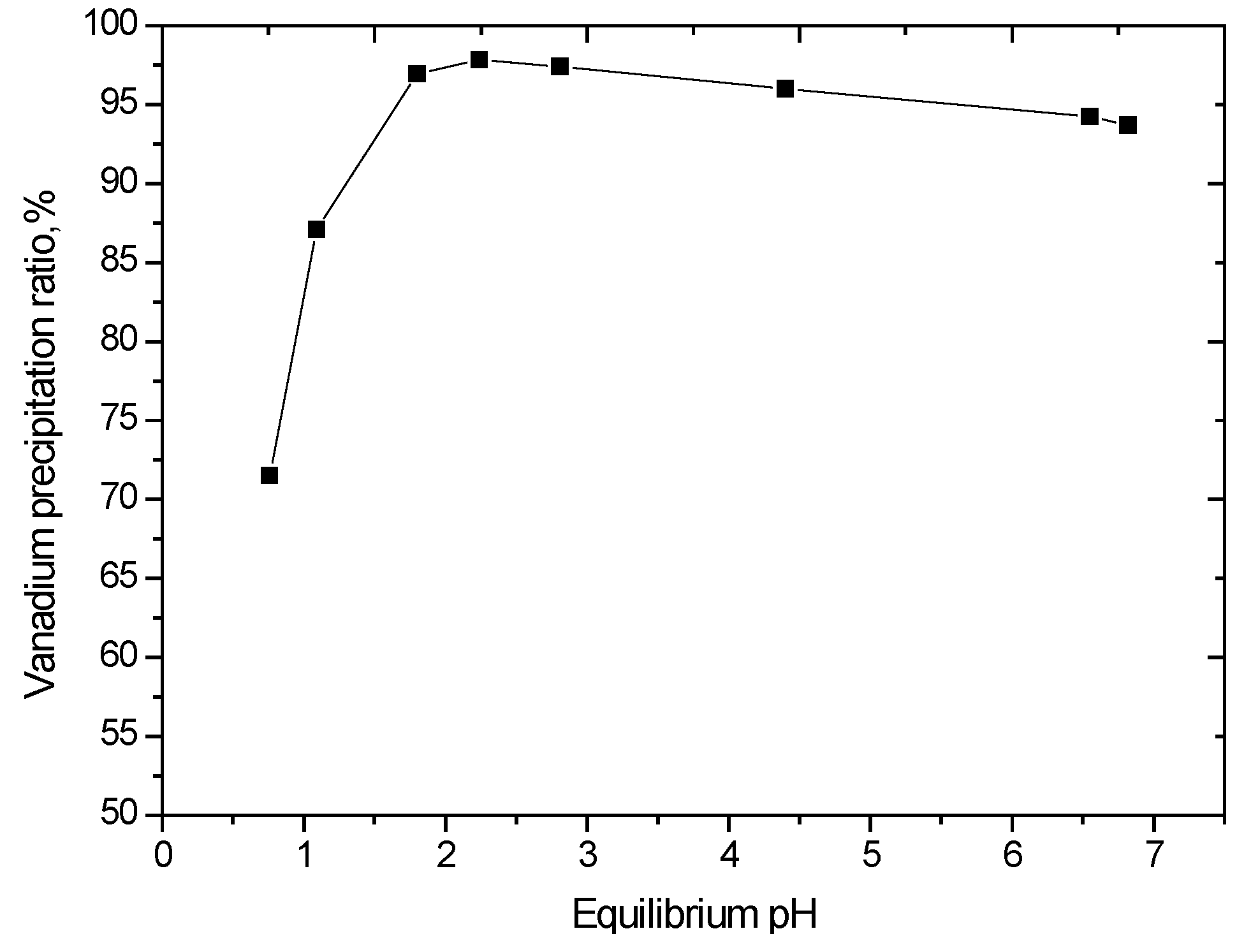
The effect of pH on vanadium precipitation was studied under conditions of 90 °C for a reaction time of 2 h. The results are shown in Figure 8. This result suggests that the precipitation reaction is promoted in the pH range of 1.8–2.2.
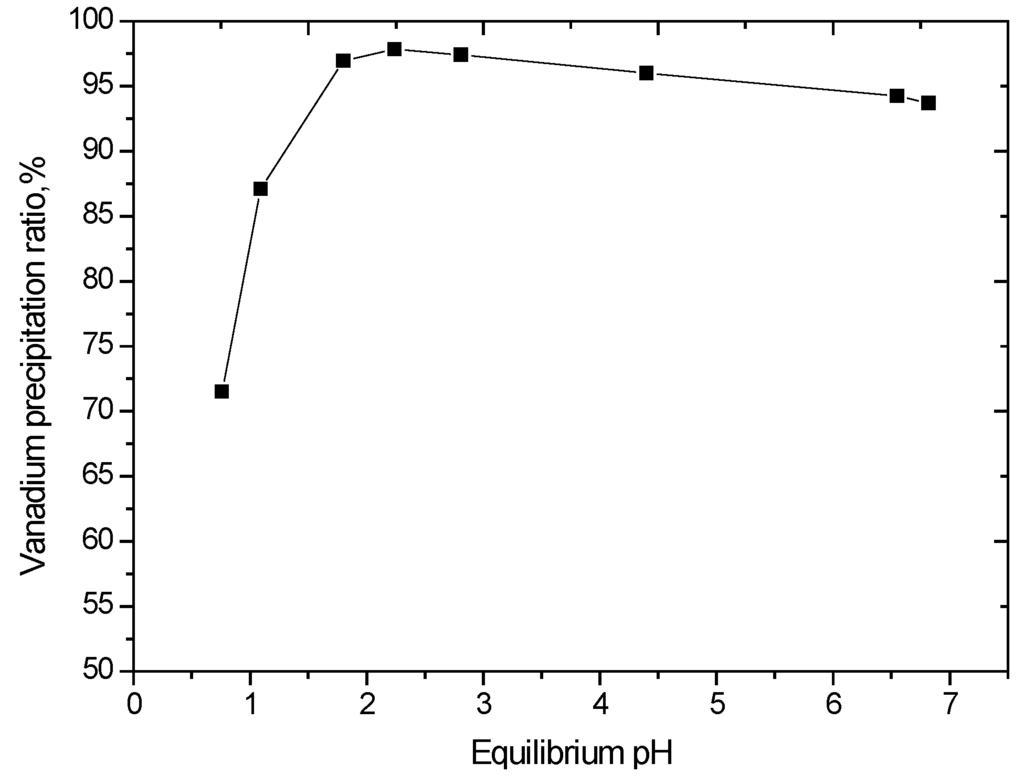
Figure 8.
Effect of equilibrium pH on vanadium precipitation (V2O5 concentration 52 g/L, reaction time of 2 h, 90 °C).
3.4. Calcination
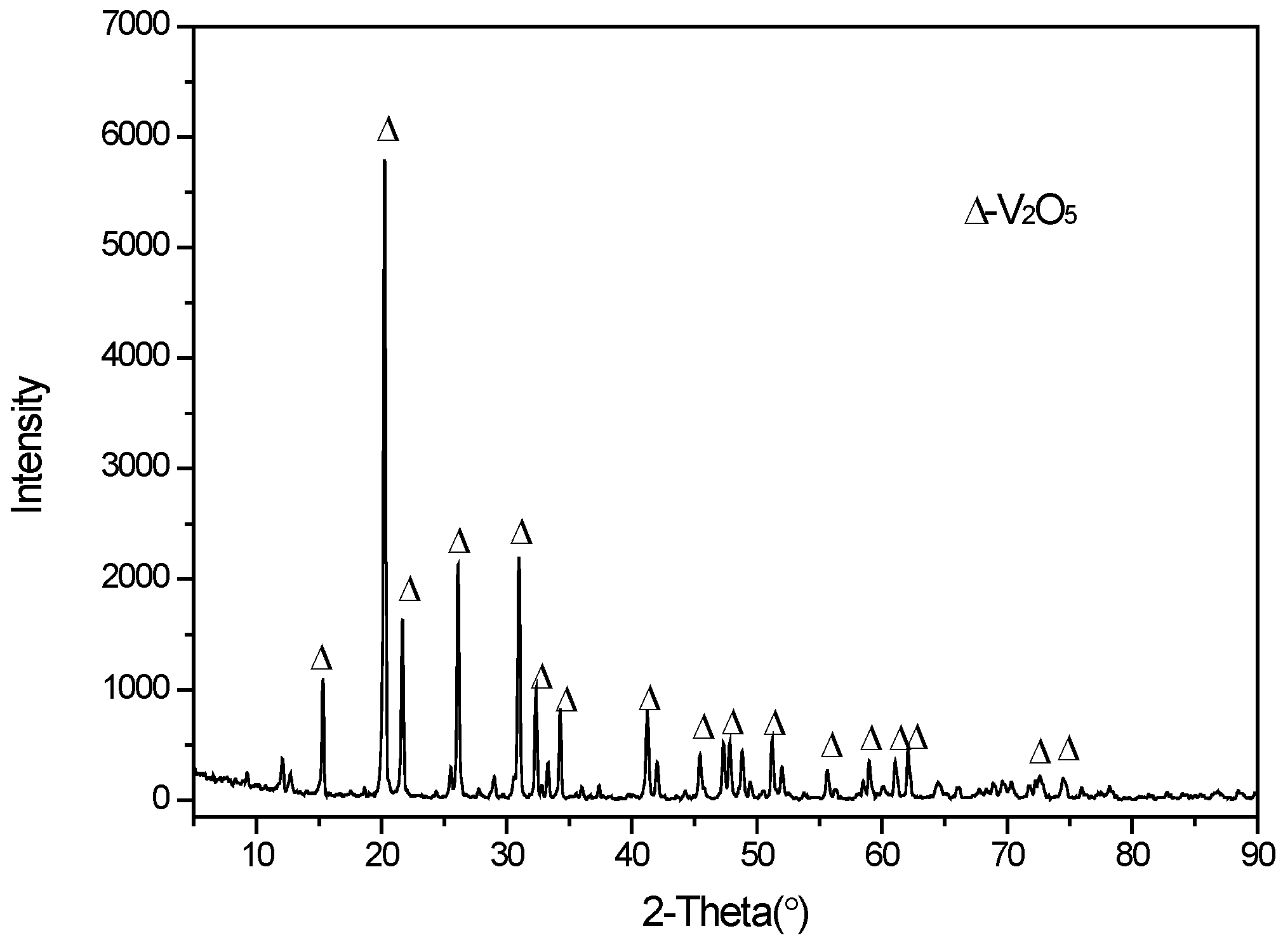
The dried ammonium polyvanadate was calcined at 550 °C for 2 h in a muffle furnace under an oxidizing atmosphere; the XRD analysis for this (Figure 9) fitted the vanadium pentoxide pattern well. The composition of the vanadium pentoxide product is shown in Table 4 and compared with the requirements of the relevant industry standard in China (YB/T5304-2011) [34]. As shown, the purity of the product reached 99.52% V2O5, with trace amounts of sodium, iron, and phosphorus impurities.
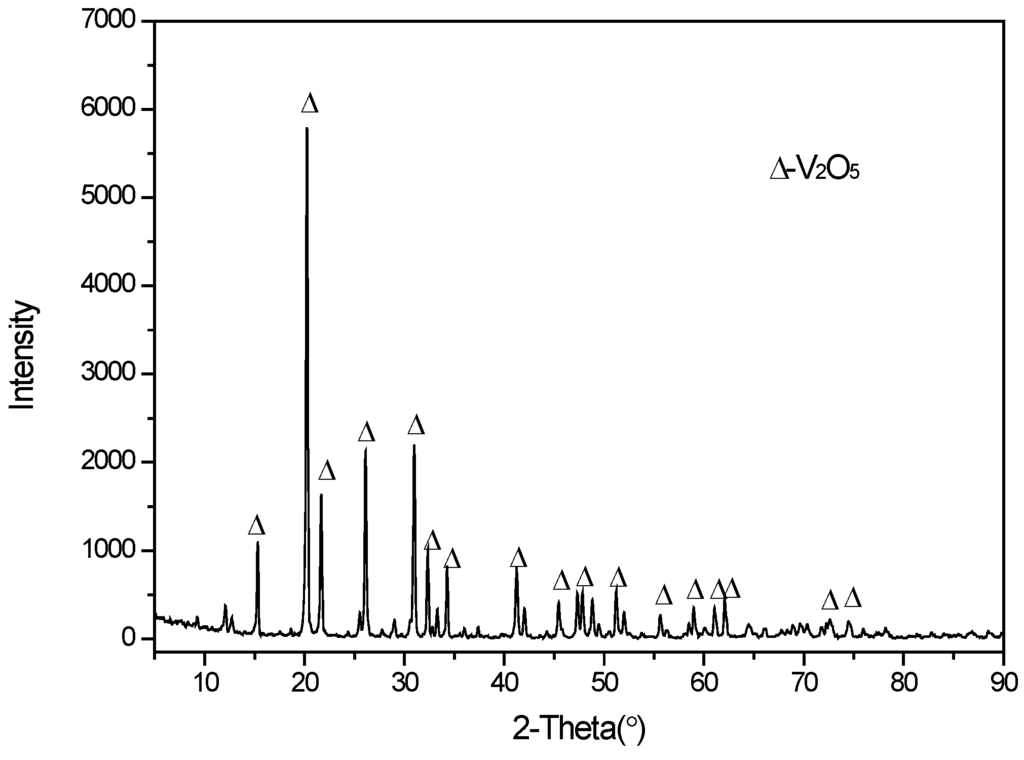
Figure 9.
XRD pattern of the V2O5 product.

Table 4.
Chemical analysis of vanadium pentoxide.
3.5. Development of a Process Flow Sheet to Recover Vanadium
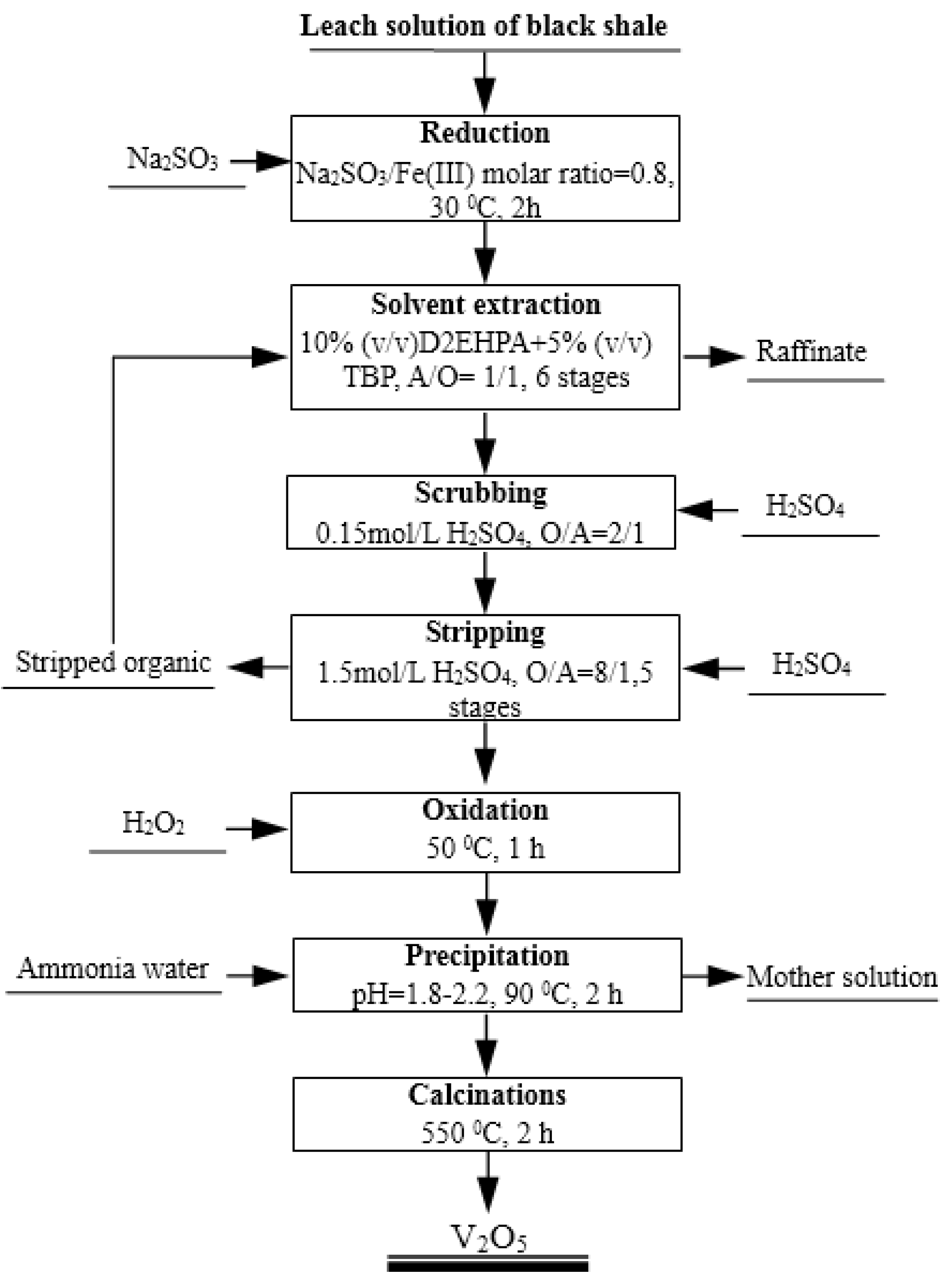
Based on the investigation and discussion above, a process flow sheet to extract and recover vanadium from the H2SO4-HF solution generated by the leaching of black shale by solvent extraction and precipitation is proposed in Figure 10. In this flow sheet, vanadium (IV) in the leach solution can be effectively extracted using 10% (v/v) D2EHPA with 5% (v/v) TBP at an aqueous pH of 2.5 and an O/A phase ratio of 1/1. With six counter-current extraction stages, 96.7% vanadium is extracted. Vanadium in the loaded organic phase can be completely stripped by 1.5 mol/L H2SO4 at an O/A phase ratio of 8/1. Then, about six times of the stoichiometric requirement of H2O2 is added to the stripping solution to oxidize vanadium (IV) to vanadium (V) at 50 °C for 1 h. Ammonium polyvanadate can be successfully precipitated from the oxidized solution by adding aqueous ammonia to adjust the pH to 1.8–2.2, at 90 °C for 2 h. The dried ammonium polyvanadate is calcined at 550 °C for 2 h to obtain a high purity of V2O5 product.
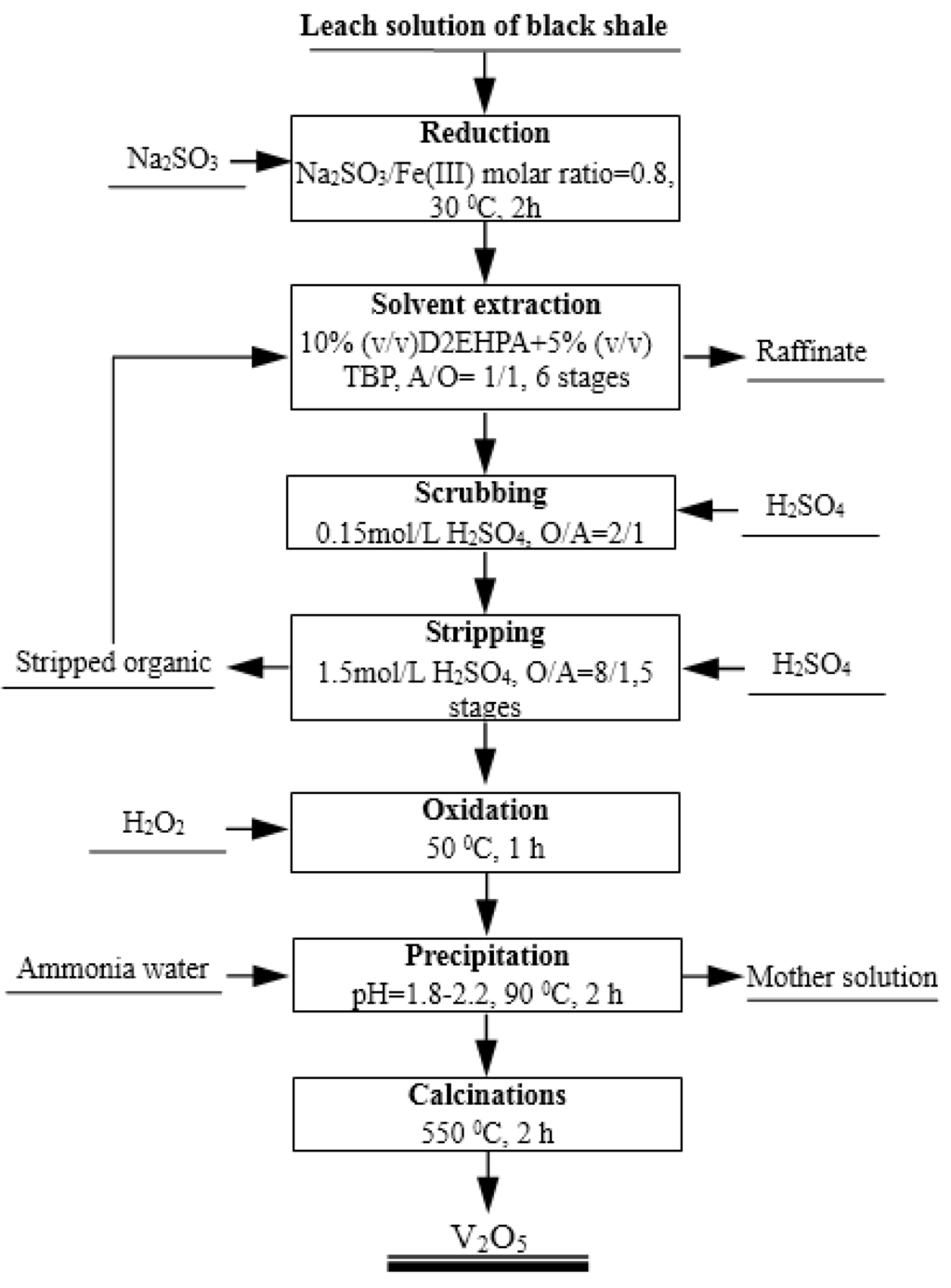
Figure 10.
Proposed flow sheet for extraction and recovery of vanadium from H2SO4-HF leach solution from black shale by solvent extraction and precipitation.
4. Conclusions
Based on the data presented in this paper, a process for extraction and recovery of vanadium from H2SO4-HF solutions generated by the leaching of black shale is suggested. The conclusions from the test work are presented below:
- (1)
- A vanadium extraction efficiency of 96.7% was achieved using 10% (v/v) D2EHPA with 5% (v/v) TBP in sulfonated kerosene in six stages of counter-current extraction at an equilibrium pH of 2.5 and an O/A phase ratio of 1/1. Fluoride ions in the leaching solution had almost no effect on vanadium (IV) extraction.
- (2)
- 99.7% of the loaded vanadium was stripped from the organic phase by 1.5 mol/L H2SO4 in five counter-current stages at an O/A phase ratio of 8/1.
- (3)
- 98% of vanadium was precipitated as ammonium polyvanadate by oxidation and precipitation. Following calcination of the dried ammonium polyvanadate at 550 °C for 2 h, a high purity 99.52% V2O5 product was obtained.
- (4)
- A flow sheet for the extraction and recovery of vanadium from a H2SO4-HF mixed leach solution by solvent extraction and precipitation is proposed.
Acknowledgments
This work has been supported by the National Basic Research Program of China (Grant No. 2014CB643404), NSFC (Natural Science Foundation of China, Grant No. 51174104, 51304093 and 51474115).
Author Contributions
Xingbin Li conducted the experiments and wrote the initial draft of the manuscript. Chang Wei designed the experiments. Zhigan Deng, Cunxiong Li and Gang Fan analyzed the data and wrote the final manuscript. Minting Li and Hui Huang reviewed and contributed to the final revised manuscript. All authors contributed to the analysis of the data and read the final paper.
Conflicts of Interest
The author declares no conflict of interest.
References
- Bin, Z. Progress of the research on extraction of vanadium pentoxide from stone coal and the market of V2O5. Hunan Nonferr. Met. 2006, 22, 16–20. (In Chinese) [Google Scholar]
- Cai, J. Development of vanadium extraction from stone coal. Rare Met. Cem. Carbides 2001, 144, 42–49. (In Chinese) [Google Scholar]
- Xu, L.; Lehmann, B.; Mao, J.; Nägler, T.F.; Neubert, N. Mo isotope and trace element patterns of lower Cambrian black shales in south China: Multi-proxy constrains on the paleoenvironment. Chem. Geol. 2012, 318–319, 45–59. [Google Scholar] [CrossRef]
- Pan, J.Y.; Ma, D.S.; Cao, S.L. Trace element geochemistry of the lower Cambrian black rock series from northwestern Hunan, south China. Prog. Nat. Sci. 2004, 14, 64–70. (In Chinese) [Google Scholar] [CrossRef]
- Pi, D.H.; liu, C.Q.; Zhou, G.A.; Jiang, S.Y. Trace and rare earth element geochemistry of black shale and kerogen in the early Cambrian Niutitang Formation in Guizhou province, south China: Constraints for redox environments and origin of metal enrichments. Precambr. Res. 2013, 225, 218–229. [Google Scholar] [CrossRef]
- Gupta, C.K.; Krishnamurthy, N. Extractive Metallurgy of Vanadium; Elsevier: Amsterdam, The Netherlands, 1992. [Google Scholar]
- Xu, G.Z. Guidance functions of vanadium valence and material composition in stone coal to extracting vanadium process. Coal Process. Compr. Util. 1989, 5, 5–8. (In Chinese) [Google Scholar]
- Guo, W.; Feng, Q.M.; Hu, C.P. Kinetics of vanadium oxidation during bone coal roasting in air. Rare Met. 1995, 14, 276–281. (In Chinese) [Google Scholar]
- Chen, T.; Qiu, G.; Zhu, D. Valence variation and oxidation kinetics of vanadium during vanadium-bearing stone coal roasting. Min. Metall. Eng. 2008, 28, 64–67. (In Chinese) [Google Scholar]
- Xu, G.; Ge, N.; Li, J. Valency study of vanadium in stone coal ash of Jiangxi province. J. China Univ. Geosci. 1990, 1, 122–130. (In Chinese) [Google Scholar]
- He, D.; Feng, Q.; Zhang, G.; Lu, Y. Mechanism of oxidizing roasting process of vanadium containing stone coal. Chin. J. Nonferr. Met. 2009, 19, 195–200. (In Chinese) [Google Scholar]
- Zhang, Y.M.; Bao, S.X.; Liu, T.; Chen, T.J.; Huang, J. The technology of extracting vanadium from stone coal in China: History, current status and future prospects. Hydrometallurgy 2011, 109, 116–124. [Google Scholar] [CrossRef]
- Lin, H.; Fan, B. Study on mechanism of phase transformation during roasting and extracting vanadium from Fangshankou stone coal. Chin. J. Rare Met. 2001, 25, 273–277. (In Chinese) [Google Scholar]
- Zhu, Y.; Zhang, G.; Feng, Q.; Lu, Y.; Ou, L.; Guang, S. Acid leaching of vanadium from roasted residue of stone coal. Trans. Nonferr. Met. Soc. China 2010, 20, s107–s111. [Google Scholar] [CrossRef]
- Jin, X.; Yang, C.; Zeng, G.; He, H.; Li, C.; Luo, Z.; Luo, S. Vanadium extraction technology from stone coal by oxidizing roasting-alkaline leaching method. Chin. J. Nonferr. Met. 2014, 24, 3177–3184. [Google Scholar]
- Li, M.; Wei, C.; Fan, G.; Li, C.; Deng, Z.; Li, X. Extraction of vanadium from black shale using pressure acid leaching. Hydrometallurgy 2009, 98, 308–313. [Google Scholar] [CrossRef]
- Dai, W.; Sun, S. Research on new process of vanadium extraction from stone coal by wet leaching. Hunan Nonferr. Met. 2009, 25, 22–25. (In Chinese) [Google Scholar]
- Deng, Z.; Wei, C.; Fan, G.; Li, M.; Li, C.; Li, X. Extracting vanadium from stone-coal by oxygen pressure acid leaching and solvent extraction. Trans. Nonferr. Met. Soc. China 2010, 20, s118–s122. [Google Scholar] [CrossRef]
- Li, C.; Wei, C.; Deng, Z.; Li, M.; Li, X.; Fan, G. Recovery of vanadium from black shale. Trans. Nonferr. Met. Soc. China 2010, 20, s127–s131. [Google Scholar] [CrossRef]
- Chen, X.; Lan, X.; Zhang, Q.; Ma, H.; Zhou, J. Leaching vanadium by high concentration sulfuric acid from stone coal. Trans. Nonferr. Met. Soc. China 2010, 20, s123–s126. [Google Scholar] [CrossRef]
- Li, M.; Wei, C.; Qiu, S.; Zhou, X.; Li, C.; Deng, Z. Kinetics of vanadium dissolution from black shale in pressure acid leaching. Hydrometallurgy 2010, 104, 193–200. [Google Scholar] [CrossRef]
- Li, M.; Wei, C.; Zhou, X.; Qiu, S.; Deng, Z.; Li, X. Kinetics of vanadium leaching from black shale in non-oxidative conditions. Miner. Process. Extr. Metall. Trans. Inst. Min. Metall. C 2012, 121, 40–47. (In Chinese) [Google Scholar] [CrossRef]
- Zhou, X.; Li, C.; Li, J.; Liu, H.; We, S. Leaching of vanadium from carbonaceous shale. Hydrometallurgy 2009, 99, 97–99. [Google Scholar] [CrossRef]
- Li, M.; Wei, C.; Fan, G.; Wu, H.; Li, C.; Li, X. Acid leaching of black shale for the extraction of vanadium. Int. J. Miner. Process. 2010, 95, 62–67. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Yang, K.; Tian, X.D.; Qin, W.Q. Vanadium leaching from carbonaceous shale using fluosilicic acid. Int. J. Miner. Process. 2011, 100, 184–187. [Google Scholar] [CrossRef]
- Zeng, L.; Li, Q.; Xiao, L.; Zhang, Q. A study of the vanadium species in an acid leach solution of stone coal using ion exchange resin. Hydrometallurgy 2010, 105, 176–178. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Y.; Liu, T.; Huang, J.; Wang, Y. Comparison of ion exchange and solvent extraction in recovering vanadium from sulfuric acid leach solutions of stone coal. Hydrometallurgy 2013, 131–132, 1–7. [Google Scholar] [CrossRef]
- Tavakoli, M.R.; Dreisinger, D.B. Separation of vanadium from iron by solvent extraction using acidic and neutral organophosporus extractants. Hydrometallurgy 2014, 141, 17–23. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, G.; Wang, X.; Zhang, J. Solvent extraction of vanadium from sulfuric acid solution. Rare Met. 2009, 28, 209–211. (In Chinese) [Google Scholar] [CrossRef]
- Li, X.; Deng, Z.; Wei, C.; Li, C.; Li, M.; Fan, G.; Huang, H. Solvent extraction of vanadium from a stone coal acidic leach solution using D2EHPA/TBP: Continuous testing. Hydrometallurgy 2015, 154, 40–46. [Google Scholar] [CrossRef]
- Rakib, M.; Durand, G. Study of complex formation of vanadium (V) with sulphate ions using a solvent extraction method. Hydrometallurgy 1996, 43, 355–366. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, C.; Li, P.; Li, S. A new process of extracting vanadium from stone coal. Int. J. Miner. Metall. Mater. 2010, 17, 381–388. [Google Scholar] [CrossRef]
- Li, X.; Wei, C.; Deng, Z.; Li, M.; Li, C.; Fan, G. Selective solvent extraction of vanadium over iron from a stone coal/black shale acid leach solution by D2EHPA/TBP. Hydrometallurgy 2011, 105, 359–363. [Google Scholar] [CrossRef]
- GB/T 8704.5–2007; Ferrovanadium-Determination of Vanadium Content—The Ammonium Ferrous Sulfate Titrimetric Method and the Potentiometric Titrimetric Method, China; the People’s Republic of China ministry and Information Technology: Beijing, China, 2007.
- YB/T 5304–2011; Vanadium Peroxide; the People’s Republic of China ministry and Information Technology: Beijing, China, 2011.
- Cheng, C.Y. Purification of synthetic laterite leach solution by solvent extraction using D2EHPA. Hydrometallurgy 2000, 56, 369–386. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).