Phase Evolution of the AlxNbTiVZr (x = 0; 0.5; 1; 1.5) High Entropy Alloys
Abstract
:1. Introduction
2. Materials and Methods
3. Results
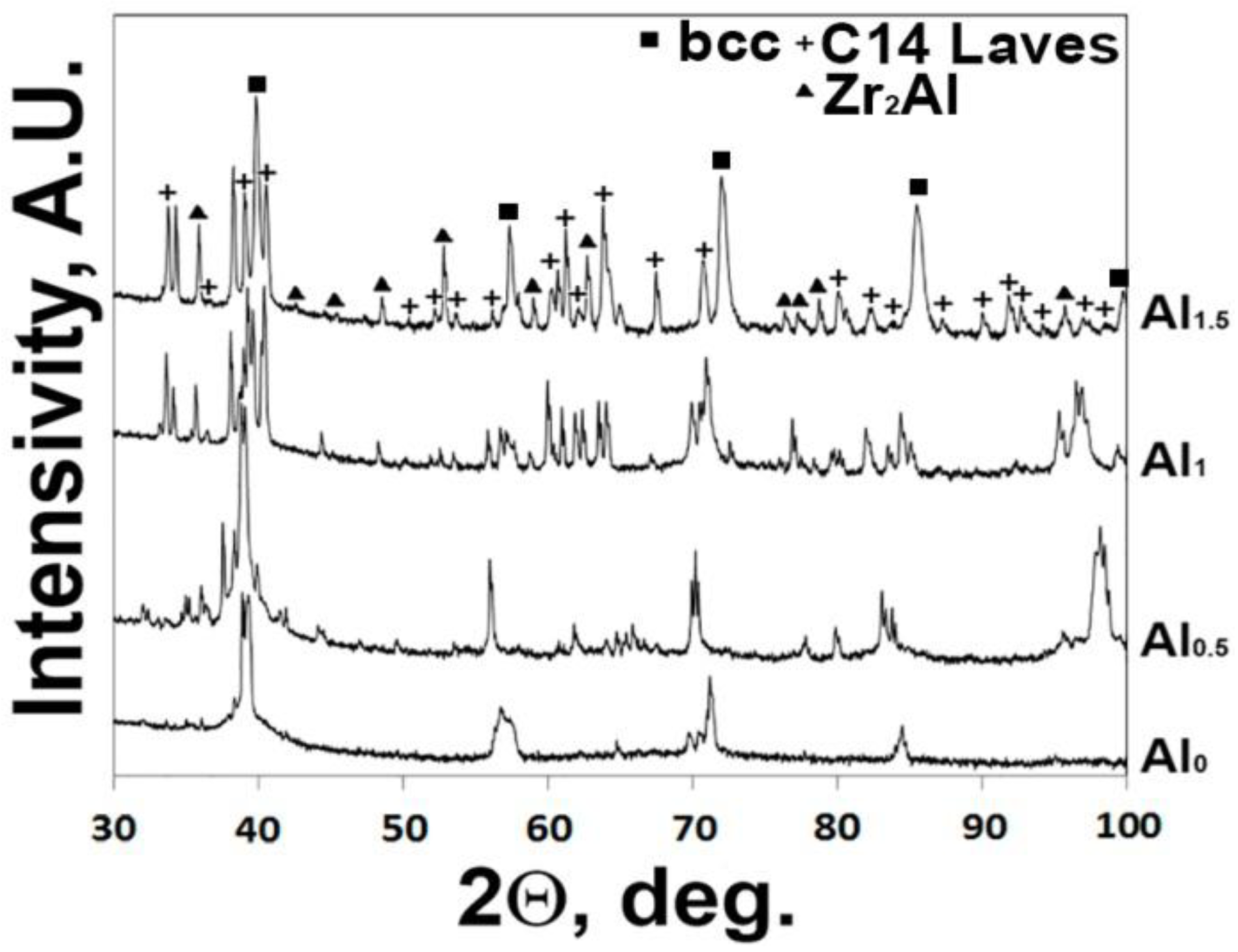
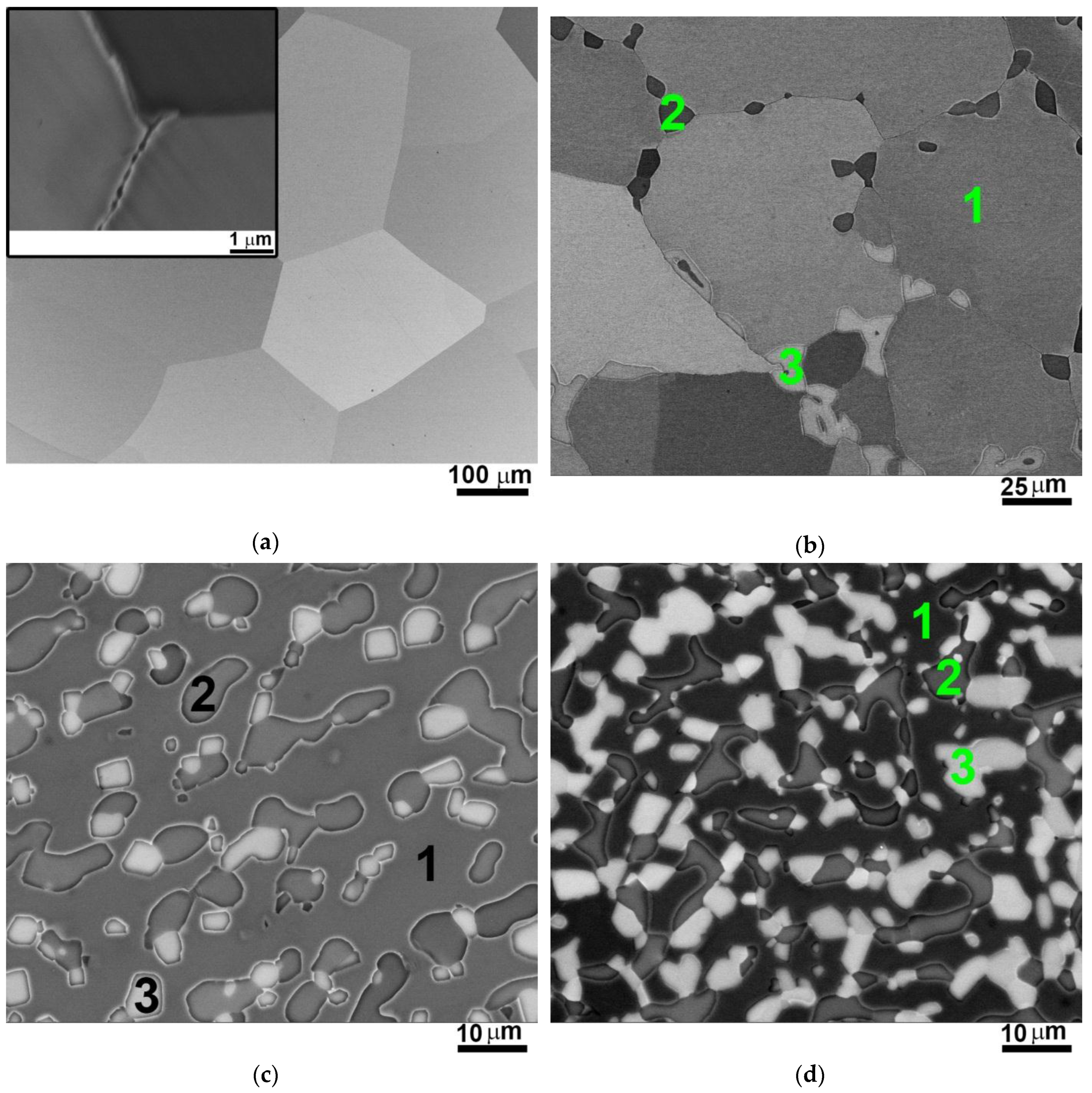
3.1. Structure of the AlxNbTiVZr (x = 0; 0.5; 1; 1.5) Alloys after Annealing at 1200 °C
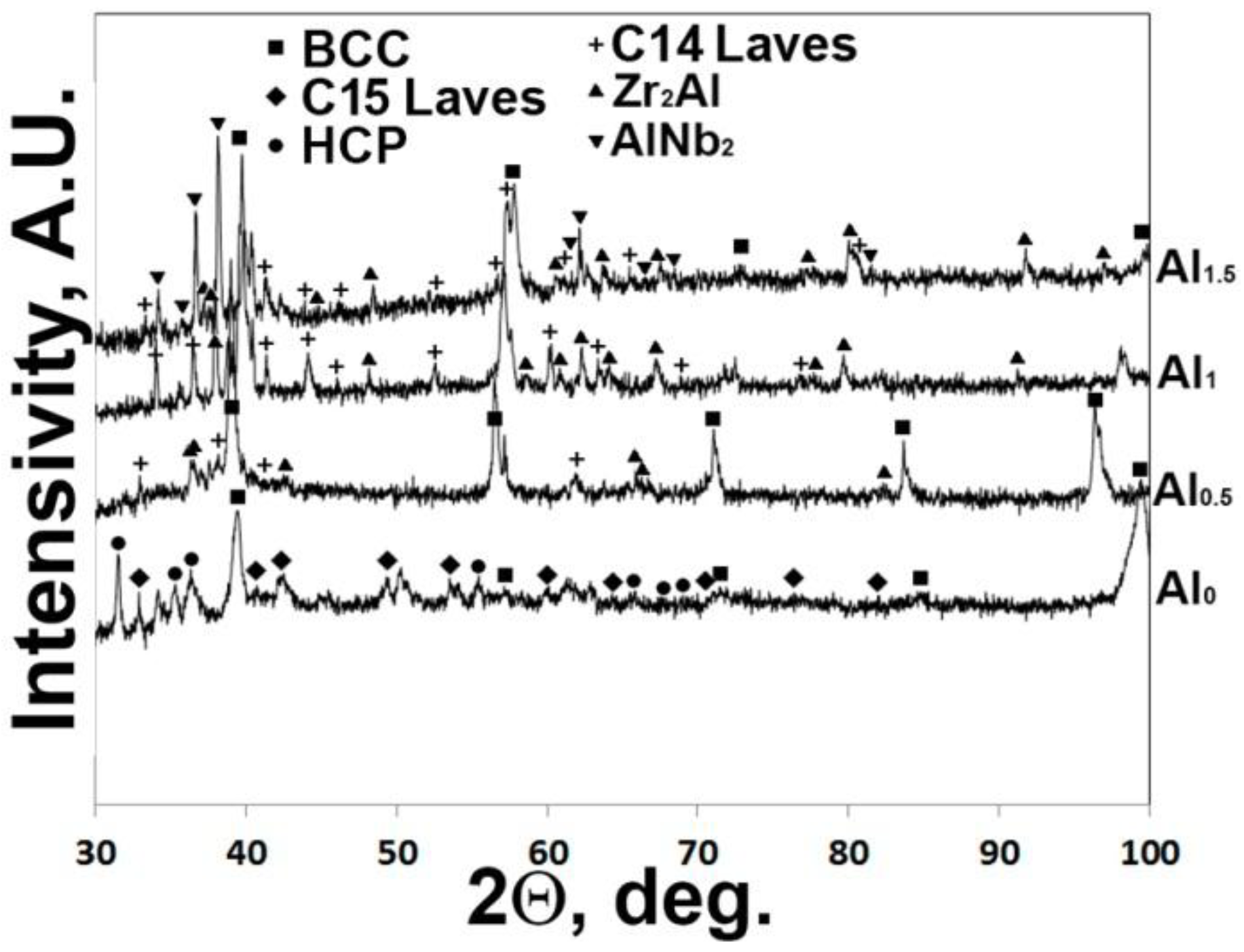
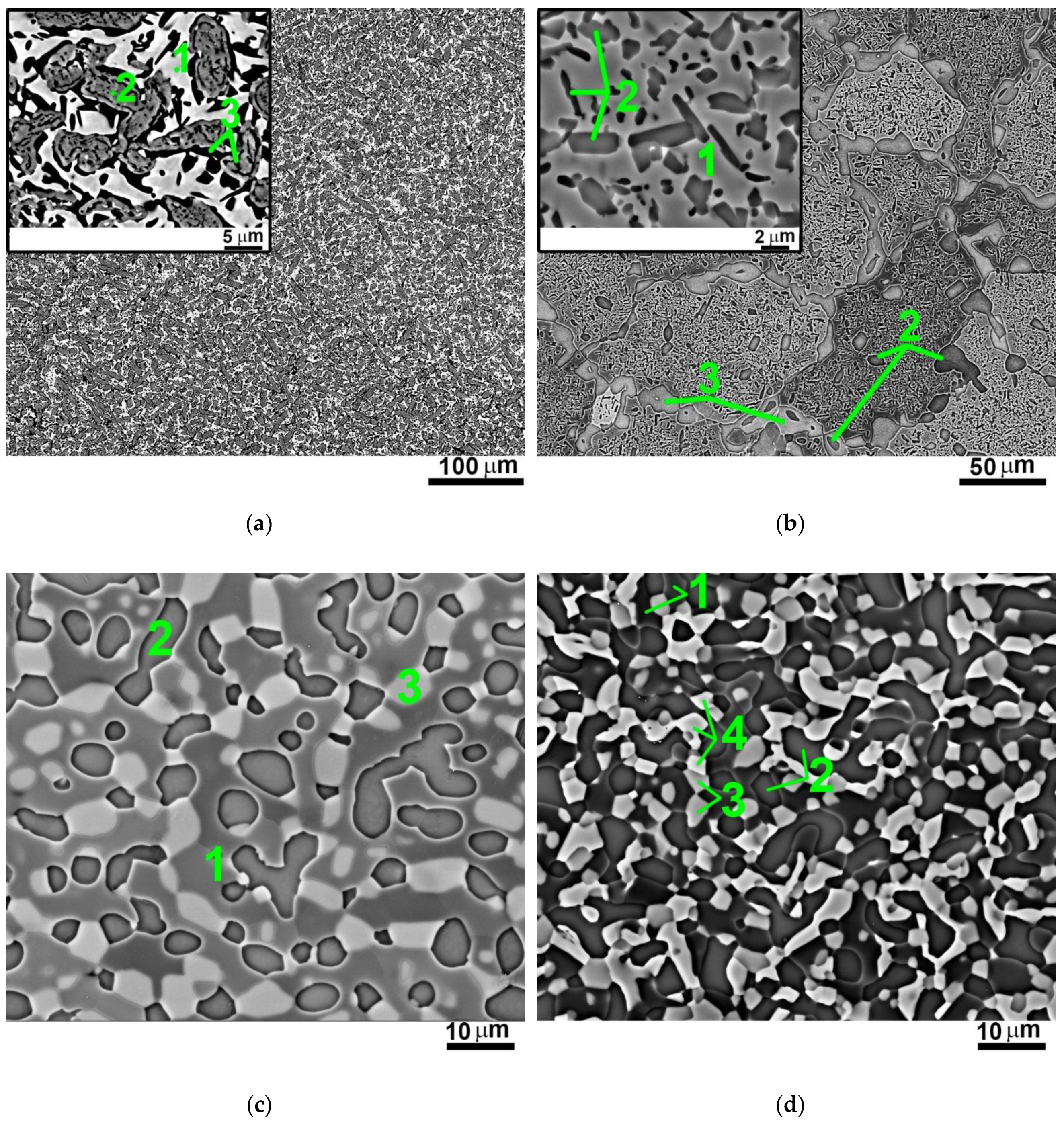
3.2. Effect of Annealing at 1000 °C on Structure of the AlxNbTiVZr (x = 0; 0.5; 1; 1.5) Alloys
3.3. Effect of Annealing at 800 °C on Structure of the AlxNbTiVZr (x = 0; 0.5; 1; 1.5) Alloys
4. Discussion
5. Conclusions
- (1)
- The structure of the AlxNbTiVZr (x = 0; 0.5; 1; 1.5) alloys after annealing at 1200 °C strongly depends on Al content. The NbTiVZr alloy has single bcc phase structure, while in the Al-containing alloys, C14 Laves and Zr2Al-type phases are found. The aggregate volume fraction of second phases increases with increase of Al content from 17% of the Al0.5NbTiVZr alloy to 46% of the Al1.5NbTiVZr alloy. The C14 Laves and Zr2Al-type phases are mostly composed of Zr and Al.
- (2)
- Annealing at lower temperatures of 800 °C and 1000 °C results in formation of additional second phases in the AlxNbTiVZr alloys. The amount and nature of second phases almost does not depend on annealing temperature (800 °C or 1000 °C) but strongly depends on the Al content in the alloy:
- in the NbTiVZr alloy, additional Zr-rich hcp and C15 Laves phases appear;
- in the Al0.5NbTiVZr alloy, the amount of C14 Laves phase increases substantially;
- in the AlNbTiVZr alloy, the amount of Zr2Al-type phase increases; and
- in the Al1.5NbTiVZr alloy, additional AlNb2-type phase appears.
- (3)
- The highest amount of second phases appeared after annealing at 800 °C and 1000 °C is found in the NbTiVZr alloy (54%), and the lowest amount (7.6% to 17.6%) is found in the AlNbTiVZr and Al1.5NbTiVZr alloys.
- (4)
- The analysis has revealed that the formation of second (intermetallic) phases in the AlxNbTiVZr (x = 0; 0.5; 1; 1.5) alloys after annealing at 1200 °C can be associated with strong chemical affinity and highly negative mixing enthalpy in Al-Zr atomic pair. At lower temperatures of 800 °C and 1000 °C, formation of second phases is mostly associated with decrease of Zr solubility in the bcc solid solution phase.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Zhang, Y.; Wang, Y.L.; Chen, G.L. Solid solution alloys of AlCoCrFeNiTix with excellent room-temperature mechanical properties. Appl. Phys. Lett. 2007, 90, 181904. [Google Scholar] [CrossRef]
- Youssef, K.M.; Zaddach, A.J.; Niu, C.; Irving, D.L.; Koch, C.C. A novel low-density, high-hardness, high-entropy alloy with close-packed single-phase nanocrystalline structures. Mater. Res. Lett. 2015, 3, 95–99. [Google Scholar] [CrossRef]
- He, J.Y.; Wang, H.; Huang, H.L.; Xu, X.D.; Chen, M.W.; Wu, Y.; Liu, X.J.; Nieh, T.G.; An, K.; Lu, Z.P. A precipitation-hardened high-entropy alloy with outstanding tensile properties. Acta Mater. 2016, 102, 187–196. [Google Scholar] [CrossRef]
- Gludovatz, B.; Hohenwarter, A.; Catoor, D.; Chang, E.H.; George, E.P.; Ritchie, R.O. A fracture-resistant high-entropy alloy for cryogenic applications. Science 2014, 345, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Gludovatz, B.; Hohenwarter, A.; Thurston, K.V.; Bei, H.; Wu, Z.; George, E.P.; Ritchie, R.O. Exceptional damage-tolerance of a medium-entropy alloy CrCoNi at cryogenic temperatures. Nat. Commun. 2016, 7, 10602. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, Y. The ultrahigh charpy impact toughness of forged AlxCoCrFeNi high entropy alloys at room and cryogenic temperatures. Intermetallics 2016, 70, 24–28. [Google Scholar] [CrossRef]
- Chuang, M.H.; Tsai, M.H.; Wang, W.R.; Lin, S.J.; Yeh, J.W. Microstructure and wear behavior of AlxCo1.5CrFeNi1.5Tiy high-entropy alloys. Acta Mater. 2011, 59, 6308–6317. [Google Scholar] [CrossRef]
- Miracle, D.B.; Miller, J.D.; Senkov, O.N.; Woodward, C.; Uchic, M.D.; Tiley, J. Exploration and development of high entropy alloys for structural applications. Entropy 2014, 16, 494–525. [Google Scholar] [CrossRef]
- Daoud, H.M.; Manzoni, A.M.; Wanderka, N.; Glatzel, U. High-Temperature Tensile Strength of Al10Co25Cr8Fe15Ni36Ti6 Compositionally Complex Alloy (High-Entropy Alloy). JOM 2015, 67, 2271–2277. [Google Scholar] [CrossRef]
- Senkov, O.N.; Senkova, S.V.; Miracle, D.B.; Woodward, C. Mechanical properties of low-density, refractory multi-principal element alloys of the Cr–Nb–Ti–V–Zr system. Mater. Sci. Eng. A 2013, 565, 51–62. [Google Scholar] [CrossRef]
- Senkov, O.N.; Woodward, C.; Miracle, D.B. Microstructure and properties of aluminum-containing refractory high-entropy alloys. JOM 2014, 66, 2030–2042. [Google Scholar] [CrossRef]
- Stepanov, N.D.; Yurchenko, N.Y.; Skibin, D.V.; Tikhonovsky, M.A.; Salishchev, G.A. Structure and mechanical properties of the AlCrxNbTiV (x = 0, 0.5, 1, 1.5) high entropy alloys. J. Alloy. Compd. 2015, 652, 266–280. [Google Scholar] [CrossRef]
- Stepanov, N.D.; Yurchenko, N.Y.; Sokolovsky, V.S.; Tikhonovsky, M.A.; Salishchev, G.A. An AlNbTiVZr0.5 high-entropy alloy combining high specific strength and good ductility. Mater. Lett. 2015, 16, 136–139. [Google Scholar] [CrossRef]
- Senkov, O.N.; Wilks, G.B.; Miracle, D.B.; Chuang, C.P.; Liaw, P.K. Refractory high-entropy alloys. Intermetallics 2010, 18, 1758–1765. [Google Scholar] [CrossRef]
- Senkov, O.N.; Wilks, G.B.; Scott, J.M.; Miracle, D.B. Mechanical properties of Nb25Mo25Ta25W25 and V20Nb20Mo20Ta20W20 refractory high entropy alloys. Intermetallics 2011, 19, 698–706. [Google Scholar] [CrossRef]
- Senkov, O.N.; Scott, J.M.; Senkova, S.V.; Miracle, D.B.; Woodward, C.F. Microstructure and room temperature properties of a high-entropy TaNbHfZrTi alloy. J. Alloy. Compd. 2011, 509, 6043–6048. [Google Scholar] [CrossRef]
- Senkov, O.N.; Scott, J.M.; Senkova, S.V.; Meisenkothen, F.; Miracle, D.B.; Woodward, C.F. Microstructure and elevated temperature properties of a refractory TaNbHfZrTi alloy. J. Mater. Sci. 2012, 47, 4062–4074. [Google Scholar] [CrossRef]
- Senkov, O.N.; Woodward, C.F. Microstructure and properties of a refractory CrNbMo0.5Ta0.5TiZr alloy. Mater. Sci. Eng. A 2011, 529, 311–320. [Google Scholar] [CrossRef]
- Senkov, O.N.; Senkova, S.V.; Woodward, C.; Miracle, D.B. Low-density, refractory multi-principal element alloys of the Cr–Nb–Ti–V–Zr system: Microstructure and phase analysis. Acta Mater. 2013, 61, 1545–1557. [Google Scholar] [CrossRef]
- Wu, Y.D.; Cai, Y.H.; Chen, X.H.; Wang, T.; Si, J.J.; Wang, L.; Wang, Y.D.; Hui, X.D. Phase composition and solid solution strengthening effect in TiZrNbMoV high-entropy alloys. Mater. Des. 2015, 83, 651–660. [Google Scholar] [CrossRef]
- Guo, N.N.; Wang, L.; Luo, L.S.; Li, X.Z.; Su, Y.Q.; Guo, J.J.; Fu, H.Z. Microstructure and mechanical properties of refractory MoNbHfZrTi high-entropy alloy. Mater. Des. 2015, 81, 87–94. [Google Scholar] [CrossRef]
- Czerwinski, F.; Jochym, P.T.; Litynska-Dobrzynska, L. Microstructure and mechanical properties of the novel Hf25Sc25Ti25Zr25 equiatomic alloy with hexagonal solid solutions. Mater. Des. 2016, 92, 8–17. [Google Scholar] [CrossRef]
- Senkov, O.N.; Senkova, S.V.; Woodward, C. Effect of aluminum on the microstructure and properties of two refractory high-entropy alloys. Acta Mater. 2014, 68, 214–228. [Google Scholar] [CrossRef]
- Stepanov, N.D.; Shaysultanov, D.G.; Salishchev, G.A.; Tikhonovsky, M.A. Structure and mechanical properties of a light-weight AlNbTiV high entropy alloy. Mater. Lett. 2015, 142, 153–155. [Google Scholar] [CrossRef]
- Stepanov, N.D.; Yurchenko, N.Y.; Shaysultanov, D.G.; Salishchev, G.A.; Tikhonovsky, M.A. Effect of Al on structure and mechanical properties of AlxNbTiVZr (x = 0, 0.5, 1, 1.5) high entropy alloys. Mater. Sci. Technol. 2015, 31, 1184–1193. [Google Scholar] [CrossRef]
- Lin, C.M.; Juan, C.C.; Chang, C.H.; Tsai, C.W.; Yeh, J.W. Effect of Al addition on mechanical properties and microstructure of refractory AlxHfNbTaTiZr alloys. J. Alloy. Compd. 2015, 624, 100–107. [Google Scholar] [CrossRef]
- Poletti, M.G.; Fiore, G.; Szost, B.A.; Battezzati, L. Search for high entropy alloys in the X-NbTaTiZr systems (X = Al, Cr, V, Sn). J. Alloy. Compd. 2015, 620, 283–288. [Google Scholar] [CrossRef]
- Poletti, M.G.; Branz, S.; Fiore, G.; Szost, B.A.; Crichton, W.A.; Battezzati, L. Equilibrium high entropy phases in X-NbTaTiZr (X = Al, V, Cr and Sn) multiprincipal component alloys. J. Alloy. Compd. 2016, 655, 138–146. [Google Scholar] [CrossRef]
- Gorr, B.; Azim, M.; Christ, H.J.; Mueller, T.; Schliephake, D.; Heilmaier, M. Phase equilibria, microstructure, and high temperature oxidation resistance of novel refractory high-entropy alloys. J. Alloy. Compd. 2015, 624, 270–278. [Google Scholar] [CrossRef]
- Yurchenko, N.Y.; Stepanov, N.D.; Shaysultanov, D.G.; Tikhonovsky, M.A.; Salishchev, G.A. Effect of Al content on structure and mechanical properties of the AlxCrNbTiVZr (x = 0; 0.25; 0.5; 1) high-entropy alloys. Mater. Charact. 2016, 121, 125–134. [Google Scholar] [CrossRef]
- Chen, H.; Kauffmann, A.; Gorr, B.; Schliephake, D.; Seemüller, C.; Wagner, J.N.; Christ, H.J.; Heilmaier, M. Microstructure and mechanical properties at elevated temperatures of a new Al-containing refractory high-entropy alloy Nb-Mo-Cr-Ti-Al. J. Alloy. Compd. 2016, 661, 206–215. [Google Scholar] [CrossRef]
- Gorr, B.; Azim, M.; Christ, H.J.; Chen, H.; Szabo, D.V.; Kauffmann, A.; Heilmaier, M. Microstructure Evolution in a New Refractory High-Entropy Alloy W-Mo-Cr-Ti-Al. Met. Mater. Trans. A 2016, 47, 961–970. [Google Scholar] [CrossRef]
- Fazakas, E.; Zadorozhnyy, V.; Varga, L.K.; Inoue, A.; Louzguine-Luzgin, D.V.; Tian, F.; Vitos, L. Experimental and theoretical study of Ti20Zr20Hf20Nb20X20 (X = V or Cr) refractory high-entropy alloys. Int. J. Refract. Met. H 2014, 47, 131–138. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.J.; Lin, J.P.; Chen, G.L.; Liaw, P.K. Solid-Solution Phase Formation Rules for Multi-component Alloys. Adv. Eng. Mater. 2008, 10, 534–538. [Google Scholar] [CrossRef]
- Guo, S.; Ng, C.; Lu, J.; Liu, C.T. Effect of valence electron concentration on stability of fcc or bcc phase in high entropy alloys. J. Appl. Phys. 2011, 109, 103505. [Google Scholar] [CrossRef]
- Guo, S.; Liu, C.T. Phase stability in high entropy alloys: Formation of solid-solution phase or amorphous phase. Prog. Nat. Sci. Mater. Int. 2011, 21, 433–446. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y. Prediction of high-entropy stabilized solid-solution in multi-component alloys. Mater. Chem. Phys. 2012, 132, 233–238. [Google Scholar] [CrossRef]
- Tsai, M.H.; Tsai, K.Y.; Tsai, C.W.; Lee, C.; Juan, C.C.; Yeh, J.W. Criterion for Sigma Phase Formation in Cr- and V-Containing High-Entropy Alloys. Mater. Res. Lett. 2013, 1, 207–212. [Google Scholar] [CrossRef]
- Poletti, M.G.; Battezzati, L. Electronic and thermodynamic criteria for the occurrence of high entropy alloys in metallic systems. Acta Mater. 2014, 74, 297–306. [Google Scholar] [CrossRef]
- Dong, Y.; Lu, Y.; Jiang, L.; Wang, T.; Li, T. Effects of electro-negativity on the stability of topologically close-packed phase in high entropy alloys. Intermetallics 2014, 52, 105–109. [Google Scholar] [CrossRef]
- Yurchenko, N.; Stepanov, N.; Salishchev, G. Laves-phase formation criterion for high-entropy alloys. Mater. Sci. Technol. 2016, 1–6. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, S.; Liu, C.T.; Yang, X. Phase formation rules. In High Entropy Alloys: Fundamentals and Applications; Gao, M.C., Yeh, J.W., Liaw, P.K., Zhang, Y., Eds.; Springer International Publishing: Basel, Switzerland, 2016; pp. 21–49. [Google Scholar]
- Senkov, O.N.; Miracle, D.B. A New Thermodynamic Parameter to Predict Formation of Solid Solution or Intermetallic Phases in High Entropy Alloys. J. Alloy. Compd. 2016, 658, 603–607. [Google Scholar] [CrossRef]
- Troparevsky, M.C.; Morris, J.R.; Kent, P.R.C.; Lupini, A.R.; Stocks, G.M. Criteria for Predicting the Formation of Single-Phase High-Entropy Alloys. Phys. Rev. X 2015, 5, 011041. [Google Scholar] [CrossRef]
- Takeuchi, A.; Inoue, A. Classification of Bulk Metallic Glasses by Atomic Size Difference, Heat of Mixing and Period of Constituent Elements and Its Application to Characterization of the Main Alloying Element. Mater. Trans. JIM 2005, 46, 2817–2829. [Google Scholar] [CrossRef]
- Yurchenko, N.Y.; Stepanov, N.D.; Shaysultanov, D.G.; Tikhonovsky, M.A.; Salishchev, G.A. Elevated temperature mechanical properties of the AlxNbTiVZr (x = 0; 0.5; 1; 1.5) high entropy alloys. Manuscript in preparation.




| Element, at. % | Al | Nb | Ti | V | Zr | Volume Fraction, % | Average Size, μm | ||
|---|---|---|---|---|---|---|---|---|---|
| Transversal | Longitudinal | ||||||||
| Constituents | NbTiVZr | ||||||||
| No. | Designation | ||||||||
| 1 | Grains | - | 25.6 | 25.5 | 24.9 | 24.0 | 100 | 240 ± 100 | |
| Alloy composition | - | 25.6 | 24.4 | 24.9 | 25.1 | - | - | ||
| Al0.5NbTiVZr | |||||||||
| 1 | Matrix | 9.5 | 25.8 | 24.7 | 20.8 | 19.2 | 83 ± 3 | 50 ± 25 | |
| 2 | Dark-grey particles | 19.5 | 11.6 | 8.6 | 28.3 | 32.0 | 14 ± 2 | 7.9 ± 4.2 | |
| 3 | Light-grey particles | 17.3 | 9.1 | 12.5 | 20.0 | 41.1 | 3 ± 1 | 8.2 ± 3.0 | |
| Alloy composition | 11.4 | 21.6 | 21.9 | 22.3 | 22.8 | - | - | ||
| AlNbTiVZr | |||||||||
| 1 | Matrix | 16.5 | 25.8 | 24.7 | 18.5 | 14.5 | 67 ± 2 | - | |
| 2 | Dark-grey particles | 28.9 | 9.9 | 8.6 | 22.3 | 30.3 | 21 ± 3 | 5.3 ± 2.1 | 14.9 ± 7.3 |
| 3 | Light-grey particles | 38.7 | 9.5 | 8.3 | 3.6 | 39.9 | 12 ± 2 | 4.5 ± 1.3 | 8.1 ± 3.1 |
| Alloy composition | 21.9 | 20.0 | 19.8 | 20.3 | 18.0 | - | - | ||
| Al1.5NbTiVZr | |||||||||
| 1 | Dark phase | 24.5 | 24.9 | 24.7 | 17.6 | 8.3 | 54 ± 3 | - | |
| 2 | Dark-grey phase | 36.2 | 7.9 | 7.6 | 19.9 | 28.4 | 16 ± 2 | 3.3 ± 1.1 | 8.1 ± 3.2 |
| 3 | Light-grey phase | 39.4 | 11.2 | 9 | 4.1 | 36.3 | 30 ± 2 | 3.8 ± 1.3 | |
| Alloy composition | 29.5 | 18.0 | 18.2 | 16.9 | 17.4 | - | - | ||
| Element, at. % | Al | Nb | Ti | V | Zr | Volume Fraction, % | Average Size, μm | ||
|---|---|---|---|---|---|---|---|---|---|
| Transversal | Longitudinal | ||||||||
| Constituents | NbTiVZr | ||||||||
| No. | Designation | ||||||||
| 1 | Light matrix | - | 45.5 | 5.8 | 46.0 | 2.7 | 46 ± 3 | 2.9 ± 1.5 | - |
| 2 | Grey phase | - | 7.5 | 10.4 | 2.2 | 79.9 | 35 ± 2 | 3.5 ± 1.6 | |
| 3 | Black phase | - | 9.2 | 77.2 | 8.8 | 4.8 | 19 ± 2 | 0.9 ± 0.3 | 3.2 ± 1.9 |
| Alloy composition | - | 25.6 | 24.4 | 24.9 | 25.1 | - | - | ||
| Al0.5NbTiVZr | |||||||||
| 1 | Matrix | 6.1 | 30.6 | 28.8 | 21.5 | 13.0 | 48 ± 3 | - | |
| 2 | Dark-grey particles | 16.8 | 14.1 | 10.8 | 28.9 | 29.4 | 39 ± 3 | 7.7 ± 4.5 (coarse); 1.8 ± 0.8 (fine) | |
| 3 | Light-grey particles | 17.7 | 12.4 | 16.6 | 16.1 | 37.2 | 13 ± 2 | 8.2 ± 3.0 | |
| Alloy composition | 11.4 | 21.6 | 21.9 | 22.3 | 22.8 | - | - | ||
| AlNbTiVZr | |||||||||
| 1 | Matrix | 12.7 | 27.8 | 28.8 | 24.8 | 5.9 | 52 ± 2 | - | |
| 2 | Dark-grey particles | 26.0 | 10.8 | 9.9 | 25.3 | 28.0 | 26 ± 3 | 5.0 ± 1.9 | 12.9 ± 6.3 |
| 3 | Light-grey particles | 35.4 | 12.7 | 10.8 | 3.9 | 37.2 | 22 ± 2 | 4.5 ± 1.3 | 8.1 ± 3.1 |
| Alloy composition | 21.9 | 20.0 | 19.8 | 20.3 | 18.0 | - | - | ||
| Al1.5NbTiVZr | |||||||||
| 1 | Dark phase | 23.6 | 19.8 | 25.2 | 16.3 | 5.1 | 36 ± 2 | - | |
| 2 | Dark-grey phase | 34.8 | 8.5 | 8.8 | 22.9 | 25.0 | 28 ± 3 | 2.9 ± 0.7 | 6.1 ± 2.8 |
| 3 | Light-grey phase | 39.3 | 13.1 | 9.7 | 4.3 | 33.6 | 16 ± 2 | 2.3±0.9 | |
| 4 | White phase | 31.8 | 30.2 | 16.8 | 9.6 | 11.6 | 20 ± 2 | 1.9 ± 0.8 | 4.5 ± 2.5 |
| Alloy composition | 29.5 | 18.0 | 18.2 | 16.9 | 17.4 | - | - | ||
| Element, at. % | Al | Nb | Ti | V | Zr | Volume Fraction, % | Average Size, μm | ||
|---|---|---|---|---|---|---|---|---|---|
| Transversal | Longitudinal | ||||||||
| Constituents | NbTiVZr | ||||||||
| No. | Designation | ||||||||
| 1 | Light matrix | - | 33.0 | 29.5 | 35.0 | 2.5 | 46 ± 3 | 2.8 ± 1.9 | >100 |
| 2 | Grey phase | - | 9.3 | 7.5 | 12.3 | 70.9 | 37 ± 3 | 2.9 ± 1.7 | >100 |
| 3 | Black phase | - | 5.3 | 56.4 | 2.6 | 35.7 | 17 ± 2 | 1.0 ± 0.4 | 2.3 ± 1.2 |
| Alloy composition | - | 25.6 | 24.4 | 24.9 | 25.1 | - | - | ||
| Al0.5NbTiVZr | |||||||||
| 1 | Decomposed matrix | 9.6 | 24.4 | 25.6 | 20.7 | 19.7 | 87 ± 3 1 | - | |
| 2 | Grey particles | 16.3 | 13.3 | 11.8 | 27.8 | 30.8 | 13 ± 1 | 6.8 ± 3.5 | |
| Alloy composition | 11.4 | 21.6 | 21.9 | 22.3 | 22.8 | - | - | ||
| AlNbTiVZr | |||||||||
| 1 | Matrix | 16.4 | 27.0 | 24.0 | 27.6 | 5.0 | 54 ± 3 | - | |
| 2 | Dark-grey particles | 30.6 | 9.3 | 7.6 | 24.3 | 28.2 | 16 ± 2 | 3.3 ± 1.0 | 7.3 ± 3.2 |
| 3 | Light-grey particles | 36.7 | 13.4 | 9.3 | 4.5 | 36.1 | 30 ± 3 | 3.3 ± 1.2 | 5.7 ± 3.1 |
| Alloy composition | 21.9 | 20.0 | 19.8 | 20.3 | 18.0 | - | - | ||
| Al1.5NbTiVZr | |||||||||
| 1 | Matrix | 24.9 | 24.3 | 26.0 | 17.3 | 7.5 | 46 ± 2 | - | |
| 2 | Dark-grey particles | 34.8 | 9.6 | 8.0 | 20.9 | 26.7 | 29 ± 3 | 3.4 ± 1.4 | 9.6 ± 5.2 |
| 3 | Light-grey particles | 38.1 | 13.6 | 11.1 | 5.3 | 31.9 | 18 ± 2 | 3.2 ± 1.5 | |
| 4 | White particles | 32.7 | 32.8 | 16.4 | 9.9 | 8.2 | 7 ± 1 | 1.2 ± 0.5 | 4.3 ± 2.5 |
| Alloy composition | 29.5 | 18.0 | 18.2 | 16.9 | 17.4 | - | - | ||
| Temperature, °C | Alloy | k1cr(T) | ΔHIM/ΔHmix | Predicted Phase Composition |
|---|---|---|---|---|
| 800 | NbTiVZr | 19.79 | −6.08 | SS |
| Al0.5NbTiVZr | 0.52 | 1.24 | SS + IM | |
| AlNbTiVZr | 0.33 | 1.31 | SS + IM | |
| Al1.5NbTiVZr | 0.27 | 1.33 | SS + IM | |
| 1000 | NbTiVZr | 23.48 | −6.08 | SS |
| Al0.5NbTiVZr | 0.62 | 1.24 | SS + IM | |
| AlNbTiVZr | 0.39 | 1.31 | SS + IM | |
| Al1.5NbTiVZr | 0.31 | 1.33 | SS + IM | |
| 1200 | NbTiVZr | 27.16 | −6.08 | SS |
| Al0.5NbTiVZr | 0.72 | 1.24 | SS + IM | |
| AlNbTiVZr | 0.45 | 1.31 | SS + IM | |
| Al1.5NbTiVZr | 0.36 | 1.33 | SS + IM |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yurchenko, N.Y.; Stepanov, N.D.; Tikhonovsky, M.A.; Salishchev, G.A. Phase Evolution of the AlxNbTiVZr (x = 0; 0.5; 1; 1.5) High Entropy Alloys. Metals 2016, 6, 298. https://doi.org/10.3390/met6120298
Yurchenko NY, Stepanov ND, Tikhonovsky MA, Salishchev GA. Phase Evolution of the AlxNbTiVZr (x = 0; 0.5; 1; 1.5) High Entropy Alloys. Metals. 2016; 6(12):298. https://doi.org/10.3390/met6120298
Chicago/Turabian StyleYurchenko, Nikita Y., Nikita D. Stepanov, Mikhail A. Tikhonovsky, and Gennady A. Salishchev. 2016. "Phase Evolution of the AlxNbTiVZr (x = 0; 0.5; 1; 1.5) High Entropy Alloys" Metals 6, no. 12: 298. https://doi.org/10.3390/met6120298
APA StyleYurchenko, N. Y., Stepanov, N. D., Tikhonovsky, M. A., & Salishchev, G. A. (2016). Phase Evolution of the AlxNbTiVZr (x = 0; 0.5; 1; 1.5) High Entropy Alloys. Metals, 6(12), 298. https://doi.org/10.3390/met6120298







